Abstract
HIV latency occurs predominantly in long-lived resting CD4+ T-cells, however latent infection also occurs in T-cell subsets including proliferating CD4+ T-cells. We compared the establishment and maintenance of latent infection in non-proliferating and proliferating human CD4+ T-cells cocultured with syngeneic myeloid dendritic cells (mDC). Resting CD4+ T-cells were labelled with the proliferation dye eFluor670 and cultured alone or with mDC, plasmacytoid DC (pDC) or monocytes in the presence of staphylococcal enterotoxin B (SEB). Cells were cultured 24 hours and infected with CCR5-tropic enhanced green fluorescent protein (EGFP)-reporter HIV. Five days post-infection, non-productively-infected (EGFP−) CD4+ T-cells that were either non-proliferating (efluor670hi) or proliferating (efluor670lo) were sorted and cultured for an additional 7 days (day 12) with IL-7 and antiretrovirals (ARV). At day 5 post-infection, sorted non-productively infected T cells were stimulated with anti-CD3/CD28 and induced expression of EGFP measured to determine the frequency of latent infection. Integrated HIV in these cells was confirmed using qPCR. By these criteria latent infection was detected at day 5 and 12 in proliferating T-cells cocultured with mDC and monocyte, but not pDC where CD4+ T-cells at day 12 was poor. At day 5 post-infection, non-proliferating T-cells expressing SEB specific TCR Vβ-17 were enriched in latent infection compared to non-SEB specific TCR Vβ-8.1. Together these data show that both non-proliferating and proliferating CD4+ T-cells can harbor latent infection during SEB stimulated T-cell proliferation and that the establishment of HIV latency in non-proliferating T-cells is linked to expression of specific TCR that respond to SEB.
Introduction
Combination antiretroviral therapy (cART) has revolutionized the treatment of HIV, however treatment remains lifelong. The main barrier to HIV cure is latent infection, which is the integration of HIV DNA into the host genome in the absence of virus expression. Latent infection was first described in long-lived, resting memory CD4+ T-cells (1–3). More recently, latent infection has been described in multiple CD4+ T-cell subsets with different half-lives and proliferative capacity, including central memory (TCM), transitional memory (TTM), effector memory (TEM), stem cell memory (TSCM) and naïve T-cells (TN) (4–8). Recent analysis of integration sites in latently infected cells in HIV-infected individuals on cART has also demonstrated enrichment of HIV integration in cancer-associated genes. This suggests that T-cell survival and proliferation may be linked to HIV persistence on cART (9–11).
Latency can be established in vitro by direct infection of resting CD4+ T-cells (12, 13), or following reversion of an activated, infected CD4+ T-cell to a resting state (14–17). It remains unclear if latency can be established in proliferating cells early after infection in vivo, or if proliferation will favor productive over latent infection. We have clearly demonstrated that latent infection can be established following direct infection of resting memory CD4+ T-cells in the presence of an additional non-activating stimuli such as coculture with chemokines (12), syngeneic myeloid dendritic cells (mDC) (18, 19) and monocytes (19). Others have shown similar results following coculture with endothelial cells (20) or using spinoculation to infect resting CD4+ T-cells (21).
Recently it has been shown that following HIV infection of activated CD4+ T-cells in vitro, a subset of T-cells that remains activated, also contains inducible virus (22, 23). Whether such cells are long-lived and can contribute to HIV persistence in HIV-infected individuals on cART remains unclear. Recent work clearly shows that intact, replication competent, latent virus can be maintained in CD4+ T-cells from HIV infected individuals on suppressive cART, even after clonal expansion has occurred in vivo (24–26). Furthermore, only a fraction of intact integrated virus can be activated ex vivo by anti-CD3/CD28 suggesting that latency can persist even during potent T-cell stimulation (26–28).
In this study, we used our previously described model of HIV latency, which involves the coculture of antigen presenting cells (APC; mDC or monocytes) with resting CD4+ T-cells, to simultaneously examine latent infection within non-proliferating and proliferating T-cells (18, 19). We demonstrated that latent infection was established in proliferating CD4+ T-cells, and latency was maintained in a subset of these proliferating cells during more extended culture in vitro. We also show that the mechanism leading to the establishment of latency in non-proliferating is different to proliferating cells whereby latency preferentially occurs in non-proliferating cells bearing a T cell receptor (TCR) that is specific to superantigen stimulation.
Materials and methods
HIV preparation
CCR5-EGFP reporter virus (NL(AD8)ΔnefEGFP or NL(AD8)IRES-EGFP) was produced from plasmid transfected into 293T-cells, concentrated and used in all experiments, as previously described (18). All cells were infected with an MOI of 0.5 as determined by limiting dilution in PHA activated PBMC (29).
Flow cytometry
Expression of surface markers was determined using specific antibodies; CD14-FITC, CD11c-APC/CD11c-V450, CD123-PE, HLA-DR-APC-Cy7/PerCP, CD69-FITC, CD25-PE, CCR7-PE-Cy7, CD27-PE, PD-1-PE (all from BD Biosciences, San Jose, CA), CD3-V450 (Pharmingen), CD45RO-ECD (Beckman Coulter, Indianapolis, IN, USA), Tim3-PE, TcR Vβ-17-PE, TcR Vβ-3-PE-Cy7 and TCR Vβ-13.1-PE (all from Biolegend).
Cells were stained in a total volume of 100ul with a previously titrated volume of antibody for 25–30 min, on ice (4°C). Cells were then washed and fixed with 100ul of 1% formaldehyde. Samples were analysed by flow cytometry (FCM) on a FACSCalibur or LSR-II (BD Biosciences), and data analyzed using Weasel (Version 2.7; WEHI, Melbourne, Australia).
Isolation of T-cells, DC subpopulations and monocytes
Resting CD4+ T-cells, mDC (HLA-DR+CD11c+CD123-) and pDC (HLA-DR+CD11c-CD123+) were isolated from PBMCs of healthy donors (Australian Red Cross, Melbourne, Vitcoria, Australia) using magnetic bead enrichment and sorting by flow cytometry on either a FACSAria (BD Biosciences) or Astrios (Beckman Coulter) as described previously (18). Isolated resting CD4+ T-cells were stained with the cytoplasmic dye eFluor670 (eBiosciences, San Diego CA) according to manufacturer’s protocol. Bulk peripheral monocytes (CD14+) were isolated from syngeneic donors using positive selection for CD14 on an autoMACS (Miltenyi Biotech, San Diego, CA). Only DC subpopulations and monocytes with a purity ≥95% and resting CD4+ T-cells with a purity of >98% were used.
Titration of SEB in DC-T cell co-cultures
The concentration of staphylococcal entertoxin B (SEB; Sigma, St Louis, MO) required to induce TCR-MHC-II specific interactions was determined by titration in a syngeneic mixed leukocyte reaction (MLR) with a 10:1 ratio of resting CD4+ T-cells: mDC/pDC (Supplemental Figure S1 A). T-cell proliferation was measured by the percentage of eFluor670lo cells, and identified an optimal SEB concentration of 10ng/ml, which was used in all infection experiments.
Measurement of HIV latency in proliferated T-cells
Resting CD4+ T-cells labelled with eFluor670 were cultured alone, or with syngeneic mDC, pDC or monocytes at a ratio of 10:1 for 24 hours in the presence of IL-2 (2U/mL, Roche diagnostics, Mannheim, Germany) and SEB (10 ng/mL) before infection with either NL(AD8)Δnef-EGFP or NL(AD8)-IRES-EGFP reporter virus for 2 hours. After washing, cells were cultured for 5 days in IL-2 (2U/mL) supplemented media without additional SEB. Productive infection was measured by FCM at day 5 post-infection. Lymphocytes were gated by forward and side scatter, then EGFP+ cells quantified against expression of eFluor670. DC and monocytes were excluded from the eFluor670lo cells by gating for HLA-DRlo CD3+ T-cells. CD4+ T-cells that were non-productively infected (EGFP−), and either non-proliferating (eFluor670hiEGFP−) or proliferating (eFluor670loEGFP−) were sorted by FCM using a FACSAria (BD Biosciences) or an Astrios (Beckman Coulter). Latent infection was determined following activation of 200,000 sorted CD4+ T-cells (eFluor670hiEGFP− or eFluor670loEGFP−) with immobilized anti-CD3 (7ug/ml; Beckman Coulter), in 10% Roswell Park Memorial Institute medium (RPMI) with antibiotics penicillin-streptomycin-glutamine (RF10), supplemented with soluble CD28 (7ug/ml; BD Biosciences) and IL-7 (50ng/ml; Sigma). Integrase inhibitor, L8 (1mM; Merck, Kenilworth, NJ) or raltegravir (1µM; NIH AIDS reagent program, Cat#11680), was added to activation media to prevent a spreading infection, and to minimize the inclusion of unintegrated virus in the analysis of latent infection in mDC and monocytes cocultures. Activation of sorted cells without integrase inhibitor allowed expression of latency that included integrated and unintegrated forms of virus. L8 and raltegravir were used at concentrations that blocked productive infection in PHA or SEB activated PBMC following incubation with NL(AD8)ΔnefEGFP at an MOI of 0.5. Sorted cells were harvested 72 hours after stimulation and expression of virus quantified by EGFP expression using FCM (FACSCalibur, BD Biosciences). Sorted T-cells (eFluor670hi EGFP− and eFluor670lo EGFP−) that were left unstimulated were used to determine background (spontaneous EGFP expression), which was then subtracted from total EGFP expression following activation (+/− integrase inhibitor) to determine induced EGFP expression (Supplemental Figure S1 C).
Extended culture of sorted non-proliferating and proliferating cells
To measure the stability of latent infection in non-proliferating and proliferating CD4+ T-cells, 500,000–1,000,000 sorted T-cells (eFluor670hiEGFP− or eFluor670loEGFP−) were cultured without stimulation for 7 days in RF10 media supplemented with IL-2 (10U/mL), IL-7 (1ng/mL), the fusion inhibitor T-20 (1ug/mL), and either L8 (1mM) or raltegravir (1µM). After 7 days (day 12 of experiment), 100,000 viable cells were stimulated with anti-CD3/CD28 for 3 days, with an integrase inhibitor, to determine latent infection. The effect of IL-7 in this coculture system was determined by analysis of viability and proliferation with different concentrations of IL-2 and IL-7 in the cultures (supplemental figure S2).
To quantify TCR Vβ expression, non-productively, non-proliferating and proliferating CD4+ T-cells were gated as described for day 5 FACSAria sort. Single TCR Vβ expression, non-proliferating and proliferating cells were visualized and quantified with eFluor670.
To quantify T-cell subsets, sorted day 5 and cultured day 12 non-proliferating and proliferating CD4+ T-cells were gated for live cells by forward and side scatter, then gated for CD45RO+ or CD45RO−.
Inhibition of HIV infection and latency with antiretrovirals (ARV)
T cells and T cells in coculture with monocytes were cultured with antiretrovirals for various times. Raltegravir (10 µm) was added at the time of infection, and the fusion inhibitor T20 (10 µg/mL; Roche Diagnostics), and NNRTI efavirenz (300 nM; NIH AIDS Reagent Program) added 24 hours post-infection. To determine kinetics of establishment of latency, T cells were cultured with antiretrovirals, raltegravir (10 uM), T20 (10 µg/mL), and efavirenz (300 nM), added in combination at the time of infection (T0), 24 hours post-infection (T1), 48 hours post-infection (T2), and 72 hours post-infection (T3). T cells were sorted at day 5 post-infection and latency measured by restimulation of EGFP− cells.
Statistical analysis
Statistical differences between conditions were determined using Wilcoxon signed-rank test (n≥6) or paired student T-test (n<6). Graphpad Prism (version 6) was used for all statistical tests. ANOVA was used to compare expression of activation markers across all cultures. P-values of <0.05 were considered significant. Bonferroni corrections for multiple comparisons were not used.
Results
Inducible latent virus in proliferating CD4+ T-cells following coculture with antigen presenting cells
We have previously shown that HIV latency can be established in non-proliferating CD4+ T-cells following coculture with syngeneic APC, including mDC and monocytes, but not pDC (18, 19). To determine whether these APCs could also establish latency in proliferating T-cells, resting CD4+ T-cells were cultured alone or with mDC, pDC or monocytes for 24 hours in the presence of the superantigen SEB (Figure 1A). SEB induces a TCR specific interaction by stabilizing the interaction between major histocompatibility complex (MHC) class-II and specific TCRs, leading to TCR Vβ specific T-cell activation and proliferation (30). Cells were infected with NL(AD8)-EGFP and cultured for 5 days. Coculture with mDC and monocytes resulted in significantly higher levels of productive infection in the CD4+ T-cells compared to those cocultured with pDC (mean of 1200, 1100 and 300 EGFP+ cells/10,000 viable cells respectively; Figure 1A, B (18).
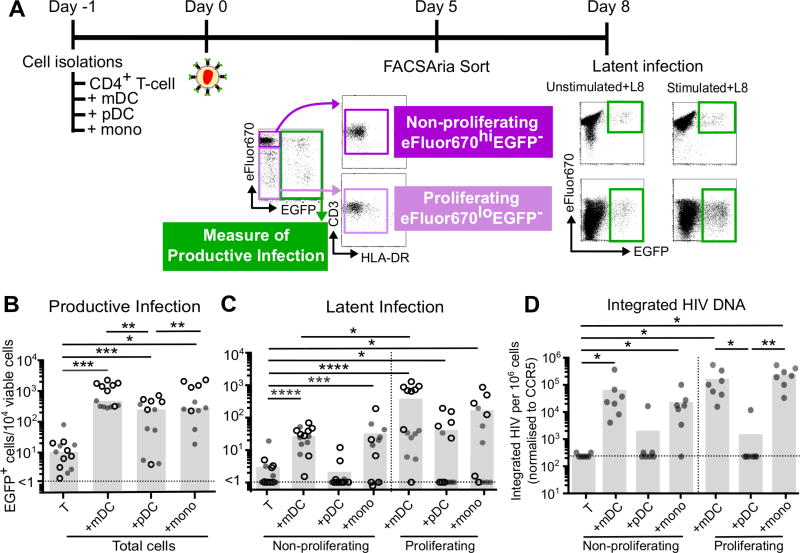
Figure 1. Productive and latent infection in non-proliferating and proliferating CD4+ T-cells.
A. eFluor670 labelled resting CD4+ T-cells were cultured alone, with mDC, pDC or monocytes at a ratio of 10:1 (T-cell:DC), in the presence of staphylococcal enterotoxin B (SEB). After 24 hours, cultures were infected for 2 hours with a CCR5-tropic EGFP reporter virus, and at day 5 post infection, productive and latent infection was quantified. B. At day 5 post-infection, productive infection was measured by EGFP expression in total cells (n=13). C. Uninfected, non-proliferating (eFluor670hiEGFP−) and proliferating T-cells (eFluor670loEGFP−) were sorted by flow cytometry and latent infection was measured by stimulation with and without anti-CD3/CD28 in the presence of the integrase inhibitor L8. Latency was determined as the difference in EGFP expression between stimulated (stimulated+L8) and unstimulated cultures (unstimulated + L8; n=14). D. Latent infection was confirmed using qPCR to measure integrated HIV DNA (n=7). Open symbols represent experiments performed at the Burnet Institute (ARIA II, BD); shaded symbols represent experiments performed at The Peter Doherty Institute (Astrios, Beckman Coulter). Columns represent median, symbols represent results from individual donors. Significance was measured by Wilcoxon signed-rank test where n≥5, *p≤0.05, **p≤0.005, ***p≤0.0005, ****p≤0.0001.
To investigate the establishment of HIV latency in proliferating CD4+ T-cells, cocultures were sorted using FCM into non-productively infected, non-proliferating (eFluor670hiEGFP−) and proliferating (eFluor670loEGFP−) CD4+ T-cells (Figure 1A). Latency was calculated as the number of cells that expressed EGFP following stimulation with anti-CD3/CD28 in the presence of an integrase inhibitor, minus background EGFP expression without stimulation (Supplemental Figure S1 C and Figure 1A). As previously reported, latency was only observed in non-proliferating CD4+ T-cells following coculture with mDC (p=0.0156, range = 10–100 cells/10,000 viable cells) and monocytes (p=0.0002, range = 10–200 cells/10,000 viable cells) but not pDC (p>0.05, range 1–10 cells/10,000 viable cells; Figure 1C) (18, 19).
Proliferating CD4+ T-cells from the mDC and monocyte cocultures had significantly more latent infection on day 5 post-infection compared to T-cells cultured alone (p= 0.0003, and 0.0134 respectively; Figure 1C) and was greater in magnitude compared to latency in non-proliferating CD4+ T-cells from mDC cocultures (p= 0.0507; Figure 1C). Integrated virus in non-proliferating and proliferating CD4+ T-cells cocultured with mDC and monocytes was further confirmed using quantitative PCR (qPCR) as previously described (Figure 1D)(31).
Together, this data demonstrates that inducible virus expression can be established in both non-proliferating and proliferating T-cells and that the frequency of latent infection was higher in proliferating T-cells compared to non-proliferating T-cells from mDC and monocyte cocultures.
Latency in non-proliferating and proliferating CD4+ T-cells is maintained in vitro
To determine whether latent infection could be maintained in this in vitro model over an extended culture period, the sorted non-proliferating and proliferating CD4+ T-cells were cultured for a further 7 days (12 days post-infection). Cells were cultured with IL-7 (1ng/mL) and IL-2 (10U/mL), to maintain cell viability, and HIV fusion (T-20) and integrase inhibitors (L8 or Raltegravir) to prevent subsequent rounds of HIV infection (Figure 2A). Initial experiments using antiretroviral drugs raltegravir, T20 and efavirenz added 24 hours after infection and in combination at 1, 2 and 3 days of culture and found that establishment of latency was delayed compared to the onset of EGFP expressing cells suggesting that the latency depended on initial productive infection in the cocultures (supplemental figure S3). The addition of antiretrovirals from day 5 during extended culture would then allow for quantitation of cells containing latent infection for at least 7 days.
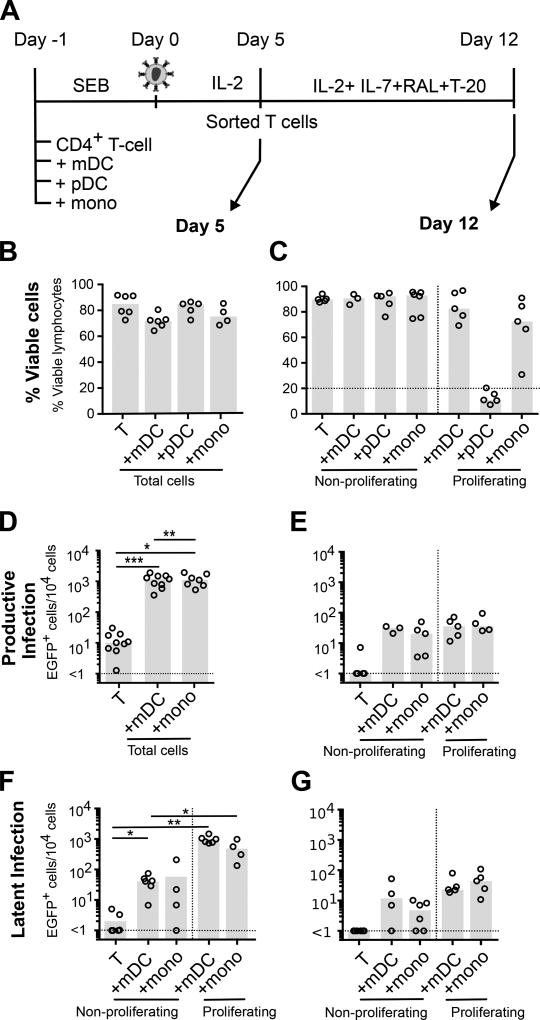
Figure 2. Latency in non-proliferating and proliferating CD4+ T-cells is maintained in a subset of cells in vitro.
Experimental conditions were as in Figure 1. A. A subset of sorted uninfected, non-proliferating and proliferating T-cells were cultured without stimulus for a further 7 days in the presence of IL-2, IL-7, HIV-1 fusion (T-20) and integrase inhibitors (L8 or Raltegravir; RAL) to measure stability of latent infection. Total cell culture viability was quantified by flow cytometry at B. day 5 (n=4–6); and C. day 12 (n=3–5). EGFP was quantified at D. day 5 (n=7–9); and E. day 12 (n=3–5) post-infection as a measure of productive infection. Latent infection was measured by quantification of EGFP expression following stimulation with anti-CD3/CD28 and IL-7 and in the presence of an integrase inhibitor at both F. day 5 (n=4–5); and G. day 12 (n=4–5). Columns represent the median; symbols represent results from individual donors. Significance was measured by a paired students T-test where n<5, and Wilcoxon signed-rank test where n≥5, *p≤0.05, **p≤0.005, ***p≤0.0005.
Lymphocyte recovery from proliferating CD4+ T-cells cocultured with pDC was lower than all other cultures at day 12 and therefore these cells were not included in further analysis.
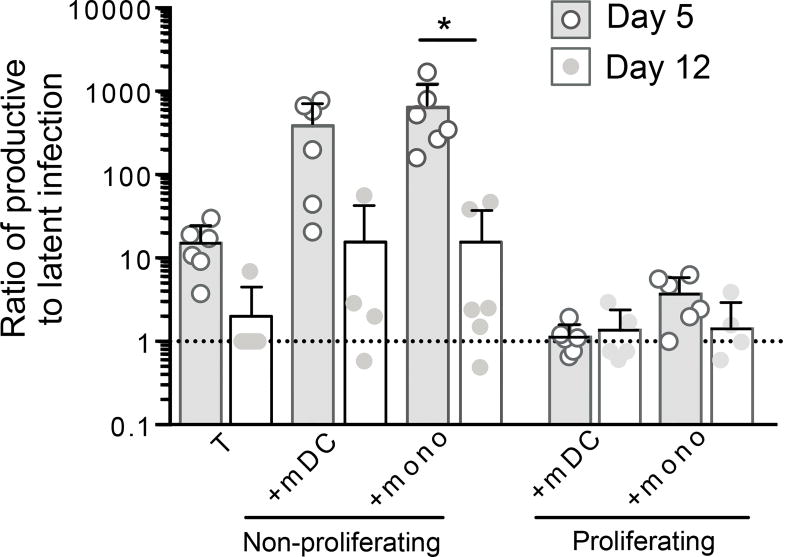
Productive infection was lower at day 12 in non-proliferating and proliferating CD4+ T-cells compared to day 5 post-infection in mDC and monocyte cocultures (Figure 2D and E). We observed the same pattern of latency at day 12 compared to day 5 in both non-proliferating and proliferating T-cells, however latent infection overall was lower at day 12 (Figure 2F, G). Following mDC coculture, latent infection in the non-proliferating and proliferating T-cells was 1.5 and 16.66 fold lower, respectively (p=0.0518) at day 12 compared to day 5. While latent infection in the non-proliferating and proliferating T-cells from monocyte cocultures was 7.04 and 4.77 fold lower respectively at day 12 compared to day 5 (p=0.0459). The reduction in latent infection in proliferating CD4+ T-cells is consistent with the hypothesis that only a subset of latently infected proliferating T-cells are long-lived (Figure 2F, G) (32). The ratio of productive to latent infection in non-proliferating T-cells cocultured with both monocytes and mDC was lower at day 12 compared to day 5 (Figure 3). In contrast, there was no change in the ratio of productive to latent infection in the proliferating T-cells. Together these data demonstrate that mDC and monocytes are able to induce latent infection in both non-proliferating and proliferating CD4+ T-cells but, in the absence of APC (upon T-cell sorting), only a subset of latently infected proliferating T-cells maintained latency in the extended culture.
Figure 3. Proportion of productive and latent infection in non-proliferating and proliferating T cells.
The frequency of EGFP expressing T-cells before (productive) and after (latent) anti-CD3/28 stimulation was used to determine the ratio of productive to latent infection at day 5 and 12 post-infection in non-proliferating and proliferating cells post coculture with mDC or monocytes (n=4–6). Columns represent median, symbols represent results from individual donors. Significance was measured by paired students T-test where n<5 and Wilcoxon signed-rank test where n≥5, *p≤0.05, **p≤0.005, ***p≤0.0005.
Phenotype of proliferating CD4+ T-cells
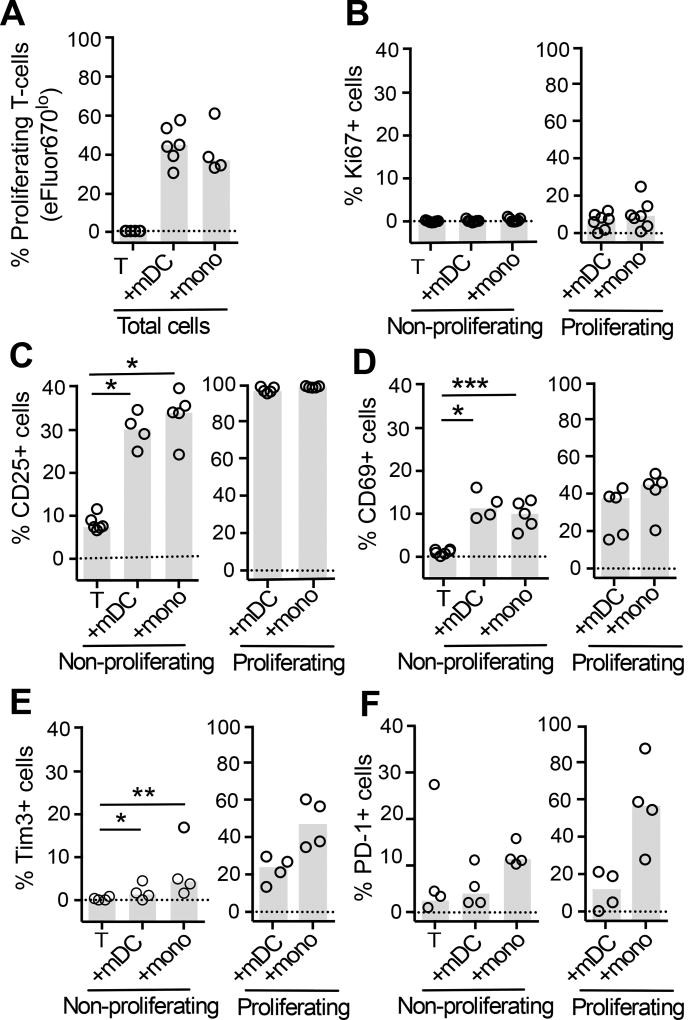
To determine the activation status of proliferating CD4+ T-cells at day 5 post-infection, we measured the cell cycle entry marker Ki67, the early activation markers CD25 and CD69, and immune checkpoint markers PD-1 and Tim-3. As expected, all APCs induced a significant increase in T-cell proliferation in the presence of SEB, as measured by a decrease in eFluor670 expression (Figure 4A). Ki67, CD25, CD69, Tim-3 and PD-1 were highly expressed on proliferating cells from all cocultures compared to non-proliferating cells (ANOVA p values: Ki67: p=0.0028, CD25: p=0.0005, CD69: p=0.0224, Tim-3: p=0.0004, PD-1: p=0.0045 respectively; Figure 4B–F). PD-1 expression was significantly higher in proliferating than non-proliferating CD4+ T-cells from mDC, but not monocyte co-cultures (ANOVA p= 0.0045; Supplemental Figure 4F). Additionally, the frequency of latency in non-proliferating CD4+ T-cells cocultured with mDC correlated with PD-1 expression (r2= 0.9218, p= 0.0419, n=4, Supplemental Figure S4 A). This correlation was not identified in proliferating T-cells from mDC coculture (r2=0.0592, p=0.7567) or in non-proliferating and proliferating T-cells post monocyte coculture (r2=0.7602, p=0.1281 and r2=0.0019, p=0.9569).
Figure 4. Expression of activation markers on non-proliferating and proliferating CD4+ T-cells.
A. Experimental conditions were as in Figure 1. Proliferation in CD4+ T-cells cultured alone, with mDC or monocytes was measured by quantification of eFluor670lo/− cells (n=6). B. Ki67 was measured to confirm entry into cell cycle (n=7). Expression of early activation markers: C. CD25 (n=6); D. CD69 (n=6), and immune checkpoints (IC): E. Tim-3 (n=4); and F. PD-1 (n=4) were measured on sorted non-proliferating (eFlour670hiEGFP−) and proliferating (eFluor670loEGFP−) CD4+ T-cells. Columns represent the median and symbols represent results from individual donors. Significance was measured by paired students T-test where n<5 or Wilcoxon signed-rank test where n≥5, *p≤0.05, **p≤0.005, ***p≤0.0005.
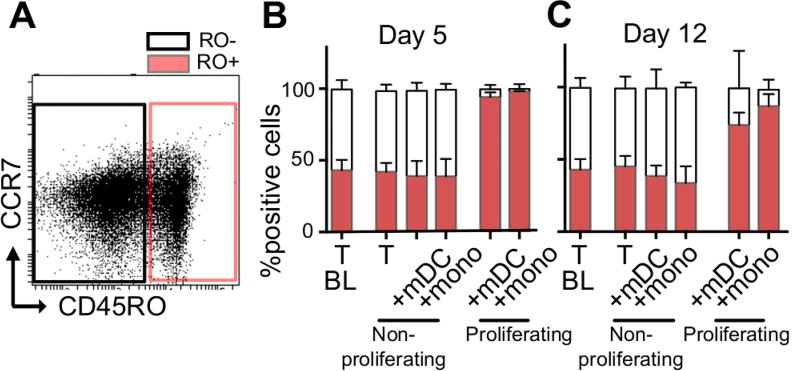
We next determined the proportion of naïve and memory CD4+ T-cell subsets within the freshly isolated resting CD4+ T-cells, as well as both the non-proliferating and proliferating CD4+ T-cells at day 5 and 12 post-infection. Naïve cells were identified based on the absence of CD45RO expression (Figure 5A, B, C). Approximately half the freshly isolated resting CD4+ T-cells were naïve T-cells and this proportion was maintained within the non-proliferating T-cell population throughout the culture period, regardless of whether the T-cells were cultured alone or with mDC/monocytes (Figure 5B, C). As expected, naïve cells were rare in the proliferating T-cell population. We quantified bulk memory cell populations using expression of CD45RO+, these cells represented approximately 50% of non-proliferating cells and were enriched in proliferating cells (Figure 5A, B, C) (5).
Figure 5. CD4+ T-cell subsets in non-proliferating and proliferating cells.
The phenotype of proliferating and non-proliferating T-cells following culture of T-cells alone, coculture with mDC or monocytes was determined at day 5 and 12 post-infection using antibodies binding to CD45RO. A. Representative dot plot of total viable T-cells showing CD45RO+ and CD45RO− gating strategy Proportion of CD45RO+ and CD45RO− T-cells at B. 5 days; and C. 12 days post infection (n=6). Columns represent the median with inter-quartile-range (IQR).
Requirements for TCR ligation for induction of HIV latency
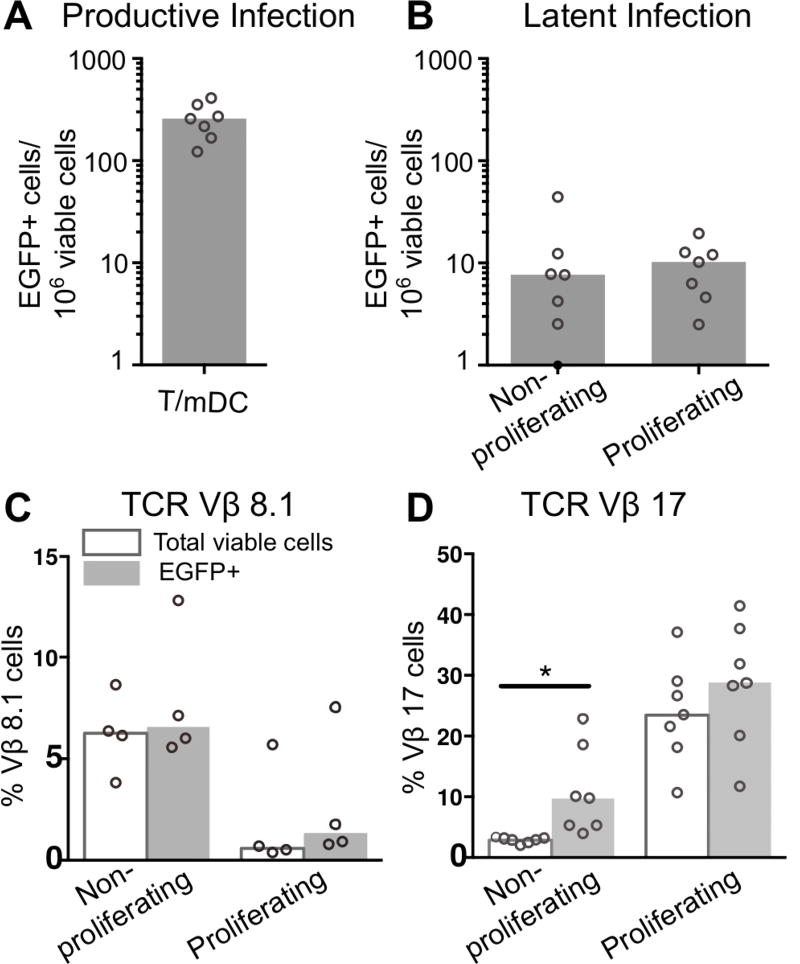
An advantage of SEB-induced T-cell proliferation is that the Vβ region of the TCR binds SEB with differential affinity. TCR Vβ-17 has a strong SEB-MHC-II interaction, while TCR Vβ-8.1 has a weak SEB-MHC-II interaction (33, 34). By exploiting the difference in SEB binding affinity for the TCR Vβ specificities, T-cells that have undergone a strong MHC-II-TCR interaction (Vβ-17) can be compared with those that have undergone a weak TCR-MHC-II interaction (Vβ-8.1). We measured Vβ expression at 2 time-points: [1] day 5 post-infection, [2] upon measurement of latent infection by anti-CD3/CD28.
Vβ-17 expression was measured, at day 5 post-infection, on productively infected (EGFP+) and non-productively infected T-cells (uninfected (EGFP−)). We found that SEB specific TCR Vβ-17 was enriched on all productively infected EGFP+ cells, including non-proliferating and proliferating EGFP+ cells (Supplemental Figure S4 B,C). The large range of Vβ-17 expression was expected due to large donor variation (34).
Vβ expression was measured on latently infected cells by co-staining for Vβ antibodies following CD3/CD28 activation of latent infection in sorted EGFP−, non-proliferating and proliferating cells. As shown in figure 1, there was more productive infection compared with latent infection in both the non-proliferating and proliferating cells in these experiments (p=0.0156; Figure 6A, B). Three days following activation we measured Vβ-17 (strong Vβ-MHC-II interaction; Figure 6C) and Vβ-8.1 (weak Vβ-MHC-II interaction; Figure 6D) expression along with quantifying latent infection (EGFP+ T-cells) on non-proliferating and proliferating cells. There were no significant changes in Vβ-8.1 expression in EGFP+ T-cells compared to total cell cultures in non-proliferating and proliferating T-cells (Figure 6C). However there was a significant increase and a trend toward an increase in Vβ-17 expression on EGFP+ T-cells from activated cultures compared to total cell cultures from non-proliferating and proliferating T-cells respective, as a percentage of the total and EGFP+ cells (Figure 6D).
Figure 6. Vβ expression in non-proliferating and proliferating latently infected CD4+ T-cells.
Resting CD4+ T-cells labelled with eFlour670 were cultured for 24 hours with mDC and infected with an EGFP reporter virus. A. Productive infection was measured at day 5 (n=7). At day 5 post-infection, cultures were sorted into non-productively infected, non-proliferating and proliferating CD4+ T-cells. B. Sorted non-proliferating and proliferating T-cells were stimulated using anti-CD3/CD28+integrase inhibitor for 72 hours, to measure latent infection (n=7), and stained with antibodies for C. weak SEB TCR Vβ-8.1 interactions (n=4) and D. strong SEB TCR Vβ-17 interaction (n=7). Columns represent the median in total (clear) or EGFP expressing T-cells (grey) and symbols represent results for individual donors. Significance was measured by paired students T-test where n<5 or Wilcoxon signed-rank test where n≥5, *p≤0.05, **p≤0.005, ***p≤0.0005.
Together these data show that HIV productive infection is associated with the Vβ alleles that favor SEB mediated strong mDC/monocyte-T-cell interactions, that also includes non-proliferating cells. More importantly, the finding of Vβ-17 enrichment in the latently infected non-proliferating T-cells suggests that latency was established in cells that had undergone TCR interactions with mDC. Proliferation (and productive infection) may have then been suppressed by immune checkpoints such as Tim-3 and PD-1. Demonstration of this would require inhibition with blocking antibodies to Tim-3 and PD-1.
Discussion
The relative contribution of latently infected non-proliferating and proliferating cells in HIV-infected individuals on cART remains unclear. However, virus can clearly persist in both activated and resting CD4+ T-cells in vivo (1, 5, 35, 36). Here, we used a novel in vitro model to assess latent infection in non-proliferating and proliferating CD4+ T-cells simultaneously. We demonstrated that following coculture with mDC or monocytes, and in the presence of SEB, inducible latent infection was detected in both non-proliferating and proliferating CD4+ T-cells. The decline in latent infection during prolonged culture indicates that only a subset of the infected, proliferating cells were long-lived in vitro. These proliferating T-cells expressed multiple markers of activation, including CD69, CD25, and Ki67; immune checkpoint markers Tim-3 and PD-1; and were predominantly memory phenotype. The enrichment of latency in non-proliferating cells indicate a strong interaction with SEB via Vβ-17, suggesting that TCR-MHC interactions are also important for induction of latency in non-proliferating T-cells. Together these data provide further evidence that proliferating CD4+ T-cells can harbor latent infection and demonstrates a key role of TCR interactions with APCs in the establishment of latent infection in both non-proliferating and proliferating T-cells.
In this study we have demonstrated latent infection, defined as integrated HIV provirus in the absence of productive infection in both non-proliferating and proliferating CD4+ T-cells. We confirmed these findings, by; [1] induction of viral expression (EGFP) following T-cell stimulation and [2] extended culture of latently infected cells, to determine maintenance of latency. Previous work using an in vitro, activated T-cell model of latency showed that latent infection was established 2 days after T-cell activation and persisted until day 9 post-infection (22). Others have shown that activated, infected cells can revert to a resting state while maintaining the integrated virus (15–17, 37). Together, we have shown that proliferating cells can harbour latent infection, during reversion to a resting state.
In general, activated, productively infected CD4+ T-cells have the cellular machinery to produce infectious virus however, we have shown that latent infection can also be maintained in these cells. Activation of intracellular signaling pathways by TCR activation, as well as interaction of co-stimulatory molecules and adhesion molecules facilitate viral entry (38–42), integration (43), and should facilitate virus replication (44). We have previously hypothesized that latent infection in non-proliferating cells is established by activation of signaling pathways that facilitate nuclear entry and integration, but in the absence of appropriate transcription factors productive infection does not occur (13, 18). In contrast, the pathways that could be maintaining latent infection in activated and/or proliferating T-cells may be those that actively lead to the suppression of T-cell activation, such as immune check point markers, including PD-1, CTLA-4 and Tim-3. Alternatively, there may be transcriptional blocks that prevent the expression of multiply spliced RNAs and viral proteins, hence preventing the expression of small viral RNAs like Tat and Rev, but not protein or virion production. Testing this hypothesis is difficult, as the tools to accurately detect intracellular Tat and Rev which drive the transcriptional machinery to complete viral production are unavailable. Using mass spectrometry Kuo et al. has shown that BRIC5, an apoptotic inhibitor is over expressed in HIV infected cells (45). Together, these data demonstrate a need for further understanding the mechanisms that restrict virus transcription in proliferating T-cells, in addition to non-proliferating cells.
Absence of viral transcription and protein expression in latently infected, proliferating CD4+ T-cells could be mediated by negative regulation of T-cell activation via immune checkpoint markers. We hypothesize that immune checkpoint markers are involved in blocking T-cell activation and virus expression in two ways. First, signaling by immune checkpoint markers leads to disruption of intracellular signaling cascades, for example CTLA-4-CD28 or PD-1-PD-L1 interaction, thereby blocking T-cell activation and early events in the viral life cycle (46–48). These processes may also be involved in establishment of latent infection in non-proliferating CD4+ T-cells. Second, immune checkpoint markers may block transcriptional activity, thereby blocking virus transcription and production of de novo virions, as shown following binding of CTLA-4 to CD28 (Reviewed in 49). This mechanism may contribute to the establishment of latent infection in proliferating T-cells, that is, the cell is activated yet the virus remains latent. Previously, others have also shown that PD-1 is upregulated on cells that have latent infection in vitro (50) and in CD4+ T-cells from HIV-1-infected patients on cART ex vivo (5). Furthermore, in a case study of an HIV-infected individual on cART who received anti-CTLA-4 (Ipilimumab 3mg/kg), we showed an increase in total peripheral T-cells, as well as an increased frequency of effector memory T-cells, and a significant increase in cell associated HIV RNA (51). An increase in cell associated HIV RNA was also demonstrated following administration of anti-PD1 (pembrolizumab) to this individual (52). Our group has further shown the importance of PD-1 and other immune checkpoint including Tim-3 and CTLA-4 in the establishment and maintenance of HIV latency (van der Sluis, personal communication). Together, these data support a potential role for immune checkpoint markers in the regulation of T-cell activation and latency in vivo and in our in vitro APC-T-cell model. However, further work is needed, with specific inhibition of these immune checkpoint markers prior to and following the establishment of latency to determine their contribution.
In this study, we generated latency in proliferating (eFluor670loEGFP−), CD4+ T-cells following mDC and monocyte co-culture. However, the majority of these cells were generally short-lived, as with prolonged in vitro culture we observed a significant decline in latency. The decrease in latently infected proliferating T-cells could be multifactorial, including: [1] progression of some latently infected, proliferating CD4+ T-cells to virus expression, thus moving to the productively infected (EGFP+) population; or [2] cell death due to proliferation and T-cell activation. Proliferating cells are activated, and therefore possess all factors necessary to produce de novo virions (53, 54). However, in vitro we observed a decline in latent infection and expected to see an increase in productive infection. We showed that there was some de novo expression of virus (EGFP expression) in the absence of additional stimulation throughout the extended culture period. Therefore it is possible that some cells that were initially EGFP− progressed to productive infection, and therefore were not latently infected. Alternatively, it is possible that upon sorting non-proliferating and proliferating T-cells from APC cocultures, the factors that maintained latent infection were removed. For example, ligation of immune checkpoint markers, which may have blocked expression of virus would no longer be active, thereby facilitating virus expression. We propose that the mDC may have provided proliferating CD4+ T-cells with signals to suppress viral expression, thereby promoting the establishment and maintenance of post-activation latency. To test this we added monocytes back to latently infected cells to measure the extent of virus suppression (55) and found that monocytes suppress the expression of latent virus, both alone and in combination of other activation stimuli including CD3/CD28 stimulation and PHA/PMA.
Productively infected CD4+ T-cells usually die due to cytopathic effects of the virus (56–59), detection by the host immune response (60–62), or bystander effects of infected cells and activation (63, 64). The population of proliferating CD4+ T-cells that contained latent infection may evade cytopathic effects due to the lack of viral protein expression and/or the expression of immune checkpoint markers, such as PD-1 and Tim-3. Immune checkpoint marker expression may mediate down regulation of T-cell activation by mDC and other APC, thereby blocking or dampening T-cell activation and the downstream cytopathic effects (47). Tim-3 is expressed on CD4+ T-cells in HIV-infected individuals on cART, however whether it is responsible for down-regulation of T-cell activation in vivo remains unclear (65–68). We only found a relationship between the expression of the immune checkpoint marker PD-1 and the frequency of latency in non-proliferating cells cocultured with mDC. This may be because we only measured immune checkpoints at two time points, and not at the times when latent infection was actually induced, or that there was a combination of immune checkpoints activated that we have not measured here.
There were some limitations in this study. This is an in vitro model, however given latency is established within days to weeks of infection in vivo, it is impossible to examine the establishment of latent infection in the absence of in vitro models (69, 70). Additionally, we measured latent infection by quantification of EGFP expression following a TCR-mediated activation stimulus. It is possible that some viruses were not activated with this stimulus and therefore, we may have under-estimated the frequency of latent infection in this coculture system. Non-inducible, intact proviruses have been described in CD4+ T-cells from HIV-1-infected patients on cART (26, 27). To fully determine whether there were intact, non-induced proviruses in this model, sequencing would be required to quantify intact integrated virus in the non-proliferating and proliferating CD4+ T-cells.
In conclusion, we have demonstrated that HIV latency can be established simultaneously in both non-proliferating and proliferating CD4+ T-cells, during in vitro coculture with mDC or monocytes, with greater frequency of latent infection in proliferating T-cells compared to non-proliferating T-cells. Together these results suggest that latent infection may not be directly linked to the proliferation and activation state of CD4+ T-cells. Instead, it may be dependent on a combination of interactions with APC, such as mDC or monocytes, which facilitate interactions through MHC and TCR, and immune checkpoint markers. The contribution of latently infected proliferating CD4+ T-cells to viral persistence on cART requires further investigation and may require targeted therapeutic strategies.
Supplementary Material
Acknowledgments
We thank Damian Purcell (University of Melbourne, Parkville, Australia) and Yasuko Tsunetsugu-Yokota (National Institute of Infectious Diseases, Tokyo, Japan) for providing us with the EGFP-reporter viruses. We thank J. Le Masurier, M. Thomson, P. Donaldson and G. Paukovics (Alfred Medical Research and Educational Precinct (AMREP) Flow Cytometry Core Facility, Melbourne, Australia), and Tina Luke, Angela Hind, Catherine Li and Daniel Blashki (University of Melbourne Flow Cytometry Facility) for flow cytometric cell sorting. We thank Ashish Nair and Jared Stern for assistance in preparation of PBMC.
Funding
NK was the recipient of the Australian Postgraduate Award (APA; fund # Q05201 6609004). S.R.L is an NHMRC practitioner fellow. This work was supported by NHMRC project grant 1041795 and National Institutes of Health Delany AIDS Research Enterprise (DARE) to find a cure Collaboratory (U19 AI096109 and U19 AI126611) and the American Foundation for AIDS Research.
Footnotes
Conflict of interest
Authors have no conflict of interest to declare.
Contributor Information
Nitasha A. Kumar, Email: asmita07@gmail.com.
Renee M. van der Sluis, Email: renee.van@unimelb.edu.au.
Talia Mota, Email: Taliamm13@gmail.com.
Rachel Pascoe, Email: Rachel.pascoe@unimelb.edu.au.
Vanessa A. Evans, Email: vanessa.evans@unimelb.edu.au.
Sharon R. Lewin, Email: sharon.lewin@unimelb.edu.au.
Paul U. Cameron, Email: paul.cameron@unimelb.edu.au.
Bibliography
- 1.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–90. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 2.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 3.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–8. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzon MJ, Martin-Gayo E, Pereyra F, Ouyang Z, Sun H, Li JZ, Piovoso M, Shaw A, Dalmau J, Zangger N, Martinez-Picado J, Zurakowski R, Yu XG, Telenti A, Walker BD, Rosenberg ES, Lichterfeld M. Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. J Virol. 2014;88:10056–10065. doi: 10.1128/JVI.01046-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartwright EK, McGary CS, Cervasi B, Micci L, Lawson B, Elliott STC, Collman RG, Bosinger SE, Paiardini M, Vanderford TH, Chahroudi A, Silvestri G. Divergent CD4+ T memory stem cell dynamics in pathogenic and nonpathogenic simian immunodeficiency virus infections. J Immunol. Baltim. Md 1950. 2014;192:4666–4673. doi: 10.4049/jimmunol.1303193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaafoura S, de Goër de Herve MG, Hernandez-Vargas EA, Hendel-Chavez H, Abdoh M, Mateo MC, Krzysiek R, Merad M, Seng R, Tardieu M, Delfraissy JF, Goujard C, Taoufik Y. Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4+ memory T Cells. Nat. Commun. 2014;5:5407. doi: 10.1038/ncomms6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, Coffin JM, Hughes SH. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345:179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner TA, McLaughlin S, Garg K, Cheung CYK, Larsen BB, Styrchak S, Huang HC, Edlefsen PT, Mullins JI, Frenkel LM. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345:570–573. doi: 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn LB, Silva IT, Oliveira TY, Rosales RA, Parrish EH, Learn GH, Hahn BH, Czartoski JL, McElrath MJ, Lehmann C, Klein F, Caskey M, Walker BD, Siliciano JD, Siliciano RF, Jankovic M, Nussenzweig MC. HIV-1 integration landscape during latent and active infection. Cell. 2015;160:420–432. doi: 10.1016/j.cell.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood. 2007;110:4161–4. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- 13.Cameron PU, Saleh S, Sallmann G, Solomon A, Wightman F, Evans VA, Boucher G, Haddad EK, Sekaly RP, Harman AN, Anderson JL, Jones KL, Mak J, Cunningham AL, Jaworowski A, Lewin SR. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc Natl Acad Sci U A. 2010;107:16934–9. doi: 10.1073/pnas.1002894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahu GK, Lee K, Ji J, Braciale V, Baron S, Cloyd MW. A novel in vitro system to generate and study latently HIV-infected long-lived normal CD4+ T-lymphocytes. Virology. 2006;355:127–137. doi: 10.1016/j.virol.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Tyagi M, Karn J. CBF-1 promotes transcriptional silencing during the establishment of HIV-1 latency. EMBO J. 2007;26:4985–4995. doi: 10.1038/sj.emboj.7601928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marini A, Harper JM, Romerio F. An in vitro system to model the establishment and reactivation of HIV-1 latency. J Immunol. 2008;181:7713–20. doi: 10.4049/jimmunol.181.11.7713. [DOI] [PubMed] [Google Scholar]
- 17.Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113:58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans VA, Kumar N, Filali A, Procopio FA, Yegorov O, Goulet J-P, Saleh S, Haddad EK, da Fonseca Pereira C, Ellenberg PC, Sekaly R-P, Cameron PU, Lewin SR. Myeloid Dendritic Cells Induce HIV-1 Latency in Non-proliferating CD4+ T Cells. PLoS Pathog. 2013;9:e1003799–1003813. doi: 10.1371/journal.ppat.1003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar NA, Cheong K, Powell DR, da Fonseca Pereira C, Anderson J, Evans VA, Lewin SR, Cameron PU. The role of antigen presenting cells in the induction of HIV-1 latency in resting CD4(+) T-cells. Retrovirology. 2015;12:76. doi: 10.1186/s12977-015-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pramanik S, Cui X, Wang H-Y, Chimge N-O, Hu G, Shen L, Gao R, Li H. Segmental duplication as one of the driving forces underlying the diversity of the human immunoglobulin heavy chain variable gene region. BMC Genomics. 2011;12:78. doi: 10.1186/1471-2164-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swiggard WJ, Baytop C, Yu JJ, Dai J, Li C, Schretzenmair R, Theodosopoulos T, O’Doherty U. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J Virol. 2005;79:14179–88. doi: 10.1128/JVI.79.22.14179-14188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Sluis RM, van Montfort T, Pollakis G, Sanders RW, Speijer D, Berkhout B, Jeeninga RE. Dendritic cell-induced activation of latent HIV-1 provirus in actively proliferating primary T lymphocytes. PLoS Pathog. 2013;9:e1003259. doi: 10.1371/journal.ppat.1003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahabieh MS, Ooms M, Brumme C, Taylor J, Harrigan PR, Simon V, Sadowski I. Direct non-productive HIV-1 infection in a T-cell line is driven by cellular activation state and NFkappaB. Retrovirology. 2014;11:17. doi: 10.1186/1742-4690-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzi JCC, Cohen YZ, Cohn LB, Kreider EF, Barton JP, Learn GH, Oliveira T, Lavine CL, Horwitz JA, Settler A, Jankovic M, Seaman MS, Chakraborty AK, Hahn BH, Caskey M, Nussenzweig MC. Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E7908–E7916. doi: 10.1073/pnas.1617789113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bui JK, Halvas EK, Fyne E, Sobolewski MD, Koontz D, Shao W, Luke B, Hong FF, Kearney MF, Mellors JW. Ex vivo activation of CD4+ T-cells from donors on suppressive ART can lead to sustained production of infectious HIV-1 from a subset of infected cells. PLoS Pathog. 2017;13:e1006230. doi: 10.1371/journal.ppat.1006230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosmane NN, Kwon KJ, Bruner KM, Capoferri AA, Beg S, Rosenbloom DIS, Keele BF, Ho Y-C, Siliciano JD, Siliciano RF. Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J Exp. Med. 2017;214:959–972. doi: 10.1084/jem.20170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho Y-C, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DIS, Lai J, Blankson JN, Siliciano JD, Siliciano RF. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bui JK, Sobolewski MD, Keele BF, Spindler J, Musick A, Wiegand A, Luke BT, Shao W, Hughes SH, Coffin JM, Kearney MF, Mellors JW. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog. 2017;13:e1006283. doi: 10.1371/journal.ppat.1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed LJ, Muench H. A Simple Method of Estimating Fifty Per Cent Endpoints. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- 30.Bhardwaj N, Friedman SM, Cole BC, Nisanian AJ. Dendritic cells are potent antigen-presenting cells for microbial superantigens. J Exp Med. 1992;175:267–73. doi: 10.1084/jem.175.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewin SR, Vesanen M, Kostrikis L, Hurley A, Duran M, Zhang L, Ho DD, Markowitz M. Use of Real-Time PCR and Molecular Beacons To Detect Virus Replication in Human Immunodeficiency Virus Type 1-Infected Individuals on Prolonged Effective Antiretroviral Therapy. J Virol. 1999;73:6099–6103. doi: 10.1128/jvi.73.7.6099-6103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat. Immunol. 2011;12:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhardwaj N, Young JW, Nisanian AJ, Baggers J, Steinman RM. Small amounts of superantigen, when presented on dendritic cells, are sufficient to initiate T cell responses. J Exp. Med. 1993;178:633–642. doi: 10.1084/jem.178.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayball JD, Robinson JH, O’Hehir RE, Verhoef A, Lamb JR, Lake RA. Identification of two binding sites in staphylococcal enterotoxin B that confer specificity for TCR V beta gene products. Int. Immunol. 1994;6:199–211. doi: 10.1093/intimm/6.2.199. [DOI] [PubMed] [Google Scholar]
- 35.Murray JM, Zaunders JJ, McBride KL, Xu Y, Bailey M, Suzuki K, Cooper DA, Emery S, Kelleher AD, Koelsch KK, PINT Study Team HIV DNA subspecies persist in both activated and resting memory CD4+ T cells during antiretroviral therapy. J Virol. 2014;88:3516–3526. doi: 10.1128/JVI.03331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hey-Cunningham WJ, Murray JM, Natarajan V, Amin J, Moore CL, Emery S, Cooper DA, Zaunders J, Kelleher AD, Koelsch KK, PINT study team Early antiretroviral therapy with raltegravir generates sustained reductions in HIV reservoirs but not lower T-cell activation levels. AIDS Lond. Engl. 2015;29:911–919. doi: 10.1097/QAD.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 37.Tyagi M, Pearson RJ, Karn J. Establishment of HIV Latency in Primary CD4+ Cells Is due to Epigenetic Transcriptional Silencing and P-TEFb Restriction. J Virol. 2010;84:6425–6437. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delon J, Bercovici N, Raposo G, Liblau R, Trautmann A. Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J Exp. Med. 1998;188:1473–1484. doi: 10.1084/jem.188.8.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popik W, Hesselgesser JE, Pitha PM. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J Virol. 1998;72:6406–6413. doi: 10.1128/jvi.72.8.6406-6413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Revy P, Sospedra M, Barbour B, Trautmann A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat Immunol. 2001;2:925–31. doi: 10.1038/ni713. [DOI] [PubMed] [Google Scholar]
- 41.Balabanian K, Harriague J, Décrion C, Lagane B, Shorte S, Baleux F, Virelizier J-L, Arenzana-Seisdedos F, Chakrabarti LA. CXCR4-tropic HIV-1 envelope glycoprotein functions as a viral chemokine in unstimulated primary CD4+ T lymphocytes. J Immunol. Baltim. Md 1950. 2004;173:7150–7160. doi: 10.4049/jimmunol.173.12.7150. [DOI] [PubMed] [Google Scholar]
- 42.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 43.Manganaro L, Lusic M, Gutierrez MI, Cereseto A, Del Sal G, Giacca M. Concerted action of cellular JNK and Pin1 restricts HIV-1 genome integration to activated CD4+ T lymphocytes. Nat. Med. 2010;16:329–333. doi: 10.1038/nm.2102. [DOI] [PubMed] [Google Scholar]
- 44.Zack JA, Kim SG, Vatakis DN. HIV restriction in quiescent CD4+ T cells. Retrovirology. 2013;10:37. doi: 10.1186/1742-4690-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo H-H, Ahmad R, Lee GQ, Gao C, Chen H-R, Ouyang Z, Szucs MJ, Kim D, Tsibris A, Chun T-W, Battivelli E, Verdin E, Rosenberg ES, Carr SA, Yu XG, Lichterfeld M. Anti-apoptotic Protein BIRC5 Maintains Survival of HIV-1-Infected CD4+ T Cells. Immunity. 2018;48:1183–1194. e5. doi: 10.1016/j.immuni.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. Baltim. Md 1950. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 47.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp. Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsson M, Shankar EM, Che KF, Saeidi A, Ellegård R, Barathan M, Velu V, Kamarulzaman A. Molecular signatures of T-cell inhibition in HIV-1 infection. Retrovirology. 2013;10:31. doi: 10.1186/1742-4690-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iglesias-Ussel M, Vandergeeten C, Marchionni L, Chomont N, Romerio F. High levels of CD2 expression identify HIV-1 latently infected resting memory CD4+ T cells in virally suppressed subjects. J Virol. 2013;87:9148–9158. doi: 10.1128/JVI.01297-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wightman F, Solomon A, Kumar SS, Urriola N, Gallagher K, Hiener B, Palmer S, Mcneil C, Garsia R, Lewin SR. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS Lond. Engl. 2015;29:504–506. doi: 10.1097/QAD.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans VA, Van Der Sluis RM, Solomon A, Dantanarayana A, McNeil C, Garsia R, Palmer S, Fromentin R, Chomont N, Sékaly R-P, Cameron PU, Lewin SR. PD-1 contributes to the establishment and maintenance of HIV-1 latency. AIDS Lond. Engl. 2018 doi: 10.1097/QAD.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 54.Brooks DG, Arlen PA, Gao L, Kitchen CMR, Zack JA. Identification of T cell-signaling pathways that stimulate latent HIV in primary cells. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12955–12960. doi: 10.1073/pnas.2233345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rezaei SD, Lu HK, Chang JJ, Rhodes A, Lewin SR, Cameron PU. The pathway to establishing HIV latency is critical to how latency is maintained and reversed. J Virol. 2018 doi: 10.1128/JVI.02225-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakai K, Dimas J, Lenardo MJ. The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3369–3374. doi: 10.1073/pnas.0509417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arokium H, Kamata M, Chen I. Virion-associated Vpr of human immunodeficiency virus type 1 triggers activation of apoptotic events and enhances fas-induced apoptosis in human T cells. J Virol. 2009;83:11283–11297. doi: 10.1128/JVI.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, Mavilio D, Planelles V, Barker E. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog. 2009;5:e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao RY, Li G, Bukrinsky MI. Vpr-host interactions during HIV-1 viral life cycle. J Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2011;6:216–229. doi: 10.1007/s11481-011-9261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hazenberg MD, Stuart JWTC, Otto SA, Borleffs JCC, Boucher CAB, de Boer RJ, Miedema F, Hamann D. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 61.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol. Rev. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doitsh G, Galloway NLK, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Muñoz-Arias I, Greene WC. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lelièvre JD, Mammano F, Arnoult D, Petit F, Grodet A, Estaquier J, Ameisen JC. A novel mechanism for HIV1-mediated bystander CD4+ T-cell death: neighboring dying cells drive the capacity of HIV1 to kill noncycling primary CD4+ T cells. Cell Death Differ. 2004;11:1017–1027. doi: 10.1038/sj.cdd.4401441. [DOI] [PubMed] [Google Scholar]
- 64.Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC. IFI16 DNA Sensor Is Required for Death of Lymphoid CD4 T Cells Abortively Infected with HIV. Science. 2014;343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kassu A, Marcus RA, D’Souza MB, Kelly-McKnight EA, Golden-Mason L, Akkina R, Fontenot AP, Wilson CC, Palmer BE. Regulation of virus-specific CD4+ T cell function by multiple costimulatory receptors during chronic HIV infection. J Immunol. Baltim. Md 1950. 2010;185:3007–3018. doi: 10.4049/jimmunol.1000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shankar EM, Che KF, Messmer D, Lifson JD, Larsson M. Expression of a broad array of negative costimulatory molecules and Blimp-1 in T cells following priming by HIV-1 pulsed dendritic cells. Mol. Med. Camb. Mass. 2011;17:229–240. doi: 10.2119/molmed.2010.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Che KF, Shankar EM, Muthu S, Zandi S, Sigvardsson M, Hinkula J, Messmer D, Larsson M. P38MAPK/STAT3 Pathway Signaling Regulates Expression of Inhibitory Molecules in T-cells Activated by HIV-1 Exposed Dendritic Cells. Mol. Med. Camb. Mass. 2012 doi: 10.2119/molmed.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leitner J, Rieger A, Pickl WF, Zlabinger G, Grabmeier-Pfistershammer K, Steinberger P. TIM-3 Does Not Act as a Receptor for Galectin-9. PLoS Pathog. 2013;9:e1003253. doi: 10.1371/journal.ppat.1003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ananworanich J, Puthanakit T, Suntarattiwong P, Chokephaibulkit K, Kerr SJ, Fromentin R, Bakeman W, Intasan J, Mahanontharit A, Sirivichayakul S, Chomont N. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS Lond. Engl. 2013 doi: 10.1097/QAD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 70.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DIS, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014 doi: 10.1038/nature13594. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.