Abstract
The Resistin-Like Molecules (RELM) α, β, and γ and their namesake, resistin, share structural and sequence homology but exhibit significant diversity in expression and function within their mammalian host. RELM proteins are expressed in a wide range of diseases, such as: microbial infections (eg. bacterial and helminth), inflammatory diseases (eg. asthma, fibrosis) and metabolic disorders (eg. diabetes). While the expression pattern and molecular regulation of RELM proteins are well characterized, much controversy remains over their proposed functions, with evidence of host-protective and pathogenic roles. Moreover, the receptors for RELM proteins are unclear, although three receptors for resistin, decorin, adenylyl cyclase-associated protein 1 (CAP1), and Toll-like Receptor 4 (TLR4) have recently been proposed. In this review, we will first summarize the molecular regulation of the RELM gene family, including transcription regulation and tissue expression in humans and mouse disease models. Second, we will outline the function and receptor-mediated signaling associated with RELM proteins. Finally, we will discuss recent studies suggesting that, despite early misconceptions that these proteins are pathogenic, RELM proteins have a more nuanced and potentially beneficial role for the host in certain disease settings.
Keywords: Resistin-like molecule, macrophage, helminth infection, T helper type 2, Toll-like Receptor 4
1. Introduction
Resistin-like molecules (RELMs) are mammalian secreted proteins, which were identified less than 20 years ago in different disease settings, leading to differing nomenclature [1–6]. RELMα (Retnla) was the first RELM protein discovered in a mouse model of asthma, where it was named FIZZ1 for Found in Inflammatory Zone. Murine resistin (Retn/FIZZ3) was subsequently identified and functionally characterized in metabolic dysfunction, where it caused “resistance” to insulin, leading to the more common nomenclature for this protein family as ‘RELMs’. Finally, RELMα was also investigated in hypoxia and named Hypoxia-Induced Mitogenic Factor (HIMF) [1]. The complex nomenclature demonstrates significant diversity in RELM expression pattern and function, however, it may cause confusion and potential bias when searching for studies on this intriguing family of proteins. Here, we provide a comprehensive summary of the RELM/FIZZ/HIMF protein family, from their discovery to more recent studies elucidating their function and putative receptors.
We will focus on the three main research areas in which RELM proteins were discovered, which include microbial infection, inflammatory diseases and metabolic dysfunction. In addition, we will highlight the existing controversies over the pathogenic versus protective function for these proteins. While early studies proposed detrimental roles for RELM proteins due to their abundant expression in pathologic settings, more recent studies suggest that these proteins can provide beneficial functions from improving metabolic homeostasis to reducing inflammation and promoting wound healing. Here, we revisit the literature on RELM proteins to (i) consolidate what is known regarding RELM genetic regulation and signaling; and (ii) delineate the function of each RELM in various disease states. We hope to highlight the versatility of these proteins and the significant role they play in host physiology. Only by fully understanding RELMs in their respective roles within their host, can we make informed decisions on the possibility of targeting these molecules and downstream pathways for new therapies in infection, inflammation and metabolic dysfunction.
2. Molecular Regulation of RELM Genes
2.1. RELM Gene and Protein Structure
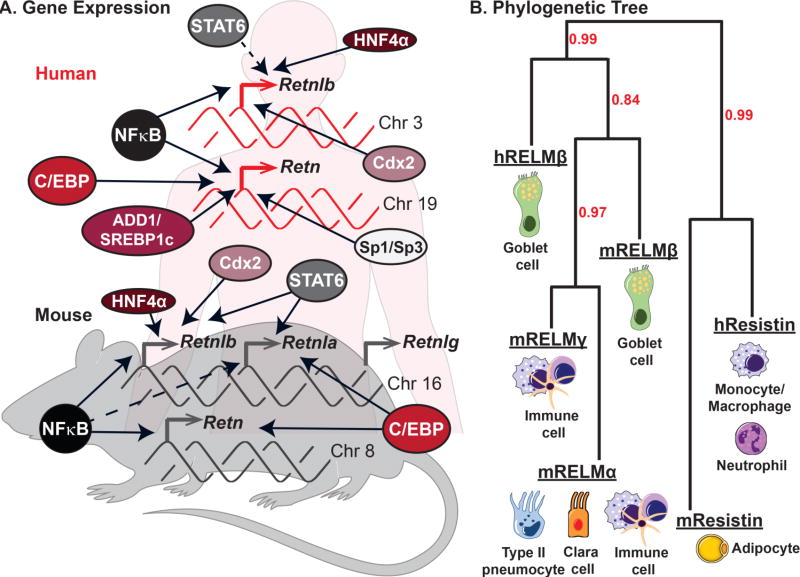
The RELM gene family (Retn) was originally identified in mice, but appears to be present in all mammals [2]. While mice and rats have four RELM genes Retn, Retnla, Retnlb, Retnlg; only Retn and Retnlb belong to a diverse taxonomic group, including humans, nonhuman primates, canines, cats and horses. In mice and rats, three of the four RELM genes, Retnlb, Retlna and Retnlg, are clustered together on chromosome 16 [3]. These genes share the most sequence homology and exhibit similar transcriptional regulation, but are differentially expressed in cell-types and tissue. In comparison, mouse Retn, human Retn, human Retnlb exhibit greater diversity in transcriptional regulation and expression pattern, and are present on different chromosomes (chromosomes 8, 19 and 3 respectively). Sequence identity is high between human and mouse RELM proteins, with ~ 60% homology in amino acid sequence [4, 5]. Figure 1 summarizes the gene expression profile and transcriptional regulation of mouse and human RELM genes.
Figure 1. RELM expression in mouse and man.
A) Genetic regulation and chomosome location of the human and murine RELM genes. Dashed arrows represent putative transcriptional regulation, while solid arrows represent molecularly confirmed transcription factors. B) Phylogenetic tree illustrating the relatedness of mouse and human RELMs was generated using http://www.phylogeny.fr software. Bootstrap values are indicated in red. The primary cell types that express each RELM are presented.
RELM genes encode secreted proteins of 105–138 amino acids in size with 3 main domains: an amino (N) terminal signal sequence, a variable middle section, and a conserved carboxyl (C) terminal. The C terminal is comprised of a cysteine signature motif sequence shared by all RELM family members (C-X11-C-X8-C-X-C-X3-C-X10-C-X-C-X-C-X9-CC-X3–6-END), which is proposed to be critical for disulfide bond formation and protein folding [3, 6, 7]. The crystal structures of mouse resistin and RELMβ have been solved, revealing that they form trimers linked together via disulfide bonds to form hexameric assemblies [8]. Dimerization of RELMβ and resistin was dependent on a cysteine in the N-terminal. This cysteine is lacking in RELMα and RELMγ, suggesting that they may exist as monomers [9, 10], however their crystal structure has not been solved. A better understanding of the RELM protein structure may provide important information for identification of the receptors, which remain unknown for many of the RELM proteins.
2.2 Genetic regulation of RELM expression
The genetic regulation and expression profile of the RELM genes have been well characterized from several human and murine studies (Figure 1A). These studies reveal both shared and distinct cellular expression profiles within the RELM gene family (Figure 1B). While some RELM genes, such as RELMα, RELMγ and human resistin, are expressed by hematopoietic cells, mouse resistin, RELMα and RELMβ are expressed in non-hematopoietic cells. All mouse and human RELM proteins are detectable in the serum, offering the potential to utilize RELM levels as biomarkers [11–13]. In this section, we summarize what is known about the cellular expression of RELM genes, the disease settings in which they are expressed, and how they are transcriptionally regulated.
2.2.1. RELMα
RELMα/Retnla exhibits the greatest heterogeneity in expression within the RELM family. Under homeostatic conditions, Retnla mRNA is present at low levels in the lung, tongue, mammary tissue, and white adipose tissue [6]. Originally discovered as a secreted protein in the bronchio-alveolar lavage of ovalbumin-challenged mice, the consensus from multiple studies using mouse asthma models is that RELMα is highly expressed by airway epithelial cells and type 2 pneumocytes [1, 14, 15]. Consistent with this, RELMα transcription is driven and critically dependent on a T helper type 2 (Th2) cytokine environment. Indeed, binding sites for the Th2 cytokine-induced transcription factor STAT6 are present within the Retnla promoter, and STAT6−/− or IL-4−/− mice exhibit reduced RELMα expression [16, 17]. In addition to expression by non-hematopoeitc cells, RELMα is also recognized as a key signature gene of M2/alternatively activated macrophages that differentiate in chronic, Th2 cytokine-skewed conditions such as helminth or chronic protozoan parasite infection [17–20]. RELMα expression by other immune cells, eosinophils and dendritic cells, has also been reported [18, 21]. RELMα is also expressed in lung and peritoneal injury models, and following hypoxic stress [1, 22, 23] In these models, RELMα expression may rely on other transcription factors, such as CCAAT/enhancer-binding protein (C/EBP), which binds to its’ specific motif adjacent to the STAT6 binding site in the Retnla promoter [16]. While RELMα induction can occur in the absence of Th2 cytokine signaling, likely through C/EBP, sustained RELMα expression requires Th2 cytokine stimulation [23]. Interestingly, functional transcription studies revealed that C/EBP binding to the Retnla promoter was necessary for IL-4/STAT6-induced Retnla expression, suggesting that the STAT6 and C/EBP work in tandem to activate the Retnla gene [16]. There are also putative binding sites for Ets family proteins and PPAR, upstream and downstream of the STAT6 binding site respectively [16]. Furthermore, transcription factor binding motifs for NF-κB, GAS, and C/EBP are present throughout the Retnla gene, both in the 5’ and 3’ flanking regions and in introns [1].
2.2.2. RELMβ
The RELMβ/Retnlb expression pattern in mouse and human studies suggests predominant expression in the secretory granules of intestinal goblet cells, however, expression by lung epithelial cells in asthmatic patients and mouse models of lung fibrosis is also observed [24, 25]. Similar to its adjacent gene Retnla, the Retnlb promoter contains STAT6 binding sites and expression is highly induced by Th2 cytokines in vivo in the lung and intestine, and in mouse and intestinal epithelial cell lines [9, 26, 27]. In addition to regulation by Th2 cytokines, Retnlb is also expressed in several intestinal colitis models driven by Th1/Th17 cytokines [28, 29]. Additionally, bacterial colonization or lipopolysaccharide (LPS) alone can induce RELMβ expression through the transcription factor Cdx2 [9, 30]. Expression in these inflammatory settings is likely through NFκB activation, given the predicted NFκB binding sites within the Retnlb promoter. Both the human and mouse Retnlb promoters also contain functional binding sites for the hepatocyte nuclear factor 4α (HNF4α), a transcription factor expressed in the liver, kidney, and intestine. Indeed, in a mouse colitis model, overexpression of the HNF4α P2 isoform promoted RELMβ expression and inflammation [31]. In addition to recent studies highlighting the functional significance of HNF4α in intestinal inflammation and cancer, HNF4α is a master regulator of liver function and metabolism [32, 33]. Future studies understanding whether RELMβ is a downstream effector of HNF4α may reveal new pathologic pathways in intestinal and metabolic disease.
2.2.3. Resistin
The Resistin/Retn gene exhibits an intriguing dichotomy in expression pattern between mouse and man, with expression of mouse Retn almost exclusively by murine adipocytes, leading to its alternative name ‘ADSF’ for adipocyte secreted factor [34]. In contrast, human Retn is expressed by immune cells, specifically macrophages, monocytes and neutrophils [13]. The mouse Retn gene is considerably larger than the human Retn gene and includes a 2279 bp long intron (intron X), with putative transcription factor binding sites: AP1, NF-κB, IRF1, IRF2, HNF3, C/EBP, and a PPAR/RXR heterodimer binding sited called IntX-PPRE [5]. The PPAR/RXR-like protein factors, which are found in differentiated adipocytes, can bind to the PPRE sequence within the mouse Retn gene [5]. This suggests that intron X could be responsible for the adipocyte tissue specific expression of Retn in mice but not in humans, which lack intron X.
The disparity in introns within the mouse and human Retn genes could explain why their expression is regulated differently. For example, TNFα decreased Retn expression in mouse adipocytes but increased Retn expression human monocytes [35, 36]. In contrast, high glucose upregulated both mouse and human Retn gene expression, while insulin treatment suppressed Retn expression in murine adipocytes and human monocyte cell lines, respectively [35, 37]. The high glucose effect was abrogated upon inhibition of MAPKs and NFκB, suggesting their roles in regulating Retn expression [37]. Further, similar to the mouse locus, the human Retn gene contains adipogenic transcription factor C/EBPα binding sites [38, 39]. Additionally, the human Retn promoter has a putative binding site for adipocyte determination and differentiation-dependent factor 1 (ADD1)/sterol regulatory element binding protein 1c (SREBP1c) [39]. In mice, binding of C/EBPα to the Retn promoter was associated with recruitment of coactivators CREB-binding protein and p300 and abundant acetylation of histones. In this setting, PPARγ ligands were able to inhibit mouse Retn expression, likely through inhibition of C/EBPα associated acetylation [38].
Single nucleotide polymorphisms of the human Retn gene have been investigated and are correlated with Retn gene expression associated with metabolic diseases such as obesity and diabetes. In a Japanese population, SNP −638 G>A and −420 C>G resulted in an increase in serum resistin levels [40, 41]. This result was partially reproduced in a Korean population that associated SNP −420C>G and the −537A>C with significantly higher plasma resistin levels [42]. At the −420 SNP, stimulatory proteins (Sp) 1 and 3 preferentially bound the −420G over −420C, and could be involved in increasing Retn expression under hyperglycemic conditions [43]. Additionally, SNP Retn SNP −358 with AG or at least 1 A allele is linked to higher risk of lung cancer than wild-type (GG) carriers in a Chinese Han population [44]. The evidence that polymorphisms may regulate human Retn expression is adding to the growing interest in human resistin for its role in immune and metabolic dysregulation. Further analyses on larger and more diverse populations will add to the current knowledge on human resistin expression and function.
2.2.4. RELMγ
RELMγ/Retnlg is by far the least studied RELM protein. It is most highly expressed in the hematopoietic system, with high expression in the bone marrow, and lower expression in white blood cells, spleen and thymus [3, 7]. While RELMγ is also expressed in the lungs and the adipose tissue, its expression is significantly lower than RELMα [3]. RELMγ has been found to have a proliferative effect on HL60 cells, but did not have an effect on cellular differentiation [7]. The genetic regulation of Retnlg expression has not yet been thoroughly examined.
3. RELM Putative Receptors and Downstream Signaling
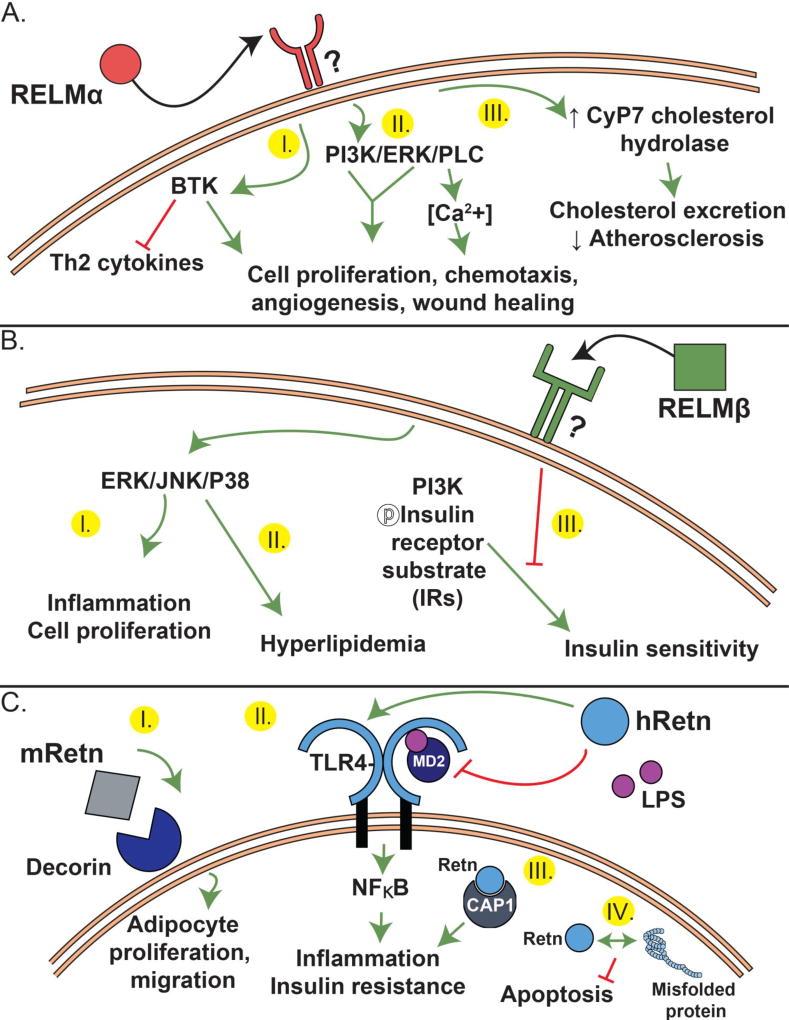
RELMs have been studied and implicated in diverse physiological functions. Surprisingly however, the RELM receptors and downstream signaling pathways are largely elusive. Of the four RELMs, resistin is the only member thus far with a confirmed receptor, whereas the rest of the members are associated with putative or unknown receptors. Figure 2 summarizes our current understanding of RELM protein signaling and function.
Figure 2.
Proposed receptor and signaling of RELMα (A), RELMβ (B) and Resistin (C).
3.1. RELMα
Although extensively studied, the search for a RELMα receptor still continues (Figure 2A). RELMα binding assays revealed that it selectively binds to CD4+ Th2 cells, dendritic cells and macrophages [45]. There is evidence that Bruton’s tyrosine kinase (BTK), an important signaling molecule in B cell maturation, is a binding partner for RELMα [45, 46] (Figure 2A, I). Immunofluorescent assays have shown that upon RELMα stimulation, BTK redistributes and anchors to the cell membrane where it then co-localizes with RELMα [46]. Given that BTK is an intracellular protein, the exact interaction between the secreted extracellular RELMα and intracellular BTK is unclear. Two distinct outcomes of RELMα binding associated with BTK signaling have been reported. First, RELMα induced BTK autophosphorylation stimulated myeloid cell chemotaxis. Second, in an in vitro CD4+ Th2 cell differentiation assay, RELMα downregulated Th2 cytokine production in a BTK-dependent manner [45, 46].
Several in vitro studies demonstrated a chemotactic and mitogenic function of RELMα, positing a function for RELMα in angiogenesis and tissue remodeling (Figure 2A, II). In human pulmonary artery smooth muscle cells, RELMα increased intracellular Ca2+ concentrations by activating inositol 1,4,5-triphosphate receptor (IP3R) in a phospholipase C dependent mechanism [47]. RELMα also stimulated proliferation of rat pulmonary microvascular smooth muscle cells via the PI3K/AKT signaling pathway, and induced expression of angiogenesis mediators: VEGF and MCP-1, and monocyte recruiting chemokine SDF-1x [1, 48]. In a lung fibrosis model, RELMα was implicated in myofibroblast differentiation during lung fibrosis, likely through activation of Notch1 and Jagged1 [49]. While fibroblast activation is detrimental in pulmonary or liver fibrosis, it is a required wound healing process following tissue injury. Recent evidence supports a critical function for RELMα in wound healing through fibroblast activation. Following skin excision wounds in mice, RELMα induced fibroblast expression of lysyl hydrolase 2, an enzyme that mediates collagen cross-linking for skin repair and tissue regeneration [50–52]. Finally, RELMα also acts as an adipokine to regulate metabolic homeostasis [53]. RELMα increased expression of cholesterol-7-α-hydroxylase (Cyp7a1) in hepatocytes by inducing the transcriptional activator liver receptor homologue-1 (LRH-1). This effect was beneficial in hyperlipidemic mice as it promoted excretion of cholesterol in the form of bile acids (Figure 2A, III).
3.2. RELMβ
Similar to RELMα, RELMβ promotes cell proliferation, but has no identified receptor. In human diabetic nephropathy mesangial cells, RELMβ induced phosphorylation of p38MAPK and JNK, and cell proliferation (Figure 2B, I) [54]. In contrast to RELMα, RELMβ contributed to metabolic dysfunction by signaling though MAPK pathways and suppressing insulin signaling in hepatocytes (Figure 2B, II–III). While RELMα binds host cells, murine RELMβ can bind the pore-like structures in the chemosensory apparatus of helminths Trichuris muris and Strongiloides stercoralis, where it inhibited helminth chemotaxis and feeding [55, 56]. Consisent with an anti-microbial function for RELMβ, a recent study showed that both mouse and human RELMβ, and human resistin, could bind gram negative bacteria and permeabilize their membranes [57]. Therefore, while the mechanism of action of RELMα is on the host, RELMβ may act both on the host and the pathogen. It is unclear whether the receptors for these proteins, spanning host and microbe, are similar, however, information from one organism may help guide identification of the receptors in others.
3.3. Resistin
In contrast to RELMα and RELMβ, mouse and human resistin receptors and downstream signaling are better characterized. In adipose stromal cells (ASC), mouse resistin bound to an isoform of decorin (DCN) with functional effects on cell proliferation and migration (Figure 2C, I) [58]. On the other hand, human resistin binds Toll-like Receptor 4 (TLR4), the innate receptor for LPS (Figure 2C, II). Human resistin signaling via TLR4 inhibited LPS binding and function and fatal endotoxic shock in a mouse sepsis model [59, 60]. This protective function of resistin can be attributed to a switch from pro-inflammatory (NFκB) to anti-inflammatory signaling pathways (TRIF/TBK-1 and STAT3) [57]. Since TLR4 signaling through Myd88/NFκB is distinct from JAK-STAT3 signaling, human resistin-TLR4 stimulation of STAT3 signaling could be indirect, perhaps through induction of IL-10. While human resistin may be anti-inflammatory in response to a fatal endotoxin challenge, other studies have shown that human resistin alone can promote inflammation [60, 61]. These conflicting finding suggest that the resulting pro-inflammatory or anti-inflammatory function of resistin is context and disease-specific. In colon cancer studies, resistin signaling through TLR4 receptor was described to increase TLR4-MyD88 signaling and SOCS3 expression [62]. The ensuing inhibition of JAK2/STAT3 signaling caused arrest of colon cells growth suggesting resistin signaling through TLR4 could delay cancer progression [62]. Although LPS is the most recognized ligand for TLR4, human resistin is not the only alternate ligand for TLR4. Indeed, TLR4 is reported to bind saturated fatty acids, the dust mite allergen Derp2, helminth antigens and the host endogenous protein high mobility group box 1 (HMGB) and heat shock proteins [63–68]. Alternate ligands for other TLRs is observed, and suggests that the evolution of TLR signaling and function was likely influenced by both microbial ligand and host endogenous molecules. Whether inflammatory or cancer disease outcomes are altered by these TLR4 alternate ligands in combination or alone is unknown, but needs to be considered when evaluating their overall function.
Adenylyl cyclase-associated protein 1 (CAP1) is also a proposed receptor for human resistin [69] (Figure 2C, III). In monocyte cell lines, resistin-CAP1 interaction led to increased cAMP levels, increased PKA, and NF-κB, and subsequent levels of inflammatory cytokines: IL-6, TNFα, and IL-1β [69]. Intriguingly, CAP1 is an intracellular receptor with no predicted transmembrane domain. Therefore, it is likely that resistin may need to bind a surface receptor, such as TLR-4, for endocytosis and presentation to CAP1. Finally, human resistin has also been described as a molecular chaperone, which binds to misfolded proteins in the cell, protecting it from stress-induced apoptosis (Figure 2C, IV) [70].
Although homologous, RELMs function through various signaling mechanisms that lead to diversified physiological phenotypes. Even though mechanistic studies on RELMs exist, the receptors for these proteins remain elusive. There are also no studies investigating RELMγ signaling. By exploring RELM signaling, it becomes evident that this family of proteins underwent a divergence in their evolution. However, by delving into their physiological functions, we can start to appreciate the similarities that RELMs retained.
4. THE LONG AND WINDING ROAD: RELM Function in Mice and Men
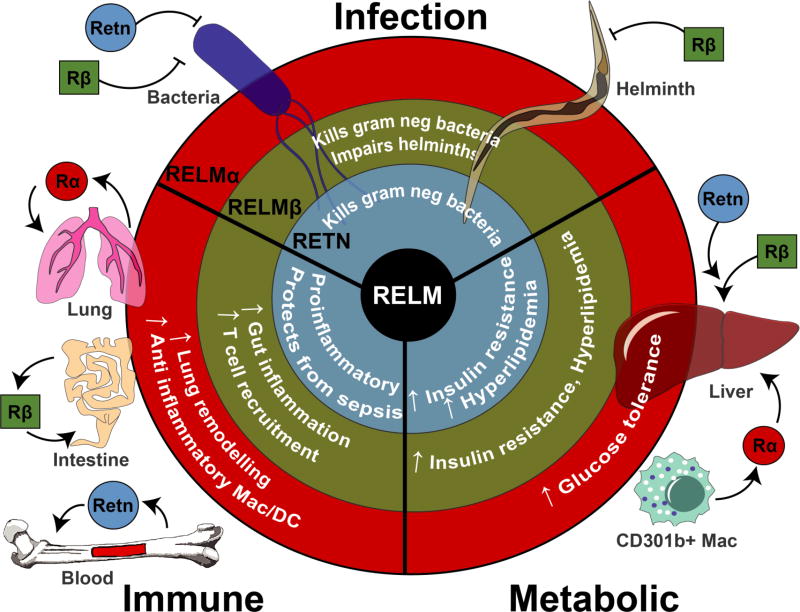
There have been a multitude of diverse studies investigating RELM protein function, some of which, reveal opposing functions. This section will review the functional studies on RELMs and their putative roles in infection, inflammatory and autoimmune diseases and metabolic function (summarized in Figure 3).
Figure 3.
The diverse roles of RELMs in infection, inflammation and metabolism.
4.1. Microbial Infection
4.1.1. RELMα
The use of RELMα−/− mice in helminth and bacterial infection as well as helminth antigen sensitization models has revealed complex host immunomodulatory roles for this protein. Following infection with the rat hookworm Nippostrongylus brasileiensis, RELMα suppressed Th2 cytokine responses through functional effects on CD4+ Th2 cells, which impaired optimal adult worm expulsion [11, 45, 71]. On the other hand, RELMα’s suppression of Th2 inflammation had beneficial effects for the host as it prevented excessive and potentially fatal lung inflammation. It seems therefore that RELMα acts as a critical rheostat in the balance between host immunity and inflammation, preserving host immune homeostasis sometimes at the expense of optimal antimicrobial immunity. In addition to direct suppression of CD4+ Th2 cells, RELMα was also able to promote IL-10 producing regulatory T cells following stimulation with Schistosoma mansoni antigen-pulsed dendritic cells [21]. In a mouse model of enteropathogenic/enterohemorrhagic bacterial infection with Citrobacter rodentium, RELMα-mediated suppression of Th2 cytokines led instead to increased Th17 cytokine-driven inflammation in the colon. Surprisingly, RELMα immunostimulatory effect had no significant effect on bacterial clearance suggesting that RELMα expression in Citrobacter rodentium infection was soley detrimental to the host [29, 72].
4.1.2. RELMβ
Infection studies with RELMβ−/− mice suggest that RELMβ promotes immunity to helminth and bacterial pathogens. RELMβ−/− mice were more susceptible to Heligmosomoides polygyrus and Nippostrongylus brasiliensis but not Trichuris muris infection [11, 55, 56, 73]. In intestinal bacterial infection with Citrobacter rodentium, RELMβ−/− mice succumbed to infection due to mucosal ulceration and deep penetration of the Citrobacter into the colonic crypts [74]. Mechanistic studies evaluating RELMβ function suggest that RELMβ’s protective effect is twofold: (i) promoting host immune cell responses and (ii) directly killing the pathogen. First, RELMβ promoted CD4+ T cell recruitment to the Citrobacter-infected intestines and increased production of antimicrobial cytokine IL-22 [74]. Additionally, recombinant RELMβ treatment of macrophage and splenocyte cultures promoted Th1 immune responses, which are protective in bacterial and viral infections, but not in chronic helminth infection [73]. Second, RELMβ binds both helminths and gram negative bacteria, leading to impaired helminth function and bacterial killing, respectively [55–57]. This microbicidal role for RELMβ is also shared by murine and human resistin.
Although not explored in the context of infection, there is strong evidence that RELMβ shapes the host microbiome. Compared to conventionally housed mice, germfree mice exhibited deficiency in intestinal RELMβ expression in the intestine that was restored upon colonization with commensal bacteria [9]. This effect was not entirely recapitulated with LPS treatment, suggesting that other bacterial factors regulate RELMβ expression [9]. However, when intestinal cell lines were directly stimulated directly with LPS, there was only a modest expression of RELMβ [9]. Between these two observations, it is likely that the microbiome within the colon can induce the expression of RELMβ, however, this expression was not purely driven by LPS expression and there are likely other bacterial factors in play. Recombinant RELMβ has antibacterial activity against gram negative bacteria, by binding to negatively charged lipids and creating multimeric pores within the membrane [57]. Because of this antimicrobial activity, RELMβ likely prunes the microbial communities present within the colon. Indeed, microbiome evaluation of wild-type and RELMβ−/− mice revealed increased gram negative bacterial invasion into the colon inner mucus layer, and increased abundance of Proteobacteria including Helicobacter [57]. Whether RELMβ’s functional effects on the bacterial and helminth infection are in part mediated by its influence on the microbiome remains to be determined.
4.1.3. Resistin
Human resistin function has been explored in helminth and viral infections. In both chronic filarial nematode infection and intestinal ascaris infection, plasma resistin levels were elevated and correlated with increased parasite burden and increased inflammatory cytokines TNFα, CCL2 and IL-6 [13]. The findings in these correlative studies were validated in vivo employing human resistin transgenic mice [13]. Here human resistin expression led to increased Nippostrongylus brasiliensis parasite burdens, correlated with increased TNFα and CCL2 gene expression. The effect of resistin in promoting a susceptible phenotype to helminth infection is similar to RELMα, however, instead of inhibiting Th2 cytokines, it seems that resistin tips the balance from a Th2-type response to a proinflammatory cytokine response. Again, similar to RELMα, it seems the role of resistin in helminth-infected hosts is counterintuitive. However, it is likely that resistin exists to direct the immune response towards combating more fatal bacterial or viral infections in an environment where bacterial-worm co-infections are common. Consistent with this, resistin has microbicidal properties and could protect mice from fatal endotoxic shock [57, 60]. Interestingly, compared to human resistin, mouse resistin did not have an impact on parasite burdens [13]. An explanation for this could be that human and mouse resistin expression are regulated differently and therefore have divergent functions in helminth infection. Mouse resistin and its role in helminth infection, if any, have yet to be uncovered.
Patients with chronic Hepatitis B viral infection had significantly elevated serum resistin levels, that were increased with progression to HBV-associated liver cirrhosis and liver failure [75]. In chronic Hepatitis C virus-infected patients, a correlation exists between resistin and disease related liver fibrosis severity [76]. In patients with HCV-induced chronic hepatitis, resistin expression was found to be higher in areas with inflammation and ongoing fibrogenisis [77]. Whether resistin is an instigator of viral-induced inflammation or is simply a result of viral infection is yet unclear.
4.2 Inflammatory and Fibrotic Disease
As in infection, the role for RELM proteins in influencing the immune response is recognized in several studies on inflammatory and fibrotic diseases. However, whether they play a pathogenic function in stimulating inflammation, or instead protect against excessive inflammatory responses is controversial. Some of this conflicting evidence may be caused by discrepancies between correlative and functional studies, while another contributor may be the use of endotoxin-contaminated bacterially-derived recombinant proteins. In addition to these caveats, it is becoming increasingly clear that the disease context and RELM protein level and expression pattern may be critical for their beneficial or pathologic outcomes.
4.2.1. RELMα and RELMβ
RELMα is highly expressed in lung injury and allergic airway inflammation models, where several studies, using recombinant RELMα administration or RELMα-expressing transgenic mice, confirmed a function in promoting chemotaxis for eosinophils and dendritic cells, and vascular inflammation [48, 78–80]. On the other hand, RELMα−/− mice exhibited similar airway and lung inflammation compared to wild-type mice following ovalbumin or Aspergillus-induced allergic airway inflammation [81]. In another study using RELMα-overexpressing transgenic mice, RELMα significantly suppressed ovalbumin-induced Th2 lung immune responses, correlated with reduced pERK signaling [14]. This is consistent with the original studies reporting RELMα−/− mice, where RELMα dampened the Th2 lung inflammatory response to sensitization and challenge with helminth Schistosoma mansoni egg antigen [45, 71].
In several studies investigating dextran sodium sulfate-induced intestinal inflammation as a mouse model of ulcerative colitis, RELMα−/− and RELMβ−/− mice exhibited reduced intestinal pathology and Th17 and TNFα cytokine responses compared to wild-type mice [29, 31, 82, 83]. In contrast, RELMβ−/− mice suffered from more severe trinitrobenzene sulfonate-induced colitis, as a model for Crohn’s disease, compared to wild-type mice [83]. The functional consequence of human RELMβ in IBD patients has yet to be determined, however, the mouse studies suggest that RELMβ’s role may be different depending on the type of inflammatory bowel disease (ulcerative colitis versus Crohn’s disease).
RELMα function in tissue repair and fibrosis is better understood with several studies showing that RELMα promotes these processes. Both tissue repair and fibrosis share similar pathways, such as stimulation by Th2 cytokines [84]. However, tissue repair is the desired outcome to injury while fibrosis, or scarring, occurs when tissue repair is not kept in check. Given that RELMα is stimulated by Th2 cytokines following both lung and skin injury, it was posited that RELMα maybe the downstream mediator of Th2 cytokine-induced tissue repair. Supportive of this, RELMα promoted myofibroblast differentiation and increased expression of type 1 collagen and α-SMA expression, leading to thickened fibrotic dermis and extracellular matrix deposition in bleomycin-induced dermal fibrosis [85]. Another study using skin biopsy-induced injury showed that IL-4 activated RELMα mediated skin healing by controlling collagen fibril assemby [50]. RELMα was profibrotic in bleomycin-induced pulmonary fibrosis through several proposed mechanisms: bone marrow cell recruitment, increased expression of VEGF, fibroblast and myofibroblast activation[86, 87]. Similar to RELMα, murine RELMβ also promoted bleomycin-induced pulmonary fibrosis, associated with increased lung fibroblast proliferation, collagen expression, and leukocyte recruitment [88]. Of clinical significance, human asthmatic patients have RELMβ deposits in the extracellular matrix is observed in human asthmatic patients, associated with increased airway remodeling [24].
4.2.2. Resistin
Increased circulating levels of resistin are observed in several human inflammatory diseases including endotoxemia, sepsis, rheumatoid arthritis and inflammatory bowel disease [89]. In vitro human monocyte culture and in vivo studies with human resistin-expressing transgenic mice suggest that resistin is a stimulatory cytokine that promotes immune cell activation and chemotaxis, neutrophil extracellular trap formation and inflammatory cytokine production [61, 69]. Intriguingly, more recent studies reveal that resistin may be anti-inflammatory in certain contexts. Resistin could impair LPS function and protect against high dose LPS-induced endotoxic shock [60]. Intriguingly, serum resistin levels were lower in patients with myalgic encephalitis/chronic fatigue syndrome, and was associated with reduced disease severity in patients with moderate to severe disease [90]. More functional studies are needed to determine the contribution of resistin to these immune-mediated diseases.
4.3 Cancer
While there are no reports on RELMα expression or function in cancer, human RELMβ expression was detected in 65.4% of 136 human cases of gastric cancers, where there was a positive correlation between RELMβ expression and patient survival [91]. Additionally, murine RELMβ was associated with reduced susceptibility to azoxymethane and DSS induced colorectal cancer, indicating that RELMβ could have a protective role in cancer [92]. Mechanistically, RELMβ decreased Th2 cytokine levels, it was suggested that RELMβ may indirectly slow the progression of colon cancer by decreasing inflammation [92]. In contrast, another study found that RELMβ overexpression in gastric carcinoma cells significantly increased invasion and migration of gastric cancer cells in a transwell assay [93]. Thus, RELMβ expression in gastric cancer may have a more complicated mechanism than initially predicted.
Resistin is reported to have indirect effects within inflammatory cancers. As previously mentioned, resistin signaling through TLR4-ERK-SOCS3 delays the progression of colonic cancers [62]. However, the arrest in cell cycle led to these cancer cells being more resistant to chemotherapeutic drugs that target highly proliferating cells. Further, another study showed that resistin blocked the apoptotic effect of the chemotherapy drugs, bortezomib or carfilzomib [94]. Here, treatment of various cancer cell lines with resistin resulted in upregulation of the transporter genes ABCC5, and ABCG2, that could act to export the drugs outside the cells [94]. In conclusion, it seems that both RELMβ and resistin are protective in inhibiting cancer progression, but may interfere with chemotherapy.
4.4 Metabolic Function
4.4.1. RELMα and RELMβ
RELMα expression is observed within white adipose tissue of high fat diet-fed mice [53]. RELMα deficiency in hyperlipidemic and atherosclerotic mice resulted in significant cholesterol increase, while overexpression of RELMα resulted in the reverse effect [53]. Mechanistically, RELMα overexpression upregulated the liver cholesterol catabolic enzymes, Cyp7a1 and Cyp8b1, which are responsible for breaking down cholesterol and converting it to bile acid. Consistent with this, RELMα overexpressing mice had increased fecal bile acid content and fecal cholesterol indicating a RELMα-mediated mechanism for cholesterol breakdown and clearance. RELMα thus induces depletion and clearance of cholesterol, and further implies that RELMα has beneficial functions in metabolism. However, in another study, RELMα expressed by CD301b+ mononuclear phagocytes within the white adipose tissue was important in maintaining healthy body weight and glucose levels [95]. When CD301b+ mononuclear phagocytes were depleted, there was a significant downregulation of metabolic genes in the liver, associated with hyperglycemia. This effect was reversed when the mice were exogenously treated with recombinant RELMα. In contrast, another study reported that RELMα−/− mice were protected from hyperglycemia [96]. It is possible that these discrepancies reflect differences in cell-specific deletion of CD301b+ cells compared to whole body RELMα−/− mice.
It is now well recognized that the immune environment, particularly the Th1/Th2 cytokine balance, is an important contributor to metabolic homeostasis or disease [97]. Given that RELMα is expressed by M2 macrophages, and regulates Th2 cytokines, it is possible that RELMα’s effect in metabolism is partly mediated through its immunoregulatory function. For example, brown adipose tissue in lean mice is more heavily populated with cells such as M2 Macs and Tregs as well as IL-4, IL-13 and IL-10 cytokines resulting in an overall Th2 immune state that maintains a noninflammatory mileu [98]. In contrast, chronically inflamed white adipose tissue associated with obesity and insulin resistance is populated with effector M1 macrophages that primarily contribute to a proinflammatory IL-1β, TNF-α and IL-6-rich environment [98]. In contrast to RELMα, however, resistin is associated with proinflammatory macrophage activation in obesity, but recent studies suggest that resistin can be anti-inflammatory in certain disease contexts [60, 89]. More specific studies investigating the immune effects of the specific RELM proteins in obesity and diabetes are necessary, however, the current data may support a therapeutic benefit in employing RELM proteins to modulate the Th1/Th2 balance to treat metabolic dysfunction.
RELMβ is highly expressed in mice fed a high fat diet [99] and RELMβ−/− mice exhibited reduced glucose tolerance [92]. In contrast, transgenic mice overexpressing RELMβ in hepatocytes suffered from increased hyperglycemia, hyperlipidemia, fatty liver, and pancreatic islet enlargement when fed a high fat diet [100]. Further, recombinant RELMβ treatment suppressed insulin signaling in cultured hepatocytes, associated with increased MAPK and reduced IRS1/2 proteins [100]. These studies suggest that, similar to RELMα, RELMβ’s effect on glucose tolerance and insulin signaling is dependent on the in vivo context and RELMβ level. Indeed, high RELMβ levels in the liver cause metabolic dysfunction, however, complete abrogation of RELMβ expression is also detrimental.
4.4.2. Resistin
Resistin’s function in metabolism has been explored thoroughly, and is discussed in a number of recent review articles [89, 101, 102]. The general consensus in mouse and human studies is that resistin plays a pathologic role in promoting insulin resistance, atherosclerosis and hypertension. Here we will focus on a few select studies investigating resistin in metabolic disease. In mice, inhibition or genetic deletion of resistin resulted in increased insulin sensitivity and glucose homeostasis [103]. Conversely administration of exogenous resistin, or transgenic expression of human resistin, promoted insulin resistance [103–106]. Some population studies have reported an association between circulating resistin levels and adiposity or increased insulin resistance [41], but other studies have challenged this link [107–109]. A bigger dataset, and more focused analysis parameters or exclusion criteria to reduce confounding factors, may address human resistin’s metabolic function. Both human and mouse resistin reduced insulin-induced glucose uptake within cardiomyocyte cells, most likely by impairing insulin-mediated GLUT4 translocation [110]. Further, in patients with coronary artery disease, elevated resistin levels were correlated with aortic stiffness [111]. It was hypothesized that resistin’s induction of insulin resistance changes glucose metabolism, which causes stress to the heart when it shifts to glucose as its energy source, and which leads to heart failure [89]. If this theory is confirmed, resistin management may prove to be a viable option for preventive treatment in patients with cardiac disorders. Given that resistin is an immunomodulatory molecule secreted by immune cells, resistin’s cross-talk with the immune system is a likely contributor to its’ metabolic effect.
5. Concluding Remarks and Perspective
Whether resistin and the RELM proteins are helpful or detrimental for the host is still under debate, however, this review has highlighted the complexity in interaction and function of these proteins, which suggests that their role cannot purely be defined as “positive” or “negative”. For example, RELMα’s chemotactic and proliferative properties may lead to inflammation but is beneficial in wound healing, which requires immune contribution. Further, RELM proteins may have therapeutic potential. For instance, RELMα and resistin’s ability to attenuate excessive inflammatory responses may be valuable in clinical settings. Conversely, RELMβ and resistin’s microbicidal properties could be harnessed as new antimicrobial agents against bacteria and helminths. Likewise, RELMβ’s inhibitory effect on cancer cell proliferation and its association with improved survival could be investigated as a potential biomarker or therapy. Last, RELMα’s beneficial effect in metabolic homeostasis could be explored to target metabolic disease.
While we have attempted to comprehensively address key studies investigating RELM protein expression and function at both the molecular and whole body level, we also highlight missing or conflicting information. First, research on the receptors and activation mechanisms for these proteins is incomplete. Second, given that RELM proteins seemingly influence metabolic, immune and microbial systems, more multidisciplinary research to understand how effects in one system may have outcomes in another system may shed light on the conflicting information in the field. It is also possible that the effects of RELM proteins are more nuanced, and overexpression or whole body knockout data represent extremes in the spectrum of RELM function. Instead, experiments investigating varied concentrations or cell-specific expression of these proteins could be useful to examine their potential as biomarkers or therapeutics. While we have come a long way in research of resistin and resistin-like molecules, there is still much work to do.
Highlights.
Resistin-like molecules (RELMs) are mammalian secreted proteins.
RELMs are homologous but have different expression patterns.
The receptor and signaling mechanisms of RELMs are not yet elucidated.
RELMα, RELMβ and resistin have immunoregulatory capabilities.
RELMs influence responses to infections and metabolic disorders.
Acknowledgments
We would like to thank Dr. Jiang Li, Jordan Lillibridge, Sarah Bobardt, Jessica Noll and Mark Wiley for comments and critique on our initial drafts of the manuscript.
Funding:
The Nair lab is supported by the NIH (1R01AI091759-01A1; 1R21AI137830-01) and the UCR School of Medicine (initial complement).
Abbreviations
- ABC
ATP-binding cassette transporter
- ADD1
adipocyte determination and differentiation dependent factor 1
- ADSF
adipocyte secreted factor
- Akt (aka PKB)
protein kinase B
- AP1
activator protein 1
- ASC
adipose stromal cells
- BTK
Bruton’s tyrosine kinase
- C/EBP
CCAAT/enhancer-binding protein
- cAMP
cyclic AMP
- CAP1
adenylyl cyclase-associated protein 1
- CCL2
chemokine (C-C motif) ligand 2
- Cdx2
caudal type homeobox 2
- CREB
cAMP-response element-binding protein
- Cyp7a1
cholesterol 7 alpha-hydroxylase
- Cyp8b1
sterol 12α-hydroxylase
- DCN
decorin
- DSS
dextran sulfate sodium
- ERK
extracellular-signal-regulated kinase
- FIZZ
found in inflammatory zone
- GAS
gamma interferon activation site
- GLUT4
glucose transporter 4
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HIMF
hypoxia-induced mitogenic factor
- HMGB
high mobility group box
- HNF
hepatocyte nuclear factor
- IBD
inflammatory bowel disease
- IL
interleukin
- IP3R
inositol 1,4,5-triphosphate receptor
- IRF1/2
interferon regulatory factor 1/2
- JAK
Janus kinase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- LRH-1
liver receptor homolog-1
- M1
classically activated macrophage
- M2
alternatively activated macrophage
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemotactic protein-1
- NF-κB
nuclear factor κB
- PI3K
phosphoinositide 3-kinase
- PKA
protein kinase A
- PPAR
peroxisome activated receptor
- PPRE
PPAR response element
- RELM
resistin-like molecule
- RXR
retinoid × receptor
- SDF-1
stromal cell-derived factor 1
- SNP
single nucleotide polymorphism
- SOCS
suppressor of cytokine signaling
- SREBP1c
sterol regulatory element binding protein 1c
- STAT
signal transducer and activator of transcription
- TBK1
serine/threonine-protein kinase 1
- Th1
T helper cell type 1
- Th2
T helper cell type 2
- Th17
T helper cell type 17
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor alpha
- TRIF
TIR domain-containing adaptor protein-inducing interferon β
- VEGF
vascular endothelial factor
- α-SMA
α-smooth muscle actin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors contributions:
GP and MGN: Concept and design, drafting of the manuscript
HMB: Drafting of the manuscript, figure design
Competing interests:
The authors have no competing interests to declare.
References
- 1.Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMα, a Novel Hypoxia-Induced Mitogenic Factor in Lung With Vasoconstrictive and Angiogenic Properties. Circulation Research. 2003;92(10):1065. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]
- 2.Horsnell WGC, Dewals BG. RELMs in the Realm of Helminths. Trends in Parasitology. 2016;32(7):512–514. doi: 10.1016/j.pt.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Gerstmayer B, Küsters D, Gebel S, Müller T, Van Miert E, Hofmann K, Bosio A. Identification of RELMγ, a novel resistin-like molecule with a distinct expression pattern☆. Genomics. 2003;81(6):588–595. doi: 10.1016/s0888-7543(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 4.Johns RA, Gao L, Rafaels NM, Grant AV, Stockton-Porter ML, Watson HR, Beaty TH, Barnes KC. Polymorphisms in Resistin and Resistin-like Beta Predict Bronchial Hyperreactivity in Human Asthma. Proceedings of the American Thoracic Society. 2009;6(3):329–329. [Google Scholar]
- 5.Ghosh S, Singh AK, Aruna B, Mukhopadhyay S, Ehtesham NZ. The genomic organization of mouse resistin reveals major differences from the human resistin: functional implications. Gene. 2003;305(1):27–34. doi: 10.1016/s0378-1119(02)01213-1. [DOI] [PubMed] [Google Scholar]
- 6.Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen XM, Wu GD, Lazar MA. A family of tissue-specific resistin-like molecules. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schinke T, Haberland M, Jamshidi A, Nollau P, Rueger JM, Amling M. Cloning and functional characterization of resistin-like molecule γ. Biochemical and Biophysical Research Communications. 2004;314(2):356–362. doi: 10.1016/j.bbrc.2003.12.100. [DOI] [PubMed] [Google Scholar]
- 8.Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-Dependent Multimeric Assembly of Resistin Family Hormones. Science. 2004;304(5674):1154. doi: 10.1126/science.1093466. [DOI] [PubMed] [Google Scholar]
- 9.He W, Wang M-L, Jiang H-Q, Steppan CM, Shin ME, Thurnheer MC, Cebra JJ, Lazar MA, Wu GD. Bacterial colonization leads to the colonic secretion of RELMβ/FIZZ2, a novel goblet cell-specific protein. Gastroenterology. 2003;125(5):1388–1397. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee RR, Lazar MA. Dimerization of Resistin and Resistin-like Molecules Is Determined by a Single Cysteine. Journal of Biological Chemistry. 2001;276(28):25970–25973. doi: 10.1074/jbc.M103109200. [DOI] [PubMed] [Google Scholar]
- 11.Chen G, Wang SH, Jang JC, Odegaard JI, Nair MG. Comparison of RELM alpha and RELM beta Single- and Double-Gene-Deficient Mice Reveals that RELM alpha Expression Dictates Inflammation and Worm Expulsion in Hookworm Infection2. Infection and Immunity. 2016;84(4):1100–1111. doi: 10.1128/IAI.01479-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shojima N, Ogihara T, Inukai K, Fujishiro M, Sakoda H, Kushiyama A, Katagiri H, Anai M, Ono H, Fukushima Y, Horike N, Viana AYI, Uchijima Y, Kurihara H, Asano T. Serum concentrations of resistin-like molecules β and γ are elevated in high-fat-fed and obese db/db mice, with increased production in the intestinal tract and bone marrow. Diabetologia. 2005;48(5):984–992. doi: 10.1007/s00125-005-1735-1. [DOI] [PubMed] [Google Scholar]
- 13.Jang JC, Chen G, Wang SH, Barnes MA, Chung JI, Camberis M, Le Gros G, Cooper PJ, Steel C, Nutman TB, Lazar MA, Nair MG. Macrophage-Derived Human Resistin Is Induced in Multiple Helminth Infections and Promotes Inflammatory Monocytes and Increased Parasite Burden. PLoS Pathogens. 2015;11(1):e1004579. doi: 10.1371/journal.ppat.1004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M-R, Shim D, Yoon J, Jang HS, Oh S-W, Suh SH, Choi J-H, Oh GT. Retnla Overexpression Attenuates Allergic Inflammation of the Airway. PLOS ONE. 2014;9(11):e112666. doi: 10.1371/journal.pone.0112666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV, Shelton DL, Hébert CC. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19(15):4046–55. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stütz AM, Pickart LA, Trifilieff A, Baumruker T, Prieschl-Strassmayr E, Woisetschläger M. The Th2 Cell Cytokines IL-4 and IL-13 Regulate Found in Inflammatory Zone 1/Resistin-Like Molecule α Gene Expression by a STAT6 and CCAAT/Enhancer-Binding Protein-Dependent Mechanism. The Journal of Immunology. 2003;170(4):1789. doi: 10.4049/jimmunol.170.4.1789. [DOI] [PubMed] [Google Scholar]
- 17.Loke P, Nair MG, Parkinson J, Guiliano D, Blaxter M, Allen JE. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair MG, Gallagher IJ, Taylor MD, Loke P, Coulson PS, Wilson RA, Maizels RM, Allen JE. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73(1):385–94. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raes G, De Baetselier P, Noël W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71(4):597–602. [PubMed] [Google Scholar]
- 20.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook PC, Jones LH, Jenkins SJ, Wynn TA, Allen JE, MacDonald AS. Alternatively activated dendritic cells regulate CD4+ T-cell polarization in vitro and in vivo. Proc Natl Acad Sci U S A. 2012;109(25):9977–82. doi: 10.1073/pnas.1121231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T, Dhanasekaran SM, Jin H, Hu B, Tomlins SA, Chinnaiyan AM, Phan SH. FIZZ1 stimulation of myofibroblast differentiation. Am J Pathol. 2004;164(4):1315–26. doi: 10.1016/S0002-9440(10)63218-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loke P, Gallagher I, Nair MG, Zang X, Brombacher F, Mohrs M, Allison JP, Allen JE. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179(6):3926–36. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 24.Fang CL, Yin LJ, Sharma S, Kierstein S, Wu HF, Eid G, Haczku A, Corrigan CJ, Ying S. Resistin-like molecule-β (RELM-β) targets airways fibroblasts to effect remodelling in asthma: from mouse to man. Clin Exp Allergy. 2015;45(5):940–952. doi: 10.1111/cea.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Baek HA, Yu H, Lee HJ, Park BH, Ullenbruch M, Liu J, Nakashima T, Choi YY, Wu GD, Chung MJ, Phan SH. FIZZ2/RELM-β induction and role in pulmonary fibrosis. J Immunol. 2011;187(1):450–61. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra A, Wang M, Schlotman J, Nikolaidis NM, DeBrosse CW, Karow ML, Rothenberg ME. Resistin-like molecule-β is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2007;293(2):L305. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 27.Grainge C, Dulay V, Ward J, Sammut D, Davies E, Green B, Lau L, Cottey L, Haitchi H-M, Davies DE, Howarth PH. Resistin-like molecule-β is induced following bronchoconstriction of asthmatic airways. Respirology. 2012;17(7):1094–1100. doi: 10.1111/j.1440-1843.2012.02215.x. [DOI] [PubMed] [Google Scholar]
- 28.Barnes SL, Vidrich A, Wang ML, Wu GD, Cominelli F, Rivera-Nieves J, Bamias G, Cohn SM. Resistin-like molecule beta (RELM beta/FIZZ2) is highly expressed in the ileum of SAMP1/YitFc mice and is associated with initiation of ileitis. Journal of Immunology. 2007;179(10):7012–7020. doi: 10.4049/jimmunol.179.10.7012. [DOI] [PubMed] [Google Scholar]
- 29.Osborne LC, Joyce KL, Alenghat T, Sonnenberg GF, Giacomin PR, Du Y, Bergstrom KS, Vallance BA, Nair MG. Resistin-like molecule (RELM) α promotes pathogenic Th17 cell responses and bacterial-induced intestinal inflammation. Journal of immunology (Baltimore, Md. : 1950) 2013;190(5):2292–2300. doi: 10.4049/jimmunol.1200706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang ML, Shin ME, Knight PA, Artis D, Silberg DG, Suh E, Wu GD. Regulation of RELM/FIZZ isoform expression by Cdx2 in response to innate and adaptive immune stimulation in the intestine. Am J Physiol Gastrointest Liver Physiol. 2005;288(5):G1074–83. doi: 10.1152/ajpgi.00442.2004. [DOI] [PubMed] [Google Scholar]
- 31.Chellappa K, Deol P, Evans JR, Vuong LM, Chen G, Briançon N, Bolotin E, Lytle C, Nair MG, Sladek FM. Opposing roles of nuclear receptor HNF4α isoforms in colitis and colitis-associated colon cancer. eLife. 2016;5:e10903. doi: 10.7554/eLife.10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayhurst GP, Lee Y-H, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte Nuclear Factor 4α (Nuclear Receptor 2A1) Is Essential for Maintenance of Hepatic Gene Expression and Lipid Homeostasis. Molecular and Cellular Biology. 2001;21(4):1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaestner KH. Making the liver what it is: The many targets of the transcriptional regulator HNF4α. Hepatology. 2010;51(2):376–377. doi: 10.1002/hep.23487. [DOI] [PubMed] [Google Scholar]
- 34.Kim KH, Zhao L, Moon Y, Kang C, Sul HS. Dominant inhibitory adipocyte-specific secretory factor (ADSF)/resistin enhances adipogenesis and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2004;101(17):6780–5. doi: 10.1073/pnas.0305905101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shojima N, Sakoda H, Ogihara T, Fujishiro M, Katagiri H, Anai M, Onishi Y, Ono H, Inukai K, Abe M, Fukushima Y, Kikuchi M, Oka Y, Asano T. Humoral Regulation of Resistin Expression in 3T3-L1 and Mouse Adipose Cells. Diabetes. 2002;51(6):1737. doi: 10.2337/diabetes.51.6.1737. [DOI] [PubMed] [Google Scholar]
- 36.Nagaev I, Andersen M, Olesen MK, Nagaeva O, Wikberg J, Mincheva-Nilsson L, Andersen GN. Resistin Gene Expression is Downregulated in CD4+ T Helper Lymphocytes and CD14+ Monocytes in Rheumatoid Arthritis Responding to TNF-α Inhibition. Scandinavian Journal of Immunology. 2016;84(4):229–236. doi: 10.1111/sji.12464. [DOI] [PubMed] [Google Scholar]
- 37.Stan D, Calin M, Manduteanu I, Pirvulescu M, Gan A-M, Butoi ED, Simion V, Simionescu M. High glucose induces enhanced expression of resistin in human U937 monocyte-like cell line by MAPK- and NF-kB-dependent mechanisms; the modulating effect of insulin. Cell and Tissue Research. 2011;343(2):379–387. doi: 10.1007/s00441-010-1092-3. [DOI] [PubMed] [Google Scholar]
- 38.Hartman HB, Hu X, Tyler KX, Dalal CK, Lazar MA. Mechanisms Regulating Adipocyte Expression of Resistin. Journal of Biological Chemistry. 2002;277(22):19754–19761. doi: 10.1074/jbc.M201451200. [DOI] [PubMed] [Google Scholar]
- 39.Seo JB, Noh MJ, Yoo EJ, Park SY, Park J, Lee IK, Park SD, Kim JB. Functional Characterization of the Human Resistin Promoter with Adipocyte Determination- and Differentiation-Dependent Factor 1/Sterol Regulatory Element Binding Protein 1c and CCAAT Enhancer Binding Protein-α. Molecular Endocrinology. 2003;17(8):1522–1533. doi: 10.1210/me.2003-0028. [DOI] [PubMed] [Google Scholar]
- 40.Azuma K, Oguchi S, Matsubara Y, Mamizuka T, Murata M, Kikuchi H, Watanabe K, Katsukawa F, Yamazaki H, Shimada A, Saruta T. Novel Resistin Promoter Polymorphisms: Association with Serum Resistin Level in Japanese Obese Individuals. Horm Metab Res. 2004;36(08):564–570. doi: 10.1055/s-2004-825762. [DOI] [PubMed] [Google Scholar]
- 41.Azuma K, Katsukawa F, Oguchi S, Murata M, Yamazaki H, Shimada A, Saruta T. Correlation between Serum Resistin Level and Adiposity in Obese Individuals. Obesity Research. 2003;11(8):997–1001. doi: 10.1038/oby.2003.137. [DOI] [PubMed] [Google Scholar]
- 42.Cho YM, Ritchie MD, Moore JH, Park JY, Lee KU, Shin HD, Lee HK, Park KS. Multifactor-dimensionality reduction shows a two-locus interaction associated with Type 2 diabetes mellitus. Diabetologia. 2004;47(3):549–554. doi: 10.1007/s00125-003-1321-3. [DOI] [PubMed] [Google Scholar]
- 43.Chung SS, Choi HH, Kim KW, Cho YM, Lee HK, Park KS. Regulation of human resistin gene expression in cell systems: an important role of stimulatory protein 1 interaction with a common promoter polymorphic site. Diabetologia. 2005;48(6):1150–1158. doi: 10.1007/s00125-005-1762-y. [DOI] [PubMed] [Google Scholar]
- 44.Hu W-W, Tang C-H, Sun Y, Lu T-T, Jiang P, Wu Y-M, Wang C-Q, Yang S-F, Su C-M. Correlation between resistin gene polymorphism and clinical aspects of lung cancer. Medicine. 2017;96(52) doi: 10.1097/MD.0000000000009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, Karow M, Stevens S, Pearce EJ, Artis D. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. The Journal of Experimental Medicine. 2009;206(4):937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su Q, Zhou Y, Johns RA. Bruton’s tyrosine kinase (BTK) is a binding partner for hypoxia induced mitogenic factor (HIMF/FIZZ1) and mediates myeloid cell chemotaxis. The FASEB Journal. 2007;21(7):1376–1382. doi: 10.1096/fj.06-6527com. [DOI] [PubMed] [Google Scholar]
- 47.Fan C, Su Q, Li Y, Liang L, Angelini DJ, Guggino WB, Johns RA. Hypoxia-induced mitogenic factor/FIZZ1 induces intracellular calcium release through the PLC-IP3 pathway. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2009;297(2):L263. doi: 10.1152/ajplung.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaji-Kegan K, Su Q, Angelini DJ, Champion HC, Johns RA. Hypoxia-induced mitogenic factor has proangiogenic and proinflammatory effects in the lung via VEGF and VEGF receptor-2. Am J Physiol Lung Cell Mol Physiol. 2006;291(6):L1159–68. doi: 10.1152/ajplung.00168.2006. [DOI] [PubMed] [Google Scholar]
- 49.Liu T, Hu B, Choi YY, Chung M, Ullenbruch M, Yu H, Lowe JB, Phan SH. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol. 2009;174(5):1745–55. doi: 10.2353/ajpath.2009.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knipper JA, Willenborg S, Brinckmann J, Bloch W, Maaß T, Wagener R, Krieg T, Sutherland T, Munitz A, Rothenberg ME, Niehoff A, Richardson R, Hammerschmidt M, Allen JE, Eming SA. Interleukin-4 Receptor α Signaling in Myeloid Cells Controls Collagen Fibril Assembly in Skin Repair. Immunity. 2015;43(4):803–16. doi: 10.1016/j.immuni.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minutti CM, Jackson-Jones LH, García-Fojeda B, Knipper JA, Sutherland TE, Logan N, Ringqvist E, Guillamat-Prats R, Ferenbach DA, Artigas A, Stamme C, Chroneos ZC, Zaiss DM, Casals C, Allen JE. Local amplifiers of IL-4Rα-mediated macrophage activation promote repair in lung and liver. Science. 2017;356(6342):1076–1080. doi: 10.1126/science.aaj2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, Gagliani N, Craft JE, Flavell RA, Ghosh S, Rothlin CV. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356(6342):1072–1076. doi: 10.1126/science.aai8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee M-R, Lim C-j, Lee Y-H, Park J-G, Sonn SK, Lee M-N, Jung I-H, Jeong S-J, Jeon S, Lee M, Oh KS, Yang Y, Kim JB, Choi H-S, Jeong W, Jeong T-S, Yoon WK, Kim HC, Choi J-H, Oh GT. The adipokine Retnla modulates cholesterol homeostasis in hyperlipidemic mice. Nature Communications. 2014;5:4410. doi: 10.1038/ncomms5410. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y-Q, Fan C-C, Chen B-P, Shi J. Resistin-Like Molecule Beta (RELM-β) Regulates Proliferation of Human Diabetic Nephropathy Mesangial Cells via Mitogen-Activated Protein Kinases (MAPK) Signaling Pathway. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research. 2017;23:3897–3903. doi: 10.12659/MSM.905381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Artis D, Mei LW, Keilbaugh SA, He WM, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HRP, Schad GA, Scott P, Wu GD. RELM beta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(37):13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herbert DR, Yang JQ, Hogan SP, Groschwitz K, Khodoun M, Munitz A, Orekov T, Perkins C, Wang Q, Brombacher F, Urban JF, Rothenberg ME, Finkelman FD. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. Journal of Experimental Medicine. 2009;206(13):2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Propheter DC, Chara AL, Harris TA, Ruhn KA, Hooper LV. Resistin-like molecule β is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(42):11027–11033. doi: 10.1073/pnas.1711395114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell. 2011;9(1):74–86. doi: 10.1016/j.stem.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 59.Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. Journal of Cellular and Molecular Medicine. 2010;14(6b):1419–1431. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jang JC, Li J, Gambini L, Batugedara HM, Sati S, Lazar MA, Fan L, Pellecchia M, Nair MG. Human resistin protects against endotoxic shock by blocking LPS–TLR4 interaction. Proceedings of the National Academy of Sciences. 2017 doi: 10.1073/pnas.1716015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang S, Park DW, Tadie J-M, Gregoire M, Deshane J, Pittet JF, Abraham E, Zmijewski JW. Human resistin promotes neutrophil pro-inflammatory activation, neutrophil extracellular trap formation, and increases severity of acute lung injury. Journal of immunology (Baltimore, Md. : 1950) 2014;192(10):4795–4803. doi: 10.4049/jimmunol.1302764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh S, Chouhan S, Mohammad N, Bhat MK. Resistin causes G1 arrest in colon cancer cells through upregulation of SOCS3. Febs Letters. 2017;591(10):1371–1382. doi: 10.1002/1873-3468.12655. [DOI] [PubMed] [Google Scholar]
- 63.Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R, Loughran P, Scott MJ, Billiar TR. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med. 2013;19:88–98. doi: 10.2119/molmed.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457(7229):585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Qian Y, Fang Q, Zhong P, Li W, Wang L, Fu W, Zhang Y, Xu Z, Li X, Liang G. Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nature Communications. 2017;8:13997. doi: 10.1038/ncomms13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al-Riyami L, Liew FY, Harnett W, Harnett MM. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J Immunol. 2005;174(1):284–93. doi: 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 67.Martin I, Cabán-Hernández K, Figueroa-Santiago O, Espino AM. Fasciola hepatica fatty acid binding protein inhibits TLR4 activation and suppresses the inflammatory cytokines induced by lipopolysaccharide in vitro and in vivo. J Immunol. 2015;194(8):3924–36. doi: 10.4049/jimmunol.1401182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu L, Wang L, Chen S. Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med. 2010;14(11):2592–603. doi: 10.1111/j.1582-4934.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee S, Lee H-C, Kwon Y-W, Lee SE, Cho Y, Kim J, Lee S, Kim J-Y, Lee J, Yang H-M, Mook-Jung I, Nam K-Y, Chung J, Lazar MA, Kim H-S. Adenylyl Cyclase-Associated Protein 1(CAP1) is a Receptor for Human Resistin and Mediates Inflammatory Actions of Human Monocytes. Cell metabolism. 2014;19(3):484–497. doi: 10.1016/j.cmet.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suragani M, Aadinarayana VD, Pinjari AB, Tanneeru K, Guruprasad L, Banerjee S, Pandey S, Chaudhuri TK, Ehtesham NZ. Human resistin, a proinflammatory cytokine, shows chaperone-like activity. Proc Natl Acad Sci U S A. 2013;110(51):20467–72. doi: 10.1073/pnas.1306145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, Urban JF, Wynn TA. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5(4):e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen G, Chan AJ, Chung JI, Jang JC, Osborne LC, Nair MG. Polarizing the T helper 17 response in Citrobacter rodentium infection via expression of resistin-like molecule α. Gut Microbes. 2014;5(3):363–368. doi: 10.4161/gmic.29100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nair MG, Guild KJ, Du Y, Zaph C, Yancopoulos GD, Valenzuela DM, Murphy A, Stevens S, Karow M, Artis D. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol. 2008;181(7):4709–15. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergstrom KSB, Morampudi V, Chan JM, Bhinder G, Lau J, Yang H, Ma C, Huang T, Ryz N, Sham HP, Zarepour M, Zaph C, Artis D, Nair M, Vallance BA. Goblet Cell Derived RELM-beta Recruits CD4(+) T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation. Plos Pathogens. 2015;11(8) doi: 10.1371/journal.ppat.1005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meng ZJ, Zhang YH, Wei ZQ, Liu P, Kang J, Ma DQ, Ke CZ, Chen Y, Luo J, Gong ZJ. High serum resistin associates with intrahepatic inflammation and necrosis: an index of disease severity for patients with chronic HBV infection. Bmc Gastroenterology. 2017;17:9. doi: 10.1186/s12876-016-0558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tiftikci A, Atug O, Yilmaz Y, Eren F, Ozdemir FT, Yapali S, Ozdogan O, Celikel CA, Imeryuz N, Tozun N. Serum Levels of Adipokines in Patients with Chronic HCV Infection: Relationship with Steatosis and Fibrosis. Archives of Medical Research. 2009;40(4):294–298. doi: 10.1016/j.arcmed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 77.Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Ginés P, Colmenero J, Parola M, Gelmini S, Tarquini R, Laffi G, Pinzani M, Marra F. Resistin as an Intrahepatic Cytokine : Overexpression during Chronic Injury and Induction of Proinflammatory Actions in Hepatic Stellate Cells. The American Journal of Pathology. 2006;169(6):2042–2053. doi: 10.2353/ajpath.2006.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doherty TA, Khorram N, Sugimoto K, Sheppard D, Rosenthal P, Cho JY, Pham A, Miller M, Croft M, Broide DH. Alternaria induces STAT6-dependent acute airway eosinophilia and epithelial FIZZ1 expression that promotes airway fibrosis and epithelial thickness. J Immunol. 2012;188(6):2622–9. doi: 10.4049/jimmunol.1101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madala SK, Edukulla R, Davis KR, Schmidt S, Davidson C, Kitzmiller JA, Hardie WD, Korfhagen TR. Resistin-like molecule α1 (Fizz1) recruits lung dendritic cells without causing pulmonary fibrosis. Respir Res. 2012;13:51. doi: 10.1186/1465-9921-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamaji-Kegan K, Takimoto E, Zhang A, Weiner NC, Meuchel LW, Berger AE, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (FIZZ1/RELMα) induces endothelial cell apoptosis and subsequent interleukin-4-dependent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;306(12):L1090–103. doi: 10.1152/ajplung.00279.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Munitz A, Cole ET, Karo-Atar D, Finkelman FD, Rothenberg ME. Resistin-like molecule-α regulates IL-13-induced chemokine production but not allergen-induced airway responses. Am J Respir Cell Mol Biol. 2012;46(5):703–13. doi: 10.1165/rcmb.2011-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Munitz A, Waddell A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha enhances myeloid cell activation and promotes colitis. J Allergy Clin Immunol. 2008;122(6):1200–1207.e1. doi: 10.1016/j.jaci.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hogan SP, Seidu L, Blanchard C, Groschwitz K, Mishra A, Karow ML, Ahrens R, Artis D, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rothenberg ME. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol. 2006;118(1):257–68. doi: 10.1016/j.jaci.2006.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gieseck RL, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18(1):62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 85.Martins V, De Los Santos FG, Wu Z, Capetozzi V, Phan SH, Liu TJ. FIZZ1-Induced Myofibroblast Transdifferentiation from Adipocytes and Its Potential Rote in Dermal Fibrosis and Lipoatrophy. American Journal of Pathology. 2015;185(10):2768–2776. doi: 10.1016/j.ajpath.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu T, Yu H, Ullenbruch M, Jin H, Ito T, Wu Z, Liu J, Phan SH. The in vivo fibrotic role of FIZZ1 in pulmonary fibrosis. PLoS One. 2014;9(2):e88362. doi: 10.1371/journal.pone.0088362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamaji-Kegan K, Su Q, Angelini DJ, Myers AC, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. J Immunol. 2010;185(9):5539–48. doi: 10.4049/jimmunol.0904021. [DOI] [PubMed] [Google Scholar]
- 88.Liu TJ, Baek HA, Yu HF, Lee HJ, Park BH, Ullenbruch M, Liu JH, Nakashima T, Choi YY, Wu GD, Chung MJ, Phan SH. FIZZ2/RELM-beta Induction and Role in Pulmonary Fibrosis. Journal of Immunology. 2011;187(1):450–461. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends in Endocrinology and Metabolism. 2011;22(7):259–265. doi: 10.1016/j.tem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Montoya JG, Holmes TH, Anderson JN, Maecker HT, Rosenberg-Hasson Y, Valencia IJ, Chu L, Younger JW, Tato CM, Davis MM. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci U S A. 2017;114(34):E7150–E7158. doi: 10.1073/pnas.1710519114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng LD, Weng MX, He J, Yang XP, Jiang GS, Tong QS. Expression of resistin-like molecule beta in gastric cancer: its relationship with clinicopathological parameters and prognosis. Virchows Archiv. 2010;456(1):53–63. doi: 10.1007/s00428-009-0861-4. [DOI] [PubMed] [Google Scholar]
- 92.Asterholm IW, Kim-Muller JY, Rutkowski JM, Crewe C, Tao C, Scherer PE. Pathological Type-2 Immune Response, Enhanced Tumor Growth, and Glucose Intolerance in Retn/beta (RELM beta) Null Mice A Model of Intestinal Immune System Dysfunction in Disease Susceptibility. American Journal of Pathology. 2016;186(9):2404–2416. doi: 10.1016/j.ajpath.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang R, Zhao CM, Wang XY, Wang SX, Sun XG, Tian Y, Song W. Resistin-Like Molecule-beta Promotes Invasion and Migration of Gastric Carcinoma Cells. Medical Science Monitor. 2016;22:937–942. doi: 10.12659/MSM.895598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pang JA, Shi QF, Liu ZQ, He J, Liu HA, Lin P, Cui JW, Yang J. Resistin induces multidrug resistance in myeloma by inhibiting cell death and upregulating ABC transporter expression. Haematologica. 2017;102(7):1273–1280. doi: 10.3324/haematol.2016.154062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumamoto Y, Camporez JPG, Jurczak MJ, Shanabrough M, Horvath T, Shulman GI, Iwasaki A. CD301b(+) Mononuclear Phagocytes Maintain Positive Energy Balance through Secretion of Resistin-like Molecule Alpha. Immunity. 2016;45(3):583–596. doi: 10.1016/j.immuni.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munitz A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-Like Molecule a Decreases Glucose Tolerance during Intestinal Inflammation. Journal of Immunology. 2009;182(4):2357–2363. doi: 10.4049/jimmunol.0803130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Odegaard JI, Chawla A. Type 2 responses at the interface between immunity and fat metabolism. Curr Opin Immunol. 2015;36:67–72. doi: 10.1016/j.coi.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shu CJ, Benoist C, Mathis D. The immune system's involvement in obesity-driven type 2 diabetes. Semin Immunol. 2012;24(6):436–42. doi: 10.1016/j.smim.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hildebrandt MA, Hoffman C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen Y-Y, Knight R, Ahima RS, Bushman F, Wu GD. High Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology. 2009;137(5):1716–24.e1. 2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kushiyama A, Shojima N Fau - Ogihara T, Ogihara T Fau - Inukai K, Inukai K Fau - Sakoda H, Sakoda H Fau - Fujishiro M, Fujishiro M Fau - Fukushima Y, Fukushima Y Fau - Anai M, Anai M Fau - Ono H, Ono H Fau - Horike N, Horike N Fau - Viana AYI, Viana Ay Fau - Uchijima Y, Uchijima Y Fau - Nishiyama K, Nishiyama K Fau - Shimosawa T, Shimosawa T Fau - Fujita T, Fujita T Fau - Katagiri H, Katagiri H Fau - Oka Y, Oka Y Fau - Kurihara H, Kurihara H Fau - Asano T, Asano T. Resistin-like molecule beta activates MAPKs, suppresses insulin signaling in hepatocytes, and induces diabetes, hyperlipidemia, and fatty liver in transgenic mice on a high fat diet. Journal of Biological Chemistry (0021-9258 (Print)) 2005 doi: 10.1074/jbc.M503065200. [DOI] [PubMed] [Google Scholar]
- 101.Al Hannan F, Culligan KG. Human resistin and the RELM of Inflammation in diabesity. Diabetol Metab Syndr. 2015;7:54. doi: 10.1186/s13098-015-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smekal A, Vaclavik J. Adipokines and cardiovascular disease: A comprehensive review. Biomedical papers. 2017;161(1):31–40. doi: 10.5507/bp.2017.002. [DOI] [PubMed] [Google Scholar]
- 103.Muse ED, Lam TK, Scherer PE, Rossetti L. Hypothalamic resistin induces hepatic insulin resistance. J Clin Invest. 2007;117(6):1670–8. doi: 10.1172/JCI30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang Y, Lu L, Hu Y, Li Q, An C, Yu X, Shu L, Chen A, Niu C, Zhou L, Yang Z. Resistin Induces Hypertension and Insulin Resistance in Mice via a TLR4-Dependent Pathway. Sci Rep. 2016;6:22193. doi: 10.1038/srep22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim SJ, Nian C, McIntosh CH. Resistin knockout mice exhibit impaired adipocyte glucose-dependent insulinotropic polypeptide receptor (GIPR) expression. Diabetes. 2013;62(2):471–7. doi: 10.2337/db12-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest. 2009;119(3):531–9. doi: 10.1172/JCI37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun. 2001;285(2):561–4. doi: 10.1006/bbrc.2001.5173. [DOI] [PubMed] [Google Scholar]
- 108.Hivert MF, Manning AK, McAteer JB, Dupuis J, Fox CS, Cupples LA, Meigs JB, Florez JC. Association of variants in RETN with plasma resistin levels and diabetes-related traits in the Framingham Offspring Study. Diabetes. 2009;58(3):750–6. doi: 10.2337/db08-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heilbronn LK, Rood J, Janderova L, Albu JB, Kelley DE, Ravussin E, Smith SR. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab. 2004;89(4):1844–8. doi: 10.1210/jc.2003-031410. [DOI] [PubMed] [Google Scholar]
- 110.Graveleau C, Zaha VG, Mohajer A, Banerjee RR, Dudley-Rucker N, Steppan CM, Rajala MW, Scherer PE, Ahima RS, Lazar MA, Abel ED. Mouse and Human Resistins Impair Glucose Transport in Primary Mouse Cardiomyocytes, and Oligomerization Is Required for This Biological Action. Journal of Biological Chemistry. 2005;280(36):31679–31685. doi: 10.1074/jbc.M504008200. [DOI] [PubMed] [Google Scholar]
- 111.Wang JH, Lee CJ, Yang CF, Chen YC, Hsu BG. Serum resistin as an independent marker of aortic stiffness in patients with coronary artery disease. Plos One. 2017;12(8) doi: 10.1371/journal.pone.0183123. [DOI] [PMC free article] [PubMed] [Google Scholar]