Figure 1.
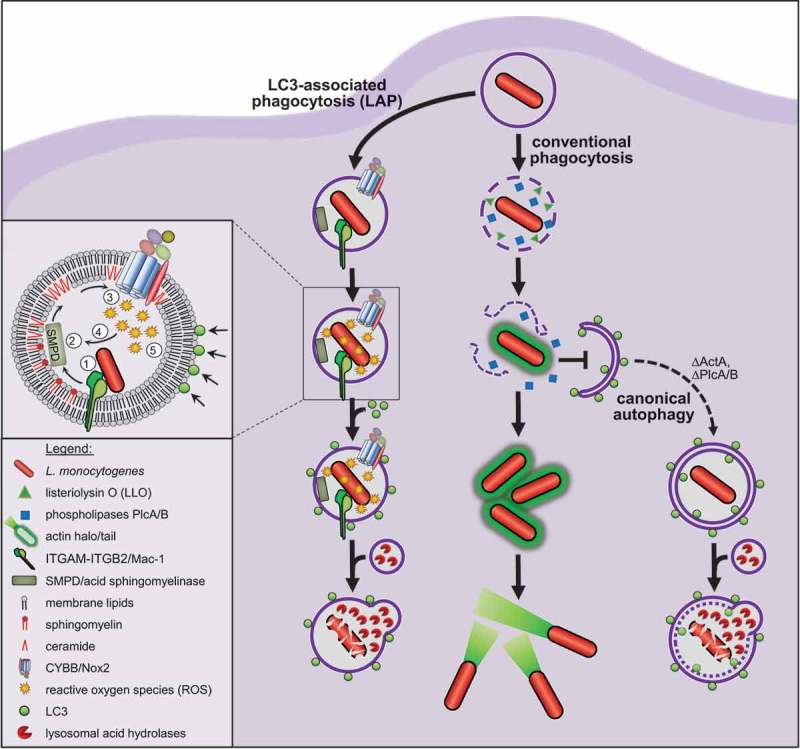
Model for LAP of Listeria. Listeria use their virulence factors LLO and PlcA/B to destroy the membrane of conventional phagosomes before these can fuse with lysosomes, and then colonize the cytosol. Cytosolic Listeria avoid targeting by canonical autophagy by surrounding themselves, in a process mediated by the virulence factor ActA, with a halo of host actin and by stalling phagophore assembly via PlcA/B. Consequently, canonical autophagy does not contribute to anti-listerial activity of macrophages. By contrast, recruitment of LC3 to Listeria-containing phagosomes by LAP markedly enhances anti-listerial activity of macrophages and immunity of mice. LAP of Listeria (1) is initiated by ITGAM-ITGB2 that induces (2) breakdown of the membrane lipid sphingomyelin into ceramide by SMPD. (3) The resulting ceramide-enriched membrane platforms facilitate CYBB assembly and activation. (4) CYBB-derived ROS amplify SMPD activity in a positive feedback loop and (5) induce recruitment of LC3 by LAP. LAP then promotes fusion of the phagosome with lysosomes, which increases exposure of Listeria to bactericidal lysosomal acid hydrolases and thus anti-listerial activity of macrophages.
