Abstract
Genetic deletion of the Src family tyrosine kinase Lyn in mice recapitulates human systemic lupus erythematosus, characterized by hyperactive B cell antigen receptor (BCR) signaling, splenomegaly, autoantibody generation and glomerulonephritis. However, the molecular regulators of autoimmunity in Lyn-deficient mice and in human lupus remain poorly characterized. Here, we report that conditional deletion of the membrane-cytoskeleton linker protein ezrin in B cells of Lyn-deficient mice (DKO mice) ameliorates B cell activation and lupus pathogenesis. B cells from DKO mice respond poorly to BCR stimulation, with severe downregulation of major signaling pathways. DKO mice exhibit reduced splenomegaly as well as significantly lower levels of autoantibodies against a variety of autoantigens, including dsDNA, histone and chromatin. Leukocyte infiltration, and deposition of IgG and complement component C3 in the kidney glomeruli of DKO mice is markedly reduced. Our data demonstrate that ezrin is a novel molecular regulator of B cell-associated lupus pathology.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an aggressive autoimmune disorder that afflicts 1.5 million Americans. B cell hyperactivity and anti-dsDNA autoantibody production are hallmarks of SLE (1–3), signifying that altered B cell function is central to the pathogenesis of SLE. Human GWAS studies and genetic knockouts have revealed a powerful biochemical pathway that keeps B cell antigen receptor (BCR) signaling under check (4–6); Lyn tyrosine kinase is a key component of this pathway. Lyn phosphorylates CD22 on inhibitory tyrosines (7), which then recruits the phosphatase Shp-1 resulting in downregulation of BCR signaling (8). Genetic variations in Lyn (9) and reduced expression of Lyn (10, 11) are associated with strong susceptibility to human SLE, and mice deficient in Lyn, CD22 or Shp-1 exhibit SLE-like disease, including hyperactive BCR signaling, autoantibody production and glomerulonephritis (12–18). As Lyn−/− mice represent a clinically relevant model of SLE, insights from characterization of proteins and processes that regulate B cell hyperactivation and autoantibody production in this model may lead to better molecular understanding of SLE.
Ezrin is a member of the ERM family of membrane-cytoskeleton cross-linking proteins. It contains an N-terminal FERM domain that binds transmembrane proteins such as CD44 (19), and Cbp/PAG (20). The C-terminal domain contains a conserved threonine residue (T567) whose phosphorylation is critical for its conformational activation and binding to filamentous actin (F-actin) (19). We have previously reported that ezrin modulates B cell function due to its ability to regulate BCR organization, signaling (21), B cell chemotaxis (22), and IL-10 production (23). Moreover, we recently showed that ezrin supports pathogenic BCR signaling in germinal center-derived diffuse large B cell lymphoma (DLBCL) (24). However, it is not known whether ezrin regulates abnormal BCR signaling and B cell-associated pathogenesis in Lyn−/− mice.
In this study we employed Lyn−/− mice in which ezrin was conditionally deleted in B cells to investigate the impact on BCR signaling, autoantibody levels and kidney pathology. Our data show that loss of ezrin in Lyn−/− B cells resolves these major hallmarks of SLE-associated autoimmune pathology.
MATERIALS AND METHODS
Mice
Ezfl/flMB1cre/+ (Ez-def), MB1cre/+, and Lyn−/− mice have been previously described (12, 13, 21). Two to eight month old animals were used, and all experiments performed in compliance with the guidelines approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Flow cytometry
Single-cell suspensions were prepared from spleens, blocked with anti-CD16/32 (clone 2.4G2), and stained with FITC- or PE-conjugated antibodies to surface IgM (BD Pharmingen). Developmental and mature B cell subsets were identified by flow cytometry based on previously described gating strategies (21). Flow cytometry data were collected on BD LSR Fortessa and analyzed using FlowJo software (Tree Star).
BCR stimulation, immunoblotting, and calcium flux
Splenic B and T cells were purified by negative selection using CD43 beads or mouse pan T cell isolation kit II (Miltenyi Biotecc), respectively. Purified B cells were stimulated with 10 μg/ml F(ab′)2 fragment of anti-mouse IgM (Jackson ImmunoResearch Laboratories) for the indicated times. B and T cell lysates were prepared and immunoblotting performed as described (21). All immunoblotting antibodies were from Cell Signaling Technology, except for actin (Santa Cruz Biotechnology), Igα (Abcam), phosphotyrosine (pY) and ezrin (EMD Millipore). To measure intracellular-free calcium levels, purified splenic B cells were loaded with Fluo-3 AM (Molecular Probes) at 37°C for 20 min. Cells were washed, resuspended in DMEM supplemented with 1% BSA (Sigma) and 20 mM HEPES (Sigma), warmed to 37°C for 5 min, and analyzed by flow cytometry. After the baseline was established for 30–40 sec, cells were stimulated with 10 μg/ml F(ab′)2 fragment of anti-mouse IgM for the indicated duration.
Autoantigen array and autoantibody ELISA
The profiling of IgM and IgG autoantibodies in sera was done using 98-plex autoantigen arrays. Arrays were hybridized with mouse sera, detected by Cy5-labeled anti-mouse IgM and Cy3-labeled anti-mouse IgG antibodies, and scanned with GenePix® 4400A Microarray Scanner to generate Tiff images. The images were analyzed using Genepix Pro 6.0 software to generate GPR files. The averaged net fluorescent intensity (NFI) of each autoantigen was normalized to internal controls (IgM or IgG). ELISA plates coated with purified dsDNA (Invitrogen) were used to quantify serum anti-dsDNA antibodies, and those coated with antibodies to IgM or IgG, respectively, to quantify total IgM and IgG levels.
Histopathology and immunofluorescent staining
Kidneys were fixed in 10% formalin (Sigma) for 24 h, washed in alcohol and embedded in paraffin. For light microscopy, sections were cut and stained with hematoxylin and eosin (H&E). IgG immune complex and complement C3 deposits in kidney were detected by direct immunofluorescence of OCT-embedded frozen kidney sections (5 μm thick) using Texas Red-conjugated goat anti-mouse IgG (Molecular Probes) or FITC-conjugated anti-C3 complement (Thermo Fisher Scientific), respectively. The slides were mounted in Vectashield (Vector Laboratories) and observed under the microscope.
Statistical analysis
All analyses were performed with Prism 4 software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical differences between any two genotypes were calculated using an unpaired t-test. P value of < 0.05 was considered significant.
RESULTS AND DISCUSSION
Reciprocal regulation of Ezrin and Lyn
Both ezrin and Lyn act at very proximal steps during B cell activation by antigen, the former through regulation of lipid raft and BCR dynamics and the latter by initiating BCR phosphorylation as well as terminating B cell activation through recruitment of tyrosine and lipid phosphatases. In order to test if ezrin and Lyn regulate each other, we employed Ez-def and Lyn−/− B cells and examined the BCR-dependent phosphorylation of Lyn and ezrin, respectively. Purified splenic B cells from 2–3 month old C57BL/6 and Lyn−/− mice (Supplemental Fig. 1A) or MB1cre/+ and Ez-def mice (Supplemental Fig. 1B) were stimulated with F(ab′)2 fragment of anti-IgM for 1 and 10 min, and the phosphorylation of ezrin and Lyn assessed in cell lysates. Lyn−/− B cells showed constitutively higher ezrin phosphorylation in unstimulated cells and a resistance to dephosphorylation upon BCR stimulation (Supplemental Fig. 1A). Similarly, phosphorylation of Lyn (p53, p56) and Fyn (p59) was increased in Ez-def B cells as compared to MB1cre/+ B cells (Supplemental Fig. 1B), indicating a reciprocal regulatory relationship between ezrin and Lyn.
Deletion of ezrin in Lyn−/− mice reduces splenomegaly
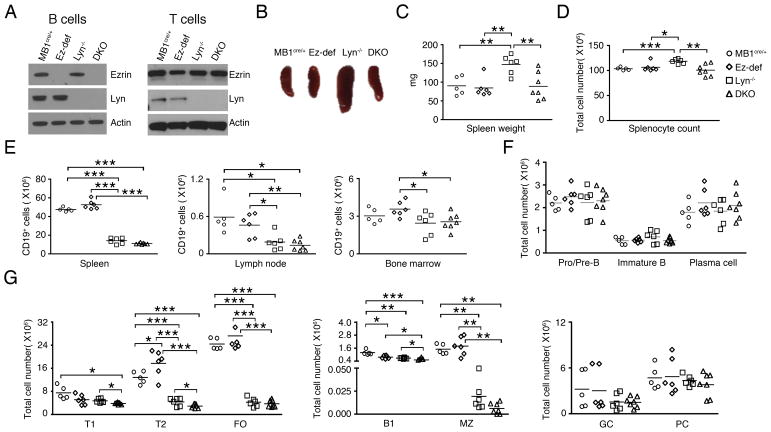
We hypothesized that ezrin may regulate BCR signaling in Lyn−/− B cells and impact downstream autoimmune pathology. To test this, we bred the Lyn−/− mice with Ez-def mice to generate Lyn−/−Ezfl/flMB1cre/+ double knockout (DKO) mice that lack the expression of Lyn systemically and ezrin exclusively in B cells. Deletion of both ezrin and Lyn expression in B cells from DKO mice was confirmed by immunoblotting of cell lysates (Fig. 1A, left panel). The expression of ezrin was intact in purified MB1cre/+, Ez-def, Lyn−/− and DKO T cells (Fig. 1A, right panel) indicating exclusive targeting in B cells. Conditional deletion of ezrin in the B cells of Lyn−/− mice (DKO) led to reduction in spleen size (Fig. 1B), weight (Fig. 1C) and the number of splenocytes (Fig. 1D). Thus, splenomegaly, which is a characteristic feature of Lyn−/− mice, was ameliorated in DKO mice. The splenic tissue in DKO mice also appeared less fibrous during mechanical disruption as compared to Lyn−/− mice. We next analyzed the B cell compartment in 2–3 month old MB1cre/+, Ez-def, Lyn−/− and DKO mice to examine if loss of ezrin in Lyn−/− mice affected bone marrow B cell development and peripheral B cell subset composition. The total number CD19+ B cells in the spleen, lymph nodes and bone marrow was not altered in DKO mice as compared to Lyn−/− mice (Fig. 1E). Bone marrow B cell development was also not affected in DKO mice, as indicated by comparable numbers of Pro/Pre-B cells and immature B cells (Fig. 1F), and long-lived plasma cells in the bone marrow were similar in number between the Lyn−/− and DKO mice (Fig. 1F). Lyn−/− mice were reported to show reduced mature follicular (FO) B cells and T1 and T2 developmental stages of B cells in the spleen (13, 25). We observed that FO B cells were not affected by deletion of ezrin in Lyn−/− mice whereas T1 and T2 B cells were further decreased in DKO mice (Fig. 1G). Splenic B1 B cell numbers were also reduced in DKO mice compared to Lyn−/− mice, while marginal zone, germinal center and splenic plasma cell numbers remained unaltered (Fig. 1G).
Figure 1. Effects of Lyn and ezrin deficiency on B cell subsets.
(A) Purified splenic B and T cell lysates from MB1cre/+, Ez-def, Lyn−/− and DKO mice were probed for ezrin, Lyn, and actin. (B) Representative photomicrograph of spleens from 2–3 month old MB1cre/+, Ez-def, Lyn−/− and DKO mice. Data are representative of 5–7 mice for each genotype analyzed. Spleen weight (C) and splenocyte count (D) from 2–3 month old mice. (E) Absolute numbers of CD19+ B cells in the spleen, lymph node, and bone marrow from 2–3 month old MB1cre/+, Ez-def, Lyn−/− and DKO mice. Absolute numbers of pro/pre, immature, and plasma cells in the bone marrow (F), and transitional 1 (T1), transitional 2 (T2), follicular (FO), B1, marginal zone (MZ), germinal center cells (GC) and plasma cells (PC) in the spleen (G). Each symbol represents an individual mouse. Data represent mean ± SEM from 5–7 mice per group. (C–G) *p < 0.05, **p < 0.01, ***p < 0.001.
Deletion of ezrin reduces activation of Lyn−/− B cells
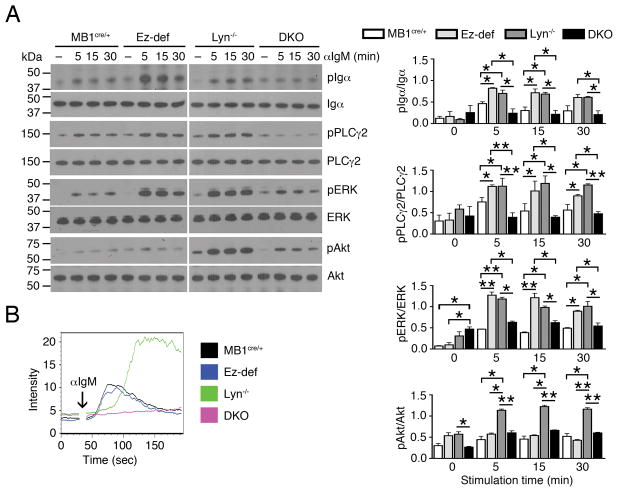
Lyn−/− B cells are known to be hyperresponsive to BCR stimulation and display hyperactive proximal and distal signaling with delayed but greatly increased magnitude of calcium flux (12). Therefore, we examined whether the loss of ezrin in Lyn−/− B cells would affect their response to BCR stimulation. Purified splenic B cells from 2–3 month old MB1cre/+, Ez-def, Lyn−/− and DKO mice were stimulated with F(ab′)2 fragment of anti-IgM for 5, 15 and 30 min to mimic ex vivo response to antigen stimulation, and the activation of major proximal and distal BCR signaling pathways analyzed in cell lysates by immunoblotting with specific antibodies. The data were quantified and statistical significance of differences is shown in Fig. 2A, right panels. Ez-def B cells exhibited stronger induced phosphorylation of Igα, PLCγ2, and ERK, but not Akt, as compared to MB1cre/+ B cells, consistent with our previous findings (21) (Fig. 2A, left panels). All of these signaling pathways were also more robustly activated in Lyn−/− B cells compared to MB1cre/+ B cells, as was Akt phosphorylation (Fig. 2A, left panels), as previously reported (12, 13). However, the DKO B cells were remarkably hyporesponsive to BCR stimulation, with signification reduction in activation of Igα and Akt, and a complete loss of induction of PLCγ2 phosphorylation (Fig. 2A, left and right panels). Interestingly, DKO B cells showed a significant increase in basal ERK phosphorylation compared to MB1cre/+ and Ez-def B cells but significantly reduced induction of ERK phosphorylation upon anti-IgM crosslinking (Fig. 2A, left and right panels). DKO B cells also failed to mobilize intracellular calcium upon anti-IgM stimulation, whereas MB1cre/+ and Ez-def B cells showed similar induction of calcium flux and Lyn−/− B cells were characteristically hyperresponsive (Fig. 2B). We tested if the reduced activation of DKO B cells was due to altered surface IgM expression. However, flow cytometry analysis revealed that the level of surface IgM was similar in MB1cre/+, Ez-def, Lyn−/− and DKO B cells (Supplemental Fig. 1C). These data indicate that loss of ezrin in Lyn−/− B cells counteracts their hyperresponsiveness to antigen stimulation.
Figure 2. B cell-specific deletion of ezrin in Lyn−/− mice inhibits BCR signaling.
(A) Purified B cells from MB1cre/+, Ez-def, Lyn−/− and DKO mice were stimulated with 10 μg/ml of anti-IgM for the indicated times, and cell lysates were probed for phosphorylated and total Igα, PLCγ2, ERK, and Akt. Representative blots from two independent experiments are shown in the left panels. The right panels depict quantification and statistical analysis of the western blotting data (mean ± SEM). *p < 0.05, **p < 0.01. (B) Intracellular calcium mobilization in MB1cre/+, Ez-def, Lyn−/− and DKO B cells after stimulation with 10 μg/ml of anti-IgM at the indicated time (arrow) for 3 min. Data are representative of three independent experiments.
B cell-specific ezrin deletion in Lyn−/− mice lowers autoantibodies
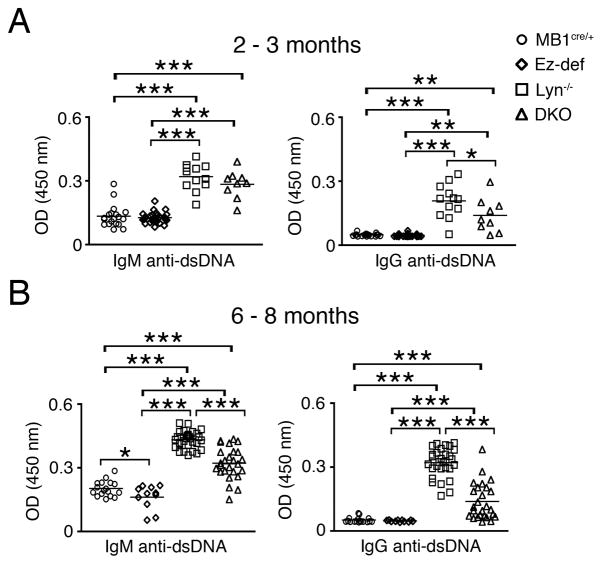
Because generation of autoantibodies to dsDNA is a hallmark clinical manifestation of autoimmune disease in human SLE as well as in aging Lyn−/− mice, and is a direct consequence of hyperactive B cells, we examined whether loss of ezrin in B cells of Lyn−/− mice would affect autoimmune disease development. ELISA was used to determine the levels of total IgM and total IgG, antibodies in the sera of 6–8 month old MB1cre/+, Ez-def, Lyn−/− and DKO mice. Total IgM levels were significantly lower in DKO mice compared to Lyn−/− mice (Supplemental Fig. 1D), whereas total IgG levels were similar between all four groups (Supplemental Fig. 1E). As reported previously (13, 14), Lyn−/− mice had detectable anti-dsDNA IgM and IgG autoantibodies within 2–3 months (Fig. 3A), which were further increased in 6–8 month old mice (Fig. 3B). Both, serum IgM and IgG levels of antibodies against dsDNA were significantly reduced in the DKO mice as compared to Lyn−/− mice at 2–3 month (Fig. 3A) as well as at 6–8 months of age (Fig. 3B). Interestingly, low levels of IgM antibodies to dsDNA were detectable in 6–8 month old MB1cre/+ mice but they were decreased in Ez-def mice (Fig. 3B). Since human SLE patients also develop antibodies to a variety of other nuclear and non-nuclear antigens, we employed a commercial autoantigen microarray to examine the effect of B cell-specific ezrin deletion in Lyn−/− mice on other autoantibody levels. Sera from randomly selected four Lyn−/− and five DKO mice were compared. Lyn−/− mouse sera contained higher than average levels of autoantibodies to most autoantigens; in contrast, most of the DKO mouse sera tested had markedly reduced IgM (Supplemental Fig. 2A) and IgG (Supplemental Fig. 2B) autoantibodies against a majority of nuclear and non-nuclear autoantigens, including histones, chromatin, ssDNA, ssRNA, snRNPs, collagen, cytochrome C and others. These data indicate that the loss of ezrin not only impacts B cell activation but also downstream autoantibody production.
Figure 3. B cell-specific ezrin deficiency in Lyn−/− mice inhibits development of pathogenic autoantibodies.
Antibodies to dsDNA in the sera of 2–3 (A), and 6–8 month old (B) MB1cre/+, Ez-def, Lyn−/− and DKO mice. Each symbol represents an individual mouse. Data represent mean ± SEM from 9–33 mice per group. *p < 0.05, **p < 0.01, ***p < 0.001.
B cell-specific ezrin deletion in Lyn−/− mice inhibits kidney pathology
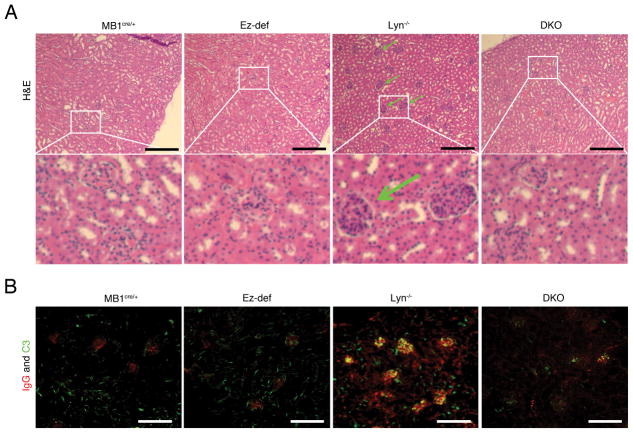
The kidneys of Lyn−/− mice have been reported to contain infiltrating inflammatory leukocytes resulting in glomerulonephritis, a clinical feature that is conserved in a proportion of human SLE patients, as well as deposition of IgG autoantibodies and fixation of complement component C3 (26). Because the DKO B cells appear to be hypoactive and do not sustain autoantibody production in DKO mice, we examined the kidney pathology in 8-month old MB1cre/+, Ez-def, Lyn−/− and DKO mice. The kidneys of Lyn−/− mice showed enlarged glomeruli filled with leukocytes (see insets marked by white boxes in Fig. 4A) while DKO mice displayed little or no evidence of glomerulonephritis (Fig. 4A). The kidneys of MB1cre/+ and Ez-def mice showed no IgG staining and only interstitial complement C3 staining, whereas in Lyn−/− kidneys both IgG deposition and C3 fixation was observed in the glomeruli. The deposition of IgG and complement component C3 was strikingly reduced in DKO mice (Fig. 4B). These data demonstrate that deletion of ezrin in the B cells of Lyn−/− mice ameliorates autoantibody production and associated severe kidney pathology, suggesting that ezrin regulates the development and progression of autoimmune disease in Lyn−/− mice.
Figure 4. B cell-specific deletion of ezrin in Lyn−/− mice leads to inhibition of glomerulonephritis.
(A) H&E stained kidney sections from 8 month old MB1cre/+, Ez-def, Lyn−/− and DKO mice. Insets marked by white boxes show more highly magnified glomerular areas showing leukocyte infiltration in Lyn−/− kidneys and lack thereof in MB1cre/+, Ez-def, and DKO kidneys. (B) Immunofluorescence images of kidneys from 8 month old MB1cre/+, Ez-def, Lyn−/− and DKO mice stained with antibodies to IgG (red) and complement fragment C3 (green). Data represent at least four mice of each genotype in two independent experiments. Scale bars, 50 μm.
Taken together, our results indicate a novel role for the membrane-cytoskeleton linker protein ezrin in controlling B cell tolerance and the development of autoimmune disease in Lyn−/− mice. Increased basal ezrin phosphorylation observed in Lyn−/− B cells may be due to higher protein kinase C expression and/or activity since ezrin is its known substrate. The absence of anti-IgM-induced dephosphorylation also indicates inability of a Ser/Thr phosphatase to gain access to or dephosphorylate ezrin. It is well known that the tyrosine and lipid phosphatase pathways (Shp-1 and SHIP, respectively) are inactive in Lyn−/− B cells. Our data suggest that the function of a Ser/Thr phosphatase such as PP1 or PP2 may be similarly compromised. The ezrin network supports the organization and integrity of BCR microclusters through its dynamic phosphorylation-dephosphorylation, and thus regulates BCR signaling (21, 27). Hyperphosphorylation of ezrin in Lyn−/− mice may regulate BCR dynamics and early activation processes that are required for appropriate initiation and termination of BCR signaling. Interestingly, hyperphosphorylation of ERM proteins in peripheral T cells of lupus patients is responsible for enhanced T cell adhesion, migration and polarization, and contributes to pathogenesis (28). Thus, altered ezrin phosphorylation in Lyn−/− mice may additionally impact B cell migration and contact with other cells within secondary lymphoid organs. A detailed analysis of Tyr, Ser/Thr and lipid phosphatase function in conjunction with microscopic analysis of BCR clustering and recruitment of different phosphatases to BCR clusters will clarify the spatial mechanisms at play.
We have previously reported that Ez-def B cells signal more strongly through the BCR (21), whereas here we demonstrate that DKO B cells signal poorly. There are at least two potential models to explain this. Higher constitutive ezrin phosphorylation observed in Lyn−/− B cells may represent an attempt by the cells to reign in BCR activation and thus serve as a negative regulator. Upon dual loss of Lyn and ezrin in DKO mice, this control is lost and autoreactive B cells may be subject to sustained overstimulation by autoantigens in vivo. Over time this may result in death of immature B cells and exhaustion-induced anergy in mature B cells, and prevent the “exhausted” B cells from supporting anti-IgM-induced BCR signaling and autoantibody generation. The reduction of T1, T2 and B1 B cell numbers in the spleens of DKO mice compared to those of Lyn−/− mice supports this model. Significantly higher basal ERK activation in DKO B cells, but failure to activate PLCγ2 and mobilize calcium upon BCR stimulation are also reminiscent of autoreactive anergic B cells (29), lending further support to this model. Alternately, higher ezrin phosphorylation may mediate hyperactivation of Lyn−/− B cells, and act as a positive regulator. In the absence of ezrin, this positive regulatory effect may be lost, leading to reduced activation of B cells. Detailed future analyses of these possibilities will elucidate the molecular pathway(s) involved in ezrin-mediated regulation of Lyn−/− B cells.
The clinical ramifications of SLE in patients are highly complex and diverse, and observed to varying degrees within the hematological, dermatological, renal, cardiac, vascular, pulmonary, ocular, gastrointestinal, musculoskeletal and neuropsychiatric systems (30). Specifically, the Lyn−/− mice develop hyperactivation of B cells, autoantibodies to double stranded DNA and other self-antigens, as well as leukocyte infiltration and immunoglobulin deposition in the kidney glomeruli. All of these features of clinical disease were ameliorated in DKO mice. Lyn−/− mice also exhibit splenomegaly (13, 18, 25), which was ameliorated in DKO mice. Given that B1 B cells are responsible for natural serum IgM, their reduction in the DKO mice may explain the reduction in total IgM levels observed in the DKO mice compared to Lyn−/− mice. Further, the remarkable lack of response of DKO B cells to polyclonal stimulation by anti-IgM ex vivo suggests that the DKO mice may mount poorer T cell-independent and/or T cell-dependent antibody responses to pathogens in vivo.
In summary, our findings indicate a novel context-dependent function of ezrin in B cell activation and pathogenesis in the autoimmune environment of Lyn−/− mice, and suggest that ezrin may be a new target of therapeutic intervention in lupus.
Supplementary Material
Acknowledgments
The authors acknowledge assistance from the Lerner Research Institute Imaging Core facility and University of Texas Southwestern Microarray Core facility.
Abbreviations used
- ERM
Ezrin/Radixin/Moesin
- DKO
Double Knockout
- GWAS
Genome wide association studies
- SLE
Systemic Lupus Erythematosus
Footnotes
This work was supported by an NIH grant (AR067705) to N.G.
References
- 1.Nashi E, Wang Y, Diamond B. The role of B cells in lupus pathogenesis. Int J Biochem Cell Biol. 2010;42:543–550. doi: 10.1016/j.biocel.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenks SA, Sanz I. Altered B cell receptor signaling in human systemic lupus erythematosus. Autoimmunity Rev. 2009;8:209–213. doi: 10.1016/j.autrev.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder K, Herrmann M, Winkler TH. The role of somatic hypermutation in the generation of pathogenic antibodies in SLE. Autoimmunity. 2013;46:121–127. doi: 10.3109/08916934.2012.748751. [DOI] [PubMed] [Google Scholar]
- 4.Pillai S, Cariappa A, Pirnie SP. Esterases and autoimmunity: the sialic acid acetylesterase pathway and the regulation of peripheral B cell tolerance. Trends Immunol. 2009;30:488–493. doi: 10.1016/j.it.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J Immunol. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornall RJ, Goodnow CC, Cyster JG. Regulation of B cell antigen receptor signaling by the Lyn/CD22/SHP1 pathway. Curr Top Microbiol Immunol. 1999;244:57–68. doi: 10.1007/978-3-642-58537-1_5. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Miller AS, Inaoki M, Bock CB, Jansen PJ, Tang ML, Tedder TF. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5:551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 8.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 9.Lu R, Vidal GS, Kelly JA, Delgado-Vega AM, Howard XK, Macwana SR, Dominguez N, Klein W, Burrell C, Harley IT, Kaufman KM, Bruner GR, Moser KL, Gaffney PM, Gilkeson GS, Wakeland EK, Li QZ, Langefeld CD, Marion MC, Divers J, Alarcon GS, Brown EE, Kimberly RP, Edberg JC, Ramsey-Goldman R, Reveille JD, McGwin G, Jr, Vila LM, Petri MA, Bae SC, Cho SK, Bang SY, Kim I, Choi CB, Martin J, Vyse TJ, Merrill JT, Harley JB, Alarcon-Riquelme ME, Nath SK, James JA, Guthridge JM. Genetic associations of LYN with systemic lupus erythematosus. Genes and immunity. 2009;10:397–403. doi: 10.1038/gene.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liossis SN, Solomou EE, Dimopoulos MA, Panayiotidis P, Mavrikakis MM, Sfikakis PP. B-cell kinase lyn deficiency in patients with systemic lupus erythematosus. J Invest Med. 2001;49:157–165. doi: 10.2310/6650.2001.34042. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Dong J, Mu R, Gao Y, Tan X, Li Y, Li Z, Yang G. MicroRNA-30a promotes B cell hyperactivity in patients with systemic lupus erythematosus by direct interaction with Lyn. Arthr Rheum. 2013;65:1603–1611. doi: 10.1002/art.37912. [DOI] [PubMed] [Google Scholar]
- 12.Chan VW, Lowell CA, DeFranco AL. Defective negative regulation of antigen receptor signaling in Lyn-deficient B lymphocytes. Curr Biol. 1998;8:545–553. doi: 10.1016/s0960-9822(98)70223-4. [DOI] [PubMed] [Google Scholar]
- 13.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 14.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 15.O’Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 16.Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 18.Lamagna C, Hu Y, DeFranco AL, Lowell CA. B cell-specific loss of Lyn kinase leads to autoimmunity. J Immunol. 2014;192:919–928. doi: 10.4049/jimmunol.1301979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta N, Wollscheid B, Watts JD, Scheer B, Aebersold R, DeFranco AL. Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat Immunol. 2006;7:625–633. doi: 10.1038/ni1337. [DOI] [PubMed] [Google Scholar]
- 21.Pore D, Parameswaran N, Matsui K, Stone MB, Saotome I, McClatchey AI, Veatch SL, Gupta N. Ezrin Tunes the Magnitude of Humoral Immunity. J Immunol. 2013;191:4048–4058. doi: 10.4049/jimmunol.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parameswaran N, Matsui K, Gupta N. Conformational switching in ezrin regulates morphological and cytoskeletal changes required for B cell chemotaxis. J Immunol. 2011;186:4088–4097. doi: 10.4049/jimmunol.1001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pore D, Matsui K, Parameswaran N, Gupta N. Cutting Edge: Ezrin Regulates Inflammation by Limiting B Cell IL-10 Production. J Immunol. 2016;196:558–562. doi: 10.4049/jimmunol.1502098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pore D, Bodo J, Danda A, Yan D, Phillips JG, Lindner D, Hill BT, Smith MR, Hsi ED, Gupta N. Identification of Ezrin-Radixin-Moesin proteins as novel regulators of pathogenic B-cell receptor signaling and tumor growth in diffuse large B-cell lymphoma. Leukemia. 2015;29:1857–1867. doi: 10.1038/leu.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross AJ, Proekt I, DeFranco AL. Elevated BCR signaling and decreased survival of Lyn-deficient transitional and follicular B cells. Eur J Immunol. 2011;41:3645–3655. doi: 10.1002/eji.201141708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu CC, Yen TS, Lowell CA, DeFranco AL. Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr Biol. 2001;11:34–38. doi: 10.1016/s0960-9822(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 27.Treanor B, Depoil D, Bruckbauer A, Batista FD. Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J Exp Med. 2011;208:1055–1068. doi: 10.1084/jem.20101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Harada T, Juang YT, Kyttaris VC, Wang Y, Zidanic M, Tung K, Tsokos GC. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol. 2007;178:1938–1947. doi: 10.4049/jimmunol.178.3.1938. [DOI] [PubMed] [Google Scholar]
- 29.Yarkoni Y, Getahun A, Cambier JC. Molecular underpinning of B-cell anergy. Immunol Rev. 2010;237:249–263. doi: 10.1111/j.1600-065X.2010.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cojocaru M, I, Cojocaru M, Silosi I, Vrabie CD. Metabolic syndrome in rheumatoid arthritis. Maedica (Buchar) 2012;7:148–152. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.