Abstract
HIV infection markedly increases the likelihood of latent tuberculosis infection progressing to active TB. Information on expression of TLR-2, myeloid differentiation factor (MyD88), IL-1R-associated kinase-4 (IRAK4) and nuclear factor kappa B (NF-kB) in HIV+ LTBI+ and HIV+ patients with active TB disease is limited. We found significantly higher percentages of CD14+TLR2+ cells in PBMCs of HIV+LTBI+ patients compared to HIV-LTBI+ individuals. γ-irradiated Mtb was unable to induce MyD88, IRAK4 expression and IL-1β, MCP-1, IP-10 production in HIV+LTBI+ patients. Pleural fluids from HIV+TB+ patients had low IL-1β, MCP-1, IP-10 and high IL-10, TNF-α production. γ-irradiated Mtb stimulated CD14+ cells from HIV+TB+ patients had low IL-1β, MCP-1,IP-10 production and MyD88, IRAK4 and similar NF-kB expression compared to those from of HIV-TB+ patients. Our results suggest defective MyD88, IRAK4 but not NF-kB inhibit IL-1β, MCP-1 and IP-10 production by CD14+ cells of HIV+ individuals with LTBI and active TB disease in peripheral blood and at the site of disease.
Keywords: Human, tuberculosis, HIV, cytokines, TLR-2, monocytes
1. Introduction
Mycobacterium tuberculosis (Mtb) infects one-third of the world’s population and causes almost 1.3 million deaths per year [1]. Approximately 90% of infected persons have latent tuberculosis infection (LTBI), have protective immunity and remain well, but 10% develop primary tuberculosis (TB) soon after infection or reactivation TB many years later [2]. HIV infection markedly increases susceptibility to TB, and HIV-infected persons with LTBI have an 800-fold greater risk of developing active TB (www.cdc.gov/tb/). TB is the leading cause of death in HIV-infected persons and more than half a million co-infected people die annually (www.avert.org/tuberculosis.htm).
Mtb can proliferate and survive in macrophages and other cells, favoring establishment and progression of infection, as well as the capacity to persist and reactivate disease many years later [2–4]. Interaction of macrophages with specific ligands, facilitates uptake of Mtb, and triggers an intracellular signaling cascade in macrophages that induces production of cytokines and chemokines essential to arrest bacterial growth [5,6]. Key macrophage receptors that sense mycobacterial antigens include toll-like receptors (TLRs), CD14 co-receptor, and C-type lectin receptors [7]. Stimulation of TLR-dependent pathways induces activation of NF-kB, with subsequent production of inflammatory cytokines and chemokines and increased expression of costimulatory molecules [8]. This in turn regulates antimicrobial mechanisms, such as induction of autophagy, and generation of nitric oxide (NO), reactive nitrogen intermediates (RNI) and reactive oxygen intermediates (ROI) all of which play critical roles in the clearance of mycobacteria [9,10]. TLR2 uses the intracellular myeloid differentiation factor (MyD88) to link receptor recognition with activation of IL-1R-associated kinase-4 (IRAK4), for translocation of NF-κB and gene transcription and production of inflammatory cytokines [11–13]. MyD88 signaling is essential in the control of Mtb infection, absence of which resulted in a dramatic reduction of host resistance to TB [14,15]. IRAK4 acts immediately downstream of MyD88 and has been shown to be essential for MyD88-dependent pathway that relays the majority of innate immune responses [16].
Limited information is available about the factors, including host immune mechanisms that induce Mtb growth and persistence, especially in HIV+LTBI+ individuals. To develop new tools to prevent tuberculosis in HIV+ individuals, it is important to identify the factors that regulate macrophage responses during Mtb infection in HIV+ individuals. In the current study, we determined TLR2, MyD88, IRAK4 and NF-kB expressions and cytokine production by CD14+ monocytes from PBMCs of HIV−LTBI+, HIV+LTBI+ individuals, HIV+TB+, HIV−TB+ patients and pleural fluids of TB patients with and without HIV infection.
2. Materials and Methods
2.1 Patient population
After obtaining written informed consent, individuals with either latent TB (LTBI+, N=40) or active pulmonary TB (AFB-positive sputum, culture-positive; N=40), both positive to HIV and 30 individuals negative to HIV with latent TB (LTBI+, N=40) and active pulmonary disease (N=40) were recruited for the study. At the time of enrollment, CD4+ cell counts were >350 in all HIV+LTBI+ individuals &<200 in HIV+ active TB patients. The study was approved by the Institutional Review Boards of Blue Peter Public Health Research Centre, and Government Chest and General Hospital, Hyderabad, India.
Pleural fluids were obtained via thoracentesis from 16 HIV-TB patients and 16 HIV+TB patients with tuberculous pleuritis with no history of anti-tuberculosis therapy. All patients had unilateral exudative effusions. The diagnosis was confirmed for all patients by culture of M. tuberculosis from pleural fluid or tissue and by histologic demonstration of granulomatous pleuritis in combination with a response to anti-tuberculosis therapy.
2.2 Antibodies and other reagents
For flow cytometry, we used FITC anti-CD14 (BD Biosciences), and PE anti-TLR2 (R&D Biosystems). We used γ-irradiated Mtb H37Rv (BEI Resources) and TLR2 agonist Pam3CSK4 (synthetic triacylated lipoprotein, Invivogen) for in vitro stimulation assays.
2.3 Determination of latent tuberculosis in the study subjects
QuantiFERON-TB gold test was used to identify subjects having a latent TB infection (LTBI). The assay was performed according to manufacturer’s instructions. Briefly, one ml of whole blood was added to each of the three QFT-TB tubes; TB antigen (ESAT-6, CFP-10 and TB 7.7), mitogen positive control (PHA) and a negative control. The tubes were treated as recommended by the manufacturer (Cellestis Ltd., Victoria, Australia) and the IFN-γ concentration (IU/mL) in plasma was measured by an ELISA reader and LTBI status was calculated by the ‘QFT-TB-analysis Software’
2.4 Isolation of CD14+ monocytes
PBMCs and PFMCs were isolated by differential centrifugation over Ficoll-Paque (Sigma Aldrich). CD14+ monocytes were isolated with magnetic beads conjugated to anti-CD14 (Miltenyi Biotec). The positively selected cells were >95% CD14+ as measured by flow cytometry.
2.5 Culture of monocytes with γ-irradiated Mtb H37Rv
Freshly isolated CD14+ cells were cultured in 12-well plates at 2 × 106 cells/well in RPMI 1640 containing 1% penicillin/streptomycin (Sigma), L-Glutamine and 10% heat-inactivated human serum, with or without γ-irradiated Mtb H37Rv or TLR2 agonist at 37°C in a humidified 5% CO2 atmosphere. After 48 h, cell-free culture supernatants were collected, aliquoted and stored at −70°C until cytokine concentrations were measured. For other experiments, RNA was extracted to perform real time PCR analysis to measure expression of MyD88 and IRAK4 genes.
2.6 Real-time PCR for quantification of MyD88, IRAK4 and NF-kB mRNA
Total RNA was extracted from cultured cells, using TRIzol reagent (Invitrogen) and reverse transcribed, using iScript cDNA synthesis kit (Bio-Rad USA). The primers sequences were: Myd88 Forward: 5′TGCCCTGAAGACTGTTCTGA3′ Reverse 3′ACTGGTTCCATGCAGGACAT5′, IRAK-4 Forward 5′TGATGGAGATGACCTCTGCT 3′, Reverse 3′GGTGGAGTACCATCCAAGCA5′and NF-kB1p65 Forward 5′ ATCCCATCTTTGACAATCGTGC Reverse 3′ CTGGTCCCGTGAAATACACCTC. Real-time PCR was performed using EVA Green (Bio-Rad, USA) on a spectrofluorometric thermal cycler (Mini Opticon, Bio-Rad). PCR was performed in duplicates as follows: 95°C for 10 min, and 45 cycles of 95°C for 15s, 60°C for 30s, and 72°C for 30s. All samples were normalized to the amount of GAPDH transcript present in each sample.
2.7 Measurement of IL-1β, TNFα, and IL-10 concentrations
Supernatants from γ-irradiated M. tuberculosis H37Rv-stimulated CD14 cells were collected after 48 h and stored at −70°C, until concentrations were measured by ELISA (eBiosciences) following manufacturer’s instructions.
2.8 Measurement of MCP-1 and IP-10 in cell culture supernatants and pleural fluids
Cytometric Bead Array (CBA): Human CBA kit (BD Biosciences, San Diego, CA) was used to quantify the levels of chemokines (MCP-1 and IP-10) following manufacturer’s instructions. Briefly, 50 μl of standard or sample was mixed with the 50μl of premixed capture beads for respective chemokine and 50 μl of PE-labeled secondary detection reagent. After 3hrs of incubation at 4°C, excess detection reagent was removed by washing. Samples were acquired on FACS calibur with cell-quest pro software. The concentrations of samples were calculated using CBA software (BD Biosciences, San Diego, CA).
2.9 Statistical analysis
Results are shown as mean ± SE. For data that were normally distributed, comparisons between groups were performed by a paired or unpaired t test, as appropriate. For data that were not normally distributed, the non-parametric Mann-U-Whitney test was performed.
3. Results
3.1 TLR2 expression by peripheral blood CD14+ monocytes
TLR2 expression on CD14low cells was higher in HIV+LTBI+ patients than in HIV−LTBI+ healthy individuals (Fig. 2A, p=0.0001) and HIV+TB+ patients (Fig. 2A, p<0.0001). The percentages these cells were higher in HIV−TB+ patients than in HIV+TB+ patients (Fig. 2A, p=0.01).
Figure 2. TLR2 expression by CD14low and CD14hi monocytes from HIV+ and HIV− individuals with latent tuberculosis infection and active tuberculosis disease.
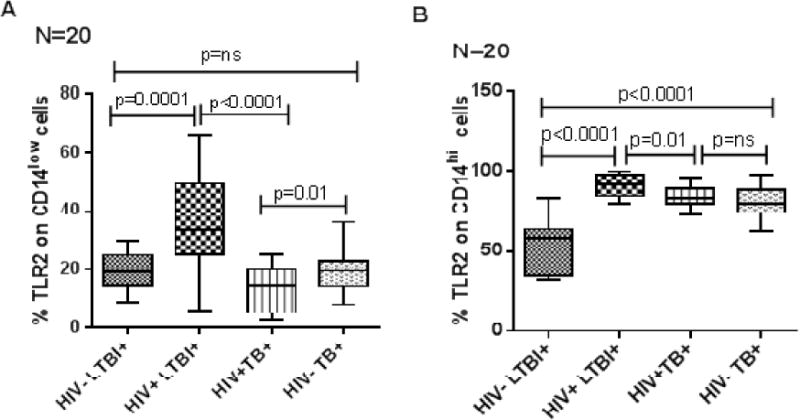
Freshly isolated PBMC (20 donors in each group) from HIV−LTBI+, HIV+LTBI+ individuals, HIV+TB and HIV-TB patients were stained with Abs to CD14 and TLR2. Flow cytometry was used to measure the percentages of TLR2+ cells on A. CD14low and B. CD14hi monocytes. Boxes show the median and interquartile range, and whiskers show the 5th and 95th percentile values.
Similarly TLR2 expression on CD14hi cells was higher in HIV+LTBI+ patients than in HIV−LTBI+ healthy individuals (Fig. 2B, p<0.0001) and HIV+TB+ patients (Fig. 2B, p=0.01). The percentages these cells were higher in HIV−TB+ patients than in HIV−LTBI+ individuals (Fig. 2B, p<0.0001).
3.2 MyD88, IRAK4 and NF-kB expression by peripheral blood CD14+ monocytes upon stimulation
γ-irradiated Mtb H37Rv significantly enhanced MyD88 and IRAK4 expression by CD14+ cells of HIV-LTBI+ healthy individuals compared to HIV+LTBI+ patients (Fig. 3A, p=0.026, p=0.0006). There is no significant difference in MyD88 expression between γ-irradiated Mtb H37Rv stimulated CD14+ cells of HIV- and HIV+ TB+ patients. But IRAK4 expression by CD14+ cells of HIV-TB+ patients was higher compared to HIV+TB+ patients (Fig. 3A p=0.029) and lower compared to HIV-LTBI+ healthy individuals (Fig. 3A p=0.03). No significant difference in NF-kB expression was observed in any of the groups.
Figure 3. MyD88, IRAK4 and NF-kB expression by peripheral blood CD14+ monocytes of HIV− and HIV+ individuals with latent tuberculosis infection and active tuberculosis disease.
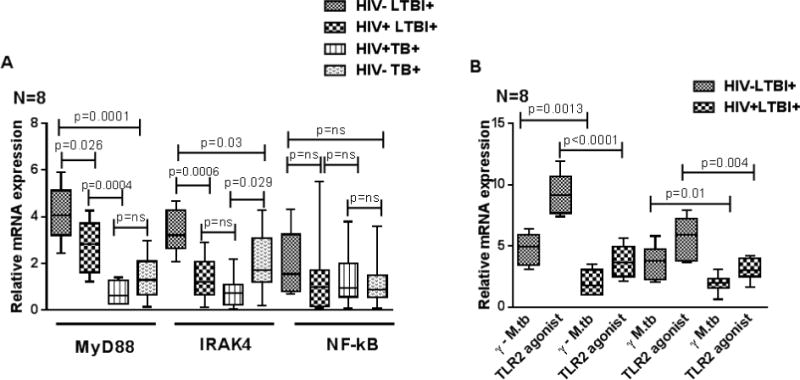
CD14+ monocytes from PBMC of HIV+LTBI+, HIV−LTBI+ individuals, HIV+TB and HIV−TB patients (8 donors in each group) were isolated by magnetic selection and cultured with or without γ-irradiated Mtb H37Rv (10 μg/ml) or TLR2 agonist Pam3CSK4 (10 μg/ml). After 48 hrs, MyD88 and IRAK4 expression was determined by PCR analysis. Boxes show the median and interquartile range, and whiskers show the 5th and 95th percentile values. A. γ-irradiated Mtb H37Rv stimulated cells. B. TLR2 agonist Pam3CSK4 stimulated cells.
Similarly, TLR2 agonist Pam3CSK4 (synthetic triacylated lipoprotein) also significantly increased MyD88 (Fig. 3B, p<0.0001) and IRAK4 (Fig. 3B, p=0.004) expression by CD14+ cells of HIV−LTBI+ healthy individuals compared to HIV+LTBI+ patients.
3.3 Cytokine production by peripheral blood CD14+ monocytes upon stimulation
CD14+ cells from HIV−LTBI+ individuals produced significantly higher amounts of IL-1β (Fig. 4A, p=0.009), lower amounts of TNF-α (Fig. 4B, p=0.02) and IL-10 (Fig. 4C, p=0.005) compared to HIV+LTBI+ individuals. Whereas HIV+TB+ patients produced significantly lower amounts of IL-1β (Fig. 4A, p=0.004), and TNF-α (Fig. 4B, p=0.002), and higher amounts of IL-10 (Fig. 4C, p=0.02), compared to HIV−TB+ patients.
Figure 4. Cytokine production by peripheral blood CD14+ monocytes of HIV− and HIV+ individuals with latent tuberculosis infection and active tuberculosis disease.
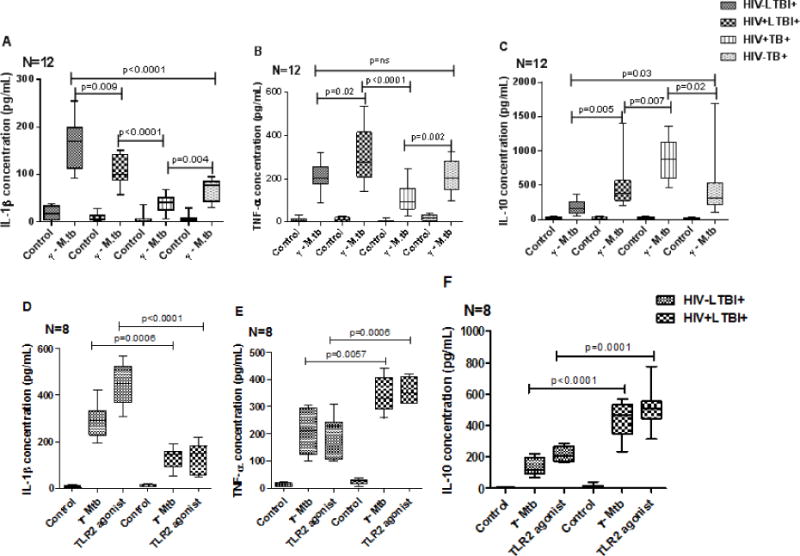
CD14+ monocytes from PBMC of HIV+LTBI+, HIV−LTBI+ individuals, HIV+TB and HIV−TB patients (12 donors in each group) were isolated by magnetic selection and cultured with or without γ-irradiated Mtb H37Rv (10 μg/ml) or TLR2 agonist Pam3CSK4 (300ng/ml) (8 donors in each group). After 48 hrs, cytokine and chemokine levels in culture supernatants were determined by ELISA. Boxes show the median and interquartile range, and whiskers show the 5th and 95th percentile values. A to C. γ-irradiated Mtb H37Rv stimulated cells. D to F. TLR2 agonist Pam3CSK4 stimulated cells.
We also stimulated CD14+ cells of HIV−LTBI+ healthy individuals and HIV+LTBI+ patients with TLR2 agonist Pam3CSK4 (synthetic triacylated lipoprotein). Similar increase in IL-1β (Fig. 4D, p<0.0001), decrease in TNF-α (Fig. 4E, p=0.0006), and IL-10 (Fig. 4F, p=0.0001) production was noted in HIV-LTBI+ healthy individuals compared to HIV+LTBI+ individuals.
3.4 Chemokine production by peripheral blood CD14+ monocytes upon stimulation
In the above culture supernatants, we also measured MCP-1 and IP-10 levels and found that CD14+ cells from HIV−LTBI+ individuals produce significantly higher amounts of MCP-1 (Fig. 5A, p=0.002), and IP-10 (Fig. 5B, p=0.003) compared to HIV+LTBI+ individuals.
Figure 5. Chemokine production by peripheral blood CD14+ monocytes of HIV− and HIV+ individuals with latent tuberculosis infection and active tuberculosis disease.
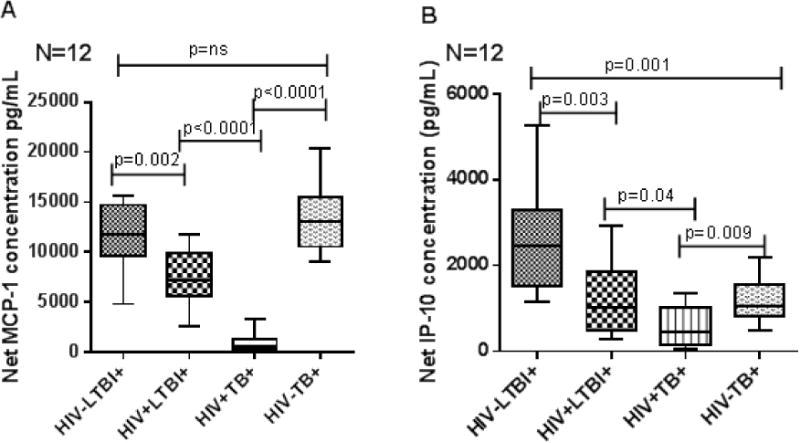
CD14+ monocytes from PBMC of HIV+LTBI+, HIV−LTBI+ individuals, HIV-TB and HIV+TB patients (12 donors in each group) were isolated by magnetic selection and cultured with or without γ-irradiated Mtb H37Rv (10 μg/ml). After 48 hrs, chemokine levels in culture supernatants were determined by ELISA. Boxes show the median and interquartile range, and whiskers show the 5th and 95th percentile values.
Our above results demonstrate that increased TLR2 expression by freshly isolated CD14+ cells of HIV+LTBI+ individuals do not correlate with the expression of MyD88 and IRAK4 and cytokine production upon γ-irradiated Mtb H37Rv or TLR2 agonist stimulation.
3.5 Cytokine and chemokine levels at the site of disease (pleural fluids) and cell cultures
Pleural fluids from HIV+TB+ patients had low, IL-1β (Fig. 6A; p=0.0001 & Fig. 6B p=0.01), MCP-1 (Fig. 6C; p =0.01 & Fig. 6D; p=0.03) and IP-10 (Fig. 6C; p =0.03 & Fig. 6D; p=0.001) concentrations compared to those from HIV-TB+ patients.
Figure 6. Cytokine and chemokine production in cell free fluids (A&C) and CD14+ pleural fluid monocytes (B&D) of HIV− and HIV+ individuals with active tuberculosis disease.
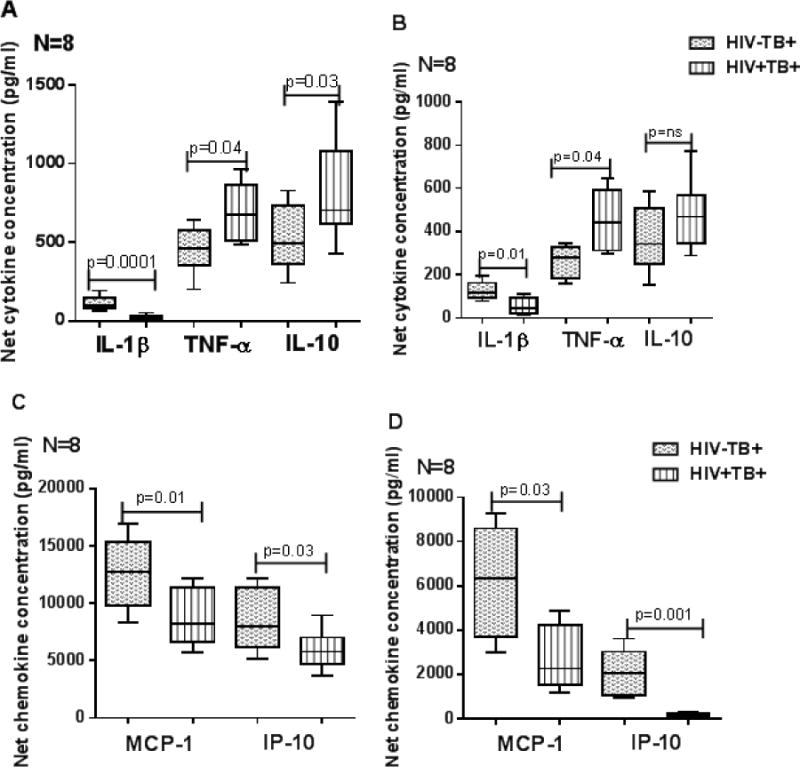
CD14+ monocytes from pleural fluids of HIV−TB+ and HIV+TB+ patients (8 donors in each group) were isolated by magnetic selection and cultured with or without γ-irradiated Mtb H37Rv (10 μg/ml). After 48 hrs, cytokine and chemokine levels in culture supernatants were determined by ELISA. Boxes show the median and interquartile range, and whiskers show the 5th and 95th percentile values.
3.6 MyD88 and IRAK4 expression by CD14+ cells in pleural fluid upon stimulation
We next determined whether cytokine and chemokine levels in pleural fluids of HIV+ and HIV− active tuberculosis patients correlate with the MyD88, IRAK4, NF-kB expressions by pleural fluid CD14+ cells upon γ-irradiated Mtb stimulation. CD14+ cells were isolated from pleural fluid by magnetic selection as mentioned in methods section and cultured with γ-irradiated Mtb. After 48 hrs expressions of MyD88, IRAK4 and NF-kB were determined by real-time PCR. γ-irradiated Mtb cultured pleural fluid CD14+ cells of HIV+TB+ patients expressed significantly less MyD88 (Fig. 7, p=0.003) and IRAK4 (Fig. 7) and similar NF-kB expression compared to HIV-TB+ patients.
Figure 7. MyD88 and IRAK4 expression by pleural fluid CD14+ monocytes of HIV− and HIV+ TB patients.
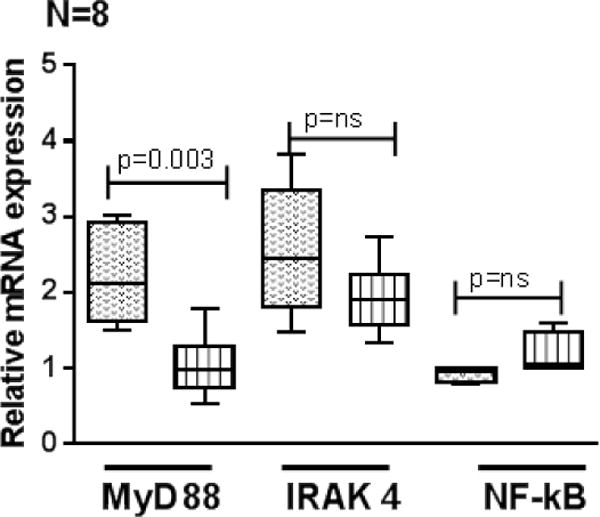
CD14+ monocytes from pleural fluids of HIV−TB and HIV+TB patients (8 donors in each group) were isolated by magnetic selection and cultured with or without γ-irradiated Mtb H37Rv (10 μg/ml). After 48 hrs, MyD88, IRAK4 and NF-kB expression was determined by PCR analysis. Boxes show the median and interquartile range, and whiskers show the 5th and 95th percentile values.
4. Discussion
Monocytes/macrophages offer first line of defense arresting bacterial replication and containing the latent Mtb infection [17]. They are a heterogeneous population and consist of CD14low and CD14hi subsets, most predominant of them, are “classical monocytes”, expressing high levels of CD14 on their surface. The more abundant “nonclassical monocytes”, are characterized by very low expression of surface CD14. These subsets are either inflammatory or phagocytic [18–21]. HIV infection dampens the body’s natural ability to fight infections and increases the likelihood of activation of latent tuberculosis infection [22, 23]. There is a limited information available about the factors that regulate monocyte/macrophage defenses against Mtb infection in HIV infected individuals [24–26]. TLR-2 expressed by antigen presenting cells is known to play an important role in production of cytokines and chemokines to induce antimicrobial defense mechanism/s [27,28]. There is no information available about expression of TLR-2 and signaling molecules (MyD88, IRAK4 and NF-kB) those are involved in TLR-2 mediated cytokines and chemokines production in HIV+ individuals with LTBI or active TB disease. In the current study, we found increased TLR2 expression on CD14low and CD14hi cells in the peripheral blood of HIV+LTBI+ individuals compared to healthy HIV-LTBI+ individuals and TB patients with or without HIV infection. Most of the CD14hi cells expressed TLR2 during HIV infection with latent or active TB disease. We speculate that this increased expression is because of on-going co-infection and indicates active inflammation in these individuals. Despite increase in TLR2 expression by monocytes of HIV+LTBI+ individuals, γ-irradiated Mtb H37Rv or TLR2 agonist were unable to enhance MyD88 and IRAK4 expression and IL-1β, MCP-1 and IP-10 production by their CD14+ cells compared to CD14+ cells of HIV-LTBI+ individuals. Pleural fluids of HIV-active TB patients have higher amounts of IL-1β, MCP-1 and IP-10 and lower amounts of IL-10 and TNF-α compared to HIV+ active TB patients. γ-irradiated Mtb H37Rv significantly enhanced IL-1β, MCP-1 and IP-10 and enhanced expression of MyD88 and IRAK4 by pleural fluid CD14+ cells of HIV-active TB patients compared to HIV+ active TB patients. Our results suggest defective MyD88 and IRAK4 not TLR-2 expression inhibits IL-1β, MCP-1 and IP-10 production and enhances TNF-α and IL-10 production by CD14+ cells of HIV+ individuals with LTBI and active TB disease. Surprisingly we found that there was no significant difference in NF-kB expression in any of our groups indicating that the regulation of the cytokine and chemokine production happens at MyD88 and IRAK4 rather than at NF-kB.
TLR2 knockout mice had defective tuberculosis granuloma formation and were susceptible to Mtb infection [29,30]. HIV infection upregulates TLR2 expression by monocytes for the production of TNF-α and IL-10 [31,32]. Increased TLR2 expression in HIV+ active TB patients has been reported previously as a biomarker in HIV+TB+ IRIS (immune reconstitution inflammatory syndrome) [33]. Our results confirm these findings and further demonstrate that HIV infection in LTBI+ individuals inhibits the expression of key molecules in TLR2 signaling pathway. Activation of TLR-2 pathway leads to anti-viral proinflammatory cytokine and chemokine production which are also essential to prevent Mtb replication. Our results also demonstrate that HIV infection prevents TLR-2 dependent proinflammatory cytokine and chemokine production in individuals with LTBI+. IRAK4 is critical for MyD88-dependent TLR signaling, and patients with IRAK-4 mutations are extremely susceptible to recurrent bacterial infections [34,35]. Upon activation of the MyD88-dependent pathway, TLR2 associated MyD88 forms “Myddosome” complexes by recruiting IRAK4 [36,37]. We found γ-irradiated Mtb H37Rv and TLR2 agonist significantly inhibit IL-1β, MCP-1 and IP-10 production by CD14+ cells of HIV+LTBI+, and HIV+ and HIV-active TB patients compared to HIV-LTBI+ healthy individuals. Our results suggest HIV infection inhibits the expression of IRAK4 and MyD88 to prevent “Myddosome” complex formation which is crucial for the production of pro-inflammatory cytokines and chemokines to contain both HIV and TB infection.
We found γ-irradiated Mtb H37Rv and TLR2 agonist significantly enhance TNF-α and IL-10 production by CD14+ cells of HIV+LTBI+, HIV+ and HIV-active TB patients compared to HIV-LTBI+ healthy individuals. Increased TNF-α production suggests immune activation for increased viral replication as found previously [38,39]. IL-10 is known to inhibit production of reactive nitrogen intermediates, causes reduced macrophage function and reactivation of TB [40–42]. Enhanced IL-10 production by CD14+ cells of HIV+LTBI+, and HIV+ and HIV− active TB patients suggest disruption of immune responses, enhanced Mtb growth and progression of LTBI to ATB.
Chemokines have been associated with numerous key processes that lead to Mtb containment, from recruitment of myeloid cells into the lung to activation of adaptive immunity and formation of protective granulomas [43–45]. MCP-1 and IP-10 have been studied in the context of immune activation and viral replication in HIV [46–48]. In the current study, we found γ-irradiated Mtb H37Rv and TLR2 agonist stimulation of CD14+ of HIV+LTBI+, and HIV+ and HIV− active TB patients produce less MCP-1 and IP-10 compared to HIV-LTBI+ healthy individuals. Our results suggest defective TLR-2 mediated signaling pathways leads to poor chemotaxis and recruitment of immune cells at the site of infection and defective control of Mtb growth in HIV+ individuals with LTBI or active disease.
We found higher levels of TNF-α and IL-10 in the pleural fluids and culture supernatants of HIV+ tuberculosis patients. HIV-tuberculosis patients have higher levels of IL-1β, MCP-1 and IP-10 in pleural fluids and in the culture supernatants. These findings are similar to those observed in CD14+ cells from peripheral blood suggesting their relevance and reiterating their importance at the site of disease.
In summary, we found HIV infection inhibits expression of molecules (MyD88 and IRAK4) involved in TLR-2 signaling pathway, regardless of TLR-2 expression in LTBI+ individuals. This leads to reduced production of cytokines and chemokines essential to control TB growth and progression of LTBI to TB in HIV+ individuals.
5. Conclusions
Understanding the mechanisms how HIV infection inhibits the expression of molecules involved in TLR-2 mediated pathways will facilitate development of immunomodulatory strategies to boost innate immunity to Mtb and prevent progression of LTBI to TB in highly susceptible HIV-infected population.
Figure 1. Representative density plots showing CD14low and CD14high cells in A. HIV−LTBI+, B. HIV+LTBI+, C. HIV+TB+ and D. HIV− TB+ individuals. E and F denote histogram plots representing TLR2 expression on CD14low and CD14high cells respectively.
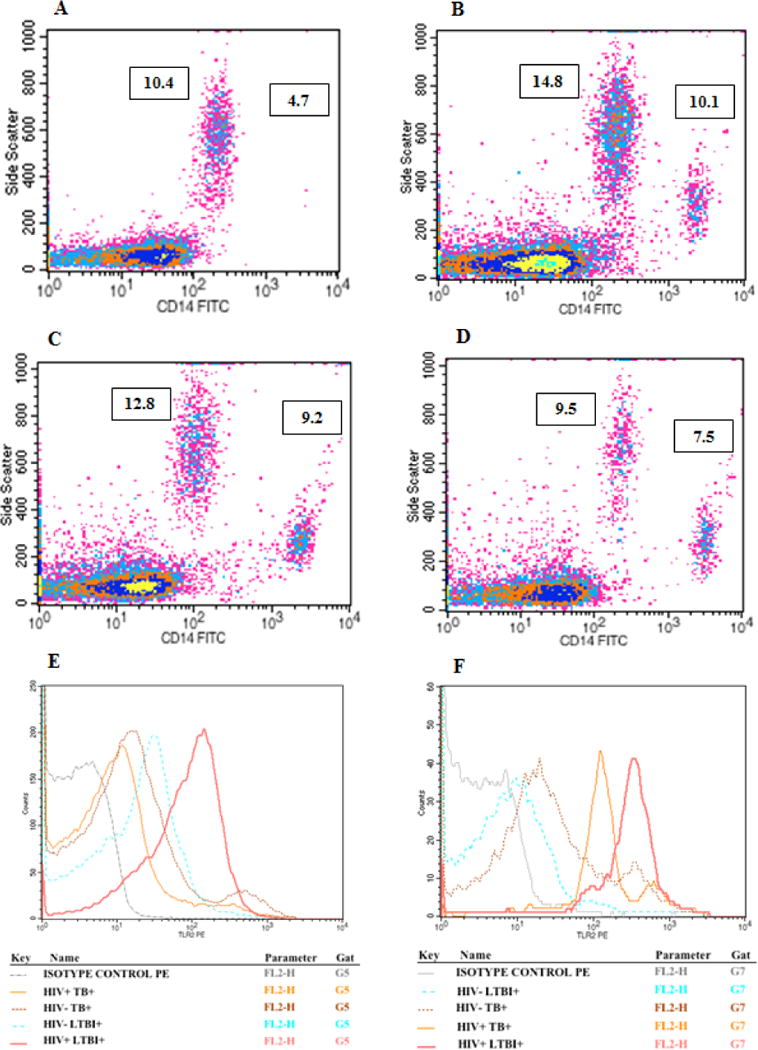
Freshly isolated PBMCs from HIV-LTBI+, HIV+LTBI+ individuals, HIV+TB and HIV-TB patients were stained with Abs to CD14 and TLR2. Flow cytometry was used to measure the percentages of CD14+TLR2+ cells.
Highlights.
This article highlights the importance of MyD88 and IRAK4 in TLR2 mediated production of IL-1β, MCP-1 and IP-10 by monocytes of HIV+ individuals with LTBI. We also demonstrated that stimulation with TLR2 agonist is unable to induce the MyD88 and IRAK4 expression and IL-1β, MCP-1, IP-10 expression in HIV infected. We hypothesize that this defect could be a reason for activation of latent TB in HIV.
Acknowledgments
The authors thank Ms. Maheshwari and Ms. Priya, ICTC counsellors at LEPRA Society, for helping with the counselling and enrolment of study subjects. We also thank all the participants and volunteers included in the study.
The γ-irradiated Mtb cells used in the study were obtained through BEI Resources, NIAID, NIH: Mycobacterium tuberculosis, Strain H37Rv, Gamma-Irradiated Whole Cells, NR-14819.
Funding
This work was supported by grants from the National Institutes of Health (AI120257 and A127178 to R.V), CRDF global, The Cain Foundation for Infectious Disease Research and The Department of Pulmonary Immunology.
Dr. VLV received a grant from Indian Council of Medical Research [No: 5/8/3(13)/2009-ECD-I (A)].
Abbreviations
- Mtb
Mycobacterium tuberculosis
- HIV
Human immunodeficiency virus
- TB
Tuberculosis
- LTBI
Latent Tuberculosis Infection
- TLR
Toll Like Receptor
- MyD88
Myeloid Differentiation Factor
- IRAK
Interleukin-1R-associated kinase
- IL
Interleukin
- CD
Cluster of differentiation
- MCP
Monocyte Chemoattractant Protein
- IP-10
Interferon gamma induced Protein
- TNF
Tumor Necrosis Factor
- NF-kB
Nuclear Factor kappaB
- NO
Nitric Oxide
- RNI
Reactive Nitrogen Intermediates
- ROI
Reactive Oxygen Intermediates
- AFB
Acid fast bacilli
- PBMC
Peripheral Blood Mononuclear Cells
- PFMC
Pleural Fluid Mononuclear Cells
- FITC
Fluorescein isothiocyanate
- PE
Phycoerythrin
- QFT
QuantiFERON
- ELISA
Enzyme Linked Immuno-Sorbent Assay
- CFP-10
Culture Filtrate Protein
- ESAT
Early Secreted Antigenic Target
- PHA
Phytohaemagglutinin
- PCR
Polymerase Chain Reaction
- RNA
Ribonucleic acid
- SD
Standard deviation
- SE
Standard error
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s contribution
VLV and RKV developed the concept, supervised the study and edited the manuscript. KPD and VSKN designed the study, performed the experiments, analyzed the data and wrote the manuscript. AV performed the experiments. RG and ABC designed the subject enrollment; KSRSS helped in analysis of data, supervised the experiments and edited the manuscript.
Disclosure
The authors have no financial or commercial conflict of interest.
References
- 1.Zumla A, Raviglione M, Hafner R, Fordham von Reyn C. Tuberculosis. N Engl J Med. 2013;368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 2.Stead WW. Pathogenesis of a first episode of chronic pulmonary tuberculosis in man: recrudescence of residuals of the primary infection or exogenous reinfection? Am Rev Respir Dis. 1967;95:729–745. doi: 10.1164/arrd.1967.95.5.729. [DOI] [PubMed] [Google Scholar]
- 3.Dhiman R, Bandaru A, Barnes PF, Saha S, Tvinnereim A, Nayak RC, Paidipally P, Valluri VL, Rao LV, Vankayalapati R. c-Maf-dependent growth of Mycobacterium tuberculosis in a CD14(hi) subpopulation of monocyte-derived macrophages. J Immun. 2011;186:1638–1645. doi: 10.4049/jimmunol.1003146. [DOI] [PubMed] [Google Scholar]
- 4.Stead WW, Kerby GR, Schlueter DP, Jordahl CW. The clinical spectrum of primary tuberculosis in adults. Confusion with reinfection in the pathogenesis of chronic tuberculosis. Ann Intern Med. 1968;68:731–745. doi: 10.7326/0003-4819-68-4-731. [DOI] [PubMed] [Google Scholar]
- 5.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 6.Rocha-Ramirez LM, Estrada-Garcia I, Lopez-Marin LM, Segura-Salinas E, Mendez-Aragon P, Van Soolingen D, Torres-Gonzalez R, Chacon-Salinas R, Estrada-Parra S, Maldonado-Bernal C, Lopez-Macias C, Isibasi A. Mycobacterium tuberculosis lipids regulate cytokines, TLR-2/4 and MHC class II expression in human macrophages. Tuberculosis. 2008;88:212–220. doi: 10.1016/j.tube.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Druszczynska M, Wlodarczyk M, Janiszewska-Drobinska B, Kielnierowski G, Zawadzka J, Kowalewicz-Kulbat M, Fol M, Szpakowski P, Rudnicka K, Chmiela M. Monocyte signal transduction receptors in active and latent tuberculosis. Clin Dev Immunol. 2013;2013 doi: 10.1155/2013/851452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heggelund L, Muller F, Lien E, Yndestad A, Ueland T, Kristiansen KI, Espevik T, Aukrust P, Froland SS. Increased expression of toll-like receptor 2 on monocytes in HIV infection: possible roles in inflammation and viral replication. Clin Infect Dis. 2004;39:264–269. doi: 10.1086/421780. [DOI] [PubMed] [Google Scholar]
- 9.Ohno H, Zhu G, Mohan VP, Chu D, Kohno S, Jacobs WR, Jr, Chan J. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell Microbiol. 2003;5:637–648. doi: 10.1046/j.1462-5822.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O’Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 13.Jefferies CA, Doyle S, Brunner C, Dunne A, Brint E, Wietek C, Walch E, Wirth T, O’Neill LA. Bruton’s tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J Biol Chem. 2003;27:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 14.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol (Baltimore, Md: 1950) 2007;179:1178–89. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 15.Scanga CA, Bafica A, Feng CG, Cheever AW, Hieny S, Sher A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun. 2004;72:2400–2404. doi: 10.1128/IAI.72.4.2400-2404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pathak S, De Souza GA, Salte T, Wiker HG, Asjo B. HIV induces both a down-regulation of IRAK-4 that impairs TLR signalling and an up-regulation of the antibiotic peptide dermcidin in monocytic cells. Scand J Immunol. 2009;70:264–276. doi: 10.1111/j.1365-3083.2009.02299.x. [DOI] [PubMed] [Google Scholar]
- 17.Raffetseder J, Pienaar E, Blomgran R, Eklund D, Patcha Brodin V, Andersson H, Welin A, Lerm M. Replication rates of Mycobacterium tuberculosis in human macrophages do not correlate with mycobacterial antibiotic susceptibility. PloS ONE. 2014;9:e112426. doi: 10.1371/journal.pone.0112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1–1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci Rep. 2015;5:13886. doi: 10.1038/srep13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kratofil RM, Kubes P, Deniset JF. Monocyte Conversion During Inflammation and Injury. Arterioscler Thromb Vasc Biol. 2017;37:35–42. doi: 10.1161/ATVBAHA.116.308198. [DOI] [PubMed] [Google Scholar]
- 21.Stansfield BK, Ingram DA. Clinical significance of monocyte heterogeneity. Clin Trans Med. 2015;4:5. doi: 10.1186/s40169-014-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okoye AA, Picker LJ. CD4(+) T cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn JL, Chan J. Tuberculosis: latency and reactivation. Infect Immun. 2001;69:4195–4201. doi: 10.1128/IAI.69.7.4195-4201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerra C, Morris D, Sipin A, Kung S, Franklin M, Gray D, Tanzil M, Guilford F, Khasawneh FT, Venketaraman V. Glutathione and adaptive immune responses against Mycobacterium tuberculosis infection in healthy and HIV infected individuals. PloS one. 2011;6:e28378. doi: 10.1371/journal.pone.0028378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwandumba HC, Russell DG, Nyirenda MH, Anderson J, White SA, Molyneux ME, Squire SB. Mycobacterium tuberculosis resides in nonacidified vacuoles in endocytically competent alveolar macrophages from patients with tuberculosis and HIV infection. J Immunol. 2004;172:4592–4598. doi: 10.4049/jimmunol.172.7.4592. [DOI] [PubMed] [Google Scholar]
- 26.Mwandumba HC, Squire SB, White SA, Nyirenda MH, Zijlstra EE, Molyneux ME, Russell DG, Rhoades ER. Alveolar macrophages from HIV-infected patients with pulmonary tuberculosis retain the capacity to respond to stimulation by lipopolysaccharide. Microbes Infect. 2007;9:1053–1060. doi: 10.1016/j.micinf.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Drage MG, Pecora ND, Hise AG, Febbraio M, Silverstein RL, Golenbock DT, Boom WH, Harding CV. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez D, Rojas M, Hernandez I, Radzioch D, Garcia LF, Barrera LF. Role of TLR2- and TLR4-mediated signaling in Mycobacterium tuberculosis-induced macrophage death. Cell Immunol. 2010;260:128–136. doi: 10.1016/j.cellimm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J, Fremond C, Wagner H, Kirschning C, Ryffel B. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiling N, Holscher C, Fehrenbach A, Kroger S, Kirschning CJ, Goyert S, Ehlers S. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002;169:3480–3484. doi: 10.4049/jimmunol.169.7.3480. [DOI] [PubMed] [Google Scholar]
- 31.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y, Fonseca SG, Van Grevenynghe J, Boulassel MR, Bruneau J, Shoukry NH, Routy JP, Douek DC, Haddad EK, Sekaly RP. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 33.Lai RP, Meintjes G, Wilkinson KA, Graham CM, Marais S, Plas H Van der, Deffur A, Schutz C, Bloom C, Munagala I, Anguiano E, Goliath R, Maartens G, Banchereau J, Chaussabel D, O’Garra A, Wilkinson RJ. HIV-tuberculosis-associated immune reconstitution inflammatory syndrome is characterized by Toll-like receptor and inflammasome signalling. Nat Commun. 2015;6:8451. doi: 10.1038/ncomms9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medvedev AE, Thomas K, Awomoyi A, Kuhns DB, Gallin JI, Li X, Vogel SN. Cutting edge: expression of IL-1 receptor-associated kinase-4 (IRAK-4) proteins with mutations identified in a patient with recurrent bacterial infections alters normal IRAK-4 interaction with components of the IL-1 receptor complex. J Immunol. 2005;174:6587–6591. doi: 10.4049/jimmunol.174.11.6587. [DOI] [PubMed] [Google Scholar]
- 35.Medvedev AE, Lentschat A, Kuhns DB, Blanco JC, Salkowski C, Zhang S, Arditi M, Gallin JI, Vogel SN. Distinct mutations in IRAK-4 confer hyporesponsiveness to lipopolysaccharide and interleukin-1 in a patient with recurrent bacterial infections. J Exp Med. 2003;198:521–531. doi: 10.1084/jem.20030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George J, Motshwene PG, Wang H, Kubarenko AV, Rautanen A, Mills TC, Hill AVS, Gay NJ, Weber ANR. Two Human MYD88 Variants, S34Y and R98C, Interfere with MyD88-IRAK4-Myddosome Assembly. J Biol Chem. 2011;286:1341–1353. doi: 10.1074/jbc.M110.159996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lederman MM, Kalish LA, Asmuth D, Fiebig E, Mileno M, Busch MP. ‘Modeling’ relationships among HIV-1 replication, immune activation and CD4+ T-cell losses using adjusted correlative analyses. AIDS. 2000;14:951–958. doi: 10.1097/00002030-200005260-00006. [DOI] [PubMed] [Google Scholar]
- 39.Rychert J, Strick D, Bazner S, Robinson J, Rosenberg E. Detection of HIV gp120 in plasma during early HIV infection is associated with increased proinflammatory and immunoregulatory cytokines. AIDS Res Hum Retroviruses. 2010;26:1139–1145. doi: 10.1089/aid.2009.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreiber T, Ehlers S, Heitmann L, Rausch A, Mages J, Murray PJ, Lang R, Holscher C. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. J Immunol. 2009;183:1301–1312. doi: 10.4049/jimmunol.0803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- 42.Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, Bancroft GJ, O’Garra A. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Imunol. 2010;40:2200–2210. doi: 10.1002/eji.201040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurashima K, Mukaida N, Fujimura M, Yasui M, Nakazumi Y, Matsuda T, Matsushima K. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med. 1997;155:1474–1477. doi: 10.1164/ajrccm.155.4.9105097. [DOI] [PubMed] [Google Scholar]
- 44.Sadek MI, Sada E, Toossi Z, Schwander SK, Rich EA. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol. 1998;19:513–521. doi: 10.1165/ajrcmb.19.3.2815. [DOI] [PubMed] [Google Scholar]
- 45.Fuller CL, Flynn JL, Reinhart TA. In situ study of abundant expression of proinflammatory chemokines and cytokines in pulmonary granulomas that develop in cynomolgus macaques experimentally infected with Mycobacterium tuberculosis. Infect Immun. 2003;71:7023–7034. doi: 10.1128/IAI.71.12.7023-7034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lane BR, King SR, Bock PJ, Strieter RM, Coffey MJ, Markovitz DM. The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology. 2003;307:122–134. doi: 10.1016/s0042-6822(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 47.Wetzel MA, Steele AD, Henderson EE, Rogers TJ. The effect of X4 and R5 HIV-1 on C, C-C, and C-X-C chemokines during the early stages of infection in human PBMCs. Virology. 2002;292:6–15. doi: 10.1006/viro.2001.1249. [DOI] [PubMed] [Google Scholar]
- 48.Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


