Abstract
A link between obesity and periodontitis has been suggested due to compromised immune response and chronic inflammation in obese patients. Here, we evaluated the anti-inflammatory properties of Kavain, an extract from Piper methysticum, on Porphyromonas gingivalis (P.gingivalis)-induced inflammation in adipocytes with special focus on peroxisome proliferation-activated receptor γ coactivator α (PGC-1α) and related pathways. 3T3-L1 mouse preadipocytes and primary adipocytes harvested from mouse adipose tissue were infected with P.gingivalis, and inflammation (TNF-α; adiponectin/adipokines), oxidative stress, adipogenic markers (FAS, CEBPα and PPARγ) expression were measured. Furthermore, effect of PGC-1α knockdown on Kavain action was evaluated. Results showed that P.gingivalis worsens adipocyte dysfunction through increase of TNF-α, IL-6, iNOS and decrease of PGC-1α and adiponectin. Interestingly, while Kavain obliterated P.gingivalis-induced pro-inflammatory effects in wild-type cells, Kavain did not affect PGC-1α-deficient cells strongly advocating for Kavain effects being mediated by PGC-1α. In vivo Adipocytes challenged with intraperitoneal injection of P.gingivalis alone or P.gingivalis and Kavain displayed the same phenotype as in vitro adipocytes. Altogether, our findings established anti-inflammatory and antioxidant effects of Kavain on adipocytes and emphasized protective action against P. gingivalis- induced adipogenesis. The use of compounds such as Kavain offer a portal to potential therapeutic approaches to counter chronic inflammation in obesity-related diseases.
INTRODUCTION
Periodontitis is an inflammatory disease of infectious origin adversely affecting tooth supporting tissues that may lead to tooth loss. Such disease is common and severe forms affect about 10% of the world population (1). Several risk factors have been described including smoking and systemic conditions. Among them, the role for overweight and obesity has been suggested as increased body mass index (BMI) and waist circumference are associated to periodontal disease worsening (2) and also to a reduced response to periodontal treatment in these individuals (3).
Several studies have explored the biological mechanisms underlying this association that may explain the aggravated periodontal destruction. Monocyte dysregulation, alteration of pro-inflammatory and anti-inflammatory cytokine secretion and impaired clearance of keystone periodontal pathogens such as Porphyromonas gingivalis (P.gingivalis) have been demonstrated (4). Bacterial spreading from the periodontal pocket to the bloodstream occurs and sustained links between periodontitis and several systemic diseases including atherosclerosis, rheumatoid arthritis and diabetes have been demonstrated (5, 6). For instance, P.gingivalis has been detected in vascular walls (7) and in inducing dysbiosis of gut microbiota (8).
In adipose tissue, impaired function of adipocytes is a key factor of obesity and type-2 diabetes and is sustained by an increased number of activated (M1) macrophages (9). Free fatty acid (FFA) deposition within the adipose tissue alters adipose cell secretory functions, induces oxidative stress and reactive oxygen species (ROS) production, causes hypertrophy and produces many vicious cytokines including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and chemokines resulting in sustained chronic inflammation (10). Several exogenous stimuli, including infection and endotoxemia, contribute to this chronic inflammatory state as demonstrated by Mycobacterium tuberculosis, Escherishia coli and P.gingivalis (11–13) through the activation of Toll-Like-Receptor (TLR)-related pathways, NOD-Like-Receptor Platforms (NLRP) and a concomitant reduction in adiponectin secretion (12, 14).
Kavain, an extract from the root of the Kava plant, Piper methysticum, exhibits anti-inflammatory properties by reducing TNF-α-induced pathophysiological conditions via down regulation of inflammatory factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) (15,16). Kavain has been evaluated in the management of several inflammatory diseases, including periodontitis, where Kavain and its derivative compound Kava-241 effectively reduced inflammatory cell infiltrate and alveolar bone loss in a P.gingivalis-induced periodontitis mouse model (17, 18). Anti-inflammatory properties were also observed in vitro in primary macrophages stimulated by E.Coli lipopolysaccharide (LPS) (17) suggesting that Kavain has therapeutic potential in the management of chronic inflammatory disease. In the context of obesity, no data are actually available regarding the putative effect of Kavain on adipocyte maturation/differentiation and release of anti-inflammatory adiponectin and pro-inflammatory adipokines in such cells.
PGC-1α is a major regulator of several key components of the adipocyte functions and adaptive thermogenesis program, including the stimulation of energy uptake, and fatty acid oxidation (19, 20). It is also considered as a major regulator of adipocyte browning and of thermogenic activation of brown fat after reduction of adipocyte hypertrophy, inflammation and generation of ROS (21). Furthermore, mice lacking PGC-1α in adipose tissue fed a high-fat diet develop insulin resistance and increased circulating lipid levels (22). These data suggest that PGC-1α is a key moderator of energy metabolism and prevents the development of metabolic syndrome or type-2 diabetes mellitus (DM2) (23–25). Interestingly, impact of P.gingivalis on PGC-1α is not described yet.
This study was designed to evaluate, in vitro and in vivo, the role of Kavain on key immunological mediator molecules associated with inflammation, oxidative stress and lipid accumulation in adipocytes infected with P.gingivalis and the mechanisms involved with the ultimate goal of reducing the periodontal disease and its social and financial costs. Furthermore, in the present study, we examined whether the Kavain-mediated attenuation of inflammatory adipokines after P.gingivalis infection is PGC-1α-dependent.
MATERIALS AND METHODS
Bacterial culture
Bacterial strain P.gingivalis ATCC 33277 (American Type Culture Collection (ATCC), Manassas, VA, USA) was cultured and maintained on enriched tryptic soy agar plates containing a mixture of defibrinated sheep blood, 5 mg/ml hemin, and 1 mg/ml menadione at 37°C under anaerobic conditions. Bacterial colonies were then transferred into brain heart infusion medium (Becton, Dickinson and Company, Sparks, MD) supplemented with the same materials. On the day of infection, bacteria were centrifuged, washed with phosphate-buffered saline (PBS) and the number of bacteria was determined by measuring the optical density at 600 nm as previously described (26).
3T3L1 derived adipocyte cells culture
3T3-L1 mouse preadipocytes were obtained from ATCC. The culture medium Dulbecco’s modified Eagle’s medium (Pan Biotech) contained 4.5 g/L glucose, 10% heat inactivated fetal bovine serum (Pan Biotech), 5mM-glutamin (Pan Biotech). The cells were cultured in a humidified 5% CO2 atmosphere at 37°C. For cell differentiation assay, preadipocytes were plated in 6-well plates at a density of 125×103 cells/well and allowed to grow until confluence. One day after confluence (day 0), preadipocytes were exposed to the culture medium supplemented with insulin (1 mg/mL, Sigma-Aldrich), isobutyl-1-methylxanthine (500 mM, Sigma-Aldrich) and dexamethasone (0.25 mM, Sigma-Aldrich) until day 2. Then, insulin-containing medium was replaced every two days (23, 24). Adipocytes were infected with P.gingivalis at a multiplicity of infection (MOI) of 25. Cells were collected at day 8 for analysis.
Primary adipocyte isolation and preparation for experiments
Mice adipose tissue were surgically removed and placed in sterile box. Isolation of primary adipocytes was performed according to Abcam Preadipocyte Isolation kit (ab196988). Adipose tissue was minced with dissecting scissors in a sterile vessel for at least 5 minutes and placed into 1 mL of collagenase/0.5 g of tissue and incubated in a heated orbital shaker at 37°C for 30 minutes at 160 rpm and then transferred to fresh preadipocyte medium (DMEM/F12, 10% FBS, P/S, amphotericin B). Preadipocytes were kept for differentiation using a 3T3-L1 differentiation media. Cells were exposed to P.gingivalis as described above and cells were collected at day 8 for analysis.
Kavain treatment
P.gingivalis infected cells were treated on the day of infection with Kavain (Millipore-Sigma) (25 to 150 ug/ml). Cells were collected at day 8 for analysis.
Generation of lentiviral vector-mediated PGC-1α deficient 3T3-L1 derived adipocytes
SMART vector lentiviral shRNA-PPARGC1A or scrambled RNA (Dharmacon, Lafayette, CO) was applied to 3T3-L1 cells to establish a stably transduced cell line. Briefly, 1×106 cells were seeded in 6-well plates 1 day prior to transduction. On the day of transduction, the transduction medium was made by 1×106 transducing units (TU) of lentiviral particles with 0.5ml α-MEM growth medium, applied to each well and incubated for 3h to maximize the contact between each cell and lentiviral particles. Cells were also treated with the transduction medium without lentiviral particles which served as un-transduced control. Growth Medium (1.5 ml) was then added to each well in the presence of 8 μg/ml polybrene (final concentration). After 48 h incubation, antibiotic selection medium (α-MEM growth medium with 10 μg/ml puromycin) was used to kill all the un-transduced cells. Cells were then cultured and maintained as outlined above (24, 27).
Analysis of intracellular ROS level
Intracellular ROS levels were measured by staining with 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Sigma-Aldrich), which is a cell permeable, no fluorescent agent that can be deacetylated by intracellular esterases to form non-fluorescent DCFH. In the presence of ROS, DCFH is highly fluorescent DCF. At day 8 following infection, cells were incubated with 25 μM DCFH-DA for 30 min at 37°C and fluorescence was observed at 200× magnification via fluorescence microscopy (28).
Real-time qPCR
Total RNA was extracted from 3T3-L1 cells after 8 days of differentiation and exposure to P. gingivalis with TRIzol® (Ambion, Austin, TX). A Biotek™ plate reader and the Take3™ plate (Biotek Winooski, VT) were used to determine RNA at an absorbance of 260nm (A260). RNA measurements were subsequently evaluated by the A260/A280 ratio. A High Capacity cDNA Reverse Transcription Kit (Applied Bio Systems) was used to synthesize cDNA from total RNA (Applied Bio Systems). TaqMan® Fast Universal Master Mix (2x) on a 7500 HT Fast Real-Time PCR System (Applied Bio Systems) was used to perform real-time PCR. Specific TaqMan® Gene Expression Assay probes for mouse CEBPa, FAS, PPRY, TLR2, TLR4, TNFα, CCL2, CCN3/NOV, MYD44, NRLP3, NFkB, iNOS, PGC-1α, HO-1, MnSOD, IL-6, adiponectin, NRLP2, NOD2 and GAPDH were used as previously described (25)((27, 29, 30).
Oil red O staining
Staining was performed using 0.21% Oil Red O in 100 % isopropanol (Sigma-Aldrich). Briefly, cells were fixed in 10 % formaldehyde for an hour, stained with Oil Red O for 10 minutes and rinsed with 60 % isopropanol (Sigma-Aldrich). The Oil Red O was then eluted by adding 100 % isopropanol for 10 minutes and the absorbance (OD) was measured at 490 nm. Lipid droplet accumulation was examined by using inverted multichannel (24).
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde at room temperature for 10 min and washed 3 times with phosphate-buffered saline (PBS). Samples were blocked and permeabilized in PBS containing 1% BSA and 0.5% Triton X-100 (Sigma-Aldrich) for 30 min at room temperature and incubated with the primary antibody diluted in PBS containing 1% bovine serum albumin (BSA) and 0.1% Triton X-100 overnight at 4°C in a moist chamber. Slides were washed 3 times with PBS, incubated with the secondary antibody diluted in PBS containing 1% BSA and 0.1% Triton X-100 at room temperature for 1h, and washed with PBS for three times (31).
Western blot analysis
For Western blot, primary adipocyte cells were first lysed in RIPA buffer supplemented with protease and phosphatase inhibitors (Complete™ Mini and PhosSTOP™, Roche Diagnostics, Indianapolis, IA) and total protein content was analyzed by the Bradford method (BIO-RAD, Hercules, CA). Immunoblotting was performed for selected Anti-phospho-Insulin Receptor (IRp-Tyr972 Cat no. I1783 Sigma), total IR (Cat no. ab95231 abcam), pAKT (S473) (Cat no. 9271cell signaling) and total AKT (Cat no.9272 cell signaling) proteins as previously described (23, 25, 27, 48).
Animal model
All animal experiments followed an NYMC IACUC institutionally approved protocol in accordance with the NIH Guidelines. DIO-B6 mice from Taconic Biosciences, Inc. were used in this study. Mice were divided into 3 groups: [1] WT (untreated control), [2] PG (infected with P.gingivalis), [3] PG+Kavain (infected with P.gingivalis and treated with Kavain). A total of 5.108 CFU’s of live P.gingivalis suspended in 100 μl of PBS was injected intraperitoneally 3 times a week for 1 week. Kavain was given at the same time at the dose of 40mg/kg. Mice were anesthetized with sodium pentobarbital (65 mg/Kg, i.p.) and, at the time of sacrifice, adipose tissue was collected. Adipose tissue was surgically removed and placed in sterile box. Isolation of primary adipocytes was performed according to Abcam Preadipocyte Isolation kit (ab196988).
Statistical analysis
Statistical significance between experimental groups was determined by Student’s t-test for pairwise comparison between groups or by ANOVA with Tukey-Kramer post-hoc analysis for comparison between multiple groups. The data are presented as means ± SEM and the null hypothesis was rejected at p<0.05.
RESULTS
Kavain counteracted increase of mRNA inflammatory gene expression induced by P.gingivalis in 3T3L1 derived adipocyte cells and primary adipocytes
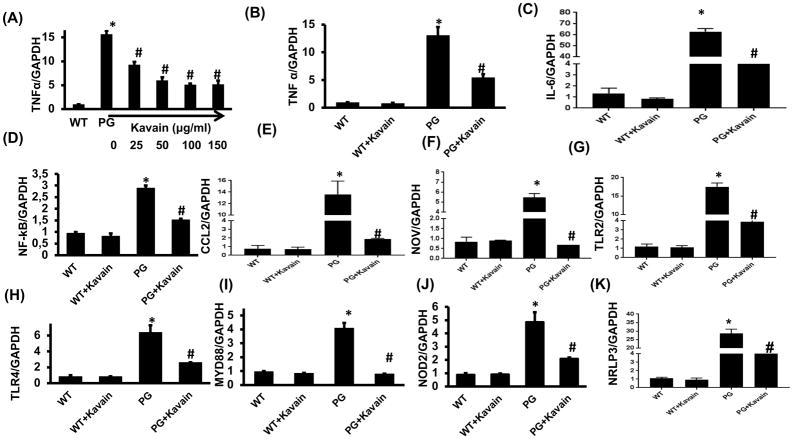
To evaluate the anti-inflammatory properties of Kavain on pro-inflammatory cytokines, RT-qPCR was performed to examine mRNA expression levels of inflammatory cytokines. As expected, P.gingivalis infection increased significantly TNF-α expression in 3T3L1-derived adipocyte cells (Figure 1A). Treatment with Kavain reduced such increase for each dose tested (25 to 150 μg/ml), reaching a plateau at 50 μg/ml (Figure 1A). Accordingly, the dose of 50 μg/ml was then selected for further experiments.
Figure 1.
Effect of Kavain treatment on 3T3-L1 derived adipocytes cells with and without exposure to P.gingivalis. RT-PCR analysis demonstrated mRNA expression of proinflammatory markers. (A) TNF-α expression in response to P.gingivalis infection and dose-response to Kavain (0–150 μg/ml) (B) TNF α, (C) IL-6, (D) NF-kβ, (E) CCL2, (F) CCN3/NOV, (G) TLR2, (H) TLR4, (I) Myd 88, (J)NOD2 and (K) NRLP3 in WT-control, WT+Kavain, P.gingivalis exposed adipocytes and P.gingivalis + Kavain exposed cells. Results are mean±SE, n=3–4, *p<0.05 vs. WT, #p< 0.05 vs. P.gingivalis.
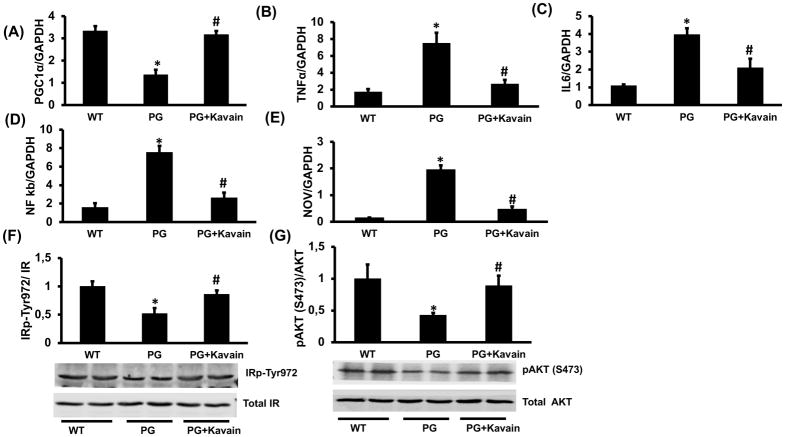
P.gingivalis also increased significantly mRNA expression levels of NFkB, IL-6, CCL2 and CCN3/NOV (nephroblastoma overexpressed) compared to control (p<0.05) (Figure 1A–E). Treatment with Kavain prevented the induced inflammatory effects (p<0.05) and reduced the expression of all markers (Figure 1A–E). Such results were also confirmed in P.gingivalis- infected primary adipocytes harvested from mice and treated with Kavain (Figure 2).
Figure 2.
Effect of Kavain treatment on PGC-1α and inflammatory markers in primary adipocytes after P.gingivalis exposure. RT-PCR investigation showed mRNA expression of (A) PGC-1α, (B) TNF-α, (C) IL-6, (D) NF-kB, (E) NOV. Representative western blots, densitometry analysis of (F) pIR972 and (G) pAKT in P.gingivalis exposed adipocytes and P.gingivalis + Kavain exposed adipocyte cells. Results are mean±SE, n=3–4, *p<0.05 vs. WT, #p< 0.05 vs. P.gingivalis.
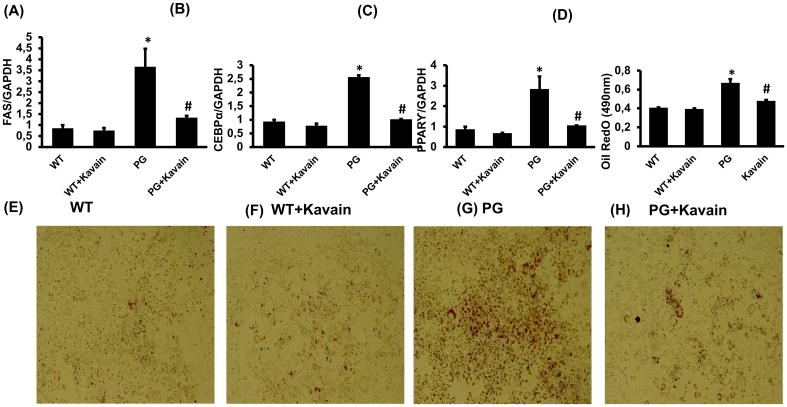
Kavain reduced the expression of adipogenic markers, oil droplet formation and insulin resistance induced by P. gingivalis
P.gingivalis enhanced (p<0.05) mRNA expression of adipogenic genes encoding CEBPα, FAS and PPARγ in 3T3L1-derived adipocyte cells. Kavain reduced the effects induced by the infection (p<0.05) (Figure 3A–C). Oil Red O staining demonstrated the increase of lipid droplet formation after exposure with P. gingivalis and treatment with Kavain significantly (p<0.05) prevented the lipid droplet formation after 8 days of differentiation (Figure 3D–H).
Figure 3.
Effect of Kavain treatment on 3T3-L1 derived adipocytes cells with and without exposure of P. gingivalis. mRNA expression of adipogenic markers (A) FAS, (B) CEBPα, (C) PPAR-γ, (D) Oil Red O absorbance measured at 490nm and lipid droplets formation (E, F, G&H) pictures presented lipid accumulation after Oil Red O staining in WT-control, WT+Kavain, P.gingivalis exposed adipocytes and P.gingivalis + Kavain exposed cells. Results are mean±SE, n=3–4, *p<0.05 vs. WT, #p< 0.05 vs. P.gingivalis.
It has been established that insulin receptor phosphorylation at tyrosine 972 activates and phosphorylates AKT at serine 473 thereby regulating metabolism and insulin-dependent gene expression. Interestingly, in isolated primary adipocytes, infection with P.gingivalis significantly decreased phosphorylation of both IRp972 and pAKT levels (p<0.05), markers of insulin resistance. Kavain treatment was effective to overcome this effect and significantly (p<0.05) induced expression of IRp972 and pAKT proteins (Figure 2F and 2G).
Kavain reduced TLRs and NOD receptors mRNA expression in 3T3L1 derived adipocyte cells
TLRs are well-described membrane receptors for pathogens. In adipocytes, P.gingivalis significantly increased mRNA expression of TLR2, TLR4 and adapter Myeloid differentiation primary response 88 (MyD88) (p<0.05) (Figure 1G–I). TLR activation was also associated with an increase of mRNA expression encoding for inflammasome platforms NOD2 and NLRP3 (Figure 1J–K). Interestingly, Kavain reduced TLR2 and -4 expressions as well as NOD2 and NLRP3 (p<0.05) (Figure 1G–K) in infected cells highlighting the ability of such compounds to reduce P.gingivalis- induced innate immune response.
Anti-oxidative properties of Kavain in 3T3L1 derived adipocyte cells
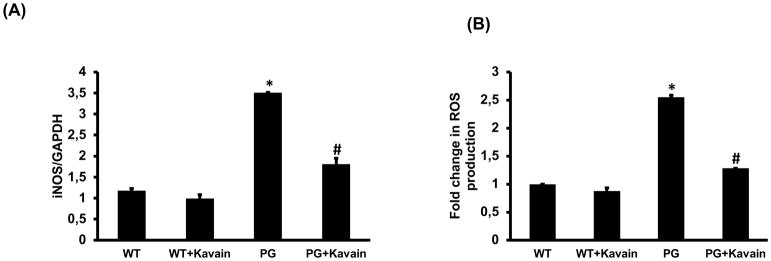
Oxidative stress is a key component of obesity-associated tissue inflammation. P.gingivalis increased iNOS mRNA expression resulting in increased intracellular ROS production (2.5 fold vs untreated cells; p<0.05) (Figure 4A–B). Kavain treatment reduced iNOS expression in infected cells but also displayed anti-oxidative properties by inducing a significant decrease in ROS production (p<0.05) (Figure 4A–B), confirming the beneficial effect of these compounds.
Figure 4.
Effect of Kavain treatment on 3T3-L1 derived adipocytes cells with and without exposure of P. gingivalis. RT-PCR analysis revealed mRNA expression of (A) iNOS, (B) ROS production measured by staining with 2′,7′-dichlorofluorescein diacetate; DCFH-DA, in WT-control, WT+Kavain, P.gingivalis exposed adipocytes and P.gingivalis + Kavain exposed cells. Results are mean±SE, n=3–4, *p<0.05 vs. WT, #p< 0.05 vs. P.gingivalis.
Kavain increased expression of PGC-1α, MnSOD, HO-1 and adiponectin
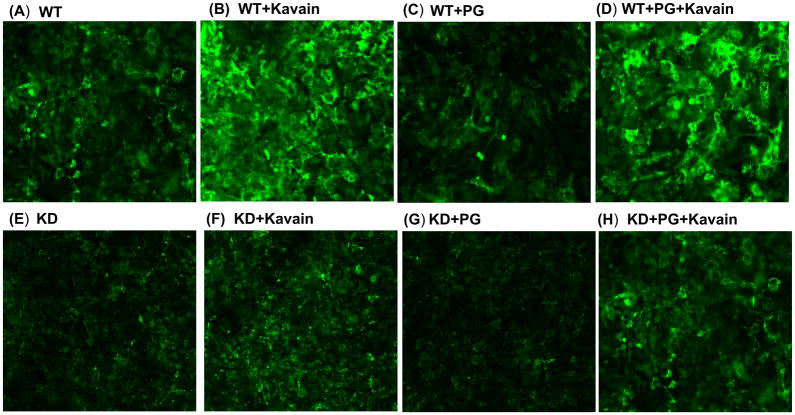
In 3T3L1- derived adipocyte cells, P.gingivalis reduced significantly mRNA expression of PGC-1α, MnSOD, adiponectin and HO-1, all involved in anti-inflammatory response (Figure 5). Interestingly, Kavain counteracted effects associated with the infection and increased significantly PGC-1α expression (p<0.05) (6-fold for Kavain) (Figure 5B). Same beneficial effects of Kavain were also observed for MnSOD, HO-1 and adiponectin mRNA expression (p<0.05) (Figure 5C–E). Furthermore, the increase in adiponectin level was confirmed by immunofluorescence (Figure 6). Same results were observed in primary adipocytes harvested from mouse adipose tissue (Figure 2).
Figure 5.
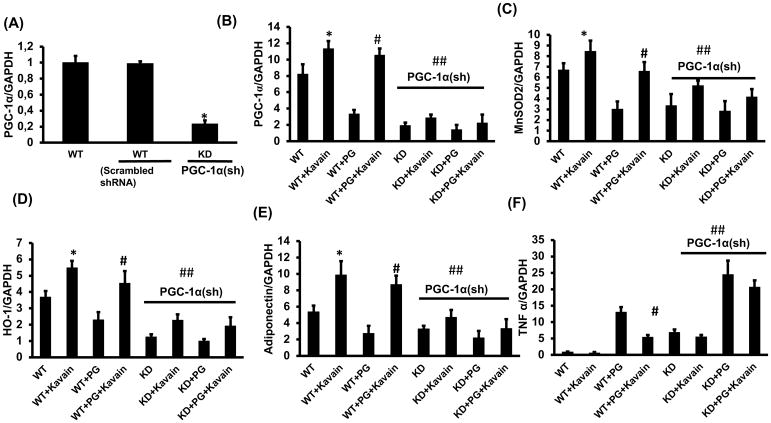
Effect of Kavain treatment on WT and PGC-1α deficient 3T3-L1 derived adipocyte cells, with and without exposure of P. gingivalis. RT-PCR assay showed mRNA expression of (A) PGC-1α in WT, Scrambled and PGC-1α deficient cells, (B) PGC-1α, (C) MnSOD2, (D) HO-1, (E) adiponectin and (F) TNF α, in WT, WT+Kavain, WT+ P.gingivalis, WT+ P.gingivalis +Kavain, PGC-1α KD, PGC-1α KD+Kavain, PGC-1α KD+ P.gingivalis, and PGC-1α KD+ P.gingivalis+ Kavain treated adipocyte cells. Results are mean ± SE, n=3–4, *p<0.05 vs. WT, #p< 0.05 vs. WT+ P.gingivalis and ## vs WT or WT+ P.gingivalis.
Figure 6.
Effect of Kavain treatment on WT and PGC-1α deficient 3T3-L1 derived adipocytes with and without exposure of P.gingivalis. Adiponectin is a protein produced by adipocytes and acts as a hormone. It has an important role in modulating glucose and lipid metabolism in insulin sensitive tissues. Immunofluorescence was performed to detect the presence of adiponectin (A) WT, (B) WT+Kavain, (C) WT+ P.gingivalis, (D) WT+ P.gingivalis +Kavain, (E) PGC-1α KD, (F) PGC-1α KD+Kavain, (G) PGC-1α KD+ P.gingivalis, (H) PGC-1α KD+ P.gingivalis+Kavain.
PGC-1α knockdown prevented Kavain mediated anti-oxidative and anti-inflammatory properties
To evaluate the impact of PGC-1α in oxidative stress and inflammatory response in 3T3L1- derived adipocyte cells, MnSOD, HO-1 and TNF-α mRNA levels were measured in cells in which PGC-1α was knockdown (Figure 5). The knockdown of PGC-1α decreased MnSOD and HO-1 mRNA, and increased TNF-α (p<0.05). Interestingly, in PGC-1α-inhibited adipocytes, Kavain did not rescue MnSOD, HO-1 mRNA expression levels but also did not reduce TNF-α expression (Figure 5). These results demonstrated that PGC-1α-related pathways are key elements in anti-inflammatory effects displayed by Kavain.
Kavain treatment reduced inflammation induced by P.gingivalis injection in mouse
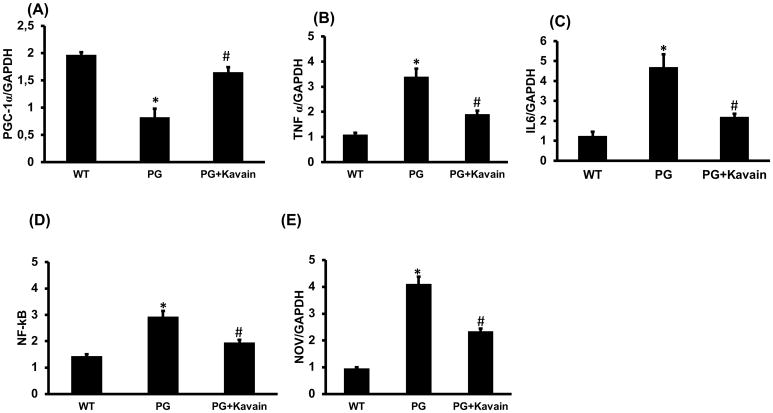
To assess the inflammatory effect induced by systemic injection of P.gingivalis on adipocytes, intraperitoneal injection was performed. Isolated primary adipocytes from infected mice exhibited an increased expression of inflammatory markers such as TNF-α, IL-6, NOV and NFkB while PGC-1α expression was decreased (Figure 7). As observed in vitro, treatment with Kavain decreased significantly inflammatory response and restored PGC-1α expression.
Figure 7.
Effect of Kavain treatment on PGC-1α and inflammatory markers on primary adipocytes isolated from adipose tissue of mice after intraperitoneal injection of P.gingivalis. RT-PCR investigation showed mRNA expression of (A) PGC-1α, (B) TNF-α, (C) IL-6, (D) NF-kB, (E) NOV, in WT-control, P.gingivalis and P.gingivalis + Kavain exposed mice. Results are mean±SE, n=3–4, *p<0.05 vs. WT, #p< 0.05 vs. P.gingivalis.
DISCUSSION
In this study, the pro-inflammatory and pro-oxidative role of P.gingivalis on adipocytes were studied in an attempt to determine more precisely the relationship between periodontitis and obesity. Moreover, the anti-inflammatory properties of Kavain counteracted such effects and appeared to improve adipogenesis by the disruption of cytokine production and an increase of mitochondrial genes, PGC-1α and adiponectin levels. This is the first study to demonstrate a direct effect of P.gingivalis infection on adipocyte inflammation in vivo and that Kavain targets PGC-1α signaling cascade. Furthermore, this process was also related to an increase of antioxidant genes MnSOD and HO-1 expression and inhibition of the progression towards terminal adipocyte differentiation as evidenced by the increase of adiponectin level. These novel findings highlighting Kavain-mediated PGC-1α-adiponectin interplay suggest a prominent role for the modulation of adipocyte maturation in the decrease of P.gingivalis induced inflammation in adipocytes and in adipose tissues.
The role of infection, mainly associated with bacterial byproducts such as LPS manifested in sustained low chronic inflammation, is considered an important factor in the development of obesity (32). For instance, specific profiles of gut microbiota are associated with increased obesity-related clinical parameters due to activation of specific molecular pathways resulting in a dysregulated inflammatory response (33). Due to a reported relationship between periodontitis and obesity, several studies focused on P.gingivalis due to its frequent occurrence in obese patients (34). P.gingivalis, as well as other pathogens from periodontal biofilms, is able to bypass the epithelial barrier of the periodontal pocket, to spread within connective tissues, to disseminate through blood flow and to overcome the innate immune response allowing its persistance within cells contributing to low-chronic inflammation at a distance from the source of infection (35). We show here that P.gingivalis infection was able to change the inflammatory phenotype of adipocytes characterized by increased TNF-α and IL-6 levels through modulation of NFkB. This is, to our knowledge, the first demonstration of a direct effect induced by such bacteria in vivo. Such inflammatory response has already been observed in adipocytes stimulated with P.gingivalis LPS (12) (14) and in other cellular models such as macrophages, epithelial cells and fibroblasts (26, 36). The secretion of TNF-α and IL-6 is of importance in the context of obesity as TNF-α is considered an important paracrine factor contributing to adipocyte insulin resistance through modulation of the NFkB and JNK signaling pathway, and IL-6 is an important determinant of increased obesity-associated cardiovascular risk through CRP induction and endothelial cell activation resulting in increased expression of endothelial adhesion molecules and therefore vascular inflammation (37).
We reported that P.gingivalis induced upregulation of adipogenic markers including FAS, CEBPα and PPAR-γ, resulting in increased inflammation and oxidative stress in adipocytes. Adipokines are key molecules of adipose tissue homeostasis and their secretion is regulated by infection-related parameters (38). Bacterial infection can modulate their physiology and alter their function as observed for Mycobacterium tuberculosis (11). The decreased level of PGC-1α in response to P.gingivalis infection may be a bacterial mechanism to subvert the innate immune response as we observed that the inhibition of PGC-1α increased significantly TNF-α.
Several pharmacological strategies aimed to reduce low-chronic inflammation exist that are associated with obesity. Here, Kavain effects were evaluated with promising results in counteracting the deleterious effects of P.gingivalis infection. This compound reversed adipogenesis and oil droplet formation and decreased proinflammatory cytokine levels, specifically TNF-α, and oxidative stress associated with ROS formation. These anti-inflammatory properties are already well established in different cell types including primary macrophages where their use was associated with a decrease of LPS-mediated TNF-α release (16)(17, 18). Regarding the reduction of oxidative stress, Kavain induced MnSOD and HO-1 expression, both primary defense mechanisms to counter the adverse effects of oxidative stress. Due to involvement of MnSOD on ROS level in obesity, several therapeutics have already been proposed to restore or enhance SOD activity (39)((40). Then, we can argue that Kavain offers potential as therapeutic approaches to reduce ROS levels in the treatment of obesity and obesity- related disease. Nevertheless, it also improved phosphorylation of IRp972 a marker of insulin sensitivity (23, 25, 27, 48). Such effect should be investigated in the future to determine which pathways are involved. In this aspect, a specific focus should be done specifically on PPAR-γ and Akt as these molecules have been described to modulate localization and activity of glucose transporters (GLUT 1 and 3) (42) and as Akt is an already described target for Kavain (43).
We report for the first time that P.gingivalis induces CCN3/NOV, which is reversed by Kavain. Ablation of NOV decreased fat mass, improved glucose tolerance, insulin sensitivity, and decreased proinflammatory cytokines and chemokines in adipose tissues of obese mice (25, 44). Elevated NOV is associated with increased obesity, plasma triglycerides and CRP (45). It has also recently been associated with obstructive sleep apnea (46), a clinical syndrome that is strongly associated with obesity, insulin resistance and cardio-metabolic disease.
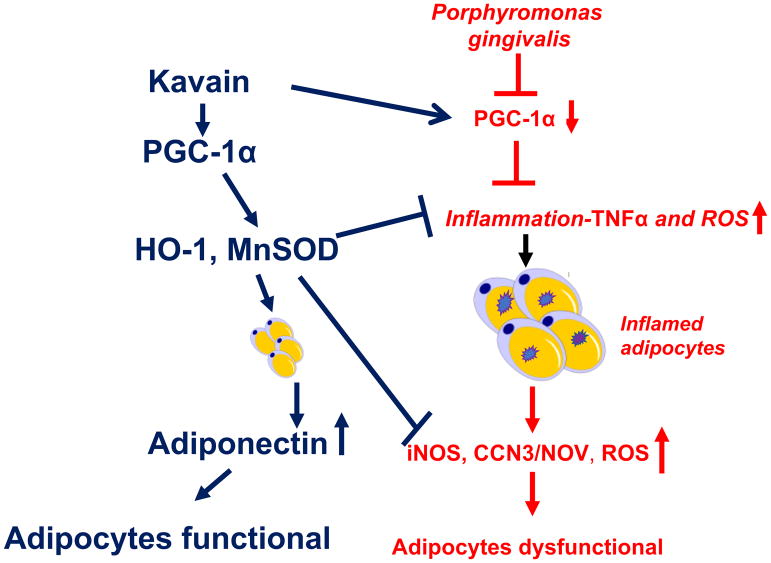
Prominently, we found that P.gingivalis reduces PGC-1α, MnSOD, HO-1 and adiponectin expression but increased TNF-α generation in adipocyte cells, which is reversed by Kavain. We postulated that Kavain mediated these beneficial effects via increase of PGC-1α signaling pathway. One key finding corroborates this conclusion. When PGC-1α was inhibited, treatment with Kavain induced reduced effects emphasizing that PGC-1α- related pathways mediated anti-inflammatory and antioxidant effects of Kavain. Recent studies reported that the decrease in PGC-1α levels increased adipocyte hypertrophy contributing to increased levels of adiposity, inflammation and generation of ROS which is also associated with development of metabolic syndrome (25, 29). PGC-1α induction improves inflammation and ROS via induction of HO-1 (47) and improves adipocyte functions and dynamics (20)(33). This is key to the development of new approaches with the treatment of obesity, diabetes, metabolic syndrome and other obesity- related diseases. Furthermore, the PGC-1α-adiponectin-HO1 module increases MnSOD, which is a critical component to attenuate superoxide induced damage. PGC-1α-mediated increase of HO-1 and Mn-SOD was efficiently reduced in PGC-1α-deficient mice corroborating that HO-1 and Mn-SOD levels are impaired in PGC-1α deficient 3T3-L1-derived adipocytes. Lack of PGC-1α and HO-1-Mn-SOD contributed to the increase of mitochondrial-derived superoxide formation and ROS (24, 48). As depicted in Figure 8, exposure of P. gingivalis increased adipogenic FAS, CEBPα and PPAR-γ expression and decreased expression of PGC-α, HO-1, MnSOD and adiponectin secretion contributing to adipocytes dysfunction. The Kavain-mediated stimulation of PGC-1α signaling induced reduction of ROS and pro-inflammatory molecules TNF-α, NF-kB and iNOS induction. The findings that Kavain activates PGC-1α and inhibits TNF-α levels that control adipocytes function emphasized these molecules as potential novel pharmacologic targets to attenuate and perhaps reverse adipocyte remodeling induced by P.gingivalis. Hence, Kavain offers a multifactorial clinical approach to the treatment of adiposity and concomitant metabolic disorders (Figure 8).
Figure 8.
Schematic description of the Kavain mediated PGC-1α-adiponectin-HO-1-MnSOD in adipocyte infected with P.gingivalis. P. gingivalis induces adipocytes expansion and remodeling evidenced by an increase in adipocyte hyperplasia and hypertrophy leading to pro-inflammatory molecules TNF-α, NFkB and iNOS pathway induction, which are associated with a decrease of PGC-1α and adiponectin. On the other hand, Kavain-mediated stimulation of PGC-1α signaling increases HO-1-adiponectin that is associated with the reduction of ROS and pro-inflammatory molecules TNF-α, NFkB and iNOS and normalization of adipocyte function.
This study confirmed the detrimental role of P.gingivalis on adipocyte homeostasis through increased levels of inflammation (TNF-α) and oxidative stress as a result of decreased levels of PGC-1α and adiponectin. Interestingly, anti-inflammatory and anti-oxidative effects of Kavain are partially dependent of the activation of PGC-1α. Future studies should be expanded to evaluate in vivo the impact of such drug on obesity-related inflammation as it will result in improved health for the patient. Fewer physician and dentist visits manifested as major savings in healthcare costs.
Acknowledgments
Funding: the study was supported by NIH/NIDCR grant R01 DE 014079
References
- 1.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global Burden of Severe Periodontitis in 1990–2010: A Systematic Review and Meta-regression. J Dent Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller A, Rohde JF, Raymond K, Heitmann BL. The Association Between Periodontal Disease and Overweight and Obesity: A Systematic Review. J Periodontol. 2015:1–15. doi: 10.1902/jop.2015.140589. [DOI] [PubMed] [Google Scholar]
- 3.Bouaziz W, Davideau JL, Tenenbaum H, Huck O. Adiposity Measurements and Non-Surgical Periodontal Therapy Outcomes. J Periodontol. 2015;86:1030–1037. doi: 10.1902/jop.2015.140734. [DOI] [PubMed] [Google Scholar]
- 4.Amar S, Leeman S. Periodontal innate immune mechanisms relevant to obesity. Mol Oral Microbiol. 2013;28:331–341. doi: 10.1111/omi.12035. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Messas E, Batista EL, Levine RA, Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation. 2002;105:861–867. doi: 10.1161/hc0702.104178. [DOI] [PubMed] [Google Scholar]
- 6.Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Clin Periodontol. 2013;40:S8–19. doi: 10.1111/jcpe.12064. [DOI] [PubMed] [Google Scholar]
- 7.Elkaim R, Dahan M, Kocgozlu L, Werner S, Kanter D, Kretz JG, Tenenbaum H. Prevalence of periodontal pathogens in subgingival lesions, atherosclerotic plaques and healthy blood vessels: a preliminary study. J Periodont Res. 2008;43:224–231. doi: 10.1111/j.1600-0765.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima M, Arimatsu K, Kato T, Matsuda Y, Minagawa T, Takahashi N, Ohno H, Yamazaki K. Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLoS ONE. 2015;10:e0134234. doi: 10.1371/journal.pone.0134234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262:134–152. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gati A, Kouidhi S, Marrakchi R, El Gaaied A, Kourda N, Derouiche A, Chebil M, Caignard A, Perier A. Obesity and renal cancer: Role of adipokines in the tumor-immune system conflict. Oncoimmunology. 2014;3:e27810. doi: 10.4161/onci.27810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayyappan JP, Vinnard C, Subbian S, Nagajyothi JF. Effect of Mycobacterium tuberculosis infection on adipocyte physiology. Microbes Infect. 2018;20(8):0–88. doi: 10.1016/j.micinf.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Sage F, Meilhac O, Gonthier MP. Porphyromonas gingivalis lipopolysaccharide induces pro-inflammatory adipokine secretion and oxidative stress by regulating Toll-like receptor-mediated signaling pathways and redox enzymes in adipocytes. Mol Cell Endocrinol. 2017;446:102–110. doi: 10.1016/j.mce.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita A, Soga Y, Iwamoto Y, Asano T, Li Y, Abiko Y, Nishimura F. DNA microarray analyses of genes expressed differentially in 3T3-L1 adipocytes co-cultured with murine macrophage cell line RAW264.7 in the presence of the toll-like receptor 4 ligand bacterial endotoxin. Int J Obes. 2008;32:1725–1729. doi: 10.1038/ijo.2008.153. [DOI] [PubMed] [Google Scholar]
- 14.Le Sage F, Meilhac O, Gonthier MP. Anti-inflammatory and antioxidant effects of polyphenols extracted from Antirhea borbonica medicinal plant on adipocytes exposed to Porphyromonas gingivalis and Escherichia coli lipopolysaccharides. Pharmacol Res. 2017;119:303–312. doi: 10.1016/j.phrs.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Pollastri MP, Whitty A, Merrill JC, Tang X, Ashton TD, Amar S. Identification and characterization of kava-derived compounds mediating TNF-alpha suppression. Chem Biol Drug Des. 2009;74:121–128. doi: 10.1111/j.1747-0285.2009.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang X, Amar S. Kavain inhibition of LPS-induced TNF-α via ERK/LITAF. Toxicol Res. 2016;5:188–196. doi: 10.1039/c5tx00164a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alshammari A, Patel J, Al-Hashemi J, Cai B, Panek J, Huck O, Amar S. Kava-241 Reduced Periodontal Destruction in a Collagen Antibody Primed Porphyromonas gingivalis Model of Periodontitis. J Clin Periodontol. 2017;44(1):123–1132. doi: 10.1111/jcpe.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan H, Zelkha S, Zelka S, Burkatovskaya M, Gupte R, Leeman SE, Amar S. Pivotal role of NOD2 in inflammatory processes affecting atherosclerosis and periodontal bone loss. Proc Natl Acad Sci. 2013;110:E5059–68. doi: 10.1073/pnas.1320862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutri. 2011;93:884S–890S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007;117:3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, Spiegelman BM. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc Natl Acad Sci. 2012;109:9635–9640. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh SP, Schragenheim J, Cao J, Falck JR, Abraham NG, Bellner L. PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: Role of epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat. 2016;125:8–18. doi: 10.1016/j.prostaglandins.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldman M, Bellner L, Vanella L, Schragenheim J, Sodhi K, Singh SP, Lin D, Lakhkar A, Li J, Hochhauser E, Arad M, Darzynkiewicz Z, Kappas A, Abraham NG. Epoxyeicosatrienoic Acids Regulate Adipocyte Differentiation of Mouse 3T3 Cells, Via PGC-1α Activation, Which Is Required for HO-1 Expression and Increased Mitochondrial Function. Stem Cells Dev. 2016;25:1084–1094. doi: 10.1089/scd.2016.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao J, Singh SP, McClung JA, Joseph G, Vanella L, Barbagallo I, Jiang H, Falck JR, Arad M, Shapiro JI, Abraham NG. EET intervention on Wnt1, NOV, and HO-1 signaling prevents obesity-induced cardiomyopathy in obese mice. Am J Physiol Heart Circ Physiol. 2017;313:H368–H380. doi: 10.1152/ajpheart.00093.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huck O, Al-Hashemi J, Poidevin L, Poch O, Davideau J-L, Tenenbaum H, Amar S. Identification and Characterization of miRNA differentially expressed in macrophages exposed to Porphyromonas gingivalis infection. Infect Immun. 2017 doi: 10.1128/IAI.00771-16. IAI.00771–16–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh SP, Bellner L, Vanella L, Cao J, Falck JR, Kappas A, Abraham NG. Downregulation of PGC-1α Prevents the Beneficial Effect of EET-Heme Oxygenase-1 on Mitochondrial Integrity and Associated Metabolic Function in Obese Mice. J Nutr Metab. 2016;2016:9039754–15. doi: 10.1155/2016/9039754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HA, Song BR, Kim HR, Kim JE, Yun WB, Park JJ, Lee ML, Choi JY, Lee HS, Hwang DY. Butanol extracts of Asparagus cochinchinensis fermented with Weissella cibaria inhibit iNOS-mediated COX-2 induction pathway and inflammatory cytokines in LPS-stimulated RAW264.7 macrophage cells. Exp Ther Med. 2017;14:4986–4994. doi: 10.3892/etm.2017.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh SP, Grant I, Meissner A, Kappas A, Abraham NG. Ablation of adipose-HO-1 expression increases white fat over beige fat through inhibition of mitochondrial fusion and of PGC1α in female mice. Horm Mol Biol Clin Investig. 2017;31:8. doi: 10.1515/hmbci-2017-0027. [DOI] [PubMed] [Google Scholar]
- 30.Singh A, Sharma RK, Siwach RC, Tewari S, Narula SC. Association of bone mineral density with periodontal status in postmenopausal women. J Invest Clin Dent. 2014;5:275–282. doi: 10.1111/jicd.12047. [DOI] [PubMed] [Google Scholar]
- 31.Singh SP, Tao S, Fields TA, Webb S, Harris RC, Rao R. Glycogen synthase kinase-3 inhibition attenuates fibroblast activation and development of fibrosis following renal ischemia-reperfusion in mice. Dis Model Mech. 2015;8:931–940. doi: 10.1242/dmm.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hersoug LG, Møller P, Loft S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes Rev. 2016;17:297–312. doi: 10.1111/obr.12370. [DOI] [PubMed] [Google Scholar]
- 33.Pekkala S, Munukka E, Kong L, Pöllänen E, Autio R, Roos C, Wiklund P, Fischer-Posovszky P, Wabitsch M, Alen M, Huovinen P, Cheng S. Toll-like receptor 5 in obesity: the role of gut microbiota and adipose tissue inflammation. Obesity. 2015;23:581–590. doi: 10.1002/oby.20993. [DOI] [PubMed] [Google Scholar]
- 34.Pataro AL, Cortelli SC, Abreu MHNG, Cortelli JR, Franco GCN, Aquino DR, Cota LOM, Costa FO. Frequency of periodontal pathogens and Helicobacter pylori in the mouths and stomachs of obese individuals submitted to bariatric surgery: a cross-sectional study. J Appl Oral Sci. 2016;24:229–238. doi: 10.1590/1678-775720150534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen I, Progulske-Fox A. Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J Oral Microbiol. 2015;7:28788. doi: 10.3402/jom.v7.28788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morand DN, Huck O, Keller L, Jessel N, Tenenbaum H, Davideau JL. Active Nanofibrous Membrane Effects on Gingival Cell Inflammatory Response. Materials. 2015;8:7217–7229. doi: 10.3390/ma8105376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 38.Schmid A, Kopp A, Hanses F, Bala M, Müller M, Schäffler A. The Novel Adipokine C1q/TNF-related Protein-3 is Expressed in Human Adipocytes and Regulated by Metabolic and Infection-related Parameters. Exp Clin Endocrinol Diabetes. 2012;120:611–617. doi: 10.1055/s-0032-1323803. [DOI] [PubMed] [Google Scholar]
- 39.Coudriet GM, Delmastro-Greenwood MM, Previte DM, Marré ML, O’Connor EC, Novak EA, Vincent G, Mollen KP, Lee S, Dong HH, Piganelli JD. Treatment with a Catalytic Superoxide Dismutase (SOD) Mimetic Improves Liver Steatosis, Insulin Sensitivity, and Inflammation in Obesity-Induced Type 2 Diabetes. Antioxidants. 2017;6:85. doi: 10.3390/antiox6040085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jankovic A, Korac A, Buzadzic B, Stancic A, Otasevic V, Ferdinandy P, Daiber A, Korac B. Targeting the NO/superoxide ratio in adipose tissue: relevance to obesity and diabetes management. Br J Pharmacol. 2017;174:1570–1590. doi: 10.1111/bph.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasgupta S, Rai RC. PPAR-γ and Akt regulate GLUT1 and GLUT3 surface localization during Mycobacterium tuberculosis infection. Mol Cell Biochem. 2018;440:127–138. doi: 10.1007/s11010-017-3161-3. [DOI] [PubMed] [Google Scholar]
- 43.Tang X, Amar S. Kavain Involvement in LPS-Induced Signaling Pathways. J Cell Biochem. 2016;117:2272–2280. doi: 10.1002/jcb.25525. [DOI] [PubMed] [Google Scholar]
- 44.Martinerie C, Garcia M, Do TTH, Antoine B, Moldes M, Dorothee G, Kazazian C, Auclair M, Buyse M, Ledent T, Marchal PO, Fesatidou M, Beisseiche A, Koseki H, Hiraoka S, Chadjichristos CE, Blondeau B, Denis RG, Luquet S, Fève B. NOV/CCN3: A New Adipocytokine Involved in Obesity-Associated Insulin Resistance. Diabetes. 2016;65:2502–2515. doi: 10.2337/db15-0617. [DOI] [PubMed] [Google Scholar]
- 45.Pakradouni J, Le Goff W, Calmel C, Antoine B, Villard E, Frisdal E, Abifadel M, Tordjman J, Poitou C, Bonnefont-Rousselot D, Bittar R, Bruckert E, Clément K, Fève B, Martinerie C, Guérin M. Plasma NOV/CCN3 levels are closely associated with obesity in patients with metabolic disorders. PLoS ONE. 2013;8:e66788. doi: 10.1371/journal.pone.0066788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weingarten JA, Bellner L, Peterson SJ, Zaw M, Chadha P, Singh SP, Abraham NG. The association of NOV/CCN3 with obstructive sleep apnea (OSA): preliminary evidence of a novel biomarker in OSA. Horm Mol Biol Clin Investig. 2017;31:1014. doi: 10.1515/hmbci-2017-0029. [DOI] [PubMed] [Google Scholar]
- 47.Abraham NG, Junge JM, Drummond GS. Translational Significance of Heme Oxygenase in Obesity and Metabolic Syndrome. Trends in Pharmacological Sciences. 2016;37:17–36. doi: 10.1016/j.tips.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh SP, McClung JA, Bellner L, Cao J, Waldman M, Schragenheim J, Arad M, Hochhauser E, Falck JR, Weingarten JA, Peterson SJ, Abraham NG. CYP-450 Epoxygenase Derived Epoxyeicosatrienoic Acid Contribute To Reversal of Heart Failure in Obesity-Induced Diabetic Cardiomyopathy via PGC-1 α Activation. Cardiovasc Pharm Open Access. 2018;7:1–11. doi: 10.4172/2329-6607.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]