Abstract
Objectives
To develop and validate an unbiased, accurate, convenient and inexpensive means of determining when an osseous defect has healed and recovered sufficient strength to allow weight-bearing.
Methods
A novel image processing software algorithm was created to analyze the radiographic images and produce a metric designed to reflect bone strength. We used a rat femoral segmental defect model that provides a range of healing responses from complete union to non-union. Femora were examined by X-ray, micro-computed tomography (μCT) and mechanical testing. Accurate simulated radiographic images at different incident X-ray beam angles were produced from the μCT data files.
Results
The software-generated metric (SC) showed high levels of correlation with both the mechanical strength (τMech) and the polar moment of inertia (pMOI), with the mechanical testing data having the highest association. The optimization analysis yielded optimal oblique angles θB of 125° for τMech and 50° for pMOI. The Pearson’s R2 values for the optimized model were 0.71 and 0.64 for τMech and pMOI, respectively. Further validation using true radiographs also demonstrated that the metric was accurate, and that the simulations were realistic.
Conclusions
The preliminary findings suggest a very promising methodology to assess bone fracture healing using conventional radiography. With radiographs acquired at appropriate incident angles, it proved possible to calculate accurately the degree of healing and the mechanical strength of the bone. Further research is necessary to refine this approach and determine whether it translates to the human clinical setting.
Keywords: image analysis software, bone healing, radiology, computer model, animal model, load bearing
INTRODUCTION
There is a lack of suitable technology for routinely assessing with precision whether or not a fractured bone has healed. Currently, orthopaedic trauma surgeons subjectively and independently determine healing based upon conventional radiography and clinical examination (1–3). Consequently, there is no consensus among clinicians as to when a fracture has healed (4) and, indeed, there is not even a standard definition of healing. These deficiencies are of considerable clinical significance as they determine key aspects of patient care. For example, if weight bearing is allowed to occur prematurely there is a high risk of re-fracture, non- or delayed union, and hardware failure. Likewise, if weight bearing is deferred, there is a risk of delayed fracture healing related to limited micro-motion at the fracture site as well as an increased risk of pin sepsis in cases where external fixators are used. Additionally, unnecessarily extended treatment in plaster or a delay in the removal of an implant can prolong the bone remodeling process and lead to muscle wasting. This has important consequences for both the patients’ quality of life and for medical costs. In fact, problems in bone healing are estimated to cost around $100 billion per year to the US economy alone (5). It is also important to know when a bone has not healed, so that appropriate intervention can be implemented in an expedient manner. Moreover, the lack of robust quantitative outcome measures hampers our ability to conduct meaningful clinical trials to compare different healing modalities (2).
The deficiencies of traditional radiography and clinical judgment are well recognized and a number of biologic (6), radiographic (7) and mechanical testing (8) methods have been explored. The use of biological biomarkers offers the prospect of measuring bone healing by a simple blood test. However, this approach is relatively new; it has yet to become a reliable predictor and remains far from clinical application (9). Measuring changes in the mechanical properties that occur during the different stages of healing are also of interest. Changes in fracture stiffness (10) have been particularly widely investigated because stiffness rises exponentially during healing (11).
Two methods have been used to measure changes in bone stiffness: vibrational analysis and mechanical testing (3). Vibrational analysis approaches include resonant frequency analysis (12), computerized sonometry (13), ultrasound velocity (14), impulse response (15) and steady state analysis (16). Although these methods are non-invasive and painless, they have been shown to be unreliable and sensitive to the interposition of soft tissue, path length, fixation and other variables. Because of this, they have not entered routine clinical practice. Unlike vibrational analysis, mechanical testing provides true measures of stiffness and strength. However, the methods used are invasive, cumbersome, and are not reliable in the presence of internal fixation devices. They may also require the removal of casts, splints and other supportive devices. Radiostereometric analysis was also employed to measure fracture micromotion, and although encouraging preliminary results have been published, the technology is expensive and not widely available (17). Thus, all of these methods suffer from one or more drawbacks including the fact that they are imprecise, invasive, clinically impractical, or very expensive. Therefore, radiological imaging in conjunction with clinical assessment remains the most commonly used approach for assessing fracture healing (18–20).
Some improvements in the accuracy of radiologic assessment has been noted since the introduction of a standardized Radiographic Union Scale for Tibial Fractures (RUST) (1). Of note, the RUST protocol involves two radiographs of the fracture site taken in anteroposterior (AP) and lateral views. However, a conventional plain radiograph is neither sufficiently specific nor sensitive enough to assess reliably the progress of bone healing. Moreover, radiographic images are generally only acquired in two different planes, AP and/or lateral, both of which might be suboptimal for determining whether a fracture is united or not. Furthermore, the RUST assessment is limited to the tibia. Computed tomography (CT), in contrast, offers true three-dimensional (3D) images of the healing bone that are far superior to plain radiography for determining fracture union. However, CT is expensive and incurs a substantially higher radiation dose to patients.
This study explores the possibility of creating a simple, safe, and inexpensive approach to assess fracture healing with novel image analysis software applied to conventional radiography. Similar image processing software has been widely used in other fields, notably to perform computer-aided detection and diagnosis of breast and lung cancer (21). A digital radiograph comprises an array of pixels where varying gray scale intensities define the image; the image analysis software attempts to find patterns or extract subtle, clinically-relevant information from these data. These algorithms are trainable, and can be optimized for maximum performance using a set of examples with known outcomes.
This project was based on the fundamental assumption that there are features visible on a digital radiographic image of a bone that can determine its healing status, but may elude a conventional radiographic assessment. Furthermore, we hypothesized that the conventional posteroanterior (PA) or lateral view radiographs may not be optimal. We have adopted a trainable algorithm under the assumption that the software approach would substantially improve assessment of bone healing once it is trained with a sufficiently large number of cases. The expectation was that the method could potentially detect subtleties in the images that would elude the human eye.
Therefore, the aim of this project was to develop and optimize a specific protocol that combines radiographic acquisitions with image processing software to produce a single metric that provides an accurate way to measure the progress of bone healing. To do this, μCT data were acquired from a rat femoral defect model (22), and used to produce simulated radiographs using a previously published method (23). The goal was to optimize the method in order to determine the ideal X-ray beam angle for the radiographic acquisition.
METHODS
Study Design
Twenty-five male, Sprague-Dawley rats, weighing 310–330g, were used for this study. Rat femoral, mid-diaphyseal, 5 mm defects were stabilized with external fixators providing four different degrees of axial stiffness (24, 25). The defect healing was enhanced using 5.5 μg of rhBMP-2 delivered on an absorbable collagen sponge, the same product as is used clinically (Infuse®, Minneapolis, MN, USA). Details of the surgical procedure are given in reference (26). Animal care and experimental protocols were followed in accordance with National Institutes of Health guidelines and approved by Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. This study was funded by a BIRT (Building Innovative Research Teams) grant from NIAMS. This mechanism is specifically designed to encourage the use of resources developed by a parent R01 grant for an additional purpose. In this case the parent R01 grant supported study of bone healing in a rat femoral defect model. This generated rats with a spectrum of different healing responses, whose μCT, radiograph, and mechanical testing data were used here.
Ex Vivo Mechanical Torsion Testing
After 8 weeks of healing, all 25 specimens were tested to failure using a torsional testing jig in conjunction with Instron MicroTester 5848 material testing system (Instron, MA, USA). Femora were tested to failure by rotating one axis of the femur at the continuous rate of 0.42 °/s using 50 N load cell. Angular deformation and applied load data were acquired at 10 Hz and used to calculate the torque (τMech) of the healed defects.
Micro-Computed Tomography
Micro-computed tomography (μCT) was performed using a desktop system (μCT40, Scanco Medical AG, Brüttisellen, Switzerland) at 70 kvp, with a 200 ms integration time producing images with an isotropic voxel spacing of 20 μm. Images were thresholded using an adaptive-iterative algorithm, and morphometric variables were computed from the binarized images using direct 3D techniques that do not rely on any prior assumptions concerning the underlying structure. A stack of cross-sectional μCT images was used to assess polar moment of inertia (pMOI, mm4). Additionally, a radiograph of each rat was also acquired with a closed cabin X-ray unit Faxitron MX 20 (Faxitron Bioptics LLC, Tucson, AZ, USA) system. Using custom software (23) realistic simulated radiographic images were produced from the raw (non-binarized) μCT data files based on the imaging configuration depicted in Figure 1. The simulated radiographs permitted the production of radiographic images at any θB, which could then be analyzed by the software. θB is defined in Figure 1. The true radiographs were acquired at θB = 0°, an X-ray beam direction perpendicular to the external fixators.
Figure 1.
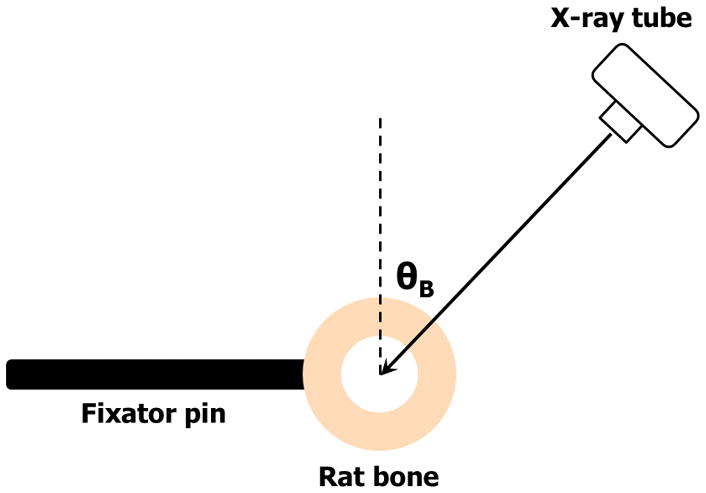
Depiction of the imaging geometry used for the simulations, and for future prospective studies of rat models. The true radiographs used in the current study were acquired at θB = 0°.
Data Analysis
A specialized image processing software algorithm was created to analyze the gray scale image intensities to produce a metric, SC, designed to reflect the bone strength. The computer model incorporated several parameters with values determined via a training and optimization procedure that selected the optimal guidelines to correlate with the independent bone strength measures. Based on a limited number of subjects (25 rats), the leave-one-out approach was employed to perform software optimization using the CVPRESS statistic (27). To further validate the use of simulations, the optimized model was applied to the true radiographs acquired at θB = 0°, measuring the correlation of SC to τMech and pMOI, and the correlation of the individual SC values obtained with simulated and true radiographs. The optimized model SC values were compared to τMech and pMOI data using the R2-value from a Pearson’s correlation analysis as a performance measure, and the dependence of R2 on θB for each comparison metric was also examined.
RESULTS
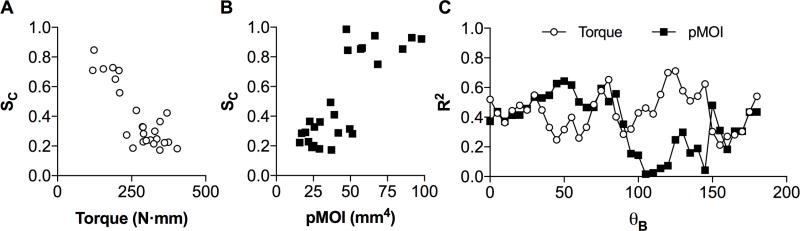
An example of a simulated image, with the corresponding true radiograph, demonstrated the realistic nature of the technique as shown in figure 2. The optimization analysis yielded an optimal angle θB of 125° for τMech and 50° for pMOI as the beam direction that produced SC having the highest correlation with the mechanical testing data. The Pearson’s R2 values for the optimized model were 0.71 and 0.64 for τMech and pMOI, respectively.
Figure 2.
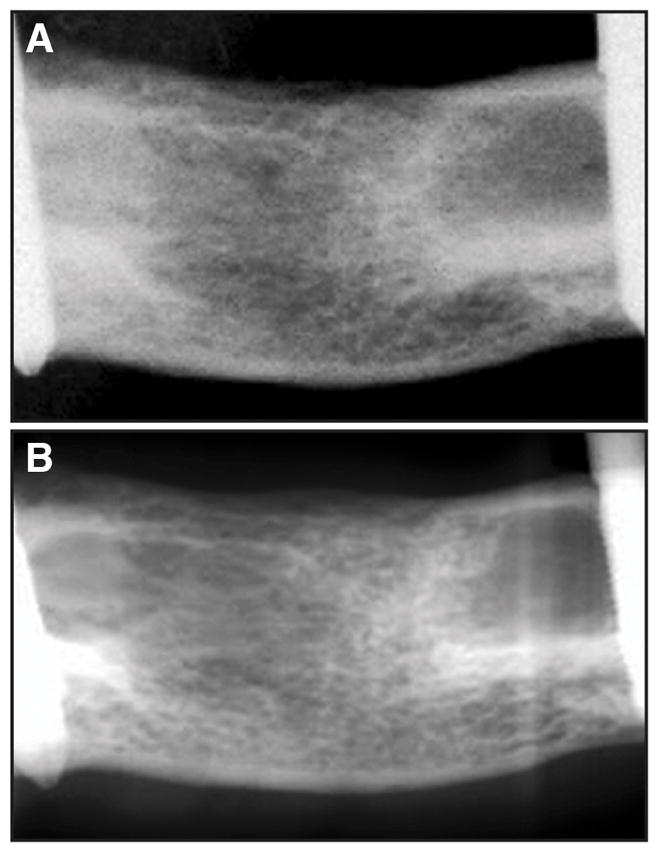
(A) True and (B) simulated radiographs of rat femora produced from micro-CT scans of bridged defects after 8 weeks. While there is not a perfect match of the image contrast, the fine structure of the simulated image, upon which SC is derived, is highly realistic.
Both bone strength parameters, from the physical testing (τMech) and μCT computational analysis (pMOI), demonstrated the strong association between SC (Figure 3a and b). Although the level of correlation for each metric was similar, the optimal angle was different.
Figure 3.
(A) Graph of SC versus τMech for the model optimized for τMech. The Pearson’s R2 was 0.71; (B) Graph of SC versus pMOI for the model optimized for pMOI. The Pearson’s R2 was 0.67; (C) Graph of the R2 values versus θB for both the torque, τMech, and pMOI showing a different behavior for each metric, and an irregular pattern which suggests the possibility of different optimal θB for each of the samples.
For the assessment of the true radiographs, the R2 values comparing SC to τMech and pMOI were 0.46 and 0.34 respectively, which is similar to the correlations from simulations (R2 = 0.52 and 0.37) for θB = 0°. SC derived from simulations at θB = 0° was correlated to SC from true radiographs with an R2 value of 0.55. To further explore the θB sensitivity, Figure 3c shows a graph of Pearson’s R2 as a function of θB for τMech and pMOI, which demonstrates an irregular dependence for both variables.
DISCUSSION
The preliminary findings suggest a very promising methodology to assess bone fracture healing using conventional radiography. This could potentially offer clinicians and researchers a fast, safe, and inexpensive method to quantify bone healing. The software metric exhibits a high level of correlation with both torque from physical testing, as well as pMOI from image analysis of the μCT data. These results indicate that it is possible to set a threshold value on SC that would have a high accuracy to distinguish between a healed and non-healed fracture (Figure 3a).
The software approach has a potential impact for both the clinical and research environments. In a clinical setting, there is an unmet need to improve bone healing assessment methods in order to reduce the risk of re-fracture from premature removal of fixator hardware. A version of this software could eventually be incorporated into digital radiology products to serve as a tool for orthopedic surgeons and musculoskeletal radiologists. In the laboratory, this method could be used in experimental in vivo studies to assess bone healing so that longitudinal assessment can be performed without the need for μCT or serial euthanasia. According to this study, radiographs acquired at an angle θB of 125° should be used for optimal performance.
The different optimal angles θB for τMech (125°) and pMOI (55°) suggest that these metrics have a degree of independence from each other, perhaps probing different mechanical properties of the bone. It is somewhat surprising that the correlation of SC to τMech is superior to that of pMOI, given that pMOI and SC are both metrics derived from the image data, while τMech is determined through independent mechanical testing. The correlation of SC to τMech is higher than the correlation of pMOI to τMech (R2 = 0.71 versus R2 = 0.64) providing further evidence that our software metric is an accurate measure of true bone strength. The irregular pattern, as shown in Figure 3c, could be an indication that the optimal value of θB may vary for different samples. To determine this will require further investigations.
There are several limitations to our study. The results using simulations from μCT will not necessarily guarantee similar behavior when the method is used on actual radiographs acquired at the optimal angle θB. However, the simulations provide a platform which will be further developed to optimize the model, thus setting the stage for more realistic testing. This weakness was mitigated by the results using true radiographs acquired at θB = 0°, which offer a realistic evaluation of the method. The similar R2 values for simulated and true radiographs, and the correlation between SC for both image types, offer concrete evidence that the method is accurate. Future work will use true radiographs made at the optimal θB values to verify the improved correlation for that angle. Moreover, the method was tested on a fracture model that did not use metal intramedullary nails implanted in the bone itself, which could potentially affect the accurate assessment of the fracture outcomes. Unlike CT however, there are no streak artifacts associated with metallic objects in a radiograph. It would be very straightforward to remove the projected intramedullary implant from the image leaving the remainder of the radiograph unaffected. This would leave the majority of the critical part of the radiograph intact and available for analysis as it is the portion specifically required by our software to assess fracture healing. Additionally, a method that functions well using laboratory-created segmental defects in the rat femur might not directly translate and perform optimally to evaluate progress of fracture healing in humans. Therefore, clinical studies will be necessary to modify the method before it can be used routinely in the clinical setting. Nevertheless, a proof-of-principle was established, and substantial problems are not anticipated in translating this method for use on human subjects.
In conclusion, this study demonstrated a novel, software-based method to accurately assess the progress of bone healing in the rat model. We predict that this method will be a promising tool in the evaluation of fracture healing in patients.
Acknowledgments
This study was supported by NIH/NIAMS R01AR050243-S. We would like to thank Dr. Anna Woloszyk for her assistance with the figures.
This project was funded by a BIRT Award (R01AR050243-09S1) from NIAMS (parent R01: R01AR050243). This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Presented as a poster at the Orthopaedic Research Society annual meeting, San Diego, CA, March 20, 2017.
Conflicts of Interest and Source of Funding: NIAMS
References
- 1.Kooistra BW, Sprague S, Bhandari M, et al. Outcomes assessment in fracture healing trials: a primer. J Orthop Trauma. 2010;24(Suppl 1):S71–75. doi: 10.1097/BOT.0b013e3181ca3fbd. [DOI] [PubMed] [Google Scholar]
- 2.Morshed S, Corrales L, Genant H, et al. Outcome assessment in clinical trials of fracture-healing. J Bone Joint Surg Am. 2008;90(Suppl 1):62–67. doi: 10.2106/JBJS.G.01556. [DOI] [PubMed] [Google Scholar]
- 3.Wade R, Richardson J. Outcome in fracture healing: a review. Injury. 2001;32:109–114. doi: 10.1016/s0020-1383(00)00126-1. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari M, Guyatt GH, Swiontkowski MF, et al. A lack of consensus in the assessment of fracture healing among orthopaedic surgeons. J Orthop Trauma. 2002;16:562–566. doi: 10.1097/00005131-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein EA, Corso PS, Miller TR. The incidence and economic burden of injuries in the United States. J Epidemiol Community Health. 2007;61:926. [Google Scholar]
- 6.Pountos I, Georgouli T, Pneumaticos S, et al. Fracture non-union: Can biomarkers predict outcome? Injury. 2013;44:1725–1732. doi: 10.1016/j.injury.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Whelan DB, Bhandari M, Stephen D, et al. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma. 2010;68:629–632. doi: 10.1097/TA.0b013e3181a7c16d. [DOI] [PubMed] [Google Scholar]
- 8.Fountain S, Windolf M, Henkel J, et al. Monitoring Healing Progression and Characterizing the Mechanical Environment in Preclinical Models for Bone Tissue Engineering. Tissue Eng Part B Rev. 2015 doi: 10.1089/ten.TEB.2015.0123. [DOI] [PubMed] [Google Scholar]
- 9.Cox G, Einhorn TA, Tzioupis C, et al. Bone-turnover markers in fracture healing. J Bone Joint Surg Br. 2010;92:329–334. doi: 10.1302/0301-620X.92B3.22787. [DOI] [PubMed] [Google Scholar]
- 10.Chehade MJ, Pohl AP, Pearcy MJ, et al. Clinical implications of stiffness and strength changes in fracture healing. J Bone Joint Surg Br. 1997;79:9–12. doi: 10.1302/0301-620x.79b1.6324. [DOI] [PubMed] [Google Scholar]
- 11.Richardson JB, Cunningham JL, Goodship AE, et al. Measuring stiffness can define healing of tibial fractures. J Bone Joint Surg Br. 1994;76:389–394. [PubMed] [Google Scholar]
- 12.Tower SS, Beals RK, Duwelius PJ. Resonant frequency analysis of the tibia as a measure of fracture healing. J Orthop Trauma. 1993;7:552–557. doi: 10.1097/00005131-199312000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Glinkowski W, Gorecki A. Clinical experiences with ultrasonometric measurement of fracture healing. Technol Health Care. 2006;14:321–333. [PubMed] [Google Scholar]
- 14.Lowet G, Van der Perre G. Ultrasound velocity measurement in long bones: measurement method and simulation of ultrasound wave propagation. J Biomech. 1996;29:1255–1262. doi: 10.1016/0021-9290(96)00054-1. [DOI] [PubMed] [Google Scholar]
- 15.Fellinger M, Leitgeb N, Szyszkowitz R, et al. Early detection of delayed union in lower leg fractures using a computerised analysis of mechanical vibration reactions of bone for assessing the state of fracture healing. Arch Orthop Trauma Surg. 1994;113:93–96. doi: 10.1007/BF00572913. [DOI] [PubMed] [Google Scholar]
- 16.Lowet G, Dayuan X, Van der Perre G. Study of the vibrational behaviour of a healing tibia using finite element modelling. J Biomech. 1996;29:1003–1010. doi: 10.1016/0021-9290(96)00002-4. [DOI] [PubMed] [Google Scholar]
- 17.Madanat R, Moritz N, Larsson S, et al. RSA applications in monitoring of fracture healing in clinical trials. Scand J Surg. 2006;95:119–127. doi: 10.1177/145749690609500207. [DOI] [PubMed] [Google Scholar]
- 18.Marsh D. Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res. 1998:S22–30. doi: 10.1097/00003086-199810001-00004. [DOI] [PubMed] [Google Scholar]
- 19.Szechinski JW, Grigorian MA, Grainger AJ, et al. Femoral neck and intertrochanteric fractures: radiographic indicators of fracture healing. Orthopedics. 2002;25:1365–1368. doi: 10.3928/0147-7447-20021201-14. discussion 1368. [DOI] [PubMed] [Google Scholar]
- 20.Whelan DB, Bhandari M, McKee MD, et al. Interobserver and intraobserver variation in the assessment of the healing of tibial fractures after intramedullary fixation. J Bone Joint Surg Br. 2002;84:15–18. doi: 10.1302/0301-620x.84b1.11347. [DOI] [PubMed] [Google Scholar]
- 21.Giger ML, Chan HP, Boone J. Anniversary paper: History and status of CAD and quantitative image analysis: the role of Medical Physics and AAPM. Med Phys. 2008;35:5799–5820. doi: 10.1118/1.3013555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glatt V, Kwong FN, Park K, et al. Ability of recombinant human bone morphogenetic protein 2 to enhance bone healing in the presence of tobramycin: evaluation in a rat segmental defect model. J Orthop Trauma. 2009;23:693–701. doi: 10.1097/BOT.0b013e3181b01b2f. [DOI] [PubMed] [Google Scholar]
- 23.Duryea J, Magalnick M, Alli S, et al. Semiautomated three-dimensional segmentation software to quantify carpal bone volume changes on wrist CT scans for arthritis assessment. Med Phys. 2008;35:2321–2330. doi: 10.1118/1.2900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glatt V, Bartnikowski N, Quirk N, et al. Reverse Dynamization: Influence of Fixator Stiffness on the Mode and Efficiency of Large-Bone-Defect Healing at Different Doses of rhBMP-2. J Bone Joint Surg Am. 2016;98:677–687. doi: 10.2106/JBJS.15.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glatt V, Evans CH, Matthys R. Design, characterisation and in vivo testing of a new, adjustable stiffness, external fixator for the rat femur. Eur Cell Mater. 2012;23:289–298. doi: 10.22203/ecm.v023a22. discussion 299. [DOI] [PubMed] [Google Scholar]
- 26.Glatt V, Matthys R. Adjustable stiffness, external fixator for the rat femur osteotomy and segmental bone defect models. J Vis Exp. 2014:e51558. doi: 10.3791/51558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SAS/STAT(R) 9.2 User’s Guide SE. 2017 https://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm-statug_glmselect_sect025.htm.