Abstract
Nerve conduits are becoming increasingly popular for the repair of peripheral nerve injuries. Their ease of application and lack of donor site morbidity make them an attractive option for nerve repair in many situations. Today, there are many different conduits to choose in different sizes and materials, giving the reconstructive surgeon many options for any given clinical problem. However, to properly utilize these unique reconstructive tools, the peripheral nerve surgeon must be familiar not only with their standard indications but also with their functional limitations. In this review, the authors identify the common applications of nerve conduits, expected results, and shortcomings of current techniques. Furthermore, future directions for nerve conduit use are identified.
Keywords: nerve injury, nerve conduit, nerve palsy, nerve gap
Introduction
Peripheral nerve injury (PNI) incurs a significant medical burden. More than 1 million people worldwide present with PNI every year. 1 After 1 year, 24 to 41% of patients with a major upper extremity PNI will remain out of work if the nerves are not repaired to adequate functional recovery. 2 3 The current gold standard treatment for PNI is nerve autografting if tensionless primary nerve repair is not possible. 4 5 6 7 However, the use nerve autograft has been hampered by limited donor nerve sources, increased operating time, donor site morbidity, and functional loss. 8 9 Furthermore, autografts are currently limited to gaps of 5 cm or less. 4 Because of these limitations, new efforts toward peripheral nerve repairs have focused on development of nerve grafting alternatives.
Nerve Conduit Materials
Nerve conduits function to enclose, or entubulate, the distal and proximal ends of the severed nerve. In doing so, these conduits provide a guide for budding axons, a barrier between the healing nerve and surrounding inflammation and fibrosis, and a space for interaction of the exudates from the severed nerve ends. This axoplasmic exudative fluid contains neurotrophic and other growth factors that will give rise to a formed fibrin matrix and basal lamina, which will in turn promote the ingrowth of axons and Schwann cells. When established in a timely fashion, these elements function to limit wallerian degeneration and reestablish the function of the severed nerves. 8 10 11 12
Nerve conduits continue to evolve as novel materials and modifications set out to address observed shortcomings of available options. One such example is silicone, which is ubiquitous in the medical field, it but presents limitations when utilized as a conduit material; specifically, silicone is nonresorbable, which translates to an increased risk of compression and decrease in axonal conduction, possibly necessitating a secondary procedure for removal. 13 Similar observations over the course of nerve conduit use suggest ideal synthetic conduit characteristics include the following: biocompatibility and nonimmunogenicity not to induce an inflammatory response; semipermeability and appropriate porosity to allow oxygen and nutrient exchange; biodegradability to prevent a second operation for removal, while also exhibiting mechanical stability during the healing process; flexibility to prevent compression of the regenerating axons and tissue ischemia; and ease of fabrication, sterilization, and implantation. 14 15 16 17 18 19 20 21 22 23
The currently available Food and Drug Administration (FDA)–approved conduits are manufactured from type I collagen, polyglycolic acid (PGA), polycaprolactone (PCL), polyvinyl alcohol (PVA), and porcine small intestine submucosa (SIS). 8 All these materials exhibit some of the aforementioned properties, but none of them have proved to exhibit perfect qualities as a nerve conduit.
Nerve Conduits in Hand Reconstruction
Though an exhaustive study of the use of nerve conduits in upper extremity peripheral nerve repair is outside the scope of this article, many authors have shown promising results for nerve conduits in small sensory nerves with small gap, especially in digital nerve repair. Furthermore, the use of conduits prevents axons from traversing two suture lines, reduces operating time, and avoids potential donor site complications ( Fig. 1 ). Mackinnon and Dellon studied 15 patients with digital nerve injuries averaging 17-mm gaps (range: 5–30 mm) repaired with PGA conduits. After a mean follow-up of 22 months, 86% of patients reported an exceptional or good outcome, judged by moving two-point discrimination (2PD) of 4 to 7 mm or static two-point discrimination 7 to 15 mm. 24 Rinker and Liau studied 36 digital nerve injuries with mean gap size of 10 mm (range: 4–25 mm) repaired with PGA conduits. Sensory testing, consisting of static and moving 2PD at 6 and 12 months, demonstrated equivalent recovery to repairs made with autologous vein grafts. 25
Fig. 1.
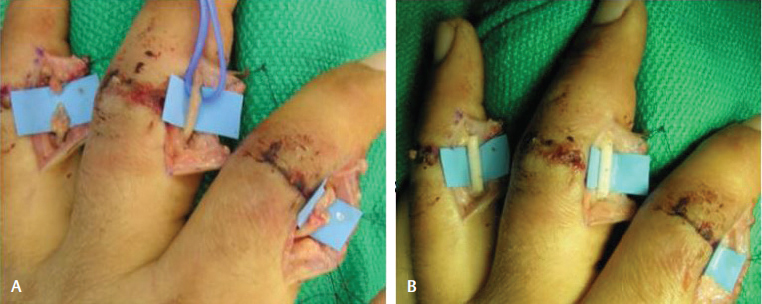
Repair of digital nerve injury with nerve conduit. (A) Evidence of injury to digital nerves with small gaps spanning the nerves. (B) Reconstruction with nerve conduits.
Similarly, Weber et al compared the use of PGA nerve conduits with primary nerve repair for deficits 4 mm or less. PGA conduits produced superior results with 91% of patients achieving excellent results with 2PD versus 49% of those repaired using an end-to-end method. For gap lengths of 5 to 7 mm, 61% of PGA conduits demonstrated excellent or good results versus 86% in the standard repair method. However, for repair of gap lengths 8 mm or greater, PGA conduits again outperformed standard repair with 42% of patients showing excellent results versus none with excellent results in the standard repair cohort. 26 Using collagen conduits, Bushnell et al followed nine patients with digital nerve injury repair for an average of 15 months. The authors reported excellent or good results with 2PD in 89% (8/9) of patients. 27 Taras et al followed 22 digital nerve injuries repaired with collagen conduits for a mean of 20 months. Seventy-three percent (16/22) of patients reported excellent or good results in tests of static and moving 2PD. 28 Lohmeyer et al studied 40 digital nerve injuries repaired with collagen conduits to 12-month follow-up. Twenty (50%) of these patients reported excellent or good 2PD and monofilament testing. 29 Haug et al followed 35 patients with 45 digital nerve repairs (mean defect length of 12 mm) to 12 months. Patients reported quality of recovery after 12 months, using a scale ranging from a score of 1 (good) to 4 (bad). Though nearly 60% reported a score of one or two for monofilament testing, around 20% reported a one or two for 2PD. 30
Problems and Limitations
Though the use of nerve conduits for peripheral nerve repairs shows promising results for small diameter nerves with small gaps, further study is needed to elucidate the efficacy of nerve conduits in cases of larger peripheral nerves with more significant gaps. Moore et al reported on two brachial plexus, one median nerve, and one ulnar nerve injury repaired with collagen and PGA nerve conduits. No patient regained either motor or sensory function. In two reexplorations of the repair site, significant neuroma formation occurred even outside of the initial repair site, necessitating neuroma resection and repair with autograft. 31 The authors hypothesized that larger diameter and length produce larger volume, thus diluting the neurotropic factors responsible for axonal budding and nerve reconstitution. Indeed, many successful reports regarding larger nerve repair with conduits use different tactics to minimize volume mismatch by using smaller diameter conduits to bridge one defect and combining a nerve conduit with a small autograft. 32 33 34 35 Alternatively, a group of authors have reported success without using these additional methods in cases of median, ulnar, and radial nerve repair using conduits. Dienstknecht et al used type 1 collagen nerve conduits to treat nine patients with acute median nerve injuries, located distal to the anterior interosseous nerve and proximal to the recurrent branch, with gaps ranging between 1 and 2 cm with the patient in wrist flexion. Their results demonstrated favorable clinical outcomes and patient satisfaction. 36 Recently, many of the same authors collaborated for a study published under Klein et al examining the treatment of 10 patients with ulnar and radial nerve defects of up to 1.2 cm in size with type 1 collagen conduits. They found the results most favorable in young patients with nondelayed nerve repair, while also recognizing that limitations remain regarding both defect size and the possibility of misdirected regrowing axons under these circumstances. 37 Modifications of existing nerve conduits to address these and other disadvantages are needed, preferably to complement future studies offering high-quality evidence. Improvements in both design and the body of literature may provide better clarification of the range of application of nerve conduits in cases of large diameter nerve injuries with significant defects.
Future Directions
The aforementioned failures with nerve conduits highlight the problems with their use. Namely, the current available nerve conduits provide nothing more than a mechanical support to the nerve. The distal and proximal ends of the nerves must supply the support cells, growth factors, and overall bridging matrix that fills the nerve conduit and promotes neuronal healing. One of the approaches to solve this problem is providing luminal fillers to bridge the gap between severed nerve ends. These luminal fillers will consist of extracellular matrix components with or without support cells and/or neurotrophic factors to both promote axonal growth and provide a luminal support structure for the budding axons. 38 39
A variety of luminal fillers have been tried with encouraging results. The results of using collagen-glycosaminoglycan (GAG) mixture as a filler are mixed. 40 41 Similarly, human hair keratin hydrogel has been used as a filler for collagen conduits. Pace et al demonstrated that in comparison to saline-filled collagen conduits, keratin-filled conduits had a larger diameter after healing, improved nerve conduction velocity, and a higher density of myofibers in the repaired nerves in a nonhuman primate model. 42 More complex fillers have also been developed. Yang et al developed a silk-fibroin scaffold seeded with bone marrow mesenchymal stem cells. Twelve weeks after creating a 10-mm defect in the rat sciatic nerve, the silk fibroin scaffold seeded with stem cells achieved much better results than the nerves repaired with the silk-fibroin scaffolds by themselves. In fact, their results were almost equivalent to those of nerve autografts. 43 Recently, Sun et al created three-dimensional (3D) nanofiber sponge-containing nerve conduits manufactured with poly (L-lactic acid-co-∊-caprolactone)/silk fibroin (PLCL/SF). Using sciatic nerve defects in rat models, the study demonstrated a better repair effect with this filler when compared with hollow nerve conduits. 44
Stem cells themselves have been highly touted as nerve conduit additives, with literally thousands of different reports being published on the matter. Hundepool et al published a meta-analysis on the topic that included 44 animal studies regarding the use of stem cells in nerve conduits. These studies mainly included adipose-derived stem cells (ADSCs), bone marrow stromal cells (BMSC), mesenchymal stem cells (MSC), and others. In all reports and with all different types of stem cells, the review noted that those nerve repairs with stem cell additives performed better than those with conduits not utilizing these additives. 45
Delivery of growth factors to the regenerating nerve is also an area of developing research. Enhancing the nerve conduit or scaffold itself with neurotrophic factors, such as glial-derived neurotrophic factor (GDNF), has proven to be an effective way to recruit Schwann cells and promote axonal regeneration. 46 47 Similarly, microspheres enhanced with nerve growth factors have been used to deliver growth factors to regenerating nerves with promising results. 48 49 Studies delivering insulin-like growth factor-1 (IGF-1) into the repair site show improved physical and functional recovery not only in young but also in aged animals. 50
Alternatives to Nerve Conduits
Autologous conduits and processed allograft serve as additional options in the hand surgeon's armamentarium for digital nerve repair. Autologous nerve autografts, most frequently using the sural nerve, are considered the gold standard of repair, with reported results showing value in up to 5 cm of injury. 4 Though they offer a viable source of Schwann cells critical to nerve regeneration, their use is not without associated problems, including the potential for size mismatch between the injured nerve and available donor nerve, risk of sensory perception loss at the donor site, limited graft material, and increased operative times associated with nerve harvesting. 37 51 In response to these shortcomings, both vascular graft conduits and muscle-in-vein conduits (MVCs) have emerged as autologous alternatives. Venous grafts, with tissue composition closely resembling that of nerve, have been the focus of studies demonstrating their effectiveness in the reconstruction of PNIs with a gap of 3 cm or less. 52 Though vein grafts for digital nerve reconstruction are most commonly harvested from the ipsilateral dorsal hand or palmar forearm, proponents highlight the wide-ranging availability of donor veins and the lesser risk of injury associated with harvesting versus that of nerve graft harvesting. 52 53 Interestingly, Chou et al demonstrated the histopathology of a saphenous vein graft successfully used to wrap a 6-cm segment of the radial branch of the median nerve in a case of recalcitrant carpal tunnel syndrome 4 years prior. 54 The biopsy demonstrated that graft neovascularization without inflammation, induced a change in vein morphology. Further, there was absence of extrinsic scar invasion, reflected by the lack of adhesion between the nerve and the intimal surface of the vein during intraoperative exploration. Despite representing only one case, these findings support the viability of vein grafts and highlight favorable characteristics that may contribute to their success. Disadvantages of the vein graft have been discussed and include the tendency of veins to collapse, thus limiting their ability to address larger defects. Therefore, MVCs have since been introduced. The added value of this option lies in its use of muscle tissue interposition to prevent vein collapse, which in turn supports nerve bridging across wider defects; however, as with all autologous options, donor site morbidity with MVC use remains a concern. 51 Eliminating this risk, processed allograft has also been used with success. Allograft production results in a nonimmunogenic material with maintenance of the structural integrity found in autografts. Studies examining its use have demonstrated benefit in digital nerve repairs with gaps up to 3 to 4 cm. 55 56 57 Using data regarding allograft use in nerve injuries collected by the RANGER registry, Cho et al analyzed outcomes associated with 35 digital nerve injuries found to have defects 5 to 40 mm in size. They found that 89% of patients in this cohort experienced improvement in sensory or motor function following repair with allograft. 57 Rinker et al also reported on allograft use in the repair of 37 digital nerve injuries documented in the RANGER registry, with gaps ranging from 5 to 15 mm in size. 58 Their outcomes were found to be favorable as well as comparable to historical controls for repairs with nerve autograft and conduits. Recently, Rinker et al used the RANGER database to study 50 digital nerve injuries with repairs, using allografts with a focus on longer nerve gaps, 25 to 50 mm in size with an average of 35 ± 8 mm, in 28 patients. 59 The results again demonstrated consistency with historical data in cases of autograft repair with a mean static 2PD of 9 ± 4 mm and recovery to an S3 or greater level using the Medical Research Council Classification (MRCC).
Conclusion
The repair of significant PNIs continues to be a challenging problem to practicing surgeons. Though nerve autografts are the gold standard of repair, their use is not without associated problems, including donor site morbidity and increased operative times. Nerve conduits are a promising alternative to nerve autograft; however, their use is currently limited to small-diameter peripheral nerves with small defects. Promising results have been achieved with modifications to the nerve conduits design, but these modifications have not yet been proven widely applicable in significant defects of human large-diameter peripheral nerves. Development of improved conduits will focus on materials that will deliver further supportive elements such as supporting scaffold structures, growth factors, or mesenchymal support. Until these new developments bear out and high-quality evidence becomes available, nerve conduit use should be limited to small peripheral nerves with short defects.
Funding Statement
Funding Partially supported by research grants from British Council's UKIERI Trilateral research partnerships 2011 (Yang, Kundu, and Li) and Department of Defense CDMRP (US) grant OR120157P1 (Smith and Li).
Footnotes
Conflict of Interest None.
References
- 1.Sachanandani N F, Pothula A, Tung T H. Nerve gaps. Plast Reconstr Surg. 2014;133(02):313–319. doi: 10.1097/01.prs.0000436856.55398.0f. [DOI] [PubMed] [Google Scholar]
- 2.Bruyns C N, Jaquet J B, Schreuders T A, Kalmijn S, Kuypers P D, Hovius S E. Predictors for return to work in patients with median and ulnar nerve injuries. J Hand Surg Am. 2003;28(01):28–34. doi: 10.1053/jhsu.2003.50026. [DOI] [PubMed] [Google Scholar]
- 3.Jaquet J B, Luijsterburg A J, Kalmijn S, Kuypers P D, Hofman A, Hovius S E. Median, ulnar, and combined median-ulnar nerve injuries: functional outcome and return to productivity. J Trauma. 2001;51(04):687–692. doi: 10.1097/00005373-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Siemionow M, Brzezicki G. Chapter 8: current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol. 2009;87:141–172. doi: 10.1016/S0074-7742(09)87008-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee S K, Wolfe S W. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8(04):243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Griffin J W, Hogan M V, Chhabra A B, Deal D N. Peripheral nerve repair and reconstruction. J Bone Joint Surg Am. 2013;95(23):2144–2151. doi: 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- 7.Li R, Liu Z, Pan Y, Chen L, Zhang Z, Lu L. Peripheral nerve injuries treatment: a systematic review. Cell Biochem Biophys. 2014;68(03):449–454. doi: 10.1007/s12013-013-9742-1. [DOI] [PubMed] [Google Scholar]
- 8.Kehoe S, Zhang X F, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43(05):553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Deumens R, Bozkurt A, Meek M F et al. Repairing injured peripheral nerves: Bridging the gap. Prog Neurobiol. 2010;92(03):245–276. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Ichihara S, Inada Y, Nakamura T.Artificial nerve tubes and their application for repair of peripheral nerve injury: an update of current concepts Injury 200839Suppl0429–39. [DOI] [PubMed] [Google Scholar]
- 11.Johnson E O, Soucacos P N.Nerve repair: experimental and clinical evaluation of biodegradable artificial nerve guides Injury 20083903Suppl 3S30–S36. [DOI] [PubMed] [Google Scholar]
- 12.Johnson E O, Zoubos A B, Soucacos P N.Regeneration and repair of peripheral nerves Injury 20053604Suppl 4S24–S29. [DOI] [PubMed] [Google Scholar]
- 13.Gerth D J, Tashiro J, Thaller S R. Clinical outcomes for Conduits and Scaffolds in peripheral nerve repair. World J Clin Cases. 2015;3(02):141–147. doi: 10.12998/wjcc.v3.i2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackinnon S E, Hudson A R, Falk R E, Kline D, Hunter D. Peripheral nerve allograft: an assessment of regeneration across pretreated nerve allografts. Neurosurgery. 1984;15(05):690–693. doi: 10.1227/00006123-198411000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Aebischer P, Guénard V, Winn S R, Valentini R F, Galletti P M. Blind-ended semipermeable guidance channels support peripheral nerve regeneration in the absence of a distal nerve stump. Brain Res. 1988;454(01)(02):179–187. doi: 10.1016/0006-8993(88)90817-7. [DOI] [PubMed] [Google Scholar]
- 16.Hudson T W, Evans G R, Schmidt C E. Engineering strategies for peripheral nerve repair. Clin Plast Surg. 1999;26(04):617–628. [PubMed] [Google Scholar]
- 17.Kokai L E, Lin Y C, Oyster N M, Marra K G. Diffusion of soluble factors through degradable polymer nerve guides: controlling manufacturing parameters. Acta Biomater. 2009;5(07):2540–2550. doi: 10.1016/j.actbio.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 18.de Ruiter G C, Malessy M J, Yaszemski M J, Windebank A J, Spinner R J. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009;26(02):E5. doi: 10.3171/FOC.2009.26.2.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Ruiter G C, Spinner R J, Yaszemski M J, Windebank A J, Malessy M J. Nerve tubes for peripheral nerve repair. Neurosurg Clin N Am. 2009;20(01):91–105. doi: 10.1016/j.nec.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Bellamkonda R V. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27(19):3515–3518. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Lietz M, Dreesmann L, Hoss M, Oberhoffner S, Schlosshauer B. Neuro tissue engineering of glial nerve guides and the impact of different cell types. Biomaterials. 2006;27(08):1425–1436. doi: 10.1016/j.biomaterials.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Griffith L G. Emerging design principles in biomaterials and scaffolds for tissue engineering. Ann N Y Acad Sci. 2002;961:83–95. doi: 10.1111/j.1749-6632.2002.tb03056.x. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Lim S H, Mao H Q, Chew S Y. Current applications and future perspectives of artificial nerve conduits. Exp Neurol. 2010;223(01):86–101. doi: 10.1016/j.expneurol.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Mackinnon S E, Dellon A L. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990;85(03):419–424. doi: 10.1097/00006534-199003000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Rinker B, Liau J Y. A prospective randomized study comparing woven polyglycolic acid and autogenous vein conduits for reconstruction of digital nerve gaps. J Hand Surg Am. 2011;36(05):775–781. doi: 10.1016/j.jhsa.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Weber R A, Breidenbach W C, Brown R E, Jabaley M E, Mass D P. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106(05):1036–1045. doi: 10.1097/00006534-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Bushnell B D, McWilliams A D, Whitener G B, Messer T M. Early clinical experience with collagen nerve tubes in digital nerve repair. J Hand Surg Am. 2008;33(07):1081–1087. doi: 10.1016/j.jhsa.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Taras J S, Jacoby S M, Lincoski C J. Reconstruction of digital nerves with collagen conduits. J Hand Surg Am. 2011;36(09):1441–1446. doi: 10.1016/j.jhsa.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Lohmeyer J A, Kern Y, Schmauss D et al. Prospective clinical study on digital nerve repair with collagen nerve conduits and review of literature. J Reconstr Microsurg. 2014;30(04):227–234. doi: 10.1055/s-0033-1358788. [DOI] [PubMed] [Google Scholar]
- 30.Haug A, Bartels A, Kotas J, Kunesch E. Sensory recovery 1 year after bridging digital nerve defects with collagen tubes. J Hand Surg Am. 2013;38(01):90–97. doi: 10.1016/j.jhsa.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Moore A M, Kasukurthi R, Magill C K, Farhadi H F, Borschel G H, Mackinnon S E. Limitations of conduits in peripheral nerve repairs. Hand (NY) 2009;4(02):180–186. doi: 10.1007/s11552-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundborg G, Dahlin L B, Danielsen N. Ulnar nerve repair by the silicone chamber technique. Case report. Scand J Plast Reconstr Surg Hand Surg. 1991;25(01):79–82. doi: 10.3109/02844319109034927. [DOI] [PubMed] [Google Scholar]
- 33.Lundborg G, Rosén B, Dahlin L, Holmberg J, Rosén I. Tubular repair of the median or ulnar nerve in the human forearm: a 5-year follow-up. J Hand Surg [Br] 2004;29(02):100–107. doi: 10.1016/j.jhsb.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Donoghoe N, Rosson G D, Dellon A L. Reconstruction of the human median nerve in the forearm with the Neurotube. Microsurgery. 2007;27(07):595–600. doi: 10.1002/micr.20408. [DOI] [PubMed] [Google Scholar]
- 35.Hung V, Dellon A L. Reconstruction of a 4-cm human median nerve gap by including an autogenous nerve slice in a bioabsorbable nerve conduit: case report. J Hand Surg Am. 2008;33(03):313–315. doi: 10.1016/j.jhsa.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Dienstknecht T, Klein S, Vykoukal J et al. Type I collagen nerve conduits for median nerve repairs in the forearm. J Hand Surg Am. 2013;38(06):1119–1124. doi: 10.1016/j.jhsa.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 37.Klein S, Vykoukal J, Felthaus O, Dienstknecht T, Prantl L. Collagen type I conduits for the regeneration of nerve defects. Materials (Basel) 2016;9(04):219. doi: 10.3390/ma9040219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenq C B, Coggeshall R E. Permeable tubes increase the length of the gap that regenerating axons can span. Brain Res. 1987;408(01)(02):239–242. doi: 10.1016/0006-8993(87)90379-9. [DOI] [PubMed] [Google Scholar]
- 39.Heath C A, Rutkowski G E. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol. 1998;16(04):163–168. doi: 10.1016/s0167-7799(97)01165-7. [DOI] [PubMed] [Google Scholar]
- 40.Lee J Y, Giusti G, Friedrich P F et al. The effect of collagen nerve conduits filled with collagen-glycosaminoglycan matrix on peripheral motor nerve regeneration in a rat model. J Bone Joint Surg Am. 2012;94(22):2084–2091. doi: 10.2106/JBJS.K.00658. [DOI] [PubMed] [Google Scholar]
- 41.Sahakyants T, Lee J Y, Friedrich P F, Bishop A T, Shin A Y. Return of motor function after repair of a 3-cm gap in a rabbit peroneal nerve: a comparison of autograft, collagen conduit, and conduit filled with collagen-GAG matrix. J Bone Joint Surg Am. 2013;95(21):1952–1958. doi: 10.2106/JBJS.M.00215. [DOI] [PubMed] [Google Scholar]
- 42.Pace L A, Plate J F, Mannava S et al. A human hair keratin hydrogel scaffold enhances median nerve regeneration in nonhuman primates: an electrophysiological and histological study. Tissue Eng Part A. 2014;20(03)(04):507–517. doi: 10.1089/ten.tea.2013.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y, Yuan X, Ding F et al. Repair of rat sciatic nerve gap by a silk fibroin-based scaffold added with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2011;17(17)(18):2231–2244. doi: 10.1089/ten.TEA.2010.0633. [DOI] [PubMed] [Google Scholar]
- 44.Sun B, Zhou Z, Wu T et al. Development of nanofiber sponges-containing nerve guidance conduit for peripheral nerve regeneration in vivo. ACS Appl Mater Interfaces. 2017;9(32):26684–26696. doi: 10.1021/acsami.7b06707. [DOI] [PubMed] [Google Scholar]
- 45.Hundepool C A, Nijhuis T H, Mohseny B, Selles R W, Hovius S E. The effect of stem cells in bridging peripheral nerve defects: a meta-analysis. J Neurosurg. 2014;121(01):195–209. doi: 10.3171/2014.4.JNS131260. [DOI] [PubMed] [Google Scholar]
- 46.Fu K Y, Dai L G, Chiu I M, Chen J R, Hsu S H. Sciatic nerve regeneration by microporous nerve conduits seeded with glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor gene transfected neural stem cells. Artif Organs. 2011;35(04):363–372. doi: 10.1111/j.1525-1594.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 47.Sivak W N, White J D, Bliley J M et al. Delivery of chondroitinase ABC and glial cell line-derived neurotrophic factor from silk fibroin conduits enhances peripheral nerve regeneration. J Tissue Eng Regen Med. 2017;11(03):733–742. doi: 10.1002/term.1970. [DOI] [PubMed] [Google Scholar]
- 48.de Boer R, Borntraeger A, Knight A M et al. Short- and long-term peripheral nerve regeneration using a poly-lactic-co-glycolic-acid scaffold containing nerve growth factor and glial cell line-derived neurotrophic factor releasing microspheres. J Biomed Mater Res A. 2012;100(08):2139–2146. doi: 10.1002/jbm.a.34088. [DOI] [PubMed] [Google Scholar]
- 49.Gu X, Ding F, Williams D F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35(24):6143–6156. doi: 10.1016/j.biomaterials.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 50.Apel P J, Ma J, Callahan M et al. Effect of locally delivered IGF-1 on nerve regeneration during aging: an experimental study in rats. Muscle Nerve. 2010;41(03):335–341. doi: 10.1002/mus.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manoli T, Schulz L, Stahl S, Jaminet P, Schaller H E. Evaluation of sensory recovery after reconstruction of digital nerves of the hand using muscle-in-vein conduits in comparison to nerve suture or nerve autografting. Microsurgery. 2014;34(08):608–615. doi: 10.1002/micr.22302. [DOI] [PubMed] [Google Scholar]
- 52.Sabongi R G, Fernandes M, Dos Santos J B. Peripheral nerve regeneration with conduits: use of vein tubes. Neural Regen Res. 2015;10(04):529–533. doi: 10.4103/1673-5374.155428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paprottka F J, Wolf P, Harder Yet al. Sensory recovery outcome after digital nerve repair in relation to different reconstructive techniques: meta-analysis and systematic review Plast Surg Int 2013. 2013704589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chou K H, Papadimitriou N G, Sarris I, Sotereanos D G. Neovascularization and other histopathologic findings in an autogenous saphenous vein wrap used for recalcitrant carpal tunnel syndrome: a case report. J Hand Surg Am. 2003;28(02):262–266. doi: 10.1053/jhsu.2003.50029. [DOI] [PubMed] [Google Scholar]
- 55.Taras J S, Amin N, Patel N, McCabe L A. Allograft reconstruction for digital nerve loss. J Hand Surg Am. 2013;38(10):1965–1971. doi: 10.1016/j.jhsa.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Karabekmez F E, Duymaz A, Moran S L. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (NY) 2009;4(03):245–249. doi: 10.1007/s11552-009-9195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho M S, Rinker B D, Weber R V et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am. 2012;37(11):2340–2349. doi: 10.1016/j.jhsa.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 58.Rinker B D, Ingari J V, Greenberg J A, Thayer W P, Safa B, Buncke G M. Outcomes of short-gap sensory nerve injuries reconstructed with processed nerve allografts from a multicenter registry study. J Reconstr Microsurg. 2015;31(05):384–390. doi: 10.1055/s-0035-1549160. [DOI] [PubMed] [Google Scholar]
- 59.Rinker B, Zoldos J, Weber R Vet al. Use of processed nerve allografts to repair nerve injuries greater than 25 mm in the hand Ann Plast Surg 201778(6S, Suppl 5)S292–S295. [DOI] [PubMed] [Google Scholar]


