Abstract
The development of HIV-preventive topical vaginal microbicides has been challenged by a lack of sufficient adherence in later stage clinical trials to confidently evaluate effectiveness. This dilemma has highlighted the need to integrate translational research earlier in the drug development process, essentially applying behavioral science to facilitate the advances of basic science with respect to the uptake and use of biomedical prevention technologies. In the last several years, there has been an increasing recognition that the user experience, specifically the sensory experience, as well as the role of meaning-making elicited by those sensations, may play a more substantive role than previously thought. Importantly, the role of the user-their sensory perceptions, their judgements of those experiences, and their willingness to use a product-is critical in product uptake and consistent use post-marketing, ultimately realizing gains in global public health. Specifically, a successful prevention product requires an efficacious drug, an efficient drug delivery system, and an effective user. We present an integrated iterative drug development and user experience evaluation method to illustrate how user-centered formulation design can be iterated from the early stages of preclinical development to leverage the user experience. Integrating the user and their product experiences into the formulation design process may help optimize both the efficiency of drug delivery and the effectiveness of the user.
Keywords: Adherence, Iterative design, Prevention product development, Sexual health, Translational science, User experience, User input
Drug discovery and formulation design rely on conventional yet flexible processes in bioengineering and biochemistry: preclinical developers work to iterate both pharmaceutical agents and a drug delivery system. Drug discovery includes designing and redesigning for a molecule with a targeted action and safety profile. Design of the drug delivery system includes determining what formulation (or device) best maps to the chemical nature of the drug for optimal delivery to targeted tissues. In addition, design includes iterating for effective dosing, efficient drug dissolution, drug transfer and availability, and excretion. With each element in the process, design decisions are made that allow developers to meet a target product profile, inclusive of manufacturing and scalability, storage constraints, dosing, and disposal requirements. A drug is identified and optimized to meet specifications within the desired drug delivery system, or vice versa. Often there is a prioritizing and balancing of outcomes which can constrain the design space.[1]
In developing drugs for the treatment of known diseases, this process has been highly effective. Those who need treatment for a specific condition are motivated by the severity of the condition or its sequelae to be as adherent as they can, given their own priorities and the known consequences. Even drug regimens that are highly toxic and have debilitating side effects, such as some cancer treatments, will be consistently used because to do otherwise would have significant health ramifications. In the case of less severe conditions, or those that are chronic, adherence becomes an important additional consideration in the prioritization and balancing of drug and formulation properties. Indeed, the field of pharmaceutics is filled with examples of medications that come to market as one dosage form, but, over the years, offers forms that optimize safety and/or adherence.
But prevention, especially prevention of a condition like HIV/AIDS that does not exact consequence for many years in an individual’s life, obscures the dimensions of perceived risk and perceived severity of consequence that frustrates a clearer prevention approach. Given the inherent dependence that exists between drug efficacy, drug delivery, and user behavior (including the confluence of the user’s behavior and a sexual partners’ behavior) what would be standard practice in product development is not likely to be sufficient to adequately design a product that potential users of HIV prevention products will incorporate into their lives and use effectively.
The dilemma is clear. Biomedical prevention products need to be used to be effective. Effectiveness is dependent on both biologic efficacy and user behavior. Biologic efficacy is dependent on drug delivery to, and retention in, target tissues. Drug delivery is dependent on either the rheological and other biophysical properties of formulations, the physical and mechanical properties of devices, or similarly characterized properties in injectable, implantable, and oral pharmaceuticals. Regardless of which drug and which drug delivery system, drug delivery itself is ultimately dependent on user behavior. But what if user behavior is also impacted by formulation (or device) properties?[2, 3]
The success of HIV prevention products - as well as multipurpose prevention technologies (MPTs: products that target HIV prevention, STI prevention, and/or pregnancy prevention) - will derive from this synergistic effect of formulation properties successfully delivering potent active pharmaceuticals to target tissues, fluids and pathogens, and successfully eliciting perceptibility and acceptability parameters conducive to human use. Despite much work on developing active microbicidal molecules and growing research in formulation science to deliver them successfully, less than optimal use adherence in later stage clinical trials has effectively stalled topical vaginal HIV prevention and has drastically reduced continued development of topical vaginal MPTs. Early topical microbicide trials exclusively evaluated semi-solid forms, specifically “gels,” which suffered in clinical trials from leakage, messiness, and other user-identified concerns. Vaginal films and suppositories are being developed as alternatives and potentially could have some success for those users who choose pericoital protection over sustained release long-acting regimens.[4–9] While deemed successful in early safety and initial efficacy studies, topical vaginal gel trials were either stopped due to futility or completed but unable to demonstrate proof of concept. Participants’ inability to adopt and sustain product use during clinical trials obviated the ability to substantiate product efficacy in intent-to-treat models: overall product adherence was critically low. However, post hoc analyses showed that, among those with high rates of adherence, incident infections were reduced.[6, 10–15] Most recently, the ASPIRE and Ring Study data documented significant reductions in HIV incidence with consistent use of an antiretroviral (ARV) intravaginal ring (IVR). But again, HIV transmission was best prevented amongst those who used the product consistently.[16] It is likely necessary that biomedical prevention be multifaceted, providing choices for users, given their circumstances, needs, and desires. This dynamic is not new to the development and marketing of sexual and reproductive health products. One only needs to examine the many formulations and devices available in the developed world for contraceptive methods to realize that there is a legitimate need for choice, a choice that bears witness both across infinitely defined and characterized user groups, as well as within an individual woman’s sexual life course.
While translational science is usually reserved for moving products and interventions with proven efficacy forward into real-world use, translational science also needs to be built backward into preclinical design. Product users require product designers to attend to elements of product design that go beyond standard indices of drug delivery and pharmacokinetic and pharmacodynamic properties. Those elements include the consideration of relevant existing and adaptive user behaviors, as well as users’ opinions about product characteristics and use parameters. When biomedical prevention technologies are being designed, whether for the prevention of sexually transmitted infections (STIs) including HIV, the prevention of unwanted pregnancies, or for multipurpose prevention, the product use experience with respect to sensory perceptions and experiences of product properties will likely impact both choice and use.[2, 3, 17] Effectiveness may also be impacted by anticipations and associations users make between formulation or device characteristics and their experiences of the product during use, whether the associations are accurate or not.[18] Further, a drug’s effectiveness may be impacted by use behaviors required for dosing, insertion, presence in the body, disposal, or hygiene.[18, 19] Users’ sensory perceptions and experiences, as well as the meanings they derive from those experiences, whether positive or negative with respect to the users’ choice to use a product, can be used to leverage use-compatible behaviors and minimize or obviate use-incompatible behaviors.
As an example of an integrated product development and user experience iterative design process, we describe here our work to develop electrospun drug-eluting fibers being designed for HIV prevention in women. Electrospun materials offer the potential for a versatile platform for microbicide and MPT development because of tunable drug delivery, material dissolution rates, and tensile properties of the final product, among other attributes.[20–24] Scalability, rate of drug delivery, dissolution and tensile properties (relating to insertion) are grossly tuned by formulation composition (e.g., polymer and solvent choice, drug-polymer combinations), and fiber architecture (achieved by adjusting the electrospinning process).[25–28] Some of these properties have been evaluated in pre-clinical studies:[23, 29, 30] however, like other candidate HIV prevention microbicides, the user perspective has not been incorporated in preclinical development and evaluation. While still a nascent technology for this application, this oversight could risk important preclinical observations toward optimization of drug efficacy and effectiveness.
In the case of electrospun fiber formulations, if formulation composition or architecture negatively impacts user sensory perceptions of the material, and hence, users engage in compensatory behaviors to avoid those sensations, this behavior could, for example, change duration of release and drug efficacy. If this problem is not addressed early in the design process, users may not adhere to the product as it proceeds through later stages of development. Thus, user input should be incorporated early in the design process to potentiate success as a function of user adherence in later stage trials.
An Example of Iterative Design and User Evaluation
In our efforts to facilitate design of a drug-eluting fiber-based vaginal microbicide for HIV prevention, we combined the goal of iterative formulation engineering with iterative user sensory perception and experience (USPE; perceptibility) evaluations (see Fig 1). To be clear, the term “iterative” has both discipline-specific and more generally recognized meanings. Here, we refer to the strategy of using successive approximations, within and between disciplines, taking into account the successes and failures of previous exemplars, to arrive at a prototype that can advance to the next stage in the drug development process. Using novel strategies being developed in perceptibility science, behavioral and user demands on formulation parameters can be incorporated into formulation design, to co-optimize use demands with efficacy demands.
Fig 1.
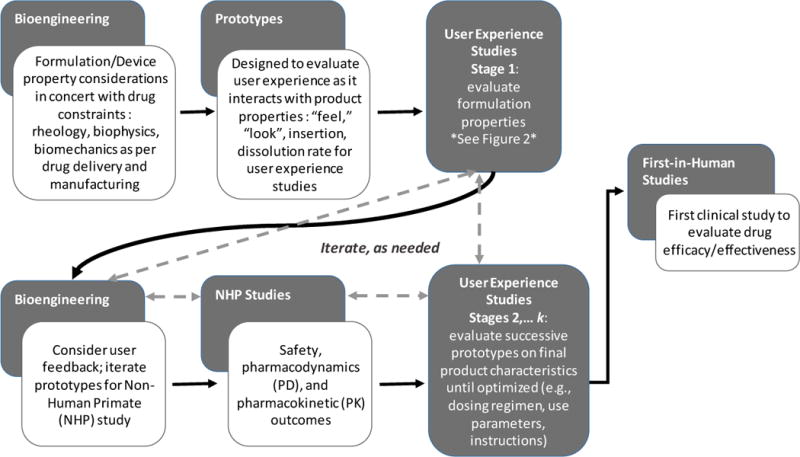
Integrated Iterative Design, combining drug development, bioengineering, and user experience evaluations. Iterations proceed as needed until target product profile and target user profile are aligned for co-optimization of drug delivery and user adherence. Dotted lines indicate potential paths for iterations
Formulation design begins with myriad possibilities. Each iteration, whether actual, computational, or theoretical, leads to a reduction in possibilities until the best solution is reached. Our goal is to consider each attribute or characteristic and weigh the decisions across efficacy and user considerations, where applicable, to determine which formulation provides the best results for all relevant outcomes in combination. In our case, once a fiber formulation was ready for initial user evaluation, the attributes that impact users and/or effectiveness could be further explored amongst users, including potential product geometries. In this case, as is true of other HIV prevention products, attributes such as scalability, dissolution rates, and effective insertion were among the outcomes considered in product design. In our case, the process began with laboratory-based formulation design, development, and testing, including consideration of conventional parameters such as scalability, dissolution, insertion attributes, and others, and then added the users’ experiences and meanings via their own voices.
To illustrate, formulation constituents were limited to electrospinning materials that were known to be safe which constrained the design to some extent. While not often articulated as an attribute critical to users’ needs, the need for materials that could be electrospun on a scaled machine to create a high number of doses at a high throughput (i.e., scalability) also has ramifications for users if those same materials constrain use parameters or elicit undesirable user experiences. Similarly, dissolution time of 15 minutes (comparable to over-the-counter vaginal contraceptive film and other contraceptive wait times for users) is a potentially acceptable wait time for topical HIV prevention products, but shorter wait times would likely increase acceptability. Further, insertion mechanics can also be characterized throughout the design process. The decision-making process could begin simply by understanding options. For instance, early decisions could be informed by inserting different materials with varying properties into ex vivo models, allowing for evaluation of potential stickiness or breakage, either of which could decrease effectiveness. Alternatively, computational models could be used to narrow the possibilities. Later, prototypes could be tested along with varying insertion procedures (e.g., digital insertion, probe insertion, etc.) to determine if further alterations are required.
In our previous work, we validated user sensory perception and experience (USPE) scales that measure specific sensations and product performance from the perspective of the user.[3] In doing so, we identified several sensory experiences critical to soft material (e.g., gel, soluble film) product design.[2] These include specific insertion (application) sensations and experiences, as well as sensations and product behavior experienced post-insertion (e.g., dissolution) and during sexual intercourse. These studies laid the foundation for identification and characterization of parallel variables in nanofiber formulation design.
From a user perspective, because the target was a pericoital product, our goals were to design a fiber-based formulation that was small and portable, easy to insert, and that realized a short dissolution-to-effect time. We also targeted a product with a reasonable effectiveness duration (i.e., preventive efficacy), and minimal disruption of the sexual experience. These later criteria are, currently, targets about which we have a limited understanding. Effectiveness duration can be modeled at this early point in the development process, but cannot be characterized fully until later in preclinical development. Additionally, it remains an empirical question which specific sensory experiences derived from fiber-based product properties play critical roles in the sexual experience, and how divergent from a “normative” experience a product’s impact can be to remain satisfactory to the user.
Thus, we began with the need to understand the most basic impact nanofiber-based formulations would have on user evaluations. What size fabric would be tolerable as users anticipate storage, preparation, insertion, and vaginal use? What shape might best facilitate insertion? How do we best characterize users’ awareness and understanding of “thickness” and “texture” of nanofiber fabric, and how do these understandings change as these parameters are varied? How long would users be willing to wait for dissolution-to-effect? How long would users need a pericoital product to be effective? And what range of sensations experienced by users might be tolerated or accepted for use during intercourse?
It bears note that efficacy is of the utmost importance in prevention product design: thus the user experience, while also critical, is in some ways secondary. This is not to minimize the import of user experience, but rather to recognize that there are at least two opportunities for user sensory perceptions and experiences to be considered. Preclinical design strategies such as this offer the best opportunity to identify those properties that can be modeled into the actual drug delivery system design. It also offers the best opportunity to identify properties or performance features of novel drug delivery systems that require attention in later stages of drug development. That is, while not all user requirements can be achieved in every formulation, behavioral scientists and engineers involved in an integrated iterative design process can identify these issues and devise ways of addressing them – either in preclinical design, or later, in user education materials, provider education materials, or adherence support programs which can determine and train appropriate compensatory behaviors that facilitate product adoption and sustained use in the context of a potentially less-than-acceptable product property or performance feature.
User experience research questions included: (1) What form (e.g., geometries) would be acceptable to potential users and why? (2) What characteristics of those forms, regardless of acceptability, would be most salient to the user experience and why? (3) Given options, what perceived properties (e.g., material strength) might be needed by users for digital insertion? (4) Would users accept, or desire, applicator insertion, and if so, under what circumstances? (5) What would the desired dissolution rate be, as well as dissolution onset, so that users could easily insert the product, as well as provide for engaging in protected sex as quickly as possible once inserted? Ultimately, if the nanofiber formulation and form were changed to accommodate a user-based perspective, what tradeoffs for other outcomes (e.g., drug efficacy, duration of effect) would need to be considered, or alternatively, could those changes be considered? As a side note, we were very aware of the existence of topical vaginal formulations for indications other than HIV prevention (e.g., vaginal contraceptive films, personal lubricants, tampons, suppositories). While familiarity can benefit new product design, it can also hinder use if familiar products have not fared well with users. Our approach was to first consider both previous (familiar) geometries, as well as novel geometries, in an effort to design a product that can appeal to the largest audience. We opted to begin with derivations on a two-dimensional geometry: flat rectangle and flat square. In adhering to a formative qualitative perspective, we chose to add novel geometries: flat circle, rolled rectangle (creating a tube), and capped tube (one end of rolled rectangle folded closed). The circle had been proposed by potential microbicide users in a previous study,[31] and the rolled rectangle and capped tube were novel strategies to address potential insertion challenges with quick-dissolving fabrics (i.e., balancing quick, easy insertion with minimal dissolution time, as well as leverage, for some, familiarity with non-applicator tampons).
Iterative User Input Facilitates Design
Overview of Iterative User Experience Data Collection Process
Figure 2 illustrates the process used to evaluate fiber based formulation prototypes through iterative qualitative methods. The formulation prototypes were evaluated for geometry, texture, and dissolution parameters, with underlying implications for application/insertion, wait time, and duration of effectiveness being queried within these discussions. As is expected, each discussion of a specific characteristic led to cognitive processes resulting in “meaning-making:” critical amongst those meanings were anticipations of sensory experiences during insertion and sexual activity, and perceptions of product efficacy. Furthermore, inherent in the purpose of the study is the objective of understanding product preference for specific prototypes, characteristics, properties, or use parameters. Preference can be established in many ways depending on the needs of the research: in our case, the qualitative data collection focused on which characteristics of the prototypes participants preferred, as well as the reasons for those preferences, and how different prototypes compared on the characteristics of import. In addition, at the end of focus groups, we asked each participant to rank their preferences. Procedural changes across focus groups are presented, as well as the primary rationale for each of these changes.
Fig 2.
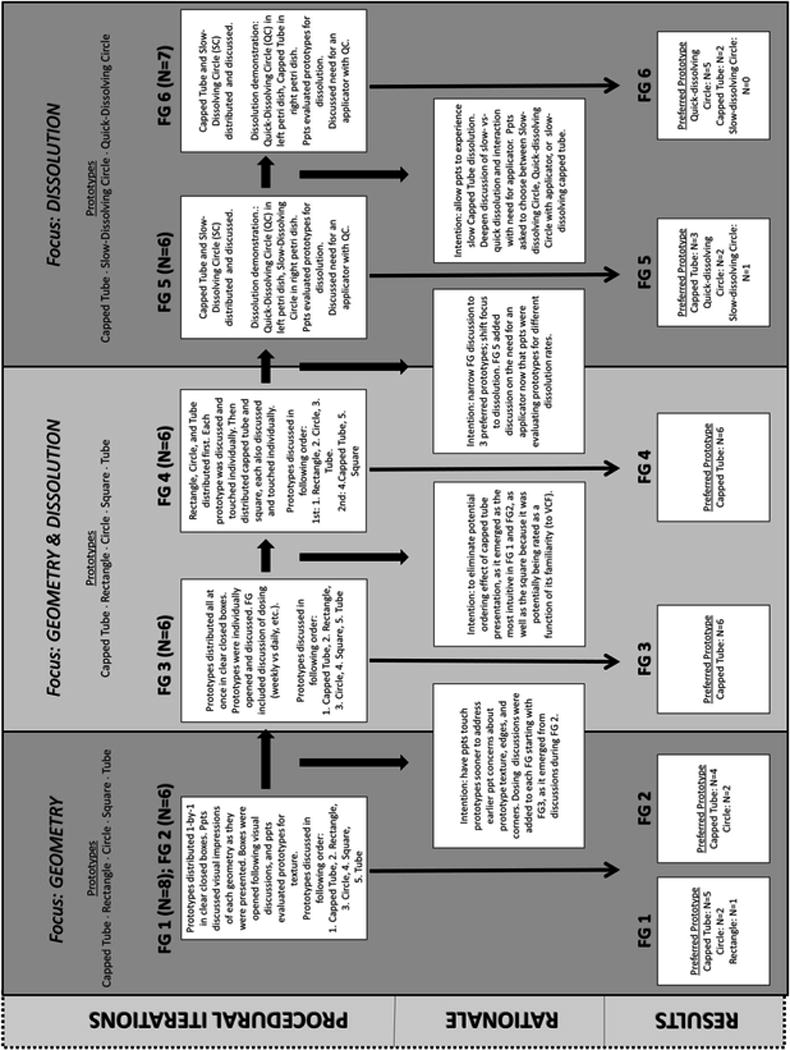
User Experience Data Collection Process Flow and Primary Results.
Procedural process, rationale for changes in properties/concepts discussed, and results of iterative qualitative methods used to evaluate fiber based formulation prototypes.
Given the novel nature of nanofiber formulation for prevention product design, we chose to begin the user evaluation study by focusing on “fabric” geometry - size and shape - for two reasons. First, all subsequent iterations of the formulation would be based on user discussions and interpretations of form characteristics, and presentations of geometry allowed for such discussions. In this particular case, we posited that the nanofiber platform would allow the ability to decouple at least some typically efficacy-dependent formulation attributes from user needs. While nanofibers may have offered a degree of flexibility, this is not always the case. With some formulations, the area and thickness of a given shape would need to align with the characteristics (e.g., molecular weight and volume) of the drug(s) to be delivered, potentially restricting the ranges of properties to be iterated. Additionally, these parameters would be impacted by, and would impact, the user: that is, the geometry, size and thickness of the formulation would be capable – or not - of being inserted in specific ways feasible and acceptable to the user. This includes the choice between digital or applicator-assisted insertion. Second, the product appearance is one of the primary characteristics that impacts initial decisions about product use. We anticipated that size and texture would also play more immediate roles in users’ thought processes with respect to geometry, so planned for these discussions, as well. We surmised that once geometry could be narrowed, additional parameters of size, thickness, and texture could be input for user consideration. Each of these would likely impact dissolution time, as well as resulting rheological indices of the dissolved form. The paired iterations of the user evaluations, therefore, mirrored the cascade of design decisions in a somewhat linear fashion: the qualitative method allowed this linearity to bend with the natural discourse of each group. The first focus group in each pair initiated discussion of specific characteristics or use parameters. Following the first focus group in each pair, we modified the subsequent focus group discussion in the pair based on participant feedback and research questions as prioritized, to further hone both input and results.
Participants
Before using the nanofiber design study process to illustrate the value-added with user input, we will provide some context on enrollment. Generalizability is not the goal in formative qualitative studies, rather relevance to key aspects of the target population in product development is. In qualitative work we seek to elicit and establish a range of experiences within the demographics of interest, without introducing so much variation as to complicate the analysis and interpretations. Because it is an iterative process, the overall design should assure as broad (and relevant) a representation as possible. In this study, our key variables for enrollment were sex (females), age (reproductive age range), and number of vaginal deliveries. Our programmatic research has typically sought these variables, as they speak to HIV epidemiology and vaginal environmental conditions that impact sensory input when using topical vaginal products.
Recruitment and Enrollment
Participants were recruited via methods typically used by the research team, including print and online advertisements, social media, recruitment logs listing members of the community interested in research participation, and informal community-based presentations. Prescreening information collected included demographics and sexual history variables typical for our studies (e.g., age; race and Latina/Hispanic ethnicity; and history of vaginal deliveries) and eligibility variables (e.g., history of vaginal sex with a male sexual partner in last 12 months; self-reported HIV status (all were HIV-negative or unknown status); and known topical allergies (none relevant to study products)). Participant vaginal product history, collected in a brief survey prior to the start of FG discussions, included use of a wide range of sexual and reproductive health products. The most reported vaginal products used among the 39 study participants were vaginal medications (24: 61.5%), vaginal lubricants (28; 71.8%), and tampons (34; 87.2%). Sixteen of 39 (41%) had ever used a vaginal douche. And, specific to our study information, 9 (23%) reported having ever used a vaginal film, the most similar product on the market to our prototype formulation. Table 1 presents data for the sample as per our key variables of age and vaginal deliveries, as well as other selected variables, illustrating the ranges present for user input.
Table 1.
Participant Selected Demographic and Sexual/Reproductive Health History Data
| Characteristic | Mean | Min | Max |
|---|---|---|---|
| Age (Years) | 29.7 | 19 | 45 |
| Sexual History (Past 12 Months) | |||
| # Male Sex Partners | 2 | 1 | 14 |
| # Vaginal Sex Episodes | 107 | 4 | 498 |
| # Anal Sex Episodes | 14 | 0 | 450 |
| Age ranges | 18-30 Years (n) | 31-45 Years (n) | |
| Pregnancy History (Ever) | |||
| # Pregnant | 6 | 8 | |
| # That Gave Birth | 3 | 7 | |
| 0 Vaginal Deliveries | 2 | 1 | |
| 1 or More Vaginal Deliveries | 1 | 6 | |
| Intravaginal Contraceptive Method Historya (Ever) | |||
| Vaginal Film | 5 | 4 | |
| Ring | 5 | 4 | |
| Spermicide | 3 | 3 | |
| Cervical Cap | 0 | 1 | |
| Diaphragm | 1 | 0 | |
| 1 | 0 | ||
| n | % | ||
| Hispanic Ethnicity | |||
| Hispanic/Latino | 4 | 10 | |
| Race | |||
| Asian | 4 | 10 | |
| Black/African American | 4 | 10 | |
| Caucasian/White | 27 | 69 | |
| More Than One Raceb | 2 | .05 | |
| Unknown or Not Reportedc | 2 | .05 | |
| Marital Status (Current) | |||
| Never Married | 28 | 72 | |
| Married | 4 | 10 | |
| Otherd | 7 | 18 | |
| Education | |||
| <College Degree | 13 | 33 | |
| College Degree | 18 | 46 | |
| Graduate/Professional Degree | 8 | 21 | |
| Income | |||
| <15k Per Year | 10 | 26 | |
| 15-36k Per Year | 15 | 39 | |
| >36k Per Year | 13 | 33 | |
| Refused to Answer | 1 | 3 | |
Not mutually exclusive
Black/African American and Caucasian/White (n=1); Asian and Caucasian/White (n=1)
Middle Eastern (n=1); Latina but did not report race (n=1)
Separated (n=1); Divorced (n=3); Widowed (n=1); Engaged (n=1); Unspecified (n=1)
Iterative qualitative focus groups
These data were collected February-May, 2015, subsequent to several other studies which focused on similar user experiences and prevention product evaluations.[2, 3, 17, 18, 32–40] We conducted three pairs of qualitative focus groups. Each of the three iterations addressed evolving and step-wise topics essential to prevention product development and design. Figure 2 illustrates this process. It is here that the interdisciplinary thought process is particularly useful. What is the most important attribute which, once understood, becomes the foundation of the design process? What is the role of engineering and what is the role of the user in that attribute? Does either restrict the range and how do we characterize that new range? What questions emerge and which are answered? Move on to the next step. To illustrate:
Results: Focus Groups 1 & 2
The first pair of discussions, FG1 and FG2, focused specifically on geometry; the structural procedures and presentation order of the prototypes remained the same across both. The prototypes were distributed and discussed one by one in clear closed boxes to allow participants to see each prototype separately and evaluate them from a visual perspective only, without yet being able to evaluate them via touch. Once their visual impressions of each geometry were captured, we asked participants to open each box and evaluate the prototypes for texture. The five geometries evaluated were capped tube, rectangle, circle, square, and tube.
In both FG1 (n=8) and FG2 (n=6), the majority of the participants (n=5 and n=4, respectively) preferred the capped tube. They noted this geometry as the most intuitive to use without the need for many instructions and liked that it could easily fit over someone’s finger for insertion. The “intuitive” nature of the prototype actually elucidates an array of characteristics and use parameters preferred by users, as well as the absence of those not preferred. This weighting or balancing of properties is the critical value added by in-depth user evaluations.
Results: Focus Groups 3 & 4
The next pair of focus groups, FG3 and FG4, specifically introduced dissolution, while still evaluating the same prototypes for any additional input on geometry. From a process perspective and as anticipated, prior to touching the prototypes (i.e., during the visual discussion on geometry) in FG1 and FG2, participants noted concerns with texture and the possibility of being injured during the insertion process because some of the geometries appeared to have sharp edges and/or corners. For FG3 and FG4, participants had the opportunity to touch the prototypes sooner to address these concerns with texture earlier in the discussion. This particular iteration, from visual to touch evaluation, illustrates both the challenge of first impressions made by consumers and the advantage of methodically exploring these to better understand how user choices are deliberated.
Thus, the same five geometries were distributed, also in clear closed boxes, and participants were asked to open the boxes, touch, and discuss the prototypes individually. This allowed participants to give feedback on texture based on what they could feel, beyond their visual impressions. In the first three focus groups, the capped tube had been discussed first. So far, it was considered the most intuitive and the preferred geometry. To see if the order of the evaluated prototypes had any effect on overall impressions and preferences, we altered the presentation order in FG4, leaving the capped tube and the square for last. Rectangle, circle, and tube were distributed first. Each of these prototypes was discussed and touched individually. Then, the capped tube and the square were distributed together. They were also discussed and touch individually. This approach added to the credibility of the data from FG1, FG2, and FG3 by accounting for potential presentation ordering effects.
Starting with FG3, discussions were included on dissolution rate and overall wait-time to product effect, asking participants to consider if and how the dissolution rate and wait-time would affect their use of a product meant for HIV/STD prevention. Participants were asked for their thoughts on dosing and how often they would prefer to use a product intended for HIV/STD prevention, i.e., weekly vs daily.
In FG3, (n=6) and FG4, (n=6) the capped tube remained the preferred prototype, in these cases, for all of the participants (N=12). Similar to the first two focus groups, they liked the intuitive nature of this geometry. Tolerable waiting times from insertion to protection (i.e., dissolution targets) were discussed, as were preferences for dosing and the reasons behind those choices; user concerns and preferences were noted so that subsequent iterations in formulation design constraints could be considered .
Results: Focus Groups 5 & 6
FG5 and FG6 procedures narrowed the scope to present only the two geometries that the previous four groups had ranked the highest, and to enhance users’ understanding of the parameter of dissolution time by providing demonstrations of quick-vs. slow-dissolving fabric. The concept of a quick-dissolving fabric prototype elicited discussion on the merits and potential challenges of quick-dissolving (≤ 10 minutes) latencies versus standard (slow-) dissolving latencies (e.g., 15 minutes). Of note, “quick-dissolving” (in total time to dissolution from a formulation parameter perspective) meant that the fabric (from an experiential perspective of the user) began to dissolve immediately upon contact with moisture, which had implications for insertion itself, as well as wait time to effect. The prototypes were a slow-dissolving capped tube, a slow-dissolving circle, and a quick-dissolving circle. They were placed in petri dishes in order to demonstrate dissolution to the participants. The strategy was to then discuss relevant dissolution parameters within the universe of characteristics and perceptions that were elicited by those geometries, as well as participants’ previous experiences with vaginal products. These concepts were then positioned within a discussion of product insertion, and on the need for use of an applicator to insert a quick-dissolving formulation. Ultimately, women expressed their preferences for geometry, texture, dissolution time, application process, and duration of effectiveness. Of note, these choices required the weighting of characteristics and use parameters for each participant, as she considered the use of the product in the context of her own life. Also of note, as in our previous work, some characteristics were deemed “non-negotiable” while others were allotted greater flexibility, sometimes within specific contexts.
The capped tube was evaluated as a slow-dissolving prototype meant to be inserted digitally. The slow-dissolving circle provided an alternative to the capped tube geometry, also to be inserted digitally, and the quick-dissolving circle introduced the option of using an applicator for insertion, as well as the possibility of a pericoital topical product without a wait time between insertion and sex. We wanted to give study participants the opportunity to see how the slow-dissolving geometries would dissolve if digitally inserted and also provide other options for those who preferred something that was quick-dissolving.
FG5 and FG6 distributed and discussed the slow-dissolving capped tube and the slow-dissolving circle together, in order to avoid confounding dissolution time and geometry. A dissolution demonstration followed and in FG5, research staff placed the quick-dissolving circle on a petri dish to the left of each participant. Then the slow-dissolving circle was placed on a petri dish to the right of each participant. A few drops of water were added to each petri dish and participants were asked to use their fingers to stir the prototypes around with the intent of dissolving them. This experience also provided them with a sensory experience of the dissolved formulation. In FG6, the slow-dissolving circle placed on the right petri dish in the previous focus group was now switched for the slow-dissolving capped tube. This allowed participants to see the capped tube dissolving.
The capped tube remained the preferred geometry in FG5 (n=6) and FG6 (n=7) when participants were asked to choose between the two slow-dissolving prototypes. In FG6, however, when asked to choose between a slow-dissolving capped tube (digital insertion), a slow-dissolving circle (digital insertion), and a quick-dissolving circle (which required insertion with an applicator), the majority of the participants (5 of 7) preferred the quick-dissolving circle with an applicator. While intuitive ease of insertion remained an important attribute, this result raised the salience of dissolution time over ease of insertion in this sample. The most important attributes – ease of insertion and dissolution time – could now be resolved in subsequent formulation iterations prior to non-human primate studies for safety, drug delivery, and initial efficacy.
Discussion
There are significant benefits to using an integrated and iterative engineering and user experience design process in the development of biomedical technologies, including those exclusively for the prevention of sexually transmitted infections (including HIV) and unwanted pregnancies, as well as multipurpose prevention technologies. Integrated iterative design allows for a step-by step, rational process toward the best possible product. In the case of formulation development, it allows for a sequential process optimizing drug delivery requirements. In the case of user experience, it allows for a similarly sequential process to optimize the user experience toward effective use. In combination, where feedback loops can integrate engineering and user input goals, a product co-optimized for efficacy and effectiveness can be realized.
There are multiple considerations throughout the process. For topical vaginal or rectal product use, initial impressions of the product made by the user can facilitate or obviate their willingness to try the product, even once. Given a user chooses to try a product, s/he must then determine whether the product is “useful” and whether it “fits” into their lives. There are a number of user experiences that impact this determination, including any product preparation required, the insertion experience, and, for any pericoital soft material form or intravaginal device (e.g., IVR), any experiences encountered following insertion, including the sexual experience and any hygiene related consequences. For non-topical products, initial impressions of the product made by the user can still facilitate or obviate their willingness to try the product, but the parameters vary.
There are some product elements and characteristics that are best evaluated during clinical trials – whether safety or efficacy. There are other product elements and characteristics that can begin to be evaluated in preclinical development.[41] Once products have advanced into clinical trials, drug delivery systems are set and will be changed only if necessary, and only once the product has failed during clinical trial or has shown poor efficacy and been returned to a preclinical development process. Integrated and iterative preclinical design inclusive of user input, therefore, provides an opportunity, not only to optimize the drug delivery system for proper dosing and safety, but, at a minimum, to identify any “no-go” elements from a user perspective. With greater care, an integrated iterative engineering and user experience design process could also identify the “go” elements of a potential product that could be leveraged to promote uptake and use.[2, 3] These include objective product design parameters, as well as subjective opinions or judgements (i.e., meaning-making) formed by users as a function of previous similar product use, cultural, educational or other concerns, or personal preference.[18]
Thus, while preclinical drug design iteration is characterized by a logical progression from an early concept to one more refined for pharmacokinetics, pharmacodynamics, safety, and efficacy studies in animal models, we believe it is imperative to also add user experience evaluation and user input as early as possible. This belief is underpinned by the growing attention to patient-centered outcomes research. While much of the burgeoning science of patient input concerns itself with treatments for diagnosed conditions, we should heed the knowledge gained there for development of prevention products, as well, thus broadening the application of translational science into preclinical development.
Acknowledgments
The authors would like to acknowledge and thank the participants in the iterative user experience studies used as the exemplar in this manuscript. We would also like to thank our colleagues who provide collaborative and thought-provoking insights and guidance as we build a science of user experience and patient input. A Stege is now affiliated with the Allen Institute for Brain Science, 615 Westlake Ave N, Seattle, WA. K.A. Smith is now at the Hassenfeld Child Health Innovation Institute, Brown University, Providence, RI. E.M. Kojic is now affiliated with Icahn School of Medicine at Mount Sinai, Division of Infectious Diseases, Mount Sinai St. Luke’s and West, 1111 Amsterdam Ave, New York, NY. Research reported in this publication was supported by the Division of AIDS, The National Institute of Allergy and Infectious Diseases of the National Institutes of Health, under award number R01 AI112002. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
Ethical Standards Statement
The human subjects research discussed in this publication complied with all current laws of the United States of America as codified in 45 CFR 46 and all applicable institutional guidelines. The study was overseen by the applicable human subjects institutional review boards. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all participants before being included in the study.
References
- 1.Stoddard RJ, et al. In pursuit of functional electrospun materials for clinical applications in humans. Ther Deliv. 2016;7(6):387–409. doi: 10.4155/tde-2016-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guthrie KM, et al. Perceptibility and the “Choice Experience”: User Sensory Perceptions and Experiences Inform Vaginal Prevention Product Design. AIDS Res Hum Retroviruses. 2016;32(10–11):1022–1030. doi: 10.1089/aid.2015.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrow KM, et al. Designing preclinical perceptibility measures to evaluate topical vaginal gel formulations: relating user sensory perceptions and experiences to formulation properties. AIDS Res Hum Retroviruses. 2014;30(1):78–91. doi: 10.1089/aid.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akil A, et al. Increased Dapivirine tissue accumulation through vaginal film codelivery of dapivirine and Tenofovir. Mol Pharm. 2014;11(5):1533–41. doi: 10.1021/mp4007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akil A, et al. Development and Characterization of a Vaginal Film Containing Dapivirine, a Non-nucleoside Reverse Transcriptase Inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res. 2011;1(3):209–222. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunge KE, et al. A Phase 1 Trial to Assess the Safety, Acceptability, Pharmacokinetics, and Pharmacodynamics of a Novel Dapivirine Vaginal Film. J Acquir Immune Defic Syndr. 2016;71(5):498–505. doi: 10.1097/QAI.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ham AS, et al. Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm Res. 2012;29(7):1897–907. doi: 10.1007/s11095-012-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunal J. Institutional Repository at the University of Pittsburgh. University of Pittsburgh; Pittsburgh: 2015. Design and Evaluation of a Topical Rectal Specific Microbicide for HIV prevention; p. 64. [Google Scholar]
- 9.Zhang W, et al. Vaginal Microbicide Film Combinations of Two Reverse Transcriptase Inhibitors, EFdA and CSIC, for the Prevention of HIV-1 Sexual Transmission. Pharm Res. 2015;32(9):2960–72. doi: 10.1007/s11095-015-1678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdool Karim Q, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdool Karim SS, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25(7):957–66. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halpern V, et al. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: results of a Phase III trial in Nigeria. PLoS One. 2008;3(11):e3784. doi: 10.1371/journal.pone.0003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer KH, et al. Safety and tolerability of BufferGel, a novel vaginal microbicide, in women in the United States. Clin Infect Dis. 2001;32(3):476–82. doi: 10.1086/318496. [DOI] [PubMed] [Google Scholar]
- 14.Van Damme L, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359(5):463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 15.van De Wijgert J, et al. Phase 1 trial of the topical microbicide BufferGel: safety results from four international sites. J Acquir Immune Defic Syndr. 2001;26(1):21–7. doi: 10.1097/00126334-200101010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Baeten JM, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016 doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrow Guthrie K, et al. The Promise of Intravaginal Rings for Prevention: User Perceptions of Biomechanical Properties and Implications for Prevention Product Development. PLoS One. 2015;10(12):e0145642. doi: 10.1371/journal.pone.0145642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen RK, et al. Meaning-making matters in product design: users’ sensory perceptions and experience evaluations of long-acting vaginal gels and intravaginal rings. Contraception. 2015;92(6):596–601. doi: 10.1016/j.contraception.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrow KM, et al. User-identified gel characteristics: a qualitative exploration of perceived product efficacy of topical vaginal microbicides. Arch Sex Behav. 2014;43(7):1459–67. doi: 10.1007/s10508-013-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28(3):325–47. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Carson D, Jiang Y, Woodrow KA. Tunable Release of Multiclass Anti-HIV Drugs that are Water-Soluble and Loaded at High Drug Content in Polyester Blended Electrospun Fibers. Pharm Res. 2016;33(1):125–36. doi: 10.1007/s11095-015-1769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Illangakoon UE, et al. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning. Int J Pharm. 2014;477(1–2):369–79. doi: 10.1016/j.ijpharm.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Kataria K, et al. In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int J Pharm. 2014;469(1):102–10. doi: 10.1016/j.ijpharm.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, et al. Rapid implantation of dissolving microneedles on an electrospun pillar array. Biomaterials. 2015;64:70–7. doi: 10.1016/j.biomaterials.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Baji A, et al. Electrospinning of polymer nanofibers: Effects on oriented morphology, structures and tensile properties. Composites Science and Tecnology. 2010;70(5):703–718. [Google Scholar]
- 26.del Valle LJ, et al. Electrospinning of polylactide and polycaprolactone mixtures for preparation of materials with tunable drug release properties. Journal of Polymer Research. 2011;18(6):1903–1917. [Google Scholar]
- 27.Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int J Pharm. 2014;475(1–2):282–91. doi: 10.1016/j.ijpharm.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okuda T, Tominaga K, Kidoaki S. Time-programmed dual release formulation by multilayered drug-loaded nanofiber meshes. J Control Release. 2010;143(2):258–64. doi: 10.1016/j.jconrel.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 29.Hu C, et al. Long-term drug release from electrospun fibers for in vivo inflammation prevention in the prevention of peritendinous adhesions. Acta Biomater. 2013;9(7):7381–8. doi: 10.1016/j.actbio.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 30.Liu KS, et al. Sustained release of vancomycin from novel biodegradable nanofiber-loaded vascular prosthetic grafts: in vitro and in vivo study. Int J Nanomedicine. 2015;10:885–91. doi: 10.2147/IJN.S78675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrow KM, et al. User Experience and Feedback for Development of Monoclonal Antibody-based Multipurpose Microbicides. unpublished. [Google Scholar]
- 32.Buckheit RW, Jr, et al. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral Res. 2010;85(1):142–58. doi: 10.1016/j.antiviral.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guthrie KM, et al. Vaginal film for prevention of HIV: using visual and tactile evaluations to design for size, color and texture among potential users. doi: 10.1080/10837450.2017.1339085. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrow K, et al. More…? Less…? Just right…? The Role of Perceived Volume in Gel and Film Perceptibility During Intercourse, and its Impact on Product Preference. 2014;30(S1):A145. [Google Scholar]
- 35.Morrow KM, Hendrix C. Clinical evaluation of microbicide formulations. Antiviral Res. 2010;88(Suppl 1):S40–6. doi: 10.1016/j.antiviral.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrow KM, et al. Vaginal Film User Evaluations: Developer Considerations from Initial Impressions and User Sensory Perceptions and Experiences During Vaginal Sex. AIDS Research and Human Retroviruses. 2014;30(S1):A145–A146. [Google Scholar]
- 37.Morrow KM, et al. “He Said, She Said.” Exploring Couples’ Sensory Perceptions and Experiences with Vaginal Gels & Film: Implications for Microbicide Development. AIDS Research and Human Retroviruses. 2014;30(S1):A86–A87. [Google Scholar]
- 38.Tolley EE, Morrow KM, Owen DH. Designing a multipurpose technology for acceptability and adherence. Antiviral Res. 2013;100(Suppl):S54–9. doi: 10.1016/j.antiviral.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Berg JJ, et al. “Set it and forget it”: women’s perceptions and opinions of long-acting topical vaginal gels. AIDS Behav. 2014;18(5):862–70. doi: 10.1007/s10461-013-0652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weld ED, et al. A Comparative Pre-Phase I Study of the Impact of Gel Vehicle Volume on Distal Colon Distribution, User Experience, and Acceptability. AIDS Res Hum Retroviruses. 2017;33(5):440–447. doi: 10.1089/aid.2016.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrow KM, Ruiz MS. Assessing microbicide acceptability: a comprehensive and integrated approach. AIDS Behav. 2008;12(2):272–83. doi: 10.1007/s10461-007-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


