ABSTRACT
Macroautophagy (hereafter autophagy) is a catabolic pathway present in all eukaryotic cells. The yeast Saccharomyces cerevisiae has been pivotal in the identification and characterization of the key autophagy-related (Atg) proteins, which play a central role in the generation of autophagosomes. The components of the core Atg/ATG machinery and their functions are highly conserved among species, although mammalian cells also have isoforms and auxiliary factors. Atg9/ATG9 is the only transmembrane protein that is part of the core Atg/ATG machinery, but it appears to have divergent localizations and molecular roles in yeast and mammals. A recent experimental analysis of the yeast endo-lysosomal system by the laboratory of Benjamin Glick, however, suggests a more simple organization of this membrane system. Although this study has not examined yeast Atg9, its findings place this protein in the same compartments as its mammalian counterpart. Here, we will discuss the implications of this conceptual change on the trafficking of yeast Atg9 and its function in autophagy.
KEYWORDS: Atg9, ATG9A, endosomes, recycling endosomes, trans-Golgi network, trafficking
Commentary
The core Atg/ATG machinery involved in the formation of autophagosomes has been subdivided into 5 functional clusters: the Atg1 kinase complex, the autophagy-specific phosphatidylinositol 3-kinase complex, the Atg9 trafficking system, and the Atg12 and Atg8 ubiquitin-like conjugation systems [1,2]. Atg9/ATG9A is the only integral membrane protein within the core Atg/ATG machinery; it has 6 highly conserved transmembrane segments with 2 cytosolically oriented termini that are involved in interactions with other ATG components [3,4].
In all organisms, the Atg9 homologs appear to principally localize to the trans-Golgi network (TGN) and endosomes or endosome-like structures, although it has also been detected at the plasma membrane and in vicinity of the mitochondria in specific organsims [4–9]. Atg9/ATG9A is sorted from one or more of these compartments to form cytoplasmic Atg9-containing membranes, which comprise vesicular and tubular structures [10–12] (Figure 1(a)). Atg9/ATG9A cycles between these locations and the site of autophagosome formation, and because of this dynamic aspect, it has been proposed that Atg9-positive structures could represent one of the membrane sources of autophagosomes [4,10,13]. In yeast, Atg9-containing membranes participate in the formation of the phagophore, the precursor cistern of autophagosomes [8,11], possibly through homo- and heterotypic fusion events [14–16]. As Atg9 is detected on the external membrane of closed autophagosomes [11,17], this implies that it is probably retrieved during the fusion of these large vesicles with the vacuole or shortly thereafter, as it is not detected on the limiting membrane of the vacuole. However, in mammalian cells ATG9A-containing structures interact with, but do not get incorporated into, the phagophore membranes [10]. It is therefore possible that ATG9A is only involved in the regulation of phagophore expansion, although ATG9A-containing vesicles may nevertheless play a key role in the early event of phagophore formation5.
Figure 1.
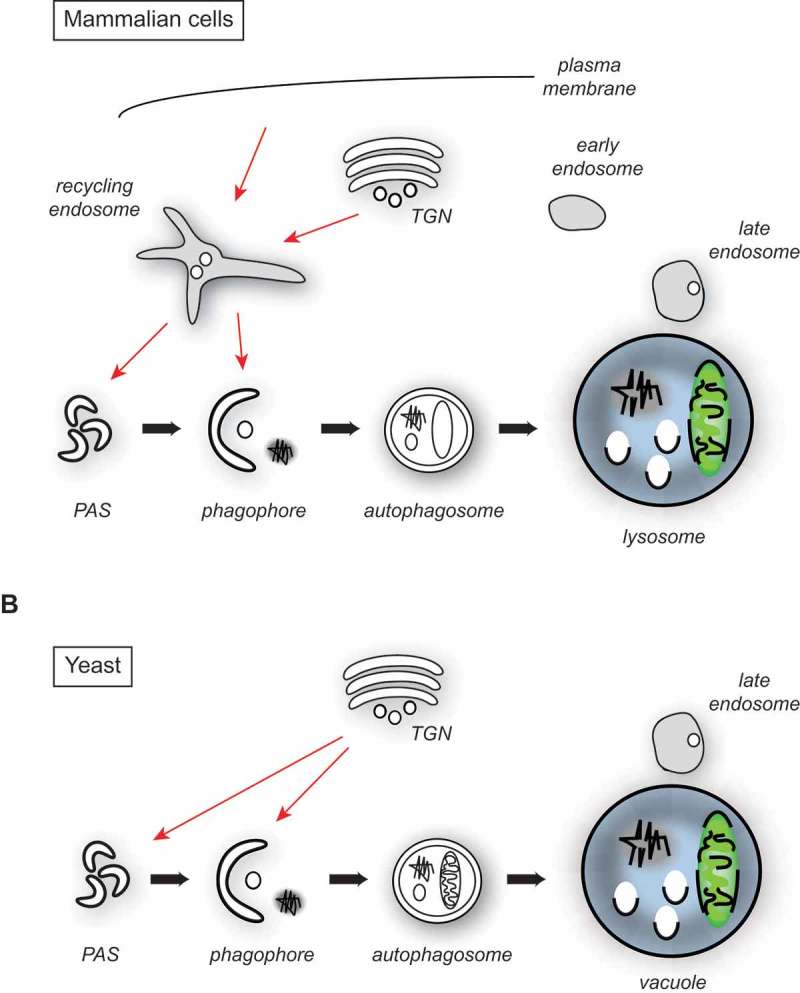
Atg9/ATG9A trafficking in yeast and mammalian cells. (A) Architecture of the endosomal system in mammalian cells and the major putative ATG9A sorting pathways to the PAS and phagophore membranes (red arrows). For simplicity, the potential fusion of the autophagosome with an endosome, resulting in the formation of an amphisome, is not depicted. (B) Proposed organization of the yeast endosomal system and the main Atg9 transport routes to the PAS and phagophore membranes (red arrows).
Trafficking of Atg9 has been mainly studied in yeast, where this protein primarily localizes to a post-Golgi compartment that appears to have unique characteristics [11,12]. In this organism, the movement of Atg9 towards the site of autophagosome biogenesis requires Atg23 and Atg27, 2 non-conserved proteins that form a complex with Atg9 [18–20]. Recent evidence shows that Atg23 and Atg27 are probably participating in the biogenesis of Atg9-containing membranes from the TGN [11,21]. Atg27 is additionally involved in retaining Atg9 in the correct compartments, as Atg9 gets mislocalized and degraded in the vacuole in its absence [11]. The autophagy-specific TRAPPIII complex is also associated with Atg9-positive membranes and involved in their trafficking [22–24]. Sorting nexins have also been implicated in Atg9 transport under specific conditions, though their exact role remains largely unknown [6,25], but they may be participating in Atg27 retrieval from the vacuole [26,27].
In mammalian cells, ATG9A is mainly distributed between the TGN and endosomes, and in part to the plasma membrane [4,28–30]. In agreement with this notion, factors such as the adaptor protein AP-1, AP-2 and AP-4 complexes, RAB11, and the RAB GAPs TBC1D5 and TBC1D14 are involved in ATG9A trafficking [28–32]. During starvation, ATG9A redistributes from the TGN towards a peripheral pool with an increased colocalization with autophagosomes [4,9,10]. The sorting of ATG9A and subsequent transport to phagophore membranes appears to occur at RAB11-positive recycling endosomes [31,33,34], where BAR domain-containing SH3GLB1/BIF-1 and sorting nexin SNX18 recruits DNM2 (dynamin 2) to induce budding of ATG9A-positive membranes directed to ATG16L1-WIPI2-positive phagophores [33,35,36]. In parallel, a TRAPPIII-like complex regulates ATG9A trafficking from a tubulovesicular intermediate that is required for autophagy, likely because it keeps ATG9A in the correct recycling endosome-Golgi compartments [37].
The mammalian endo-lysosomal system has been morphologically and functionally subdivided into recycling, early and late endosomes (Figure 1(a)). Membranes are lipid bilayers with embedded proteins, and enter this system mainly from the TGN and via endocytosis, and they can ultimately be delivered to lysosomes, when late endosomes, also known as multivesicular bodies, fuse with these degradative organelles [38]. The organization of the yeast endo-lysosomal system, in contrast, is less well defined despite the fact that it has been of fundamental importance in the identification and characterization of central and conserved factors involved in its biogenesis such as phosphatidylinositol kinases, ESCRTs, the retromer, and fusion factors [39]. Although it possesses late endosomes/multivesicular bodies, yeast appears to not have distinct recycling endosomes, and early endosomes remain poorly characterized [40] (Figure 1(b)). The major 2 difficulties in uncovering the exact architecture of the yeast endo-lysosomal system have been the lack of 1) well-defined marker proteins; and 2) a distinct morphology associated with each compartment. In a recent work, Day and co-workers have employed a series of elegant 4D live-cell imaging approaches to follow the dynamics of endocytosis and trafficking of a few endosomal and TGN marker proteins [40]. In particular, they observed that markers of the TGN strongly colocalized with endocytic cargos such as the endocytosed dye FM 4–64, whereas endosomal proteins were all found in a prevacuolar endosome. Moreover, they showed that endocytic vesicles fused directly with the TGN. With their approach, they discovered that the yeast TGN also combines the function of the early and the recycling endosome, implying that the organization of its endo-lysosomal system is simpler and probably reflects an ancestral design (Figure 1(b)).
This conceptual change also has a few implications for autophagy, although this pathway and its components were not directly studied. In particular for our understanding of Atg9/ATG9A trafficking, it places Atg9 in the same compartment, i.e. the recycling endosomes, as its mammalian homolog, because the yeast TGN bears this function as well (Figure 1(b)). A similar origin could also explain the morphological similarity of the Atg9- and ATG9A-positive membranes that emerge from the secretory pathway [10,12]. Moreover, it could clarify why multiple genes involved in sorting from the yeast late Golgi compartments/TGN such as the phosphatidylinositol 4-kinase Pik1, the conserved oligomeric Golgi (COG) complex, the GTPases Arf1, Arf2, Sec4, Arl1, and Ypt6, and some of their guanine nucleotide exchange factors including Sec7, Gea1, Gea2 and Sec2, are essential for autophagy progression [6,41–45]. Consistent with this hypothesis, several of the indicated proteins play a role in Atg9 trafficking to the PAS [41–44]. The involvement of mammalian recycling endosomes is not restricted to the sorting and transport of ATG9A. For example, ULK1-positive subdomains, which are distinct from those containing ATG9A and are involved in ULK1 transport to LC3-positive phagophores in a TBC1D14-RAB11-mediated manner, have been observed [31]. Analogously, one should keep in mind that the yeast TGN/endosome hybrid compartment could carry out functions critical for autophagy other than Atg9 sorting, as autophagosomes also need to acquire the appropriate SNARE machinery for their fusion with vacuole [46].
Although the study from Benjamin Glick’s group clarifies the identity of the compartments where Atg9 and ATG9A originate from, it does not reconcile other differences between the yeast and mammalian proteins, in particular their dynamic connection with autophagosomal membranes. ATG9A associates very transiently with phagophores throughout its formation [10], whereas its yeast counterpart gets incorporated into phagophores at their nucleation, and remains inserted in the external membrane of the forming autophagosomes until their closure [11,17], at least in mutant strains that delay or block fusion of the autophagosome with the vacuole. Thus, not all the intriguing aspects of Atg9/ATG9A have been solved yet, and their study will certainly help to unveil general conserved molecular principles underlying the biogenesis of an autophagosome but also to understand differences between autophagy in different model organisms.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft [UN111/7-3]; Deutsche Forschungsgemeinschaft [SFB 944]; Deutsche Forschungsgemeinschaft [Project P11]; H2020 Marie Skłodowska-Curie Cofund [713660]; H2020 Marie Skłodowska-Curie ITN [765912]; ALW Open Programme [ALWOP.310]; ZonMW VICI [016.130.606].
Acknowledgments
We thank Franziska Kriegenburg for the critical reading of the manuscript. F.R. is supported by ZonMW VICI (016.130.606), ALW Open Programme (ALWOP.310), Marie Skłodowska-Curie Cofund (713660) and Marie Skłodowska-Curie ITN (765912) grants. C.U. is supported by the DFG (SFB 944, Project P11 and UN111/7-3).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Lamb CA, Yoshimori T, Tooze SA.. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759–774. [DOI] [PubMed] [Google Scholar]
- [2].Mizushima N, Yoshimori T, Ohsumi Y.. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. [DOI] [PubMed] [Google Scholar]
- [3].Noda T, Kim J, Huang W-P, et al. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J Cell Biol. 2000;148:465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Young ARJ, Chan EYW, Hu XW, et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119:3888–3900. [DOI] [PubMed] [Google Scholar]
- [5].Puri C, Renna M, Bento CF, et al. Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell. 2013;154:1285–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ohashi Y, Munro S. Membrane delivery to the yeast autophagosome from the Golgi-endosomal system. Mol Biol Cell. 2010;21:3998–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reggiori F, Shintani T, Nair U, et al. Atg9 cycles between mitochondria and the pre-autophagosomal structure in yeasts. Autophagy. 2005;1:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mari M, Reggiori F. Atg9 reservoirs, a new organelle of the yeast endomembrane system? Autophagy. 2010;6:1221–1223. [DOI] [PubMed] [Google Scholar]
- [9].Ravikumar B, Moreau K, Jahreiss L, et al. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12:747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Orsi A, Razi M, Dooley HC, et al. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Mol Biol Cell. 2012;23:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yamamoto H, Kakuta S, Watanabe TM, et al. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J Cell Biol. 2012;198:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mari M, Griffith J, Rieter E, et al. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol. 2010;190:1005–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reggiori F, Tucker KA, Stromhaug PE, et al. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev Cell. 2004;6:79–90. [DOI] [PubMed] [Google Scholar]
- [14].Rao Y, Perna MG, Hofmann B, et al. The Atg1-kinase complex tethers Atg9-vesicles to initiate autophagy. Nat Commun. 2016;7:10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nair U, Jotwani A, Geng J, et al. SNARE proteins are required for macroautophagy. Cell. 2011;146:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hurley JH, Young LN. Mechanisms of autophagy initiation. Annu Rev Biochem. 2017;86:225–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cebollero E, van der Vaart A, Zhao M, et al. Phosphatidylinositol-3-phosphate clearance plays a key role in autophagosome completion. Curr Biol. 2012;22:1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tucker KA, Reggiori F, Dunn WA Jr., et al. Atg23 is essential for the cytoplasm to vacuole targeting pathway and efficient autophagy but not pexophagy. J Biol Chem. 2003;278(48):48445–48452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Legakis JE, Yen W-L, Klionsky DJ. A cycling protein complex required for selective autophagy. Autophagy. 2007;3:422–432. [DOI] [PubMed] [Google Scholar]
- [20].Yen W-L, Legakis JE, Nair U, et al. Atg27 is required for autophagy-dependent cycling of Atg9. Mol Biol Cell. 2007;18:581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Backues SK, Orban DP, Bernard A, et al. Atg23 and Atg27 act at the early stages of Atg9 trafficking in S. cerevisiae. Traffic. 2015;16:172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kakuta S, Yamamoto H, Negishi L, et al. Atg9 vesicles recruit vesicle-tethering proteins, Trs85 and Ypt1, to the autophagosome formation site. J Biol Chem. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lynch-Day MA, Bhandari D, Menon S, et al. Trs85 directs a Ypt1 GEF, TRAPPIII, to the phagophore to promote autophagy. Proc Natl Acad Sci U S A. 2010;107:7811–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shirahama-Noda K, Kira S, Yoshimori T, et al. TRAPPIII is responsible for vesicular transport from early endosomes to Golgi, facilitating Atg9 cycling in autophagy. J Cell Sci. 2013;126:4963–4973. [DOI] [PubMed] [Google Scholar]
- [25].Nice DC, Sato TK, Stromhaug PE, et al. Cooperative binding of the cytoplasm to vacuole targeting pathway proteins, Cvt13 and Cvt20, to PtdIns(3)P at the pre-autophagosomal structure is required for selective autophagy. J Biol Chem. 2002;277:30198–30207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ma M, Burd CG, Chi RJ. Distinct complexes of yeast Snx4 family SNX-BARs mediate retrograde trafficking of Snc1 and Atg27. Traffic. 2017;18:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Suzuki SW, Emr SD. Membrane protein recycling from the vacuole/lysosome membrane. J Cell Biol. 2018;217:1623–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mattera R, Park SY, De Pace R, et al. mediates export of ATG9A from the trans-Golgi network to promote autophagosome formation. Proc Natl Acad Sci U S A. 2017;114:E10697–E706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Popovic D, Dikic I. TBC1D5 and the AP2 complex regulate ATG9 trafficking and initiation of autophagy. EMBO Rep. 2014;15:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Imai K, Hao F, Fujita N, et al. Atg9A trafficking through the recycling endosomes is required for autophagosome formation. J Cell Sci. 2016;129:3781–3791. [DOI] [PubMed] [Google Scholar]
- [31].Longatti A, Lamb CA, Razi M, et al. TBC1D14 regulates autophagosome formation via Rab11- and ULK1-positive recycling endosomes. J Cell Biol. 2012;197:659–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou C, Ma K, Gao R, et al. Regulation of mATG9 trafficking by Src- and ULK1-mediated phosphorylation in basal and starvation-induced autophagy. Cell Res. 2017;27:184–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Knaevelsrud H, Soreng K, Raiborg C, et al. Membrane remodeling by the PX-BAR protein SNX18 promotes autophagosome formation. J Cell Biol. 2013;202:331–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Corcelle-Termeau E, Vindelov SD, Hamalisto S, et al. Excess sphingomyelin disturbs ATG9A trafficking and autophagosome closure. Autophagy. 2016;12:833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Soreng K, Munson MJ, Lamb CA, et al. SNX18 regulates ATG9A trafficking from recycling endosomes by recruiting Dynamin-2. EMBO Rep. 2018;19:e44837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Takahashi Y, Tsotakos N, Liu Y, et al. The Bif-1-Dynamin 2 membrane fission machinery regulates Atg9-containing vesicle generation at the Rab11-positive reservoirs. Oncotarget. 2016;7:20855–20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lamb CA, Nuhlen S, Judith D, et al. TBC1D14 regulates autophagy via the TRAPP complex and ATG9 traffic. EMBO J. 2016;35:281–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. [DOI] [PubMed] [Google Scholar]
- [39].Balderhaar HJ, Ungermann C. CORVET and HOPS tethering complexes - coordinators of endosome and lysosome fusion. J Cell Sci. 2013;126:1307–1316. [DOI] [PubMed] [Google Scholar]
- [40].Day KJ, Casler JC, Glick BS. Budding yeast has a minimal endomembrane system. Dev Cell. 2018;44:56–72 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yen WL, Shintani T, Nair U, et al. The conserved oligomeric Golgi complex is involved in double-membrane vesicle formation during autophagy. J Cell Biol. 2010;188:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yang S, Rosenwald AG. Autophagy in Saccharomyces cerevisiae requires the monomeric GTP-binding proteins, Arl1 and Ypt6. Autophagy. 2016;12:1721–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Geng J, Nair U, Yasumura-Yorimitsu K, et al. Post-Golgi Sec proteins are required for autophagy in Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:2257–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang K, Yang Z, Liu X, et al. Phosphatidylinositol 4-kinases are required for autophagic membrane trafficking. J Biol Chem. 2012;287:37964–37972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].van der Vaart A, Griffith J, Reggiori F. Exit from the Golgi is required for the expansion of the autophagosomal phagophore in yeast Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:2270–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Reggiori F, Ungermann C. Autophagosome maturation and fusion. J Mol Biol. 2017;429:486–496. [DOI] [PubMed] [Google Scholar]


