ABSTRACT
Endoribonuclease toxins (ribotoxins) are produced by bacteria and fungi to respond to stress, eliminate non-self competitor species, or interdict virus infection. PrrC is a bacterial ribotoxin that targets and cleaves tRNALys UUU in the anticodon loop. In vitro studies suggested that the post-transcriptional modification threonylcarbamoyl adenosine (t6A) is required for PrrC activity but this prediction had never been validated in vivo. Here, by using t6A-deficient yeast derivatives, it is shown that t6A is a positive determinant for PrrC proteins from various bacterial species. Streptococcus mutans is one of the few bacteria where the t6A synthesis gene tsaE (brpB) is dispensable and its genome encodes a PrrC toxin. We had previously shown using an HPLC-based assay that the S. mutans tsaE mutant was devoid of t6A. However, we describe here a novel and a more sensitive hybridization-based t6A detection method (compared to HPLC) that showed t6A was still present in the S. mutans ΔtsaE, albeit at greatly reduced levels (93% reduced compared with WT). Moreover, mutants in 2 other S. mutans t6A synthesis genes (tsaB and tsaC) were shown to be totally devoid of the modification thus confirming its dispensability in this organism. Furthermore, analysis of t6A modification ratios and of t6A synthesis genes mRNA levels in S. mutans suggest they may be regulated by growth phase.
KEYWORDS: Modified nucleosides, RNA maturation, translation, t6A detection
Abbreviations
- ASL
Anticodon Stem Loop
- t6A or t6A37
threonylcarbamoyladenosine
- TCTC
Threonyl Carbamoyl Transferase Complex
Introduction
tRNA molecules are heavily modified post-transcriptionally to ensure translational accuracy and cell survival.1 tRNA Anticodon Stem Loops (ASLs) (Fig. 1) make excellent targets for ribotoxins (or anticodon nucleases, ACNases) in toxin/antitoxin (TA) modules that can be used by organisms to eliminate competition or for suicidal functions under stress or phage attack.2,3 Different ACNases have been characterized with very specific tRNA cleavage patterns. The Escherichia coli toxins Colicin E5 and Colicin D target tRNAs with a QUN anticodon (Q being the modified base Queuosine)4 and tRNAArg isoacceptors, respectively.5 The Mycobacterium tuberculosis toxin VapC-mt4 targets tRNAAla UGC, tRNASer GCU and tRNASer GGA6 while the enteric VapC proteins target tRNAiniMet CAU7 MazF-mt9, from the same organism, targets the ASL of tRNALys UUU8 Whereas none of these toxins require the presence of tRNA modifications for cleavage, there are other ACNases for which activity is dependent on such modifications. For example, the PaT toxin from Pichia acacia targets the methyl group of 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U34) containing tRNAGln UUG, as evidenced by the resistance of the mcm5s2U34 deficient Saccharomyces cerevisiae trm9Δ strain.9 Another example is the γ-toxin from Kluyveromyces lactis, which recognizes the wobble modification mcm5U of tRNAGlu UUC. 10
Figure 1.
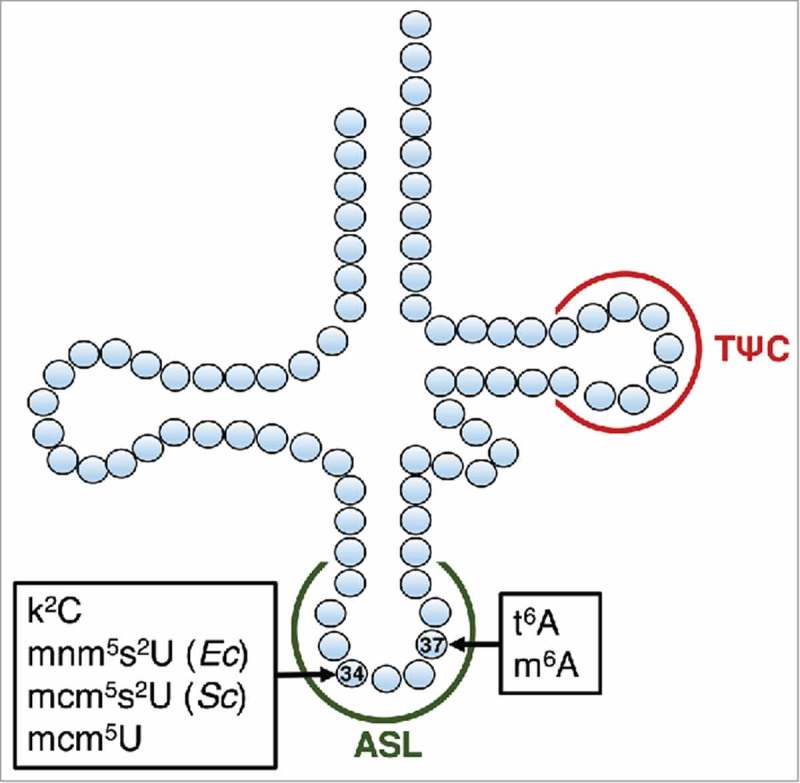
Mapping of nucleoside modifications and targets for oligo probes on tRNA. Modifications on positions 34 and 37 are listed in their respective boxes. Target for ASL probe is depicted in green and target for the TΨC probe is depicted in red.
One of the first identified ACNases was E. coli PrrC (PrrCEc).11 Infection of E. coli by bacteriophage T4 activates PrrC, which then cleaves the ASL of tRNALys UUU to disrupt translation of late T4 proteins (primarily structural and assembly viral proteins)12 and contain the infection in a suicidal gesture.2,13 PrrC has been shown to cleave native tRNALys UUU in vitro but not the corresponding unmodified transcript,14-16 leading to the hypothesis that a post-transcriptional modification on tRNALys was a requirement for PrrC activity. Two complex post-transcriptional modifications are found in the ASL of tRNALys in both E. coli and S. cerevisiae.17 First, the U at position 34 is modified to 5-methylaminomethyl-2-thiouridine (mnm5s2U) in E. coli and to 5-methoxycarbonylmethyluridine (mcm5U) in S. cerevisiae. Second, the A at position 37 is modified to threonylcarbamoyl adenosine (t6A) in both organisms (Fig. 1). In some organisms, t6A is further modified into complex derivatives such as cyclic-t6A (ct6A), N6-methyl-t6A (m6t6A),6,7 and 2-methylthio-t6A (ms2t6A).18 Unlike the γ-toxin from K. lactis, PrrC was still toxic when expressed in S. cerevisiae elp3Δ and trm9Δ derivatives (lacking mcm5U34).16 This left the t6A modification of tRNALys as a logical candidate for the missing positive determinant for cleavage by PrrC.
t6A is one of the few universal modifications on the ASL found in most tRNAs that decode ANN codons.17 The t6A synthesis pathway has recently been elucidated in all domains of life (see19 for review). In bacteria, the first enzyme L-threonylcarbamoyladenylate synthase (EC 2.7.7.87), which can be of type 1 (TsaC) or type 2 (TsaC2), produces L-threonylcarbamoyladenylate (TC-AMP). In yeast, the equivalent protein is encoded by the TCS2 (SUA5) gene. The TC-AMP intermediate is then used by the bacterial tRNA adenosine (37) threonylcarbamoyltransferase complex, which is composed of the 3 subunits TsaD, TsaB and TsaE to transfer the threonylcarbamoyl moiety to the target adenosine-37 of the target tRNA. In the yeast cytoplasm, the transferase complex is composed of the KEOPS complex subunits (encoded by the TCS3/KAE1, TCS5/BUD32, TCS6/PCC1, TCS7/CGI121, TCS8/GON7 genes) whereas TCS4 (QRI7) fulfills that role in yeast mitochondria. The tsaBCDE genes are generally essential in bacteria,20 and in E. coli, it was shown that t6A is a strict determinant for the bacterial-type isoleucyl-tRNA synthetase (IleRS) and possibly for lysidine synthase (TilS), roles that easily explain the essentiality phenotype. However, the t6A synthesis genes can be deleted in some bacteria, including Streptococcus mutans, an oral pathogen that thrives in multi-species biofilms on tooth surfaces.20 In this organism, the ΔtsaE (brpE/SMU.409) strain is viable although growth rate was affected. The mutant is also compromised in biofilm formation, and is more sensitive than the wild-type to low pH and oxidative stress.21 The tsaE/brpE gene is co-transcribed in S. mutans UA159 with brpA, which encodes a surface-associated protein with global effects on S. mutans biofilm formation and tolerance to antibiotic, acid, and oxidative stresses.22,23 In a systematic study of a gene deletion library of S. mutans, tsaC (SMU.1083c) was found to be dispensable and its deletion also led to poor acid survival and biofilm formation deficiencies.24 Using an HPLC based assay, we previously showed that the S. mutans ΔtsaE strain lacked t6A,20 but the t6A levels in the S. mutans tsaC mutant have not been analyzed. More generally, the molecular rationale underlying the pH sensitivity and biofilm defects of t6A deficient S. mutans strains is unknown.
Toxin/antitoxin modules such as MazE/F or RelB/E have been suggested to play a role in the survival of S. mutans in its harsh environment, possibly by triggering dormancy states.25,26 A prrC homolog is also found in S. mutans (SMU.893/PrrCSm) just upstream of the relBE toxin/antitoxin genes.25 The corresponding PrrCSm protein is comparably active as a cytotoxin in yeast as its E. coli counterpart.27 However, the role of PrrC in S. mutans physiology is not clear. The prrC gene is co-transcribed in an operon with the genes hdsMSR that encode the subunits of the type Ic DNA Restriction-Modification (R-M) complex prrI.28 PrrCEc is kept in an inactive state by binding to an EcoprrI/DNA complex, but the toxin can be activated by several mechanisms.28 One such example is during infection by T4 phage, which results in the binding of EcoprrI by T4-encoded polypeptide STP, thus releasing active PrrCEc. Other stresses that affect the activity of type I DNA restriction endonucleases also appear to activate PrrCEc and possibly disable protein synthesis.28 Although, it is known that increased levels of dTTP combined with GTP hydrolysis activates the PrrCEc ACNase, the triggers in other organisms such as S. mutans remain a mystery. Furthermore, it is unclear whether the PrrC toxin plays a role in dormancy or programed cell death (PCD), states known to be important for S. mutans survival.29
In this work, we show that t6A is a positive determinant for both the E. coli and S. mutans PrrC toxins. Using a newly developed and more sensitive than HPLC t6A detection method (based on the described previously “positive hybridization in the absence of i6A37” (PHA6) assay30-32), we demonstrate that t6A is not absent in the S. mutans ΔtsaE strain as previously thought, but is present at greatly reduced levels compared with wild type. The ΔtsaC and ΔtsaB mutants, however, were also viable but appeared to completely lack this modification. We also showed that in S. mutans, expression of the tsaBCE genes and t6A levels are growth-phase dependent, being greatly reduced in stationary phase.
Results
The modification t6A is a positive determinant for cleavage of tRNA by PrrC
If t6A is a determinant for PrrC, then t6A− yeast strains should be resistant to the PrrC-induced growth inhibition previously observed in WT cells.16 To test this hypothesis, a plasmid containing the E. coli prrC gene under the control of galactose inducible promoter (pYCPrrCEc)16 was transformed into wild-type (WT) and t6A deficient yeast strains. As shown Fig. 2A, the BY4741 (pYCPrrCEc) cells did not grow in the presence of galactose whereas the t6A deficient tcs3Δ (pYCPrrCEc) strain grew. The tcs4Δ strain (containing a deletion for the mitochondrial-specific t6A synthesis gene but with a fully functional cytosolic t6A biosynthesis machinery) was sensitive to the PrrC toxin similar to that observed in the WT strain. The strains carrying the control vector (pRS415) showed no difference in growth between induced and non-induced conditions.
Figure 2.

Resistance of t6A deficient yeast strains to PrrC toxins. A. The tcs3Δ is resistant to PrrCEc while mitochondrial tcs4Δ is not. B. tcs8Δ is resistant to both PrrCEc and PrrCSm, while the PrrCEc K46A variant has no effect on growth. Cells were grown in synthetic minimal media containing agar and leucine dropout supplement (SD-Leu). Galactose (2% w/v) and raffinose (1% w/v) were added where necessary (SD-Leu Gal/Raf). Strains were incubated at 30°C for ∼72 hours.
A difficulty when working with most t6A deficient yeast strains is their poor growth on minimal media supplemented with carbon sources other than glucose.33 The tcs8Δ strain is an exception as it is devoid of t6A but its growth on minimal media is robust enough for reproducible growth tests.33 This strain was thus used to compare the toxicity of the PrrCEc and S. mutans PrrC (PrrCSm) toxins in yeast and the role of t6A in this toxicity. The empty vector pYCplac111 and a catalytically inactive variant of PrrCEc bearing a Lys to Ala mutation at position 46 (PrrCEc K46A)16 were used as negative controls. As shown in Fig. 2B, tcs8Δ is resistant to the expression of PrrC toxins from both E. coli and S. mutans while BY4741 is not. As expected, overexpression of any of the control plasmids did not affect growth of the BY4741or tcs8Δ strains. These results confirmed the prediction that t6A is a positive determinant for the PrrC toxins from both E. coli and S. mutans.
Development of a sensitive hybridization based t6A detection assay
To explore the role of t6A in S. mutans physiology, a more sensitive detection assay was required. Inspired by the assay developed for the detection of i6A by Northern blot (PHA6),30 a Northern blot assay was developed for the detection of t6A.34 The premise of the assay is based on the carbamoylthreonyl group preventing hybridization of an ASL probe spanning position 37 (Fig. 1). It is predicted that the extent of hybridization will increase as the levels of t6A modification decrease. A probe spanning the TΨC loop of the tRNA can be used as an internal control for tRNA quantification. For this assay, purified bulk tRNAs were spotted and cross-linked onto neutral nylon membranes and detected with biotinylated probes (Table S1) to allow detection with streptavidin-labeled Horseradish Peroxidase (HRP).
tRNA extracted from yeast BY4741, tcs2Δ, tcs4Δ, and tcs3Δ strains, were spotted onto nylon membranes in duplicate. One membrane was treated with a biotinylated probe annealing to the TΨC loop and the second membrane was treated with the probe for tRNAIle IAU. As shown in Fig. 3A, only tRNAs from t6A deficient strains tcs2Δ and tcs3Δ hybridized with the ASL probe, while no hybridization is observed with BY4741 and minimal hybridization with tcs4Δ. tRNAs from all strains hybridized with the TΨC probe showing relative quantities of tRNAs spotted on the membrane. These results are consistent with t6A detection via HPLC.33 The limit of detection of this method for yeast bulk tRNA was found to be 10 ng (Fig. 3B).
Figure 3.
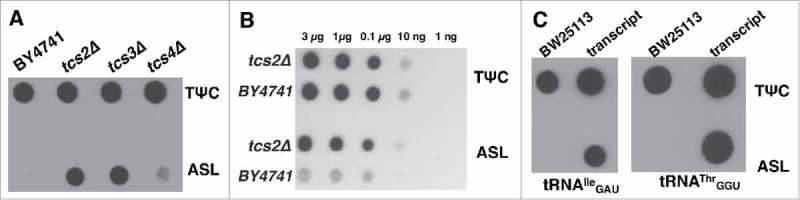
PHAt6A assay with yeast and E. coli bulk tRNAs. A. PHAt6A with tRNA isolated from wild type yeast and t6A deficient strains. B. Sensitivity of the PHAt6A method using yeast tRNAs. C. PHAt6A with wild type E. coli and in vitro transcribed tRNAs.
This assay was then extended to tRNAs from the bacterial model E. coli and to different tRNA isoacceptors. t6A is essential in E. coli making it impossible to isolate tRNAs from a t6A deficient strain, therefore in vitro transcribed tRNA was used as negative control using probes specific for tRNAIle GAU and tRNAThr GGU. As shown Fig. 3C, both wild type and in vitro transcribed tRNAs anneal to the TΨC probe, while only the transcripts anneal to the ASL probe. Similar results were obtained with probes specific for with E. coli tRNAThr GGU. This new assay was named Positive Hybridization in the Absence of t6A, or PHAt6A Assay. This method allows one to detect t6A in specific tRNAs with a sensitivity that now allows the exploration of the role of t6A in bacterial physiology generally, and more specifically in S. mutans.
t6A is not essential in S. mutans but the tsaE mutant does contain residual amounts of the modification
Previous analysis of bulk tRNA extracted from the S. mutans ΔtsaE strain using HLPC indicated that no t6A was present in that background.20 However, when using the PHAt6A method with probes specific for S. mutans tRNAIle GAU, faint annealing was observed, which made us consider that t6A may still be present (Fig. 4A). To investigate this result further, each corresponding tRNA preparation was analyzed by mass spectrometry (MS), which demonstrated that t6A was present in the ΔtsaE strain but in lower amounts (∼7% t6A modified) than the wild-type (Fig. 4B and Fig. S1A). This result made us question our prior conclusions on the dispensability of t6A in S. mutans.20 We therefore obtained the S. mutans UA159 ΔtsaC and ΔtsaB strains (kind gift from Robert Quivey, University of Rochester),24 and analyzed t6A levels using PHAt6A with the probe targeting tRNAIle GAU. As shown in Fig. 4C, strong annealing of the ASL probe was observed in the tRNA extracted from the ΔtsaC and ΔtsaB strains but also from the ΔtsaE mutant. This ambiguity in the PHAt6A necessitated MS analysis (Fig. 4B) of the tRNA samples. Indeed ΔtsaC and ΔtsaB are devoid of t6A while ΔtsaE maintains trace amounts of t6A modified tRNA. With only ∼7% of the WT t6A levels present in the ΔtsaE strain, we are at the limit of the detection of the PHAt6A modification and under the limit of regular HPLC.20 Among the platforms for t6A detection, only MS offers the sensitivity to detect such low levels of the modification.
Figure 4.
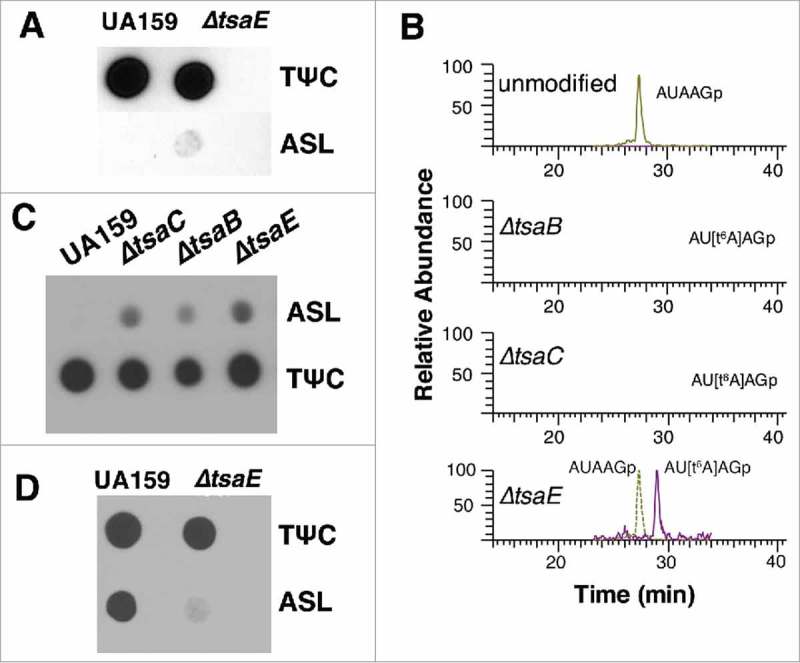
Detection of t6A in S. mutans wild-type and mutant strains. A. PHAt6A with S. mutans ΔtsaE. B. Mass spec analysis of t6A modification in tRNAIle GAU showing no t6A in ΔtsaC and ΔtsaB but trace amounts are detected in ΔtsaE. C. PHAt6A with t6A deficient strains ΔtsaC, ΔtsaB, ΔtsaE using oligonucleotides specific to tRNAIle GAU. D. PHAt6A with wild type UA159 and ΔtsaE using probes for tRNAiniMet CAU.
We extended the method to other S. mutans tRNAs and found that tRNAiniMet CAU, a tRNA known to not be t6A modified in bacteria,35 showed reduced annealing in the ΔtsaE strain compared with WT. This suggests tsaE might play a role in tRNA discrimination in the t6A insertion machinery. It is possible that tRNAiniMet CAU is targeted for t6A modification by mistake when tsaE is absent. Further analysis is required to fully elucidate this observation.
Analysis of tsaBCDE expression levels and t6A content in S. mutans in different growth conditions
At the time of the analysis (March 2017 release), 22 transcriptomics experiments for the S. mutans U159 strain had been integrated in the PATRIC36 and 25 in the Microbesonline databases.37 Using the transcriptomics heatmap analysis tools of pathway databases, we surveyed expression profiles of prrC (SMU.893) and of the four t6A synthesis genes (tsaC/SMU.1083c; tsaB/SMU.385; tsaD/SMU.387; tsaE/SMU.409) and found a few conditions where these genes were differentially-expressed, such as biofilm vs. planktonic growth or when co-cultured with other oral bacteria (Table S2).
As mentioned above, the available transcriptomic data did suggest that the genes encoding for the t6A synthesis proteins might be differentially expressed in biofilm vs planktonic growth. To explore this further, cells were grown in rich (BHI) media and in semi-defined media containing sucrose (which promotes biofilm growth).38 Cells were harvested from these two culture conditions at four different growth phases: early exponential (4 h growth), mid exponential (6 h growth), late exponential (8 h growth), and stationary phase (12 h growth) (Fig. S2). tRNA was extracted from cells harvested from each growth phase and media type, and analyzed via PHAt6A and by LC-MS/MS. The PHAt6A showed an increase in signal in the ASL from early exponential (4 h) to stationary phase (12 h) (Fig. 5), suggesting that t6A levels decrease in a growth-phase dependent manner. The samples were analyzed via MS on two separate occasions and both analyses showed a similar trend of decreasing t6A levels along the growth curve for both BHI and biofilm media. However, there was no statistically significant difference in the degree of t6A modification for each of these time points (Figure S3). This seeming disparity in results between the two methods arises from the scope of each analysis. MS quantifies t6A modification from all tRNA isoacceptors where subtle variations in t6A levels of a few tRNA species may be undetectable. In contrast, PHAt6A reports only on a specific tRNA species allowing for isoacceptor-specific monitoring of t6A levels. Expression of prrC and of the t6A biosynthesis genes were also measured by quantitative real-time PCR (qPCR) in the same set of biological samples. Expression of the t6A synthesis genes correlated well with the PHAt6A results, whereby expression of most t6A biosynthesis genes and of prrC decreased during stationary phase in both BHI and biofilm media (Fig. 6A). The only exception to this pattern was in tsaD expression, which increased in stationary phase relative to exponential growth phase. The same pattern of growth phase dependent gene expression was observed in both BHI and biofilm media cultures (Fig. 6A). Moreover, when directly comparing expression of each gene between growth conditions at each time point (Fig. 6B), stationary phase tsaC expression was increased 2.5-fold in biofilm media relative to BHI.
Figure 5.

Detection of t6A in S. mutans UA159 tRNA grown in Biofilm and Rich Media. PHAt6A of tRNA isolated from the following time points: 4h – Early-exponential, 6h – Mid-exponential, 8h – Late-exponential, and 12h – Stationary phase.
Figure 6.

S. mutans UA159 gene expression profile for ΔtsaC, ΔtsaB, ΔtsaD, ΔtsaE, and prrC genes during growth in Biofilm and Rich media (BHI). A. Fold change difference of each of the genes with respect to 4h time point (calibrator). * indicates statistical significance (P < 0.05, Student-Newman-Keuls Test) relative to calibrator sample. B. Fold change difference in gene expression at each time point in Biofilm media with respect to Rich media (calibrator). * indicates statistical significance (P < 0.05, Two-tailed T-Test) relative to calibrator sample.
Discussion
Recent studies in different model organisms have shown that levels of tRNA modification can be fine-tuned to specifically regulate the translation efficiency of specific genes.39,40 To date, no such regulation has been observed with t6A dependent codons, and it is not known whether t6A levels are regulated in any model system. Indeed, the identity of the complete set of genes involved in t6A synthesis were only discovered within the last 5 years.41,42 In addition, the methods available for t6A detection did not allow, until very recently, any physiologic studies that could address potential regulation mechanisms. Advances in MS analytical methods, as well as the hybridization based PHAt6A assay, described in this present paper, have now solved this issue. Using these tRNA modification profiling platforms, the ratios of specific modifications found in bulk tRNA extracted from different conditions can be captured. Recent studies have shown that levels of t6A and ms2t6A vary accordingly to fluctuations in tRNA modification profiles in Mycobacterium tuberculosis under hypoxic conditions,40 and yeast under different stress conditions.43 The MS platform is quantitative for the total amount of t6A but is unable to discriminate between the different tRNAs. To identify the specific tRNA modifications in a sequence context, another MS-based platform called the RNA modification mapping approach can be used. This method involves base-specific RNase digestion to the oligonucleotide level. These RNase digestion products are separated and analyzed by LC-MS/MS from which the identity and site of modification can be determined.44 Whereas MS-based platforms are accurate and robust, the cost of, and access to, such detection platforms is a major challenge. Herein lies the advantage of the PHAt6A assay, a cost-effective and universally accessible method for t6A detection. This method allows detection of t6A levels in specific tRNAs in very low sample quantities (only 10 ng of bulk tRNA are required) therefore facilitating analysis of samples from limited biological sources. Moreover, PHAt6A enables one to survey t6A levels in many replicates over multiple growth conditions. In fact, this assay has already been used to monitor t6A variations in TCS3 mutants in Drosophila melanogaster.34 With the recent discovery that mutations in the Human Tcs4 (Kae1) gene lead to severe disease,45 methods to easily detect t6A levels in human cells could have diagnostic value.
The PHAt6A assay was used to analyze t6A levels in different tRNAs extracted from S. mutans WT, ΔtsaE, ΔtsaC and ΔtsaD strains and showed that: 1) t6A is dispensable in S. mutans; 2) tsaC and tsaB are strictly required for t6A synthesis; 3) the absence of tsaE significantly reduces the amounts of t6A, and TsaE is not strictly required for t6A synthesis, instead possibly playing a role in specificity (targeting the correct tRNA) or in regulation of t6A levels.
The first study on regulation of t6A synthesis genes was recently published in the Mycobacterium tuberculosis model.46 The tsaD, tsaB and tsaE genes are in the same operon with quite a complex regulation. Specific expression of tsaD was observed under a few conditions such as H2O2 exposure.46 The S. mutans tsaE (brpB) gene is co-transcribed with the regulator gene brpA22 (Figure S4), which plays a role in resistance to various antibiotic and environmental stressors.22,23 The S. mutans tsaB and tsaD genes are in a predicted operon that encodes a MarR-type regulator (SMU.384) of unknown function37 (Figure S4). Results from available transcriptomic data (Table S2) and the qPCR results presented here (Fig. 6 and Supplemental data 1), do suggest that the expression of S. mutans t6A genes is growth-phase dependent, but further experiments will be required to fully understand the nature of this regulation.
The PrrC ACNases from E. coli or S. mutans were only toxic in yeast strains harboring t6A. Combined with the prior results that showed that PrrC was toxic to strains missing the mcm5U34 modification,47 it is clear t6A is a positive determinant for PrrC. Attempts to purify recombinant WT PrrC for structure/function studies are confounded by the inhibition of protein synthesis elicited when PrrC begins to accumulate. The tcs8Δ yeast strain is resistant to PrrC but grows better than the other t6A deficient yeast strains and could be used as a host for the large-scale expression of the toxin.
The presence of PrrC in S. mutans, an organism where t6A is dispensable, raises questions and avenues for future work. Recent single cell analysis experiments have shown that subpopulations of S. mutans cells in biofilm have different fates: growth, dormancy or death depending on the expression of specific toxins.48 Given that both prrC and tsaE expression are downregulated in a brpA mutant (Table S2) and BrpA is thought to respond to cell envelope stress,22 could downregulation of t6A levels protect a subpopulation from stress-induced PrrC? Further studies are required to explore the physiologic role of PrrC in S. mutans and involvement of t6A in mechanisms of toxin resistance.
Methods
Strains and growth conditions
A list of all organisms used in this study can be found in Table S3. Yeast strains were grown on YPD (DIFCO Laboratories) at 30°C. Synthetic minimal media, with or without agar, with or without dropout supplements (-uracil, -ura; -leucine, -leu; -histidine, -his) were purchased from Clontech (Palo Alto, CA) and prepared as recommended by the manufacturer. Glucose (Glu, 2% w/v), Glycerol (Gly, 4% w/v), 5-fluoro-orotic acid (5-FOA, 0.1% w/v) and G418 (300 μg/mL) were used when appropriate. Yeast transformations were performed using frozen competent cells as described49 with plating onto the appropriate media. E. coli strains were grown in LB (1% tryptone w/v, 0.5% yeast extract w/v, and 1% salt w/v; 1.5% agar w/v was added for plates) at 37°C, unless otherwise stated. When necessary, LB was supplemented with kanamycin (Kn, 50 μg/mL), ampicillin (Ap, 100 μg/mL), or chloramphenicol (Cm, 35 μg/mL). S. mutans was grown in Brain Heart Infusion media (semi-defined media containing sucrose as described in [Ref 38]) at 37°C in a CO2 incubator. When necessary, 5 µg/mL of erythromycin, 50 μg/mL kanamycin, and 50 μg/mL spectinomycin was added. For phenotype screens, yeast cultures were grown in the media listed in the figure to saturation, washed, normalized to an OD600 of 1.0 and 5 µL of 1:10 serial dilutions were spotted on the listed media with the supplements listed in the figure and text. Galactose (2% w/v) and Raffinose (1% w/v) was added when needed.
Bioinformatics
Transcriptomics data was taken from the PATRIC database where as of March 2017, 22 experiments for S. mutans were available.36 Microbesonline was also used as a resource for microarray data and operon prediction for S. mutans.37 Resources at the National Center for Biotechnology Information (NCBI) and BLAST tools were used.50 tRNA gene sequences were taken from the GtRNAdb: Genomic tRNA Database.51
Extraction of bulk tRNAs and preparation of in vitro transcribed tRNA
Bulk tRNA were prepared as described previously using acid buffered-phenol (phenol saturated with 50 mM sodium acetate, pH 5.8) and alcohol precipitation.52 The template for producing E. coli tRNAIle GAU transcript was produced via a Klenow extension reaction53 with the oligonucleotides 5′-AATTCCTGCAGTAATACGACTCACTATAAGGCTTGTAGCTCAGGT GGTTAGAGCGC-3′ and 5′-TGGTAGGCCTGAGTGGACTTGAACCACCGACCTCACCCTT ATCAGGGGTGCGCTCTAAC-3′. The template for E. coli tRNAThr GGU used the plasmid pCDI147 which had been linearized by MvaI to allow for run-off transcription. pCDI147 was generated using 2 ligation events. First, the ligation of 6 oligonucleotides 5′-AGCTTTAATA CGACTCACTATAGGGGCTGATATGGCTCAG-3′, 5′-TTGGTAGAGCGCACCCTTGGTAG GGGTGGGGTCCCCAGTTCGACTCTGGG-3′, 5′-TATCAGCACCATATGCTAGTTATTGC TCAGG-3′, 5′-GATCCCTGAGCAATAACTAGC-3′, 5′-ATATGGTGCTGATACCCAGAGTC GACTGGGGACCCCACCCCTACCAAGGG-3′, and 5′-TGCCTCTACCAACTGAGCCATAT CAGCCCCTATAGTGAGTCGTATTAA-3′. This oligonucleotide was subsequently digested with BamHI/HindIII before a second ligation into a similarly treated pUC18 plasmid to generate pCDI147. Transcription reactions were run for 4 hours at 37°C in 80 mM HEPES (pH 7.4), 2.0 mM spermidine, 24 mM MgCl2, 2.0 mM ribonucleotide triphosphates (NTPs), 3 μM template, and 2.5 μg/mL of T7 polymerase. The RNA products generated in the transcription reactions were precipitated by the addition of 0.1 volume 8.0 M ammonium acetate, 3 volumes of 100% ethanol, and cooling at −80°C for 30 minutes, then pelleted by centrifugation at 15,000 RCF for 30 minutes at 4°C, and resuspended in 50 mM HEPES (pH 7.4), 2.0 mM EDTA. The solutions were mixed 1:1 with formamide, heated at 90°C for 5 minutes, and snap cooled on ice before being purified via Urea-PAGE electrophoresis (10%). The RNA was extracted by cutting the excised band from the Urea-PAGE gel, slicing it into 1 cm cubes, followed by adding 10 mL HEPES (pH 7.4) 2 mM EDTA per 1 g of gel. This suspension was then placed at 4°C with agitation overnight. The soluble portion of the suspension was then precipitated as described previously and resuspended in water. The tRNA solution was then frozen at −80°C before lyophilization.
Positive Hybridization in the absence of t6A Assay
Blotting. tRNAs were diluted to the appropriate concentration (3 µg – 1ng/µL) to which 3 volumes of denaturing solution (500 µL formamide, 162 µL 37% formaldehyde, 100 µL RNase-free 10X MOPS) was added. tRNAs were denatured at 85°C for 15 min and cooled to 4°C for 2 min and the final volume was adjusted to 30 µL with 10X SSC (1.5 M sodium chloride, 0.15 M sodium citrate). Biodyne® A membrane (Thermo Scientific) was rehydrated in 10X SSC for at least 10 min and placed in a dot blot vacuum manifold (Bio-Rad). Each of the wells were rinsed twice with 0.5 mL 10X SSC before applying the denatured tRNA samples. The wells were rinsed twice with 10X SSC before removing the membrane from the apparatus. The membrane was dried at in an 80°C incubator for 30 seconds followed by RNA crosslinking at 120 mJ/cm2 (optimal crosslink mode in Fisher Biotech UV Crosslinker FB-UV XL-1000).
Hybridization. The membrane was rehydrated in 10X SSC for 1 minute and pre-hybridized for 30–60 min at 42°C with pre-warmed Dig Easy Hyb (Roche). One µL of 100 µM biotinylated probes (Listed in Table S1) per 5 cm2 membrane was added to 80 µL DIG Easy Hyb and heat denatured at 95°C for 10 minutes followed by cooling to 4°C for 2 min. The denatured probes were applied to the pre-hybridized membrane and incubated at 39°C for at least 16 hours with moderate rocking.
Washing. Hybridization solution was poured off and 2 types of washes was implemented: The low stringency wash consists of 3 washes with wash buffer (2x SSC/0.2% SDS) for 10 min while the high stringency wash adds 1 additional wash at room temp for 10 min and 1 final wash at 55°C for 15 min. For S. cerevisiae tRNAIle GAU, membrane probed with TΨC was subjected to low stringency washing while the membrane probed with ASL required the high stringency wash to reduce background. For E. coli and S. mutans tRNAs, low stringency washing for both membranes were sufficient. Visualization was performed as described by the manufacturer of North2South Chemiluminescent Detection Kit (Thermo Scientific No. 17097)
Quantitative real-time PCR (qPCR)
All S. mutans UA159 cultures were grown at 37 °C, 0 RPM in a 5% CO2 incubator. For each experiment, S. mutans was freshly streaked from a 40% (vol vol−1) glycerol stock (stored at −80°C) onto Brain heart infusion (BHI) agar and grown for 48 h. A single colony was then inoculated into 40 ml BHI broth, and grown for 18 hours. For each growth experiment (n = 3), the S. mutans 18 hour culture was diluted to an optical density at 600 nm (OD600) = 0.05, in a 0.4 media/flask volume ratio, and grown in BHI or Biofilm Media containing 11 mM glucose and 10 mM sucrose.38 Culture samples were collected from each flask at 4, 6, and 12 hours growth (corresponding to early exponential, late exponential, and stationary phase), harvested by centrifugation, and cell pellets were stored at −80 °C in RNAlater (Thermo Fisher Scientific). RNA was subsequently isolated with the RNeasy Kit (Qiagen) and FASTPREP lysing matrix B tubes (MP Biomedical) using previously-described methods.54,55 Each RNA sample was then subjected to a second DNAse treatment using the TURBO DNA-free™ Kit (Thermo Fisher Scientific) per the manufacturer's protocols. Lack of contaminating genomic DNA in each RNA sample was determined using PCR and S. mutans gyrB primers. RNA samples (0.750 µg) were subsequently converted to cDNA using the iScript Reverse Transcriptase kit (BioRad). Expression of genes of interest was measured in the cDNA from each sample by qPCR using iQ SYBR green supermix (BioRad) and the CFX Connect System (BioRad) following previously-published qRT-PCR protocols.56 The Livak method (2−ΔΔCt)57 was used to calculate the relative fold change between the calibrator samples (indicated in each figure legend) and test samples. Primers specific to the housekeeping gene gyrB (gyrB-F/gyrB-R) were used as the reference gene (Table S4).
Mass spectrometry
Total tRNA samples were digested with purified RNase T1 (50 U/μg tRNA) in a 220 mM ammonium acetate buffer for 2 h at 37°C. Samples were vacuum dried and resuspended with 10 μL mobile phase A for LC-MS/MS analysis. The RNase digestion products were separated on a Poroshell 120 EC-C18 column (1 × 50 mm and 2.7 μm pore size, column oven at 30°C) using a Thermo Surveyor HPLC attached to a Thermo LTQ-XL (Thermo Scientific, Waltman, MA) linear ion trap mass spectrometer. Mobile phase A (MPA) consists of 8 mM TEA/200 mM HFIP, pH 7 and mobile phase B is 50% MPA and methanol with a flow rate set at 50 μL/min. The LC gradient initiated at 10%B then increased linearly to 60%B for 32 min, followed by 95%B for 5 min before a minimum 20 min re-equilibration period at 10%B. The source was set at the following conditions: capillary temperature was set at 275°C, spray voltage of 4 kV and 35, 14 and 10 arbitrary flow units of sheath, auxiliary and sweep gas, respectively. The mass spectra were recorded in negative polarity. The entire run was divided into two segments, each with five scan events. The product ion's sequence information was obtained by collision induced dissociation (CID) in scan events 2–5. Data acquisition was through Thermo Xcalibur software.
Analysis of t6A modifications in S. mutans grown in rich media and biofilm media
Bulk tRNA (1 μg) from each sample was hydrolyzed to ribonucleosides in a reaction containing Benzonase (0.375 U), calf intestine alkaline phosphatase (8.5 U), phosphodiesterase I (0.05 U), coformycin (3.5 μm; nucleobase deaminase inhibitor), deferoxamine (3 mm; antioxidant), butylated hydroxytoluene (0.3 mm; antioxidant) HEPES (500 mM, pH 8) and MgCl2 (5 mm) in a final reaction volume of 50 μl. The reaction was allowed to proceed for 2 h at 37 °C and was stopped by removal of the enzymes by microfiltration with 10,000 Da spin filters. Following the addition of [15N5]-2′-deoxyadenosine as an internal standard for data normalization, ribonucleosides were resolved on a Synergy Fusion RP HPLC column (2.5 μm particle size, 100 Å pore size, 100 mm length, 2 mm inner diameter; Phenomenex, Torrance, CA, USA) mounted on an Agilent 1290 series HPLC system equipped with a diode array detector (DAD). The ribonucleosides were eluted at a flow rate of 0.35 ml/min and a column temperature of 35 °C with a gradient consisting of 5 mm ammonium acetate (A) and acetonitrile (B) as follows: 0–1 min 100% A, 1–10 min 0–10% B, 10–24 min 10–40% B, 24–44 min 40–80% B, and 44–49 min 100% A to regenerate the column. The column, with its eluent directed through the DAD to record the 260 nm absorbance of canonical ribonucleosides, was coupled to an Agilent 6430 triple quadrupole mass spectrometer operated in positive ion mode with the following parameters: electro-spray ionization (ESI-MS), fragmentor voltage (average) 80 V, cell accelerator voltage 2 V, N2-gas temperature 350 °C, N2-gas flow 10 l/min, nebulizer 40 p.s.i., capillary 3500 V. Using dynamic multiple reaction monitoring (MRM), modified ribonucleosides were identified based on retention time (t6A at 7.9–8.3 min) and mass transition (m/z 413→281 for loss of ribose from t6A). The signal for t6A was normalized by dividing by the peak area of the [15N5]-dA standard (inter-sample variation) and by the summed MRM peak areas of the canonical ribonucleosides (input RNA variation). The normalized peak areas of 3 biologic replicates were then averaged.
Supplementary Material
Disclosure of potential conflict of interest
No potential conflict of interest were disclosed.
Acknowledgments
We would like to thank Robert Quivey (University of Rochester) for the S. mutans ΔtsaC and ΔtsaB strains, and Tom Wen (Louisiana State University Health Sciences Center) for the S. mutans ΔbrpB strain. We would also like to thank Lin Zheng and Robert Burne (University of Florida) for sharing their transcriptomics results on S. mutans.
Funding
This work was supported by the National Institutes of Health (NIH) under grant R01 GM70641 to Valérie de Crécy-Lagard, partially under grant AI118999 to Kelly C. Rice, and the Florida Education Fund McKnight Doctoral Fellowship awarded to Silvia S. Orsini.
References
- 1.El Yacoubi B, Bailly M, de Crécy-Lagard V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Ann Rev Gen 2012; 46:69-95; https://doi.org/ 10.1146/annurev-genet-110711-155641 [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann G. Anticodon nucleases. Trends Biochem Sci 2000; 70-74; https://doi.org/ 10.1016/S0968-0004(99)01525-X [DOI] [PubMed] [Google Scholar]
- 3.3. Cruz JW, Woychik NA. tRNAs taking charge. Pathogens Dis 2016; 74:ftv117; https://doi.org/ 10.1093/femspd/ftv117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa T, Tomita K, Ueda T, Watanabe K, Uozumi T, Masaki H. A cytotoxic ribonuclease targeting specific transfer RNA anticodons. Science 1999; 283:2097-100; https://doi.org/ 10.1126/science.283.5410.2097 [DOI] [PubMed] [Google Scholar]
- 5.Tomita K, Ogawa T, Uozumi T, Watanabe K, Masaki H. A cytotoxic ribonuclease which specifically cleaves four isoaccepting arginine tRNAs at their anticodon loops. Proc Natl Acad Sci 2000; 97:8278-83; https://doi.org/ 10.1073/pnas.140213797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz JW, Sharp JD, Hoffer ED, Maehigashi T, Vvedenskaya IO, Konkimalla A, Husson RN, Nickels BE, Dunham CM, Woychik NA. Growth-regulating Mycobacterium tuberculosis VapC-mt4 toxin is an isoacceptor-specific tRNase. Nat Commun 2015; 6:7480; https://doi.org/ 10.1038/ncomms8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winther KS, Gerdes K. Enteric virulence associated protein VapC inhibits translation by cleavage of initiator tRNA. Proc Natl Acad Sci 2011; 108:7403-7407; https://doi.org/ 10.1073/pnas.1019587108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schifano JM, Cruz JW, Vvedenskaya IO, Edifor R, Ouyang M, Husson RN, Nickels BE, Woychik NA. tRNA is a new target for cleavage by a MazF toxin. Nucleic Acids Res 2016; 44(3):1256-70; https://doi.org/ 10.1093/nar/gkv1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klassen R, Paluszynski JP, Wemhoff S, Pfeiffer A, Fricke J, Meinhardt F. The primary target of the killer toxin from Pichia acaciae is tRNAGln. Mol Microbiol 2008; 69:681-97; https://doi.org/ 10.1111/j.1365-2958.2008.06319.x [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Huang B, Esberg A, Johansson MJO, Byström AS. The Kluyveromyces lactis gamma-toxin targets tRNA anticodons. RNA 2005; 11:1648-54; https://doi.org/ 10.1261/rna.2172105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levitz R, Chapman D, Amitsur M, Green R, Snyder L, Kaufmann G. The optional E. coli prr locus encodes a latent form of phage T4-induced anticodon nuclease. EMBO J 1990; 9:1383-9; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC551823/pdf/emboj00232-0051.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabussay D, Geiduschek EP. Phage T4-modified RNA polymerase transcribes T4 late genes in vitro. Proc Natl Acad Sci U S A 1977; 74:5305-9; https://doi.org/ 10.1073/pnas.74.12.5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann G, David M, Borasio GD, Teichmann A, Paz A, Amitsur M. Phage and host genetic determinants of the specific anticodon loop cleavages in bacteriophage T4-infected Escherichia coli CTr5X. J Mol Biol 1986; 188:15-22; https://doi.org/ 10.1016/0022-2836(86)90476-6 [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Meidler R, Amitsur M, Kaufmann G. Specific interaction between anticodon nuclease and the tRNALys wobble base. J Mol Biol 2001; 305:377-88; https://doi.org/ 10.1006/jmbi.2000.4282 [DOI] [PubMed] [Google Scholar]
- 15.Meidler R, Morad I, Amitsur M, Inokuchi H, Kaufmann G. Detection of anticodon nuclease residues involved in tRNALys cleavage specificity. J Mol Biol 1999; 287:499-510; https://doi.org/ 10.1006/jmbi.1999.2634 [DOI] [PubMed] [Google Scholar]
- 16.Meineke B, Schwer B, Schaffrath R, Shuman S. Determinants of eukaryal cell killing by the bacterial ribotoxin PrrC. Nucleic Acids Res 2011; 39:687-700; https://doi.org/ 10.1093/nar/gkq831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Machnicka MA, Milanowska K, Oglou OO, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al.. MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res 2013; 41:262-7; https://doi.org/ 10.1093/nar/gks1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarin LP, Leidel SA. Modify or die?–RNA modification defects in metazoans. RNA Biol 2014; 11:1555-67; https://doi.org/ 10.4161/15476286.2014.992279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thiaville PC, Iwata-Reuyl D, de Crécy-Lagard V. Diversity of the biosynthesis pathway for threonylcarbamoyladenosine (t6A), a universal modification of tRNA. RNA Biol 2014; 11:1529-39; https://doi.org/ 10.4161/15476286.2014.992277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiaville PC, Yacoubi BE, Kohrer C, Thiaville JJ, Deutsch C, Iwata-Reuyl D, Bacusmo JM, Armengaud J, Bessho Y, Wetzel C, et al.. Essentiality of threonylcarbamoyladenosine (t6A), a universal tRNA modification in bacteria. Mol Microbiol 2015; 98:1199-221; https://doi.org/ 10.1111/mmi.13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bitoun JP, Liao S, Xie GG, Beatty WL, Wen ZT. Deficiency of BrpB causes major defects in cell division, stress responses and biofilm formation by Streptococcus mutans. Microbiology 2014; 160:67-78; https://doi.org/ 10.1099/mic.0.072884-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bitoun JP, Liao S, Yao X, Ahn S-J, Isoda R, Nguyen AH, Brady LJ, Burne RA, Abranches J, Wen ZT. BrpA Is Involved in Regulation of Cell Envelope Stress Responses in Streptococcus mutans. Appl Environ Microbiol 2012; 78:2914-22; https://doi.org/ 10.1128/AEM.07823-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen ZT, Burne RA. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl Environ Microbiol 2002; 68:1196-203; https://doi.org/ 10.1128/AEM.68.3.1196-1203.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quivey RG, Grayhack EJ, Faustoferri RC, Hubbard CJ, Baldeck JD, Wolf AS, MacGilvray ME, Rosalen PL, Scott-Anne K, Santiago B, et al.. Functional profiling in Streptococcus mutans: construction and examination of a genomic collection of gene deletion mutants. Mol Oral Microbiol 2015; 30:474-95; https://doi.org/ 10.1111/omi.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemos JA, TA, Brown JA Jr, Burne RA. Characteristics of Streptococcus mutans strains lacking the MazEF and RelBE toxin-antitoxin modules. FEMS Microbiol Lett 2005; 253:251-7; https://doi.org/ 10.1016/j.femsle.2005.09.045 [DOI] [PubMed] [Google Scholar]
- 26.Syed MA, Koyanagi S, Sharma E, Jobin MC, Yakunin AF, Levesque CM. The chromosomal mazEF locus of Streptococcus mutans encodes a functional type II toxin-antitoxin addiction system. J Bacteriol 2011; 193:1122-30; https://doi.org/ 10.1128/JB.01114-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meineke B, Shuman S. Determinants of the cytotoxicity of PrrC anticodon nuclease and its amelioration by tRNA repair. RNA 2012; 18:145-54; https://doi.org/ 10.1261/rna.030171.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uzan M, Miller ES. Post-transcriptional control by bacteriophage T4: mRNA decay and inhibition of translation initiation. Virol J 2010; 7:360; https://doi.org/ 10.1186/1743-422X-7-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung V, Dufour D, Celine M. Lévesque. Death and survival in Streptococcus mutans: Differing outcomes of a quorum-sensing signalling peptide. Front Microbiol 2015; 6:1176; https://doi.org/ 10.3389/fmicb.2015.01176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamichhane TN, Blewett NH, Crawford AK, Cherkasova VA, Iben JR, Begley TJ, Farabaugh PJ, Maraia RJ. Lack of tRNA modification isopentenyl-A37 alters mRNA decoding and causes metabolic deficiencies in fission yeast. Mol Cell Biol 2013; 33:2918-29; https://doi.org/ 10.1128/MCB.00278-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamichhane TN, Mattijssen S, Maraia RJ. Human cells have a limited set of tRNA anticodon loop substrates of the tRNA Isopentenyltransferase TRIT1 Tumor Suppressor. Mol Cell Biol 2013; 33:4900-8; https://doi.org/ 10.1128/MCB.01041-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarham JW, Lamichhane TN, Pyle A, Mattijssen S, Baruffini E, Bruni F, Donnini C, Vassilev A, He L, Blakely EL, et al.. Defective i6A37 modification of mitochondrial and cytosolic tRNAs results from pathogenic mutations in TRIT1 and its substrate tRNA. PLoS Genet 2014; 10:e1004424; https://doi.org/ 10.1371/journal.pgen.1004424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiaville PC, Legendre R, Rojas-Benítez D, Baudin-Baillieu A, Hatin I, Chalancon G, Glavic A, Namy O, de Crécy-Lagard V. Global translational impacts of the loss of the tRNA modification t6A in yeast. Microb Cell 2016; 3:29-45; https://doi.org/ 10.15698/mic2016.01.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojas-Benitez D, Thiaville PC, de Crécy-Lagard V, Glavic A. The levels of a universally conserved tRNA modification regulate cell growth. J Biol Chem 2015; 290:18699-707; https://doi.org/ 10.1074/jbc.M115.665406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jühling F, Mörl M, Hartmann RK, Sprinzl M, Stadler PF, Pütz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res 2009; 37:D159-62; https://doi.org/ 10.1093/nar/gkn772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wattam AR, Davis JJ, Assaf R, Boisvert S, Brettin T, Bun C, Conrad N, Dietrich EM, Disz T, Gabbard JL, et al.. Improvements to PATRIC, the all-bacterial Bioinformatics database and analysis resource center. Nucleic Acids Res 2017; 45:D535-42; https://doi.org/ 10.1093/nar/gkw1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dehal PS, Joachimiak MP, Price MN, Bates JT, Baumohl JK, Chivian D, Friedland GD, Huang KH, Keller K, Novichkov PS, et al.. MicrobesOnline: an integrated portal for comparative and functional genomics. Nucleic Acids Res 2010; 38:D396-400; https://doi.org/ 10.1093/nar/gkp919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas I, Drake L, Biswas S. Regulation of gbpC expression in Streptococcus mutans. J Bacteriol 2007; 189:6521-31; https://doi.org/ 10.1128/JB.00825-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duechler M, Leszczyńska G, Sochacka E, Nawrot B. Nucleoside modifications in the regulation of gene expression: focus on tRNA. Cell Mol Life Sci 2016; 73:3075-95; https://doi.org/ 10.1007/s00018-016-2217-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chionh YH, McBee M, Babu IR, Hia F, Lin W, Zhao W, Cao J, Dziergowska A, Malkiewicz A, Begley TJ, et al.. tRNA-mediated codon-biased translation in mycobacterial hypoxic persistence. Nat Commun 2016; 7:13302; https://doi.org/ 10.1038/ncomms13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deutsch C, El Yacoubi B, de Crécy-Lagard V, Iwata-Reuyl D. Biosynthesis of threonylcarbamoyl adenosine (t6A), a universal tRNA nucleoside. J Biol Chem 2012; 287:13666-73; https://doi.org/ 10.1074/jbc.M112.344028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin C-J, Smibert P, Zhao X, Hu JF, Ramroop J, Kellner SM, Benton MA, Govind S, Dedon PC, Sternglanz R, et al.. An extensive allelic series of Drosophila kae1 mutants reveals diverse and tissue-specific requirements for t6A biogenesis. RNA N Y N 2015; 21:2103-18; https://doi.org/ 10.1261/rna.053934.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai WM, Chionh YH, Hia F, Gu C, Kellner S, McBee ME, Ng CS, Pang YLJ, Prestwich EG, Lim KS, et al.. A platform for discovery and quantification of modified ribonucleosides in RNA: Application to stress-induced reprogramming of tRNA modifications. Methods Enzymol 2015; 560:29-71; https://doi.org/ 10.1016/bs.mie.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross R, Cao X, Yu N, Limbach PA. Sequence mapping of transfer RNA chemical modifications by liquid chromatography tandem mass spectrometry. Methods San Diego Calif 2016; 107:73-8; https://doi.org/ 10.1016/j.ymeth.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edvardson S, Prunetti L, Arraf A, Haas D, Bacusmo JM, Hu JF, Ta-Shma A, Dedon PC, de Crécy-Lagard V, Elpeleg O. tRNA N6-adenosine threonylcarbamoyltransferase defect due to KAE1/TCS3 (OSGEP) mutation manifest by neurodegeneration and renal tubulopathy. Eur J Hum Genet EJHG 2017; 25:545-51; https://doi.org/ 10.1038/ejhg.2017.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhat AH, Pathak D, Rao A. The alr-groEL1 operon in Mycobacterium tuberculosis: an interplay of multiple regulatory elements. Sci Rep 2017; 7:43772; https://doi.org/ 10.1038/srep43772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meineke B, Schwer B, Schaffrath R, Shuman S. Determinants of eukaryal cell killing by the bacterial ribotoxin PrrC. Nucleic Acids Res 2011; 39:687-700; https://doi.org/ 10.1093/nar/gkq831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shields RC, Burne RA. Growth of Streptococcus mutans in biofilms alters peptide signaling at the sub-population level. Front Microbiol 2016; 7:1075; https://doi.org/ 10.3389/fmicb.2016.01075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gietz RD, Schiestl RH. Frozen competent yeast cells that can be transformed with high efficiency using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2007; 2:1-4; https://doi.org/ 10.1038/nprot.2007.17 [DOI] [PubMed] [Google Scholar]
- 50.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403-10; https://doi.org/ 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 51.Chan PP, Lowe TM. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res 2016; 44:D184-9; https://doi.org/ 10.1093/nar/gkv1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El Yacoubi B, Lyons B, Cruz Y, Reddy R, Nordin B, Agnelli F, Williamson JR, Schimmel P, Swairjo MA, de Crécy-Lagard V. The universal YrdC/Sua5 family is required for the formation of threonylcarbamoyladenosine in tRNA. Nucleic Acids Res 2009; 37:2894-909; https://doi.org/ 10.1093/nar/gkp152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sherlin LD, Bullock TL, Nissan TA, Perona JJ, Lariviere FJ, Uhlenbeck OC, Scaringe SA. Chemical and enzymatic synthesis of tRNAs for high-throughput crystallization. RNA 2001; 7:1671-8 [PMC free article] [PubMed] [Google Scholar]
- 54.Ahn SJ, Qu MD, Roberts E, Burne RA, Rice KC. Identification of the Streptococcus mutans LytST two-component regulon reveals its contribution to oxidative stress tolerance. BMC Microbiol 2012; 12:187; https://doi.org/ 10.1186/1471-2180-12-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patton TG, Rice KC, Foster MK, Bayles KW. The Staphylococcus aureus cidC gene encodes a pyruvate oxidase that affects acetate metabolism and cell death in stationary phase. Mol Microbiol 2005; 56:1664-74; https://doi.org/ 10.1111/j.1365-2958.2005.04653.x [DOI] [PubMed] [Google Scholar]
- 56.Lewis AM, Rice KC. Quantitative Real-Time PCR (qPCR) Workflow for analyzing Staphylococcus aureus gene expression. Methods Mol Biol Clifton NJ 2016; 1373:143-54; https://doi.org/ 10.1007/7651_2014_193 [DOI] [PubMed] [Google Scholar]
- 57.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods San Diego Calif 2001; 25:402-8; https://doi.org/ 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


