Despite antiretroviral therapy (ART), a latent reservoir of replication-competent HIV-1 persists in resting memory CD4+ T cells and precludes a cure1–6. Lorenzo-Redondo et al.7 analysed HIV-1 sequences collected from three individuals during the first six months of ART, discovered specific patterns of sequence evolution, and concluded that viral replication persists during therapy. We believe that these evolutionary patterns are artefacts of rapidly decaying viral subpopulations present during the first months of therapy and are not characteristic of the long-lived reservoir. We therefore argue that ref. 7 does not provides evidence that ongoing replication is an additional barrier to a cure for treated individuals who consistently maintain low viral loads. There is a Reply to this Brief Communication Arising by Lorenzo-Redondo, R. et al. Nature 551, http://doi.org/10.1038/nature24635 (2017).
Lorenzo-Redondo et al.7 collected samples before and three and six months after treatment initiation, when labile viral populations dominate and change rapidly. Before treatment, most HIV-1 DNA in resting CD4+ T cells exists in an unintegrated state, decaying with a half-life of days8,9. Another major population of infected resting cells decays with a half-life of weeks10. The latent reservoir of integrated proviruses, observed in blood and lymphoid tissue1, is smaller and decays with half-life of approximately four years5,6. Lifelong persistence of this reservoir is determined by the longevity and proliferation of the infected cells11. Initiation of ART blocks new infection from replenishing these populations, revealing their different lifespans. Differential decay causes marked shifts in infected cell populations in the first six months of ART, bringing into question any conclusions about viral evolution gleaned from this period. Below, we support our claim by simulating differential decay and replicating the analysis of ref. 7 on the simulated data. We find that false signals of viral evolution—and ongoing viral replication—often appear.
To estimate the size and decay of labile compartments, we examined a cohort of seven early-treated individuals, which we consider comparable to the two early-treated participants in ref. 7. The quantitative viral outgrowth assay (qVOA) has been used on blood samples10 to detect resting CD4+ T cells harbouring replication-competent HIV-1. At the onset of ART, infected cell frequencies greatly exceeded those of individuals on long-term ART. A multi-log, multi-phasic decay over the first year of therapy reduced frequencies to levels typically observed during long-term ART. Fitting to the most extensively sampled individual, we inferred a large, fast-decaying population, a smaller population with slower decay, and a very small, persistent reservoir, which was approximated as constant (Fig. 1). At zero, three, and six months after ART initiation, labile populations comprised 99.99%, 96.2%, and 76% of infected resting cells, respectively, masking the persistent reservoir. The RNA-based assays performed by Lorenzo-Redondo et al.7 on lymphoid tissue paint a similar picture: the infection decays rapidly over the first three to six months, eventually dwindling to a more stable state that is more than three orders of magnitude smaller than the pretreatment population (Extended Data Fig. 1 of ref. 7). Regardless of sequencing depth, the limited number of infected cells in a blood draw or tissue biopsy is likely to prevent the persistent reservoir from being sequenced at early time points. The genetic diversity of this reservoir emerges only later. Latency studies are therefore generally restricted to participants who have received suppressive ART for more than six months, a precaution not taken by Lorenzo-Redondo et al.7.
Figure 1. For the first six months of ART, the persistent latent reservoir makes up a minority of sampled sequences.
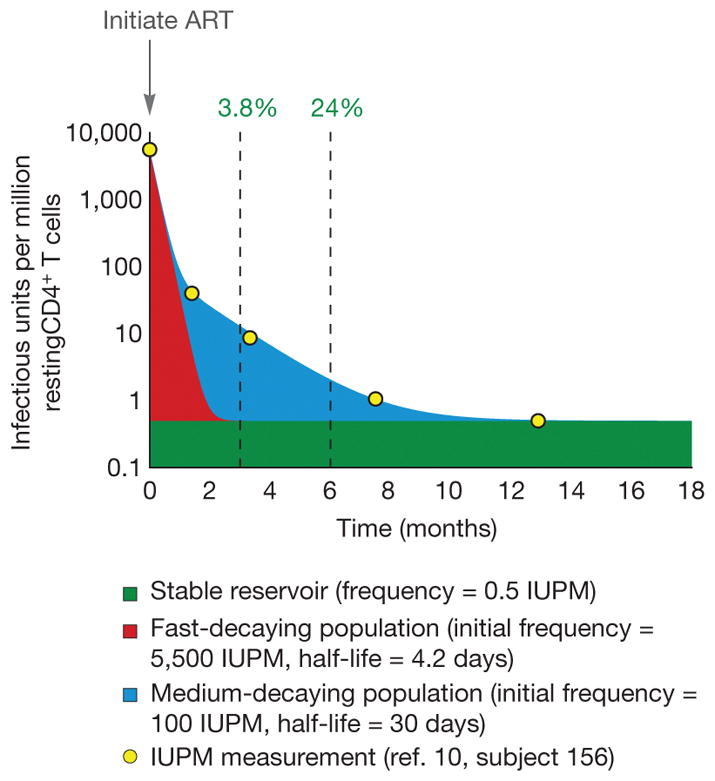
Yellow dots show longitudinal measurements of inducible, replication-competent HIV-1 in resting CD4+ T cells from one patient10. Infection frequency was measured by qVOA and is reported as infectious units per million cells (IUPM). Intact, unintegrated HIV-1 DNA in recently infected cells can be detected in this assay because cellular activation stimulates completion of the viral life cycle1,8,10. The solid-coloured regions show estimated sizes of the underlying populations that combine to yield the observed triphasic decay of IUPM. A latent reservoir (green, proportion shown at three and six months) is established before treatment and persists despite ART. This reservoir decays at a very slow rate5,6 that can be approximated as constant over the one-year period represented here. Initially, there is a large, rapidly decaying viral population (red) that is likely to include unintegrated viral genomes. A smaller population of infected cells decays at a moderate rate (blue). Consequently, at therapy initiation, less than 0.01% of resting CD4+ T cells with replication-competent virus belong to the stable reservoir, while 98% belong to the large, fast-decaying population. Only after a year of therapy would the stable reservoir exceed 95%. All patients in the study showed similar decay, in which the proportion of infected cells belonging to the latent reservoir did not stabilize within the first six months of therapy10. Note that cells with infectious provirus are generally outnumbered by orders of magnitude by cells containing defective provirus. The percentages given here therefore overestimate the proportion of all sampled HIV-1 DNA that represents the persistent latent reservoir of replication-competent virus.
Brodin et al12 suggested that the decay of labile populations may produce false signals of evolution during treatment, even in the absence of viral replication. We used computer simulations of viral populations during acute infection and treatment to confirm this hypothesis. Simulated virus replicated and seeded subpopulations for four months, and treatment then blocked replication for six months. During treatment, labile subpopulations decayed, while a stable reservoir persisted, as in Fig. 1. Nearly 12,000 simulations were subjected to the tests performed by Lorenzo-Redondo et al.7: genetic divergence from start to end of therapy, evolutionary rate calculations, and measurement of clock-like signal in maximum-likelihood trees (Supplementary Tables 1, 2).
We tested a range of parameters defining growth and competition in the pre-treatment viral population and selected 8,000 simulations with realistic viral diversity. Depending on parameter values, up to 57% of simulations produced a false impression of clock-like evolution according to all three tests used by Lorenzo-Redondo et al.7 (Supplementary Methods). For comparison, of the 17 gene–tissue combinations studied by Lorenzo-Redondo et al.7, 11 (65%) produced a signature of evolution according to the two tests for which statistics were explicitly presented (Extended Data Tables 1, 2 of ref. 7). Decay of labile compartments can remove positively selected variants that arose before treatment, revealing ancestral genotypes, which masquerade as the product of new mutation during treatment. Strong positive selection (anywhere in the genome, not only in the sequenced region) is required to generate a false impression of evolution. We believe that this mechanism is realistic, as rapid selective sweeps, caused by cytotoxic-T-lymphocyte escape mutations with selective coefficients of 20% or more, typify acute infection13.
To support our argument with sequence data and without assuming a selection coefficient, we simulated reservoir seeding and post-treatment decay using HIV-1 gag sequences obtained from three individuals during untreated acute infection14. The appearance of post-treatment ‘evolution’ again depended on pre-treatment dynamics. The individual with the most extensive sequence changes, suggesting strong pre-treatment selection, passed a phylogenetic test of forward evolution in 92 of 100 replicate simulations (Supplementary Methods, Supplementary Table 3, Extended Data Table 1).
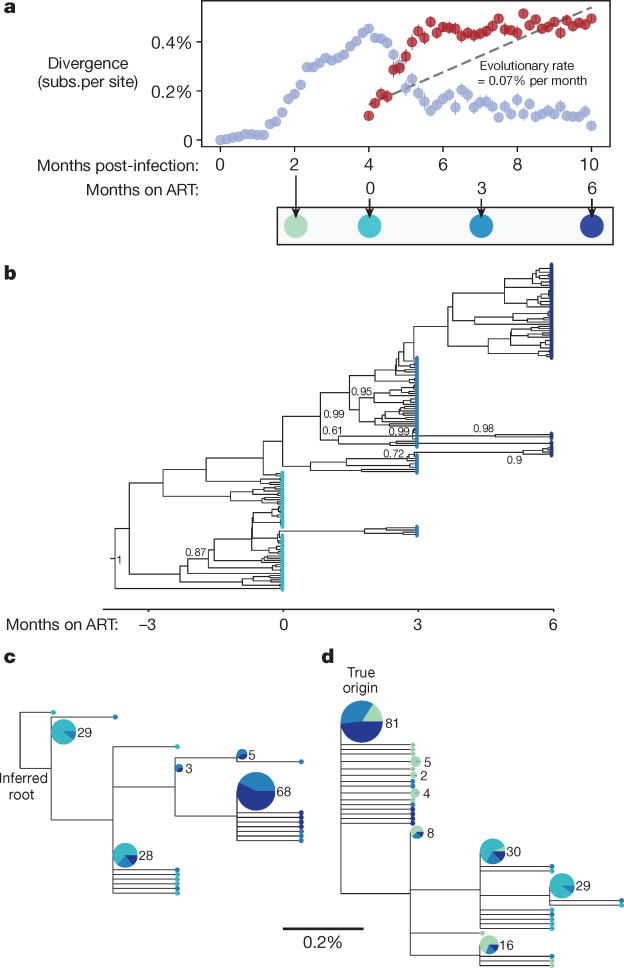
Figure 2 illustrates how the decay of labile compartments produces the appearance of evolution in an example simulation (Replicate 91 of Parameter Set 48, Supplementary Table 2). Before treatment, the population diverges through mutation and selection. When treatment starts, the most diverged sequences die out, resulting in a decline in divergence. If divergence is measured not from the origin of infection, but rather (as in ref. 7) from the most common genotype at treatment initiation, this retreat towards the origin is misinterpreted as increasing divergence (Fig. 2a). After approximately two months of treatment, this trend slows, as labile compartments (the source of more recently produced virus) decay. If a time-structured tree is constructed only from sequences collected during treatment (Fig. 2b), then a pattern of forward evolution appears. Maximum-likelihood phylogenetic analysis suggests the same pattern, and there are even three internal branches (three separate ‘novel mutations’) leading exclusively to sequences sampled at the two later time points (Fig. 2c). Including sequences sampled before the start of treatment and rooting at the actual infection origin reveals the truth (Fig. 2d): sequences diverge for four months and then the pattern of divergence reverses (that is, sequences sampled at treatment initiation tend towards the rightmost leaves of the tree).
Figure 2. Simulated decay of labile infected cell population creates misleading appearance of viral replication and evolution in first six months of treatment.
A simulated sample of 50 sequences of length 587 base pairs (bp) is shown at each time point (see Methods); these values were chosen for consistency with the number and length of haplotypes presented in the phylogenetic analysis of ref. 7. a, Genetic divergence is measured, as in ref. 7, as the average fraction of sites differing from the most common genotype found at treatment initiation (red symbols). Although simulated treatment halts all viral replication, decay of labile infected cells over the first year of treatment causes the sampled viral population to diverge genetically from this common genotype. If divergence is instead measured from the infection origin (light blue symbols), the true pattern is unmasked: evolution proceeds before treatment, but then reverses during early treatment as an increasing number of ancestral reservoir sequences are sampled. Bars show s.e. b, A time-structured tree, constructed as in Fig. 1 of ref. 7 from sequences sampled at and after initiation of ART, creates the misleading appearance of clocklike evolution. Posterior clade probabilities >60% shown. c, A maximum-likelihood tree, constructed and rooted as in Extended Data Fig. 2 of ref. 7, recapitulates this pattern. A clock-like evolutionary signal is detected (0.07% substitutions per site per month, R2 = 0.54, P < 10−25). d, When sequences sampled before initiation of ART are also included in the maximum-likelihood tree and the root is placed at the true origin of infection, no clock-like signal is detected. Posterior clade probabilities >60% shown. In c and d, leaf sizes and labels indicate multiplicity of each genotype in the sample; leaves without numbers occur only once; and segments show the proportion sampled at each time, using the colour scheme below a.
We have not attempted to show that 100% of viral replication ceases during suppressive ART; this ‘absolute negative claim’ is neither believable nor strictly necessary. The question relevant to HIV cure research is not whether any replication occurs, but whether sufficient replication occurs to fuel viral persistence during ART. Insufficient or subcritical replication may contribute to residual viraemia, but does not cause long-term persistence and re-seeding of the latent reservoir. Most importantly, subcritical replication is not a barrier to HIV cure15.
What we do claim is that, even in the complete absence of viral replication, misleading evolutionary signatures of high-level replication are expected to appear in the first six months of ART. Adding multiple anatomical compartments or other elaborations to our model could increase realism but would not alter this basic conclusion. The decay of labile populations confounds evolutionary analysis, and so the observations of Lorenzo-Redondo et al. are insufficient evidence for ongoing replication. We thus remain unconvinced that ongoing replication contributes to reservoir stability during ART, and we encourage repetition of these studies using replication-competent viruses from HIV-1-infected individuals after more than one year of suppressive ART.
D.I.S.R. acknowledges support from amfAR Fellowship no. 109511-61-RKRL and National Institutes of Health grant R01GM117591. A.L.H. acknowledges support from National Institutes of Health grant DP5OD019851 and Bill & Melinda Gates Foundation award OPP1148627.
Methods
Simulations used a stochastic model of birth, death, and mutation of infected cells. Upon birth, a productively infected cell gives rise to another infected cell, which may experience mutation. A productively infected cell may transition to any state identified in Fig. 1. Maximum-likelihood trees were constructed using PhyML (HKY85 model; single rate category; estimation of base frequencies, transition/transversion ratio, and proportion invariant sites; best of NNI/SPR). The inferred root was chosen from the earliest time point to maximize R2 of the root-to-tip regression. The time-structured tree was constructed using BEAST v2.4.4. Details are provided in Supplementary Methods.
Extended Data
Extended Data Table 1.
Analysis of post-treatment simulated samples based on sequences obtained before treatment by Novitsky et al.14.
| Participant Code14 | ||||
|---|---|---|---|---|
| Number of replicates out of 100 that pass test(s) (p < 0.05) | B | H | OU | |
| Test 1: Divergence | Forward evolution | 10 | 0 | 36 |
| Reverse evolution | 4 | 30 | 0 | |
| Test 2: Evolutionary rate | Forward evolution | 24 | 0 | 41 |
| Reverse evolution | 2 | 29 | 0 | |
| Test 3: Root-to-tip regression | Forward evolution | 20 | 9 | 92 |
| Reverse evolution | 56 | 45 | 1 | |
| Passes at least one test; not contradicted by other tests | Forward evolution | 22 | 9 | 92 |
| Reverse evolution | 42 | 46 | 1 | |
| Passes all three tests | Forward evolution | 4 | 0 | 27 |
| Reverse evolution | 1 | 27 | 0 | |
Supplementary Material
Footnotes
Author Contributions Wrote the manuscript: D.I.S.R., A.L.H., S.B.L., R.F.S. Simulations and phylogenetic analyses: D.I.S.R., A.L.H., R.F.S. Estimation of labile compartments: S.B.L., R.F.S.
Competing Financial Interests: Declared none.
References
- 1.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 2.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 5.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 6.Crooks AM, et al. Precise quantitation of the latent HIV-1 reservoir: implications for eradication strategies. J Infect Dis. 2015;212:1361–1365. doi: 10.1093/infdis/jiv218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenzo-Redondo R, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530:51–56. doi: 10.1038/nature16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierson TC, et al. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J Virol. 2002;76:8518–8531. doi: 10.1128/JVI.76.17.8518-8531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blankson JN, et al. Biphasic decay of latently infected CD4+ T cells in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000;182:1636–1642. doi: 10.1086/317615. [DOI] [PubMed] [Google Scholar]
- 11.Hosmane NN, et al. Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J Exp Med. 2017;214:959–972. doi: 10.1084/jem.20170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodin J, et al. Establishment and stability of the latent HIV-1 DNA reservoir. eLife. 2016;5:1312. doi: 10.7554/eLife.18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, et al. Selection on the human immunodeficiency virus type 1 proteome following primary infection. J Virol. 2006;80:9519–9529. doi: 10.1128/JVI.00575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novitsky V, Wang R, Rossenkhan R, Moyo S, Essex M. Intra-host evolutionary rates in HIV-1C env and gag during primary infection. Infect Genet Evol. 2013;19:361–368. doi: 10.1016/j.meegid.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conway JM, Perelson AS. Residual viremia in treated HIV+ individuals. PLOS Comput Biol. 2016;12:e1004677. doi: 10.1371/journal.pcbi.1004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.