Abstract
Purpose/Background:
Prolactin-related side effects contribute to nonadherence and adverse health consequences, particularly in women with severe mental illness. Treating these side effects may improve treatment acceptability, adherence and long-term outcomes.
Methods/Procedures:
Premenopausal women with a DSM-IV-TR diagnosis of schizophrenia, schizoaffective disorder or bipolar disorder were recruited for a randomized double-blind placebo-controlled 16-week trial of adjunct aripiprazole (5 to 15 mg/day). Participants had elevated prolactin (> 24 ng/mL) and were experiencing galactorrhea, amenorrhea, oligomenorrhea or sexual dysfunction on a prolactin-elevating antipsychotic. Participants were evaluated bi-weekly for prolactin elevation and, galactorrhea and completed a menstrual diary review. Psychiatric symptoms and adverse effects were closely monitored.
Findings/Results:
Forty-six women were randomized (N=25 aripiprazole, N=21 placebo). Thirty-seven completed at least 8 weeks of the study (N= 20 (80%) aripiprazole and N=17 (81%) placebo). Aripiprazole (mean dose 11.7 ± 2.4 mg/day) was effective for lowering prolactin relative to placebo (p=0.04). Additionally, 45% (9/20) of the aripiprazole group had a normalized prolactin (<24 mg/mL) compared to 12% (2/17) of the placebo group (p=0.028). Galactorrhea resolved in 77% (10/13) of the aripiprazole-treated participants compared to 33% (4/12) in the placebo group (p=0.028). Normalization of sexual function (<16 on ASEX) occurred in 50% on aripiprazole (7/14) vs. 9% (1/11) on placebo (p=0.030). No differences between groups in symptoms or side effects were noted. Overall, women rated a mean score of 4.6 ± 0.6 on a 5-point Likert Scale for sexual function improvement, suggesting their particular satisfaction with improvement in this domain.
Implications/Conclusions:
Building upon prior studies, this rigorous evaluation confirms the utility of adjunctive aripiprazole as a strategy for improving prolactin and managing prolactin-related side effects in premenopausal women with psychosis.
Keywords: Aripiprazole, Women, Prolactin, Clinical Trial, Sexual dysfunction
Introduction
There are many sex differences in people with schizophrenia. For example, illness presentation is often 3–5 years later, women have fewer negative symptoms and more affective symptoms, and women have slower metabolism of some antipsychotic treatments as compared to men.1,2 Side effects are also known to be different in women and men with schizophrenia treated with antipsychotics.1 Women may be more distressed by weight gain, have higher rates of cardiovascular disease1,3 and have significantly higher rates of hyperprolactinemia.1,4,5
Hyperprolactinemia is associated with many symptoms and side effects, some of which can be serious. Various consequences include menstrual abnormalities, galactorrhea, and sexual dysfunction. More long-term risks include breast cancer, pituitary tumors, and decreased bone mineral density.6 A case-control study from the UK found that the use of a prolactin-elevating antipsychotics resulted in an increased odds of sustaining a hip fracture.7 In people experiencing prolactin-related side effects, nonadherence to antipsychotic treatment is common. And, in a nationwide survey of almost 900 people with schizophrenia taking antipsychotic medications, almost 60% reported nonadherence, with prolactin- and endocrine-related effects being one of the commonly cited reasons, decreasing the odds of adherence by about 30%.8 In addition, many of these symptoms are embarrassing or difficult for people to discuss with healthcare providers, and may go unaddressed for years.9 Furthermore, symptomatic hyperprolactinemia has been found to significantly increase the risk of suicide in people with schizophrenia.10
Hyperprolactinemia is relatively common during antipsychotic treatment in women. One study found that, among 194 people with schizophrenia or bipolar disorder receiving antipsychotics from a single community mental health facility in the UK, 38% had prolactin levels above the upper limit of normal, two thirds of whom had significantly elevated levels with potential clinical consequences. Women were particularly at risk for elevations and associated consequences, as over 50% of women in the study had abnormal prolactin levels.9 Women in general have higher levels than men, and women are often more prone to elevations with dopamine antagonist treatment.11 Nonetheless, many studies do not separate prolactin levels in men and women when reporting results. A review by Bostwick summarized that mean prolactin levels in women during 3–6 weeks of treatment with risperidone, paliperidone and first-generation antipsychotics was over 100 ng/ml, with normal ranges generally considered < 24 ng/mL.6
Antipsychotics with tight binding to D2 receptors are associated with the highest rates of hyperprolactinemia,12 though there are interpersonal variations as well. The degree of prolactin elevation may be a dose-related effect, at least for some antipsychotics.13 Risperidone and paliperidone are the biggest culprits among prolactin-elevating antipsychotics due to their low blood-brain barrier penetration, resulting in higher concentration, and subsequent effect, at the pituitary gland.14 Other antipsychotics may result in slight or transient elevations in prolactin, but including the higher potency first generation antipsychotics, such as haloperidol, but not to the extent to which is observed with risperidone and paliperidone. Low potency first generation agents and some of the newer second-generation antipsychotics are prolactin-sparing. Female patients and clinicians alike are eager for options to better manage these distressing and less acceptable side effects seen with some antipsychotics.
In contrast to the D2 antagonism of most other antipsychotics, aripiprazole is an antipsychotic with partial dopamine agonist activity at D2 receptors. Aripiprazole has been shown to act as an agonist in pituitary cells at the molecular level and thus, lactotroph cells may not become blocked by aripiprazole treatment.15 In addition, aripiprazole treatment has been associated with significant decreases in serum prolactin levels. In a double-blind, six-week, randomized trial of aripiprazole or perphenazine to treat symptoms for treatment-resistant schizophrenia by Kane and colleagues, mean prolactin levels decreased in the aripiprazole group from 33.4 ng/mL to 5.2 ng/mL, while prolactin levels in the perphenazine group remained unchanged at 35.8 ng/mL to 35.5 ng/mL.16 Another recent four-week double-blind randomized trial comparing the efficacy and safety of risperidone or aripiprazole in persons with schizophrenia or schizoaffective disorder reported that prolactin levels were significantly decreased in the aripiprazole group (−9.0 ng/mL) compared to a mean increase of 55.4 ng/mL in the risperidone group. In this study, the percentage of participants with schizophrenia who had an abnormal prolactin levels (>25 ng/mL) at endpoint was significantly higher in the risperidone group (93%) than in the aripiprazole group (5%).17 Our group has generated pilot data suggesting that up to 30 mg of aripiprazole resolves elevated prolactin in women with amenorrhea on haloperidol.5 Twenty-one of 24 (88%) women in another previous open-label study of adjunct aripiprazole given to women with risperidone-induced amenorrhea experienced recurrence of menstrual cycles between 8–16 weeks, as well as significantly decreased prolactin levels from a mean of 96.6 ng/mL at baseline to 28.1 ng/mL.18
The objective of this randomized double-blind placebo-controlled trial was to test the efficacy and tolerability of adjunct aripiprazole compared to placebo in premenopausal women with symptomatic hyperprolactinemia treated with antipsychotics on important and understudied outcomes such as galactorrhea, sexual dysfunction and amenorrhea. This study is referred to the DAAMSEL study, Dopamine Partial Agonist Aripiprazole for the Management of Symptomatic ELevated Prolactin.
Materials and Methods
Participants:
This study was conducted at the Maryland Psychiatric Research Center, University of Maryland School of Medicine in Baltimore, Maryland and at Georgia Regents University in Augusta, Georgia. It was conducted between 2011 and 2016. It was approved by Institutional Review Boards at the University of Maryland, Georgia Regents, Sheppard Pratt Health System and a reliance for the UMB IRB to review was granted from the State of Maryland Department of Health and Mental Hygiene IRB. The study was review annually by a Data Safety and Monitoring Board. The following inclusion and exclusion criteria were used:
Inclusion Criteria
Premenopausal women (ages 18 to 50 years) who met DSM-IV-TR criteria for schizophrenia, schizoaffective disorder, or bipolar disorder were eligible. Women were required to be taking a stable dose of an antipsychotic for at least two months and considered to have stable symptoms by the treating psychiatrist. Only those taking prolactin-elevating antipsychotics, including risperidone, paliperidone, or high potency first-generation antipsychotics (i.e., haloperidol, perphenazine, loxapine, or fluphenazine) were recruited. Those enrolled also were required to have a prolactin > 24 ng/mL and evidence of a prolactin-related hormonal side effect (amenorrhea, oligomenorrhea or galactorrhea), or significant sexual dysfunction (Arizona Sexual Experience Survey, ASEX >16). The presence of these side effects were determined by participant report/history and medical record/clinician interview. Oligomenorrhea was defined as infrequent, irregularly timed episodic bleeding occurring at intervals of more than 35 days from the previous menstrual cycle, and amenorrhea was defined as absence of menstruation for three menstrual cycles or six months.19 Galactorrhea was defined as lactation or copious milk secretion as evaluated by trained clinicians upon breast exam. Additionally, all women were required to score at least a 10/12 on the Evaluation to Sign Consent (ESC), which is used to document capacity to provide informed consent.
Exclusion criteria
Women were excluded if they were considered postmenopausal. Any potential participant > 45 years was assessed for menopausal symptoms, including but not limited to: hot flashes, depression, excitability and fatigue using the modified Menstrual Symptom Rating Scale (MRS). A medical doctor evaluated and assessed all participants and did not permit enrollment of those with apparent peri or post-menopausal symptoms. Other exclusion criteria included a history of a pituitary tumor (microadenoma, macroadenoma, neoplasm) or Cushing’s disease, pregnancy or current post-pregnancy lactation, DSM-IV-TR criteria for alcohol or substance abuse within the last month (excluding caffeine or nicotine) or medications which may affect prolactin or cause sexual dysfunction through dopaminergic effects (e.g. metoclopramide, methyldopa, reserpine, amoxapine, droperidol, prochlorperazine, promethazine, bromocriptine, and cabergoline). Medications that may affect sexual function unrelated to dopamine transmission were permitted only if receiving > 4 weeks (including, but not limited to, SSRIs, mood stabilizers, diuretics, antihypertensives, H2 antagonists, buproprion). MRIs were not completed for the evaluation of pituitary tumors, however consultation to endrocrinology was available for potential patients if they had very high prolactin levels (>300 n/mL) or were suspect for possible tumor.
Study Procedures
Participants were screened for eligibility and then randomized to 16 weeks of double-blind treatment with adjunct aripiprazole or placebo, while continuing their prior antipsychotic treatment.
Screening and Baseline
Screening involved one to two visits, and a variety of assessments to determine eligibility. Participants were educated about the study and possible medication side effects and completed the ESC prior to signing the informed consent. Psychiatric diagnosis was confirmed by the Structured Clinical Interview for Diagnosis (SCID).20 All participants received a physical exam and reviewed their medical history with a medically-accountable physician who assessed the participant for evidence of prolactin-related side effects, menopausal status, and study eligibility. Participants also completed a number of assessments including electrocardiograph (EKG), vital signs, pregnancy test, and blood work. Participants had a breast examination with a trained female clinician to determine if galactorrhea was present. Documented birth control was required during the study with new prescription oral contraceptive not permitted and only long-term OC use permitted.
All participants were seen every two weeks for study evaluations and phone contact occurred twice weekly to ensure completion of the menstrual diary. Salivary hormone assessments were completed weekly at home.
Study Assessments
Hormonal side effects
DAAMSEL is noteworthy for the rigor in clinical assessment of hormonal effects directly in relation to menstrual cycle. To measure hormonal side effects, specifically menstrual periods and galactorrhea, participants completed menstrual diaries throughout the study. Each woman was taught to document daily in her menstrual diary whether or not she experienced bleeding or pain. The diary was formatted as a monthly calendar that prompted participants to indicate presence and degree of menstrual flow (spotting = 1, light = 2, moderate = 3, heavy = 4). If galactorrhea was detected at baseline, the participant was instructed to also note daily lactation in the menstrual diary. Participants were reminded to fill out this diary via phone twice weekly. This diary was then reviewed with research staff at biweekly study appointments to verify correct use of the diary, and to track occurrence of menstrual bleeding. If the diary was found to be incomplete, staff would review a calendar with the participant and ask her to recall any menstrual bleeding (and lactation, if applicable) in the past two weeks. Staff then followed up with the participant over the phone more frequently to be sure the participant completed the diary prior to the next visit. Menstrual bleeding for 3–8 days followed by another 3–8 days of menstrual bleeding 21–35 days later constituted regaining a regular menstrual cycle.
Pilot data determined that a menstrual period should take 8–12 weeks to return after aripiprazole initiation. Thus, a 16-week trial was devised to allow time for two regular menstrual cycles. Participants experiencing galactorrhea also had breast exams performed by clinical staff at their biweekly appointments. An absence of any lactation or milk discharge for at least two consecutive breast exams was characterized as galactorrhea resolution. To control the effects of menstrual cycles on prolactin and hormone levels, menstruating participants were randomized to begin treatment on the 7th day after starting their menstrual period.
Sexual function
Sexual function was assessed using the Arizona Sexual Experience Scale (ASEX)21. The Female Sexual Distress Scale-Revised (FSDS-R)22 and Female Sexual Function Index (FSFI)23 were administered as secondary measures. The ASEX is a valid and reliable scale in people with schizophrenia or schizoaffective disorder consisting of five items rated 1–6 with higher scores indicating more sexual dysfunction. Total scores on the ASEX can range from 5–30, and a score of >16 indicates sexual dysfunction. These scales were administered monthly.
Psychiatric symptoms
Psychiatric symptoms were measured by the Brief Psychiatric Rating Scale (BPRS)24; Scale for the Assessment of Negative Symptoms (SANS)25; Calgary Depression Scale (CDS)26; Clinical Global Impression Scale (CGI).27 The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)28 was used to assess neuropsychological status. We measured positive symptoms using the BPRS conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content symptom items. To assess negative symptoms, we subtracted the global items, inappropriate affect, poverty of speech, and attention items from the total score of the SANS.29 These assessments were administered monthly.
Subject wellness and quality of life
We measured wellness, quality of life, and satisfaction with treatment. The 36-item Short Form (SF-36) scale,30 which incorporates physical and mental concepts, such as behavioral functioning, perceived wellbeing, social and role disability, and personal evaluations of overall health, was used to measure general health status. The Psychological General Well Being Schedule (PGWB),31 a 22-item scale with a focus on inner personal experience consisting of 6 subscales of anxiety, depressed mood, positive well-being, self-control, general health and vitality, was used to measure quality of life. The SF-36 and PGWB were performed at baseline and at weeks 4, 8, 12, and 16. At the conclusion of the study, participants were asked if they were 1) more likely to stay on their primary medication with the addition of aripiprazole, 2) happy that their menstrual period resumed (if irregularity present at baseline) and 3) happy that their sexual function improved (if dysfunction present at baseline). These were recorded on a 5-point Likert Scale.
Side effect measures
To assess side effects, we administered the Simpson-Angus Extrapyramidal Symptom Rating Scale (SAS)32, Barnes Akathisia Rating Scale (BARS),33 Abnormal Involuntary Movement Scale (AIMS)34 and the Side Effect Checklist (SEC) at biweekly appointments.
Laboratory assessments
Fasting serum prolactin was drawn every two weeks during the study. Normalization of prolactin was defined as a prolactin level < 24 ng/mL. Salivary estradiol and progesterone were collected at screening, and then weekly after enrollment (kits were sent home). Salivary cortisol was collected at baseline, midpoint (week 8) and endpoint (week 16). Plasma osteocalcin and bone specific alkaline phosphatase (measures of osteoblastic activity), as well as urinary N-telopeptide of type I collagen crosslinks (NTx) and C-telopeptide of collagen crosslinks (CTx) (measures of osteoclastic activity), were measured at baseline and then at weeks 4, 8 and 16. We measured the CBC, fasting glucose, lipids, thyroid stimulating hormone (TSH), parathyroid hormone, homocysteine and 25-hydroxy-vitamin D level at baseline, midpoint, and endpoint. All fasting laboratory measurements were drawn the morning of testing prior to breakfast. All laboratory measures were performed by a CLIA (Clinical Laboratory Improvement Amendments) certified laboratory, LabCorp, with the exception of salivary estradiol, progesterone and cortisol which were sent to ZRP Laboratory. Details on all laboratory assessments are found in Supplemental Table 1.
Study medication and Randomization procedures
Participants were randomized using a permuted block randomization sequence based on inpatient/outpatient status. Aripiprazole or matched placebo tablets were given in a double-blind fashion starting at 5 mg PO daily, and increased to 10 mg/day at the end of week 2. The dosage was increased at the end of week 8 to 15 mg/day in any participant who had not had any response in prolactin-related symptoms. The study medication was added to the ongoing antipsychotic regimens. Study physicians were instructed to avoid changing doses of other somatic and psychotropic medications throughout the study. Anticholinergic medications for extrapyramidal side effects (e.g. benztropine and diphenhydramine), propranolol for akathisia, and benzodiazepines for anxiety or agitation (e.g. lorazepam) could be prescribed as needed.
Statistical Analysis
All analyses were conducted on an intention-to-treat basis according to randomly assigned treatment. The primary outcome of the study, resolution of amenorrhea, oligomenorrhea, or galactorrhea, was analyzed at two-sided alpha = 0.05. We compared percentages in whom hormonal side effects (galactorrhea and oligo/amenorrhea) had remitted by end of study (week 16) in the aripiprazole versus placebo groups using a Mantel-Haenszel chi-square test at two-sided alpha = 0.05. Sexual functioning, general well-being, psychiatric symptoms, and laboratory measures were assessed using mixed models analysis of covariance for repeated measurement of the form follow-up measurement = baseline measurement + treatment + week + treatment x week, where within-subject correlation of repeated measurements is assessed with an unstructured covariance model and “week” is not treated as a continuous measure, but as a set of categorical indicators of the week at which a measurement is collected. With this model, the “treatment” term estimates the average (across follow-up weeks difference between treatments and the treatment x week term assesses whether this difference varies over time. We performed a Kaplan-Meier survival curve analysis to examine time to normalization of prolactin, time to resolution of galactorrhea and time to remission of sexual dysfunction using a Log-Rank Chi-Square. The sample size of 46, while slightly smaller than the preplanned 25 per group, provided power > 0.80 to detect large differences in prolactin change and resolution of galactorrhea (e.g. 60% remission on aripiprazole versus 10% on placebo). It is important to note that the primary outcome could not be observed conclusively in those who withdrew before week 16. To assess sensitivity to dropout, we looked at how results would be altered if participants who withdrew before response was observed were imputed to be non-responders.
Results
Participants
Sixty participants with a DSM-IV-TR diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder were enrolled into the study. Fourteen were excluded prior to randomization for not meeting inclusion and exclusion criteria, and so, a total of 46 were randomized. See Supplemental Figure 1 for the participant CONSORT flow diagram for participant withdrawals and completions.
The mean age was 36.6 ± 9.4 years, and 62% were African American. About 70% reported having two prolactin-related side effects present at baseline, and 30% had all three. Detailed demographic information for all randomized participants exposed to study drug for longer than 2 weeks are provided in Table 1. No statistically significant differences were noted in baseline characteristics. The mean aripiprazole dose used in the study was 11.7 ± 2.4 mg/day and was 14.4 ± 1.7 mg/day for placebo (F=9.66, df=112, p<0.001). The mean baseline prolactin concentration was 84.3 ± 56.6 ng/mL (88.5 ± 68.1 ng/mL in the aripiprazole group and 79.5 ± 40.6 in the placebo group). Among those completing at least 8 weeks of follow-up, at baseline, 23/37 (62%) had galactorrhea, 24/37 (65%) had oligomenorrhea/amenorrhea and 25/37 (68%) had sexual dysfunction (see Table 1).
Table 1.
Demographic and Clinical Information
| Aripiprazole (N=24) | Placebo (N=18) | |
|---|---|---|
| Race | 62% AA, 33% W | 56% AA, 35% W |
| Age (years) | 37.8 ± 8.9 | 36 ± 10.1 |
| Age of Illness Onset (years) | 17.9 ± 7.9 | 20.9 ± 5.8 |
|
Diagnosis Schizophrenia Schizoaffective Disorder Bipolar Disorder |
16 (67%) 7 (29%) 1 (4%) |
13 (72%) 4 (22%) 1 (6%) |
| Educational Level (years) | 12.3 ± 1.7 | 11.5 ± 1.4 |
| Age at Menstruation (years) | 12.1 ± 2.1 | 11.9 ± 2.2 |
| Number of Pregnancies | 1.2 ± 1.7 | 1.7 ± 2.1 |
| Number of Children | 0.8 ± 1.3 | 1.1 ± 1.3 |
| Age at First Sexual Encounter (years) | 14.4 ± 3.4 | 13.6 ± 5.6 |
| Mean Number of Partners | 15.4 ± 25.6 | 7.9 ± 12.9 |
| Current Galactorrhea | 65% | 71% |
| Current Oligo/Amenorrhea | 65% | 65% |
| Current Sexual Dysfunction | 70% | 65% |
| Prevalence of Prolactin-Related Effects | 100% 1, 70% 2, 40% 3 | 100% 1, 71% 2, 29% 3 |
|
Primary Antipsychotic Treatment |
||
| Risperidone | 13 (54%) | 9 (50%) |
| Paliperidone | 5 (21%) | 4 (22%) |
| Haloperidol | 4 (17%) | 2 (22%) |
| Fluphenazine/loxapine | 2 (8%) | 1 (6%) |
| Time on primary antipsychotic (days) | 658.4 ± 741.5 | 812.1 ± 865.8 |
|
Other Medications |
||
| Antidepressants | 12 (50%) | 15 (83%) |
| Mood Stabilizers | 6 (25%) | 5 (28%) |
| Anticholinergic Medications | 6 (25%) | 8 (44%) |
| Oral contraceptives | 1 (4%) | 1 (6%) |
This table presents demographic and clinical information for the DAAMSEL cohortsDemographic Information included for those randomized and exposed to drug > 2 weeks (N=42); Prolactin-related outcomes reported only for those with >8 weeks drug exposure (N=37)For baseline variables there were no significant differences noted in demographic measures, clinical history or medications (p>0.05).
Prolactin
As shown in Figure 1, the prolactin level significantly decreased in the aripiprazole group compared to placebo over 16 weeks (estimated difference −26.3 ±12.6, df=35, p=0.04), with the greatest drop occurring in the first week. It is important to note that even with this significant difference, normalization was not achieved in all women on aripiprazole. Kaplan-Meier Survival Curve Analysis estimated that at week one, 20% of aripiprazole participants had normalization of prolactin compared to 6% of placebo participants, and this increased to 35% at week 4 and week 12 compared to 6% of placebo. At week 16, 45% of aripiprazole participants versus 12% of placebo participants had complete normalization of prolactin levels (Log Rank Chi-Square= 4.8, df =1, p=0.028).
Figure 1.
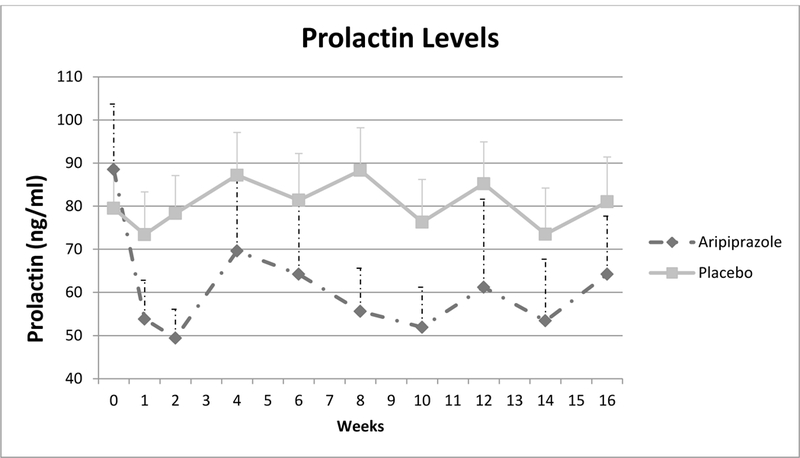
Prolactin Levels over the 16-week Clinical Trial t=−26.3 ±12.6, df=35, p0.04; Effect size=0.40 Legend Figure 1: This figure depicts the mean (solid line) and standard error (dotted line) of prolactin levels in the aripiprazole and placebo groups during the 16-week clinical trial.
Prolactin-Related Side Effects
Seventy-seven percent (10/13) of participants with galactorrhea in the aripiprazole group had improved or remitted symptoms versus 33% (4/12) in the placebo group (Chi-Square=4.8, df =1, p=0.028). In the Kaplan-Meier Survival Curve analysis, the estimated rate of normalization of galactorrhea in the aripiprazole and placebo groups respectively was 29% versus 17% at week 4, 57% versus 33% at week 8, 71% versus 33% at week 12 and 79% versus 33% at week 16 (Log Rank Chi-Square= 3.8, df =1, p=0.05). The difference in resumption of normal regular menstruation in the aripiprazole (6/13, 46%) versus placebo groups (3/11 27%) was not statistically significant (p=0.34). At any point during the study, menstrual bleeding occurred in 85% (11/13) with aripiprazole and 64% (7/11) in the placebo group (p= 0.24).
Normalization of sexual dysfunction (< 16 on ASEX) occurred in 50% (7/14) with aripiprazole versus 9% (1/11) with placebo (Chi-Square=4.7, df=1, p=0.03). Also, there were a significantly greater percentage of women who experienced a 20% reduction on the ASEX (25% (5/20) vs. 0%) on aripiprazole compared to placebo (Chi-Square=4.9. df=1, p=0.027). There were no significant group differences in the FSDS-R; the baseline and endpoint scores were 13.3 ±11.8 and 9.0 ± 8.7 respectively for aripiprazole and 7.6 ±10.2 and 6.5 ±11.3 for placebo (F=0.0, df=1,36, p=0.97). There were also no significant group differences in the FSFI (p>0.05). While not significant, the relationship between change in prolactin and change in the ASEX in the aripiprazole group was r=0.31, p=0.18. There was no correlation noted between change in prolactin and change in the ASEX in the placebo group (r=0.016, p=0.95).
Clinical Symptoms
There were no significant treatment group differences in BPRS, SANS, or CGI scores (p>0.05; see Table 2). Robust response on the BPRS total score (>30% response) was noted in 3/24 (13%) in the aripiprazole group versus 0/20 in the placebo group (Chi-Square=2.42, df=1, p=0.12). There was a significant treatment effect for the CDS total score (F=4.4, df=1,38.4, p=0.04), which favored aripiprazole. There were no significant differences in RBANS composite score or the SF-36 or PGWB total scores. On the PGWB subscales of depressive symptoms and positive well-being there was no change noted in the aripiprazole group and a significant improvement noted in the placebo group. One participant in the aripiprazole group, as noted earlier, discontinued due to worsening of positive symptoms.
Table 2.
Psychiatric Symptoms
| Aripiprazole (N=24) |
Placebo (N=18) | Statistics Treatment Effects |
|||
|---|---|---|---|---|---|
| Baseline | Endpoint | Baseline | Endpoint | ||
| BPRS | 35.3 ± 10.0 | 31.8 ± 10.9 | 33.6 ± 10.4 | 30.1 ± 9.0 | F=1.56, df=1, 37,9, p=0.22 |
| Psychosis | 10.1 ± 4.5 | 9.0 ± 4.6 | 10.1 ± 5.3 | 8.1 ± 4.3 | F=0.75, df=, 1,38.7, p=0.39 |
| Activation | 4.0 ± 1.5 | 3.9 ± 1.4 | 4.3 ± 1.6 | 3.7 ± 1.2 | F=3.07, df=1,40, p=0.09 |
| Hostility | 5.0 ± 2.1 | 5.0 ± 2.5 | 5.3 ± 1.8 | 4.4 ± 2.0 | F=0.60, df=1, 36.6, p=0.44 |
| Anxiety/Depression | 6.8 ± 2.7 | 5.9 ± 3.2 | 6.4 ± 2.7 | 5.6 ± 2.5 | F=0.95, df=1,39.1, p=0.34 |
| Negative Symptoms | 5.6 ± 2.8 | 5.2 ± 2.4 | 4.79 ± 2.7 | 5.1 ± 2.4 | F=0.10, df=1,36, p=0.75 |
| SANS Total | 24.0 ± 13.4 | 21.2 ± 12.0 | 21.4 ± 10.3 | 20.8 ± 10.7 | F=0.14, df=1, 33.3, p=0.71 |
| CDS | 3.5 ± 4.0 | 2.1 ± 3.0 | 2.5 ± 3.0 | 1.3 ± 1.3 | F=4.41, df=1,38.4, p=0.04 |
| CGI | 3.8 ± 0.9 | 3.6 ±0.9 | 3.7 ± 1.1 | 3.5 ± 1.0 | F=0.42, df=1,39.3, p=0.52 |
| RBANS Total | 69.7 ± 17.8 | 72.2 ± 17.0 | 67.6 ± 10.0 | 90.9 ± 80.5 | F=1.23, df=1,35, p=0.28 |
| DAI Total | 18.7 ± 7.9 | 20.2 ± 6.2 | 16.4 ± 7.6 | 20.4 ± 6.3 | F=0.62, df=1, 38.4, p=0.44 |
| PGWB global | 76.5 ± 14.1 | 77.7 ± 14.5 | 78.7 ± 18.7 | 86.7 ± 14.3 | F=7.2, df=1,35.5, p=0.01 |
| Anxiety | 17.7 ± 4.9 | 18.2 ± 4.6 | 18.1 ± 5.9 | 20.5 ± 5.1 | F=3.48, df=1, 36, p=.07 |
| Depressed mood | 12.4 ± 2.4 | 12.9 ± 2.9 | 11.8 ± 3.3 | 13.7 ± 1.8 | F=5.41, df=1, 30, p=0.03 |
| Positive well being | 12.0 ± 4.1 | 12.0 ± 3.4 | 13.6 ± 4.3 | 14.4 ± 3.3 | F=10.25, df=1, 37.8, p=0.003 |
| Self-control | 11.3 ± 3.0 | 11.4 ± 2.8 | 12.7 ± 3.0 | 13.4 ± 2.0 | F=3.23, df=1, 33.3, p=0.08 |
| General health | 10.9 ± 3.2 | 11.4 ± 2.6 | 11.5 ± 3.1 | 12.5 ± 3.7 | F=0.02, df=1, 36.3, p=0.89 |
| Vitality | 12.3 ± 2.9 | 11.9 ± 2.7 | 11.1 ± 3.3 | 12.3 ± 2.1 | F=2.79, df=1, 34.7, p=0.10 |
| SF-36 General/Global Health | 65.9 ± 21.5 | 70.2 ± 18.4 | 70.8 ±19.6 | 72.9 ±27.1 | F=0.09, df=1, 38.1, p=0.77 |
This table reports the baseline and endpoint scores for the psychiatric symptoms reported in the studyThere was a statistical improvement in the PGWB global scale seen in the placebo group with no difference noted in the aripiprazole groupThis improvement was seen in depressed mood and positive well-being.
Laboratory Results and Side Effects
The only noted difference in laboratory measures was a decrease in alkaline phosphatase with aripiprazole compared to placebo (see Table 3); however, this was not observed in the bone specific alkaline phosphatase. There were no significant group differences in any measures of bone health. There were no significant group differences seen in side effects, indicating that aripiprazole was well tolerated (Table 4). Since akathisia is a side effect of aripiprazole, the BARS was used to assess this side effect. The baseline and endpoint scores on the BARS were 0.8 ± 2.2 and 0.5 ± 1.5 for aripiprazole and 0.6 ± 1.3 and 0.3 ± 1.2 for placebo (F=0.35, df=1,41, p=0.56). There were also no significant group differences in change in SAS or AIMS total scores; baseline and endpoint SAS scores were 1.3 ± 1.9 and 0.8 ± 1.0 for aripiprazole and 0.9 ± 1.3 and 0.5 ± 0.7 for placebo (F=0.19, df=1,41, p=0.67) and baseline and endpoint AIMS scores were 0.7 ± 1.5 and 0.5 ± 1.8 for aripiprazole and 0.5 ±1.2 and 0.3 ± 0.8 for placebo (F=0.65, df=1,38, p=0.43). Two participants were withdrawn due to side effects; one in the aripiprazole group due to nausea and one in the placebo group due to abdominal pain.
Table 3.
Laboratory Measures
| Aripiprazole (N=24) | Placebo (N=18) | Statistics Treatment Effects |
|||
|---|---|---|---|---|---|
| Baseline | Endpoint | Baseline | Endpoint | ||
| Estradiol (pg/mL) | 0.9 ± 0.5 | 1.1 ± 0.6 | 1.0 ± 0.5 | 0.8 ± 0.7 | F=0.31, df=1, 36.5, p=0.58 |
| Progesterone (pg/mL) | 26.2 ± 27.7 | 31.8 ± 28.4 | 28.3 ± 35.1 | 19.6 ± 30.1 | F=0.03, df=1, 38, p=0.86 |
| Cortisol (μg/dL) | 3.4 ± 2.0 | 3.6 ± 3.3 | 2.3 ± 1.2 | 1.9 ± 1.6 | F=0.39, df=1, 17.5, p=0.39 |
| C-telopeptide (pg/ml) | 427.9 ± 254.9 | 369.6 ± 232.5 | 373.0 ± 199.1 | 383.9 ± 282.2 | F=0.15, df=1, 28.5, p=0.70 |
| N-telopeptide (mM BCE/mmol) | 34.6 ± 14.8 | 34.1 ± 15.7 | 27.5 ± 10.2 | 27.3 ± 11.3 | F=0.23, df=1, 35.6, p=0.64 |
| Thyrotropin-releasing hormone (TRH) (pg/ml) | 13.9 ± 6.1 | 13.0 ± 9.5 | 13.7 ± 3.3 | 17.0 ± 5.2 | F=2.65, df=1, 23.3, p=0.12 |
| Parathyroid hormone (pg/ml) | 30.1 ± 9.9 | 35.4 ± 13.1 | 32.9 ± 14.6 | 34.2 ± 10.6 | F=1.61, df-1, 27.3, p=0.22 |
| Homocysteine (μmol/L) | 9.5 ± 3.7 | 8.8 ± 2.9 | 8.3 ± 3.5 | 8.1 ± 2.7 | F=0, df=1, 36.2, p=0.95 |
| Vitamin D (ng/ml) | 20.9 ± 9.0 | 24.4 ± 9.9 | 19.0 ± 6.5 | 20.1 ± 7.2 | F=0.64, df=1, 34.6, p=0.43 |
| Osteocalcin (ng/ml) | 15.7 ± 9.4 | 15.0 ± 9.6 | 14.7 ± 8.0 | 16.2 ± 10 | F=0.07, df=1, 33.4, p=0.79 |
| Bone specific alkaline phosphatase (μg/L) | 17.3 ± 5.9 | 15.9 ± 6.0 | 15.8 ± 6.6 | 14.5 ± 5.1 | F=1.03, df=1, 37.6, p=0.32 |
| Alkaline phosphatase (U/L) | 79.8 ± 17.2 | 76.8 ± 20.9 | 78.2 ± 35.7 | 78.5 ± 32.6 | F=4.88, df=1, 32.9, p=0.034 |
| Total Cholesterol (mg/dL) | 182.7 ± 37.2 | 172.4 ± 34.3 | 182.4 ± 32.0 | 175.4 ± 37.8 | F=0.01, df=1, 33.7, p=0.91 |
| High Density Lipoprotein (mg/dL) | 52.7 ± 17.1 | 50.9 ±19.0 | 48.3 ±15.7 | 48.1 ±15.7 | F=0.04, df=1, 33.4, p=0.85 |
| Low Density Lipoprotein (mg/dL) | 107.9 ±34.2 | 98.4 ± 26.4 | 109.6 ± 33.5 | 103.4 ± 29.6 | F=0.01, df=1, 32.8, p=0.91 |
| Fasting Glucose (mg/dL) | 102.1 ± 47.2 | 101.5 ± 22.6 | 99.9 ± 25.2 | 97.4 ± 17.2 | F=0.06, df=1, 34.1, p=0.81 |
| Weight (kg) | 103.0 ± 23.2 | 101.2 ± 20.7 | 98.3 ± 34.0 | 96.9 ±34.1 | F=0.35, df=1,42, p=0.56 |
| Waist Circumference (in) | 45.1 ± 7.5 | 45.2 ± 7.6 | 44.4 ± 8.0 | 42.4 ± 7.8 | F=4.15, df=1, 37.3, p=0.049 |
| BMI (kg/m2) | 37.4 ± 8.5 | 36.7 ±7.5 | 35.3 ± 11.2 | 35.1 ± 11.4 | F=0.22, df=1, 39.7, p=0.64 |
This table shows baseline and endpoint values for all laboratory measuresAlkaline phosphatase decreased in the aripiprazole group relative to placeboThe placebo group had a significantly greater change in waist circumference in the placebo group compared to aripiprazole while no change was noted in the aripiprazole group.
Table 4.
Side Effect Incidence
| Side Effect | Aripiprazole (N=24) |
Placebo (N=18) |
|---|---|---|
| Abdominal Pain | 7 (29%) | 6 (33%) |
| Anorexia (loss of appetite) | 4 (17%) | 4 (22%) |
| Bruising easily | 2 (8%) | 1 (6%) |
| Constipation | 6 (25%) | 5 (28%) |
| Diarrhea | 9 (38%) | 7 (39%) |
| Dizziness | 5 (21%) | 4 (22%) |
| Dry Mouth | 6 (25%) | 4 (22%) |
| Enuresis | 5 (21%) | 2 (11%) |
| Fever | 1 (4%) | 2 (11%) |
| Headache | 6 (25%) | 5 (28%) |
| Insomnia | 8 (33%) | 3 (17%) |
| Malaise (weakness, fatigue) | 7 (29%) | 5 (28%) |
| Mucosal ulceration | 4 (17%) | 2 (11%) |
| Nausea | 10 (42%) | 4 (22%) |
| Rash | 2 (8%) | 3 (17%) |
| Restlessness | 5 (21%) | 2 (11%) |
| Salivation | 4 (17%) | 5 (28%) |
| Sedation | 7 (29%) | 6 (33%) |
| Sore Throat | 2 (8%) | 4 (22%) |
| Stiffness | 5 (21%) | 5 (28%) |
| Tinnitus | 7 (29%) | 2 (11%) |
| Tremor | 8 (33%) | 2 (11%) |
| Urticaria (hives, itching) | 1 (4%) | 1 (6%) |
| Vomiting | 10 (42%) | 7 (39%) |
| Weight Loss | 4 (17%) | 3 (17%) |
This table shows the incidence of new onset or worsening of adverse events in the study by treatment groupNo differences were noted in incidence between groups on any side effect (p>0.05).
Participant Perceptions
While a small sample, 80% of women on aripiprazole answered “yes” to feeling more inclined to take their risperidone with this add-on treatment (4/5 aripiprazole and 0/3 placebo; Chi-Square=4.8, df=1, p=0.029). Overall, women were asked about satisfaction with the resolution of their menstrual periods and improvement with sexual dysfunction on a Likert Scale of 1–5. Women rated a mean score of 3.6 ± 1.5 when asked how happy they were that menstrual periods resumed and a mean score of 4.6 ± 0.6 when asked about improvements in sexual function. This did not differ by group.
Discussion
The 16-week DAAMSEL study, employing multiple clinical and hormonal measures, reveals that the addition of relatively low dose aripiprazole can achieve meaningful reductions in serum prolactin levels, as well as facilitate demonstrable improvements in sexual function and galactorrhea among females who experience distressing antipsychotic- induced hyperprolactinemia. Adjunctive aripiprazole has been shown in a handful of randomized double-blind trials and a meta-analysis35 to decrease prolactin, however, this is one of few published studies examining add-on aripiprazole for the treatment of symptomatic hyperprolactinemia in only female participants. Supplemental Table 2 lists all randomized controlled double blind trials found in English databases. Most other published studies with prolactin as primary endpoint were shorter in duration (i.e., 8 weeks) than our study of 16 weeks. The beneficial results of short term trials may be due, in part, to the discontinuation of prolactin elevating antipsychotics. In addition, the DAAMSEL study controlled for phase of the menstrual cycle, weekly pill counts and completed breast exams biweekly. Few other studies also collected hormonal information.
With regard to galactorrhea, we see a significant reduction in this side effect with about 80% of aripiprazole-treated participants having normalization at study endpoint. It is notable that while prolactin decreases in the first weeks, galactorrhea does take longer to resolve with the majority of resolution of galactorrhea occurring at 2–3 months. Very few other studies have addressed this side effect through the use of regular standardized breast examinations. One other randomized trial that we previously completed found an improvement in galactorrhea.5 Additionally, an open-label study by Ranjbar and colleagues36 found that prolactin normalization with add-on aripiprazole took approximately 84 days, but that resolution of galactorrhea was seen in 29% of patients at week 2, 57% at week 4 and then almost 80% by week 8, also confirming that it takes some time to resolve galactorrhea even after immediate prolactin decreases. This finding is new and important as breast evaluation for galactorrhea rarely occurs in both clinical practice and research, and this draws attention to the critical need to examine this side effect.
Our study did not detect a significant improvement in participant menstrual status, similar to other RCTs and open-label studies.4,37,38 This may be due to the lack of normalization of prolactin in all participants, or that both those with amenorrhea and oligomenorrhea were included in the study. Menstrual periods were randomly occurring in the majority of participants, making it difficult to operationalize an improvement definition. Also, we did examine time to any bleeding and time to the reinstatement of two regular menstrual periods, however it may take longer periods of time to see normalization to regularly occurring menses in people lacking regular menstrual periods for years or decades.
Improvement in sexual function was a major finding of our study. Not only did we see significantly greater number of people on adjunct aripiprazole with a significant drop in ASEX scores, we also saw a greater number of those on adjunct aripiprazole with normalization of sexual functioning (50%). Only a few other randomized studies addressed sexual dysfunction using a standardized rating scale,4,39 however there is scant information on the effects of prolactin reduction on sexual function in women specifically. A few open-label studies have found that aripiprazole monotherapy or as an adjunct reduces sexual dysfunction in people with schizophrenia.40–42 Sexual dysfunction is a significant under-addressed problem both in schizophrenia and in women. In fact, sexual dysfunction has been rated as one of the most distressing and frequently reported side effects with antipsychotic treatment43 and associated with a poor quality of life.44
In a few studies in schizophrenia, add-on aripiprazole was found to improve psychiatric symptoms, particularly negative symptoms, which we did not see in the current study. However, these studies were designed with psychiatric symptoms as the primary endpoint, enrolled participants with baseline symptom thresholds, and used doses as high as 30 mg, which is higher than those used in our study.4,5,45–48 In the most recent meta-analysis evaluating add-on aripiprazole, statistically significant reductions in Positive and Negative Symptom Scale (PANSS) and BPRS total scores were reported, with reduction in negative symptom scores driving most of these findings. The results of the studies in the meta-analysis were highly heterogeneous (I2 = 78–91%), and the GRADE analyses revealed overall low quality of evidence for these outcomes. In addition, most of the included studies were in Chinese participants, potentially affecting generalizability49. An earlier meta-analysis found no statistically significant improvement in any clinical scales, and still reported low quality evidence48. Potential improvement of clinical symptoms, especially negative symptoms, with aripiprazole requires further investigation. We saw mixed results on depressive symptoms: aripiprazole favoring treatment was associated with greater reductions on the CDS total score, whereas placebo was associated with greater reductions in PGWB depressive symptoms. The PGWB depression subfactor is composed of only 3 items (depressed, blue and sad) while the CDS consists of 9 items that include other depressive aspects such as guilt hopelessness and self-depreciation. Aripiprazole may have more effect on other domains of depressive symptoms such as observed in this study.
Given the high incidence of nonadherence in serious mental illnesses such as schizophrenia, the increase in desire to continue antipsychotic therapy is a major step towards maintaining psychiatric stabilization.50 The benefit of reversing hyperprolactinemia with aripiprazole has had much recognition; however the benefits to the women being treated with this strategy remain unclear. This study helps establish aripiprazole as a treatment in women with symptomatic hyperprolactinemia. We better understand rates of side effect occurrence, estimated treatment response and time course to reduction in prolactin in this study by having employed detailed assessments on menstrual cycle, breast examinations and sexual function assessments. In summary, benefits of the strategy include improvement in prolactin, side effects and satisfaction with treatment. This in turn could improve adherence and other longer term consequences not studied. With employing the strategy as monotherapy instead of adjunct treatment, it may decrease the risk of other long-term side effects, increased costs and the burden of additional pills but has less evidence base and switching from one antipsychotic to another has been associated with psychiatric exacerbation. Thus, there is a stronger evidence base for the efficacy and safety of adjunct aripiprazole therapy than switching to aripiprazole monotherapy. It is noteworthy that 16 weeks of adjunct aripiprazole treatment did not worsen metabolic side effects or movement disorder symptoms
Our study is not without limitations. We were not able to objectively verify menstrual status and had to rely on self-report. Also, the study may not have been long enough to detect attenuation of bone changes or menstrual changes. It is also noteworthy that the study participants were overweight and obese as often seen with this population. Sexual dysfunction, galactorrhea and amenorrhea at baseline may be partly contributed to other factors such as obesity, hormone imbalance or other unknown factors. We were not able to assess causality of prolactin-related side effects which may be why direct correlations between prolactin and sexual function were not significantly correlated. In addition, we did not assess bone density using dual-energy X-ray absorpitometry (DXA) to measure bone loss. Lastly, polypharmacy is not without risk of other side effects. We do not observe side effects in this study, however, long-term side effects may occur.
Conclusion
Our study demonstrates that aripiprazole can be used in premenopausal women to not only reduce serum prolactin levels, but also prolactin-related side effects. Through this, patients are more likely to remain adherent to medications, and therefore remain more stable psychiatrically. It is important to personalize treatment and this strategy may help reduce untoward side effects in women stabilized on treatment and experiencing prolactin-related side effects. Longer-term studies are needed to examine the risks and benefits of long-time use.
Supplementary Material
Acknowledgements:
This study was supported by NIMH R01MH090071–01A1 (Kelly PI) and aripiprazole and matching placebo were supplied by Bristol-Myers Squibb/Otsuka. We thank David A. Gorelick, Bernard Fisher, Elaine Weiner and William R. Keller for their roles as medically accountable physicians on the study. We thank Margaret Altemus for her consultation on the study design.
Funding Source: This study was supported by NIMH R011R01MH090071–01A1 (Kelly PI)
Aripiprazole and matching placebo supplied by Bristol Myers Squibb/Otsuka
Footnotes
DAAMSEL: (Dopamine Partial Agonist, Aripiprazole, for the Management of Symptomatic ELevated Prolactin)
Conflict of Interest: Dr. Buchanan has served on the advisory boards of Astellas Pharma; Boehringer Ingelheim-RCV; ITI, Inc.; and Lundbeck; he has also been a consultant for Takeda; Upsher-Smith Laboratories, Inc.; and been on the DSMB for Pfizer. Dr. McEvoy received honoraria for advisory boards with Neurocrine and TEVA during the past two years. Dr. Kelly served on the advisory board for Lundbeck and a consultant for HLS Therapeutics. For the remaining authors none were declared.
Contributor Information
Deanna L. Kelly, Maryland Psychiatric Research Center (MPRC), Department of Psychiatry, University of Maryland School of Medicine.
Megan M. Powell, Department of Psychiatry, University of Maryland School of Medicine.
Heidi J. Wehring, Department of Psychiatry, University of Maryland School of Medicine.
MacKenzie A. Sayer, Department of Psychiatry, University of Maryland School of Medicine.
Ann Marie Kearns, Department of Psychiatry, University of Maryland School of Medicine.
Ann L. Hackman, Department of Psychiatry, University of Maryland School of Medicine.
Robert Buchanan, Department of Psychiatry, University of Maryland, School of Medicine.
Rebecca B. Nichols, Department of Psychiatry, Georgia Regents University.
Heather A. Adams, Spring Grove Hospital Center and MPRC, Department of Psychiatry, University of Maryland School of Medicine.
Charles M. Richardson, Department of Psychiatry, Spring Grove Hospital Center and MPRC, University of Maryland School of Medicine.
Gopal Vyas, Department of Psychiatry, Spring Grove Hospital Center and MPRC, University of Maryland School of Medicine.
Robert P. McMahon, Department of Psychiatry, University of Maryland School of Medicine.
Amber K. Earl, Thread, Inc..
Kelli M. Sullivan, Cystic Fibrosis/Pumonary Research and Treatment Center, University of North Carolina at Chapel Hill.
Fang Liu, Department of Psychiatry, University of Maryland School of Medicine.
Sarah Luttrell, Department of Pharmacy, University of Maryland Eastern Shore School of Pharmacy.
Faith B. Dickerson, Department of Psychology, Sheppard Pratt Health System.
Stephanie M. Feldman, Department of Psychiatry, University of Maryland School of Medicine.
Supriya Narang, Mosaic Community Services, Sheppard Pratt Health System.
Maju M. Koola, Department of Psychiatry and Behavioral Sciences, George Washington University School of Medicine and Health Sciences.
Peter F. Buckley, Department of Psychiatry, Virginia Commonwealth University School of Medicine.
Jill A. RachBeisel, Department of Psychiatry, University of Maryland School of Medicine.
Joseph P. McEvoy, Department of Psychiatry, Georgia Regents University.
References
- 1.Aichhorn W, Whitworth AB, Weiss EM, et al. Second-generation antipsychotics: is there evidence for sex differences in pharmacokinetic and adverse effect profiles? Drug Saf. 2006;29:587–598. [DOI] [PubMed] [Google Scholar]
- 2.Kelly DL Treatment considerations in women with schizophrenia J Womens Health (Larchmt). 2006;15:1132–1140. [DOI] [PubMed] [Google Scholar]
- 3.Pucci G, Alcidi R, Tap L, et al. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature Pharmacol Res. 2017;120:34–42. [DOI] [PubMed] [Google Scholar]
- 4.Raghuthaman G, Venkateswaran R, Krishnadas R Adjunctive aripiprazole in risperidone-induced hyperprolactinaemia: double-blind, randomised, placebo-controlled trial BJPsych Open. 2015;1:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shim JC, Shin JG, Kelly DL, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial AJ Psychiatry. 2007;164:1404–1410. [DOI] [PubMed] [Google Scholar]
- 6.Bostwick JR, Guthrie SK, Ellingrod VL Antipsychotic-induced hyperprolactinemia Pharmacotherapy. 2009;29:64–73. [DOI] [PubMed] [Google Scholar]
- 7.Howard L, Kirkwood G, Leese M Risk of hip fracture in patients with a history of schizophrenia Br J Psychiatry. 2007;190:129–134. [DOI] [PubMed] [Google Scholar]
- 8.Barnes TR, Harvey CA Psychiatric drugs and sexuality Sexual pharmacology. 1993;1:176–196. [Google Scholar]
- 9.Bushe C, Shaw M Prevalence of hyperprolactinaemia in a naturalistic cohort of schizophrenia and bipolar outpatients during treatment with typical and atypical antipsychotics J Psychopharmacol. 2007;21:768–773. [DOI] [PubMed] [Google Scholar]
- 10.Brugnoli R, Novick D, Haro JM, et al. Risk factors for suicide behaviors in the observational schizophrenia outpatient health outcomes (SOHO) study BMC Psychiatry. 2012;12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torre DL, Falorni A Pharmacological causes of hyperprolactinemia Ther Clin Risk Manag. 2007;3:929–951. [PMC free article] [PubMed] [Google Scholar]
- 12.Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review CNS Drugs. 2014;28:421–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinberg DL, Davis JM, de Coster R, et al. Prolactin levels and adverse events in patients treated with risperidone J Clin Psychopharmacol. 1999;19:57–61. [DOI] [PubMed] [Google Scholar]
- 14.Ajmal A, Joffe H, Nachtigall LB Psychotropic-induced hyperprolactinemia: a clinical reviewPsychosomatics. 2014;55:29–36. [DOI] [PubMed] [Google Scholar]
- 15.Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release Brain Res. 2004;1003:9–17. [DOI] [PubMed] [Google Scholar]
- 16.Kane JM, Meltzer HY, Carson WH, Jr., et al. Aripiprazole for treatment-resistant schizophrenia: results of a multicenter, randomized, double-blind, comparison study versus perphenazine J Clin Psychiatry. 2007;68:213–223. [PubMed] [Google Scholar]
- 17.Chan HY, Lin WW, Lin SK, et al. Efficacy and safety of aripiprazole in the acute treatment of schizophrenia in Chinese patients with risperidone as an active control: a randomized trial J Clin Psychiatry. 2007;68:29–36. [DOI] [PubMed] [Google Scholar]
- 18.Jung DU, Kelly DL, Kong BG, et al. Adjunctive Treatment with Aripiprazole for Risperidone-Induced Amenrrhea Schizophr Bull. 2011;37:307. [Google Scholar]
- 19.Berek JS Berek and Novaks Gynecology 15th ed Philadelphia: Lippincott Williams and Wilkins; 2011:991–1034. [Google Scholar]
- 20.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders- Patient Edition (SCID-I/P, 11/2002 revisionNew York, New York: Biometrics Research Department New York State Psychiatric Insititute; 2002. [Google Scholar]
- 21.McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity J Sex Marital Ther. 2000;26:25–40. [DOI] [PubMed] [Google Scholar]
- 22.Derogatis LR, Rosen R, Leiblum S, et al. The Female Sexual Distress Scale (FSDS): initial validation of a standardized scale for assessment of sexually related personal distress in women J Sex Marital Ther. 2002;28:317–330. [DOI] [PubMed] [Google Scholar]
- 23.Wiegel M, Meston C, Rosen R The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores J Sex Marital Ther. 2005;31:1–20. [DOI] [PubMed] [Google Scholar]
- 24.Overall JE, Gorham DR The brief psychiatric rating scale Psychol Rep. 1962;10:799–812. [Google Scholar]
- 25.Andreasen NC Negative symptoms in schizophreniaDefinition and reliability Arch Gen Psychiatry. 1982;39:784–788. [DOI] [PubMed] [Google Scholar]
- 26.Addington D, Addington J, Maticka-Tyndale E Assessing depression in schizophrenia: the Calgary Depression Scale Br J Psychiatry Suppl. 1993:39–44. [PubMed] [Google Scholar]
- 27.Guy W The clinical global impression scaleThe ECDEU assessment manual for psychopharmacology revised (VolDHEW Publ No ADM 76–338, pp218–222) Rockville, MD: US Department of Health, Education, and WelfarePublic Health Service, Alcohol, Drug Abuse, Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research; 1976. [Google Scholar]
- 28.Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity J Clin Exp Neuropsychol. 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 29.Buchanan RW, Carpenter WT Domains of psychopathology: an approach to the reduction of heterogeneity in schizophrenia J Nerv Ment Dis. 1994;182:193–204. [PubMed] [Google Scholar]
- 30.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36)IConceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 31.Dupuy H The Psychological General Well-being (PGWB) IndexW: Assessment of quality of life in clinical trials of cardiovascular therapiesRed Wenger NK, Mattsson ME, Furberg CD, Elinson J New York: Le: Jacq Publishing Inc; 1984. [DOI] [PubMed] [Google Scholar]
- 32.Simpson GM, Angus JW A rating scale for extrapyramidal side effects Acta Psychiatr Scand Suppl. 1970;212:11–19. [DOI] [PubMed] [Google Scholar]
- 33.Barnes TR A rating scale for drug-induced akathisia Br J Psychiatry. 1989;154:672–676. [DOI] [PubMed] [Google Scholar]
- 34.Guy W ECDEU assessment manual for psychopharmacologyUS Department of Health, and Welfare Rockville, MD: 1976:534–537. [Google Scholar]
- 35.Li X, Tang Y, Wang C Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials PLoS One. 2013;8:e70179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranjbar F, Sadeghi-Bazargani H, Niari Khams P, et al. Adjunctive treatment with aripiprazole for risperidone-induced hyperprolactinemia Neuropsychiatr Dis Treat. 2015;11:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics Prog Neuropsychopharmacol Bol Psychiatry. 2010;34:1495–1499. [DOI] [PubMed] [Google Scholar]
- 38.Trives MZ, Llácer J-MB, Escudero M-AG, et al. Effect of the addition of aripiprazole on hyperprolactinemia associated with risperidone long-acting injection J Clin Psychopharmacol. 2013;33:538–541. [DOI] [PubMed] [Google Scholar]
- 39.Kane JM, Correll CU, Goff DC, et al. A multicenter, randomized, double-blind, placebo-controlled, 16-week study of adjunctive aripiprazole for schizophrenia or schizoaffective disorder inadequately treated with quetiapine or risperidone monotherapy J Clin Psychiatry. 2009;70:1348–1357. [DOI] [PubMed] [Google Scholar]
- 40.Mir A, Shivakumar K, Williamson RJ, et al. Change in sexual dysfunction with aripiprazole: a switching or add-on study J Psychopharmacol. 2008;22:244–253. [DOI] [PubMed] [Google Scholar]
- 41.Fujioi J, Iwamoto K, Banno M, et al. Effect of Adjunctive Aripiprazole on Sexual Dysfunction in Schizophrenia: A Preliminary Open-Label Study Pharmacopsychiatry. 2017;50:74–78. [DOI] [PubMed] [Google Scholar]
- 42.Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia J Clin Psychopharmacol. 2010;30:596–599. [DOI] [PubMed] [Google Scholar]
- 43.Bobes J, Garc APMP, Rejas J, et al. Frequency of sexual dysfunction and other reproductive side-effects in patients with schizophrenia treated with risperidone, olanzapine, quetiapine, or haloperidol: the results of the EIRE study J Sex Marital Ther. 2003;29:125–147. [DOI] [PubMed] [Google Scholar]
- 44.Bushong ME, Nakonezny PA, Byerly MJ Subjective quality of life and sexual dysfunction in outpatients with schizophrenia or schizoaffective disorder J Sex Marital Ther. 2013;39:336–346. [DOI] [PubMed] [Google Scholar]
- 45.Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial J Clin Psychiatry. 2008;69:720–731. [DOI] [PubMed] [Google Scholar]
- 46.Lee BJ, Lee SJ, Kim MK, et al. Effect of aripiprazole on cognitive function and hyperprolactinemia in patients with schizophrenia treated with risperidone Clin Psychopharmacol Neurosci. 2013;11:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen JX, Su YA, Bian QT, et al. Adjunctive aripiprazole in the treatment of risperidone-induced hyperprolactinemia: A randomized, double-blind, placebo-controlled, dose-response study Psychoneuroendocrinology. 2015;58:130–140. [DOI] [PubMed] [Google Scholar]
- 48.Qiao Y, Yang F, Li C, et al. Add-on effects of a low-dose aripiprazole in resolving hyperprolactinemia induced by risperidone or paliperidone Psychiatry Res. 2016;237:83–89. [DOI] [PubMed] [Google Scholar]
- 49.Zheng W, Zheng YJ, Li XB, et al. Efficacy and Safety of Adjunctive Aripiprazole in Schizophrenia: Meta-Analysis of Randomized Controlled Trials J Clin Psychopharmacol. 2016;36:628–636. [DOI] [PubMed] [Google Scholar]
- 50.Wade M, Tai S, Awenat Y, et al. A systematic review of service-user reasons for adherence and nonadherence to neuroleptic medication in psychosis Clin Psychol Rev. 2017;51:75–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


