Abstract
Phenotypic differences among substrains of laboratory mice due to spontaneous mutations or pre-existing genetic variation confound the interpretation of targeted mutagenesis experiments, and contribute to challenges with reproducibility across institutions. Notably, C57BL/6 Hsd mice and gene-targeted mice that have been backcrossed to this substrain have been reported to harbor a duplication in exons 28 and 29 of Dock2. Here, we demonstrate the presence of this Dock2 variant in the widely used Nod2−/− mice. NOD2 is a cytosolic innate immune receptor associated with inflammatory bowel disease (IBD) susceptibility. Consistent with a role of NOD2 in an immunological disorder, Nod2−/− mice bred at our institution displayed multiple B cell defects including deficiencies in recirculating B cells, marginal zone B cells and B1a cells in vivo, as well as defects in class switch recombination in vitro. However, we found that these effects are due to the Dock2 variant and are independent of Nod2 deletion. Despite originating from the same gene-targeted founder mice, Nod2−/− mice from another source did not harbor the Dock2 variant or B cell defects. Finally, we show that Dock2−/− mice display the same B cell defects as mice harboring the Dock2 variant, confirming that the variant is a loss-of-function mutation and is sufficient to explain the alterations to the B cell compartment observed in Nod2−/− mice. Our findings highlight the effects of confounding mutations from widely-used inbred strains on gene-targeted mice and reveal new functions of DOCK2 in B cells.
INTRODUCTION
Inbred laboratory mice are essential animal models that facilitate comparison of experimental outcomes observed across institutions. They are commonly used to identify the function of a gene product following a targeted mutagenesis event such as a gene knockout or the expression of a transgene. However, spontaneous mutations, that eventually contribute to genetic drift, can arise from maintaining breeding stocks separately at different vendors and institutions, and the effect of genetic variability among a given inbred strain is rarely considered. These concerns may have contributed to the recent controversy regarding reproducibility of findings using preclinical mouse models (1).
Among inbred mice, C57BL/6 is the most commonly used genetic background in immunology research. Although C57BL/6 substrains are known to harbor genetic differences (2), they are often treated as equal and are not distinguished in publications. The discovery of a mutation within the coding region of dedicator of cytokinesis 2 (Dock2) in certain gene-targeted C57BL/6 lines highlights this issue. DOCK2 mediates actin polymerization and intracellular signaling as a guanine nucleotide exchange factor (GEF) for the small GTPase Rac (3). Consistent with its expression in the hematopoietic compartment, DOCK2 mutations have been identified in immunocompromised children who display early onset invasive infections (4). Also, Dock2−/− mice display several immune defects including loss of marginal zone (MZ) B cells and impaired migration of lymphocytes, neutrophils, and plasmacytoid dendritic cells (pDCs) (3, 5–7). Similar immune defects observed in mice with other targeted mutations have now been attributed to a spontaneous mutation in Dock2. Loss of MZ B cells and reduced type I interferon (IFN) production by pDCs in Irf5−/− mice were shown to be independent of Irf5 deletion and instead due to a duplication of exons 28 and 29 of Dock2 that leads to reduced Dock2 expression (8). Likewise, defects in B cell development observed in a subset of mice deficient in sialic acid acetyl esterase (Siae−/−) were due to a similar exon duplication in Dock2. This mutation in Dock2 was traced to the C57BL/6 Hsd substrain that was used to backcross the Siae−/− line onto the C57BL/6 background (9). Nonetheless, not all the B cell defects in these mutant mice were a consequence of the defective Dock2 allele, as another study noted that differences in IgG isotype switching were found in Irf5−/− mice with and without the Dock2 variant (10). Decreased Dock2 expression has also been suggested to explain why a subset of mice deficient in the inflammasome adaptor ASC (Apoptosis-associated speck-like protein containing a CARD, Pycard−/−) display defects in antigen presentation and lymphocyte migration, although it is unclear whether these mice harbor the same duplication event as Irf5−/− mice (11).
Here, we demonstrate that B cell defects in a Nod2−/− inbred strain are due to Dock2 mutation and independent of Nod2 deficiency. NOD2 (nucleotide-binding oligomerization domain-containing protein 2) is a cytosolic pattern recognition receptor best known for controlling an antimicrobial gene expression program in response to peptidoglycan (12). Loss of function mutations in NOD2 are among the strongest susceptibility factors for Crohn’s disease, a major type of inflammatory bowel disease (IBD) characterized by chronic relapsing inflammation of the gastrointestinal tract (13). Inconsistent results obtained with Nod2 mutant mice have created a major barrier to understanding the role of NOD2 in Crohn’s disease. Nod2−/− mice were originally shown to display defective defensin expression by Paneth cells (14), antimicrobial epithelial cells in the small intestine (15). However, this defect was not observed in commercially available Nod2−/− mice that were backcrossed onto the C57BL/6J background (16). Also, early findings demonstrating increased cytokine production and a T cell-intrinsic function in Nod2 mutant mice were not reproduced in subsequent studies, potentially due to the presence of unintended mutations in the original mice that were characterized (17–22). Variation in the microbiota can also explain disparate results. Helicobacter species that are eradicated in some animal facilities induce an enhanced Th1 response in Nod2−/− mice that leads to inflammatory lesions in the small intestine (23). Although Nod2−/− mice are susceptible to colonization by Bacteroides species, control wild-type (WT) mice co-housed with Nod2−/− mice acquire a similar microbiota (24–26). We previously demonstrated that a particular Bacteroides species that is not present in commercially available Nod2−/− mice, Bacteroides vulgatus, mediates goblet cell defects in Nod2−/− mice and not WT mice raised in our vivarium (24, 27). Thus, genetic background and microbiota composition have profound influence on results obtained with Nod2 mutant mice.
In this study, we identify deficiencies in populations of recirculating B cells in the bone marrow, MZ B cells, and splenic and peritoneal B1a B cells in Nod2−/− mice. These B cell defects were not present in mice deficient in the NOD2 signaling adaptor RIP2 (receptor interacting protein kinase 2, Rip2−/−) or a second Nod2−/− line that we acquired. We found that differences in phenotype were driven by the presence of the aforementioned Dock2 mutation. Importantly, we demonstrate that independently generated Dock2−/− mice display similar B cell defects. All together, these findings reveal new functions of DOCK2 and show that certain lymphocyte-defects observed in Nod2−/− mice are independent of NOD2 function.
MATERIALS AND METHODS
Mice
Nod2−/− mice backcrossed to the C57BL/6 background for at least 12 generations were previously described (28). These mice were imported to Washington University School of Medicine and subsequently rederived into the animal facility at NYU School of Medicine where they have been maintained until present. Nod2−/− mice (Nod2−/−Jax mice) derived from the original gene targeting experiment (14) were obtained from The Jackson Laboratory and bred on-site. Wild-type C57BL/6J mice were purchased from The Jackson Laboratory and bred on-site. Tail clippings from Cd11a−/− mice (Jackson Laboratory stock #005257 that were originally back-crossed to C57BL/6 Hsd strain) were obtained from Dr. Susan Schwab at the New York University School of Medicine. Rip2−/− mice bred on-site were previously described (24, 29). All on-site animals were maintained in a specific pathogen-free facility at the New York University School of Medicine. Experiments were approved by the Institutional Animal Care and Use Committee of the New York Unversity School of Medicine. Dock2−/− mice and wild-type littermates used for experiments were generated from Dock2+/− breeders and were a gift of Dr. Yoshinori Fukui of Kyoto University (3). These mice were bred and housed at a specific pathogen-free facility at Charles River Laboratories. Experiments were approved by the IACUC of the Icahn School of Medicine at Mount Sinai.
Bone marrow chimeras
Bone marrow chimeras were generated by irradiating 8-week-old female Rag1−/− mice (1,100 CGy once) followed by intravenous injection of 5 × 106 T-cell-depleted bone marrow cells mixed in a 1:1 ratio from donor WT and Nod2−/− female mice. Recipient Rag1−/− mice were treated with antibiotics in their drinking water one week prior to irradiation.
In vitro B cell assays
Single cell suspensions were harvested from the cervical, axillary, inguinal, and para-aortic lymph nodes of WT, Nod2−/−, and Nod2−/− Dock2mut mice. Mature B cells were isolated using CD43+ Dynabeads (Thermo Fisher Scientific) and stained with Cell Trace Violet dye (BD Biosciences) to track proliferation. 1×106 B cells were plated in 500μL of B cell media with 20μg/mL of lipopolysaccharide (Sigma) for plasma cell differentiation assays, 25μg/mL of IL-4 (R&D) and 2μg/mL of anti-CD40 (eBioscience) for IgG1 class switch recombination assays, or 0.4μg/mL of BAFF (R&D) to enable in vitro survival and act as negative control in the proliferation assays. Cultured cells were harvested on day four and extent of proliferation was assessed via FACS.
Flow cytometry
Single cell suspensions from spleen, bone marrow, and cells from the peritoneal cavity were stained for cell surface markers. Antibodies to the following cell surface markers were used: anti-B220 (eBioscience and Biolegend), CD19 (eBioscience and Biolegend), CD25 (eBioscience), c-kit (eBioscience), AA4.1 (CD93) (eBioscience), CD21 (eBioscience), CD23 (eBioscience), CD3 (eBioscience and Biolegend), CD19 (Biolegend), CD5 (eBioscience), CD1d (Biolegend), IgM (μ chain specific) (Jackson ImmunoResearch), CD138 (BD Biosciences), IgG1 (BD Biosciences), CD11b (Biolegend), CD11c (Biolegend), BST2 (Biolegend), and MHCII (Biolegend). FACS analysis was performed on an LSR Fortessa cytometer. Data were analyzed using FlowJo software. Events are gated on total lymphocytes and singlets. Further gating is described in the figure legends when appropriate.
PCR
Genomic DNA from mouse tails was harvested by NaOH extraction and analyzed for the presence of the Dock2 duplication by PCR as previously described (9, 30). PCR primers used for the Dock2 duplication were ln29.4F 5′-GACCTTATGAGGTGGAACCACAACC-3′ and lnR22.3.1R 5′-GATCCAAAGATTCCCTACAGCTCCAC-3′. PCR primers for the internal control (CD19) were oIMR1589 5′-CCTCTCCCTGTCTCCTTCCT-3′ and oIMR1590 5′-TGGTCTGAGACATTGACAATCA-3′.
Statistical analysis
Statistical analysis was performed using GraphPadPrism 7. Differences in median values were analyzed by Mann-Whitney U test to compare two groups.
RESULTS
Nod2−/− mice are deficient in several B cell subsets
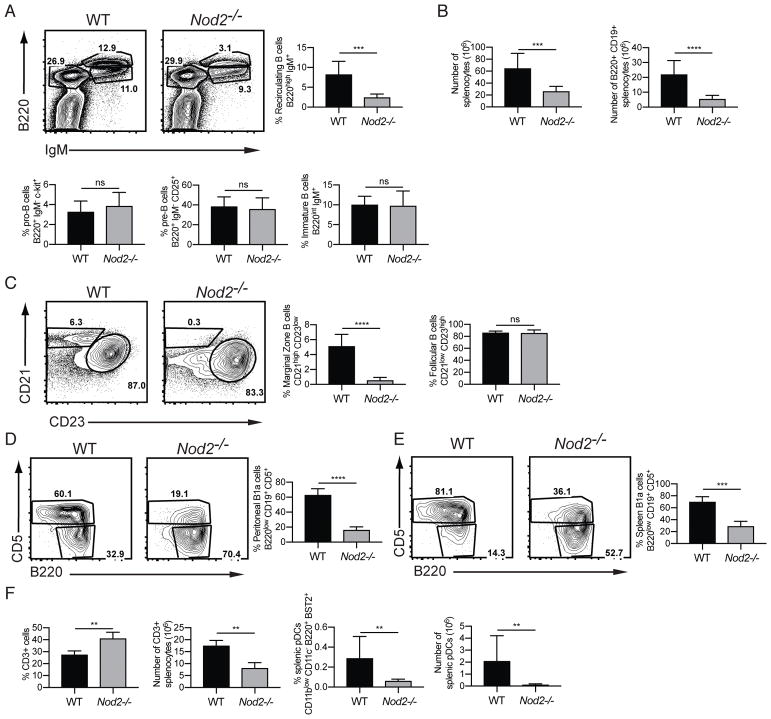
Nod2−/− mice are susceptible to disease caused by intestinal pathogens and pro-inflammatory members of the microbiota (23, 24, 26, 31). Given the fundamental role of B cells in mucosal immunity, and the role of antibodies in intestinal homeostasis and immune exclusion, we performed a broad flow cytometry-based analyses of B cell populations in the bone marrow and spleen of Nod2−/− mice to evaluate the contribution of these cells to colonization resistance. The Nod2−/− mice maintained in our animal facility were originally generated through gene targeting of 129/SvJ ES cells and backrossed over 10 generations onto the C57BL/6 background (see Methods). We found that these Nod2−/− mice lacked recirculating B cells defined as B220hi IgM+ cells in the bone marrow, despite displaying similar proportions of pro- and pre-B cells as well as immature B lymphocytes compared to wild-type (WT) C57BL/6J controls (Fig 1A). Nod2−/− mice also displayed marked splenocytopenia with a reduction in total B220+CD19+ B cell numbers compared to WT controls (Fig. 1B). Although the follicular B cell compartment remained intact, MZ B cells were entirely absent in the spleen of Nod2−/− animals (Fig. 1C). Additionally, there was an increase in the proportion of CD3+ T cells in the spleen, although the number of cells was lower than in wild-type (Fig. 1F). The Nod2−/− mice also displayed a defect in proportion and number of plasmacytoid dendritic cells, while other myeloid lineage cells remained unaffected (Fig. 1F, data not shown).
Figure 1.
Nod2−/− mice have defects in recirculating B cells, marginal zone B cells, B1a cells. (A) Representative FACS plots and percentage of recirculating B cells (B220high IgM+) and pro-B, and pre-B, and immature B cells in the bone marrow. (B) Number of total live cells, number of B220+ CD19+ B cells, and ratio of immature to mature B cells in the spleen. (C) Representative plots and percentages of marginal zone (CD21 high CD23 low) and follicular (CD21 low CD23 high) B cells in the spleen. Populations are gated on B220+ CD19+ AA4.1-mature B cells. (D–E) Representative plots and percentages of B1a cells (B220 low, CD19+ CD5+) from peritoneal lavage (D) and spleen (E). (F) Percentage and numbers of CD3+ T cells and plasmacytoid dendritic (CD11blow CD11c− B220+ BST2+) cells in the spleen. For A–E, data is from is representative of >5 independent experiments from a total of WT (n = 9) and Nod2−/− (n = 9) mice. For F, data is representative of two independent experiments from a total of WT (n = 4) and Nod2−/− (n = 8) mice. ns p > 0.05, ***p ≤ 0.001, and ****p ≤ 0.0001 by two-tailed Mann-Whitney.
B1 B cells participate in early immune responses as a first line of defense against a wide range of pathogens and are critical contributors to mucosal IgA responses (32, 33). Because of the shared signaling pathways downstream of Ig engagement between MZ and B1 B cells (34–36), defects in MZ development are often accompanied by a reduction in B1a B cells. We found that B1a B cells were reduced in the spleen and the peritoneal cavity of Nod2−/− compared to WT mice (Fig. 1D, E). These findings indicate that Nod2−/− mice maintained in our facility are deficient in several key B cell populations, specifically recirculating B cells in the bone marrow, MZ B cells in the spleen, and B1a B cells in the spleen and peritoneum.
B cell defects in Nod2−/− mice are cell-autonomous
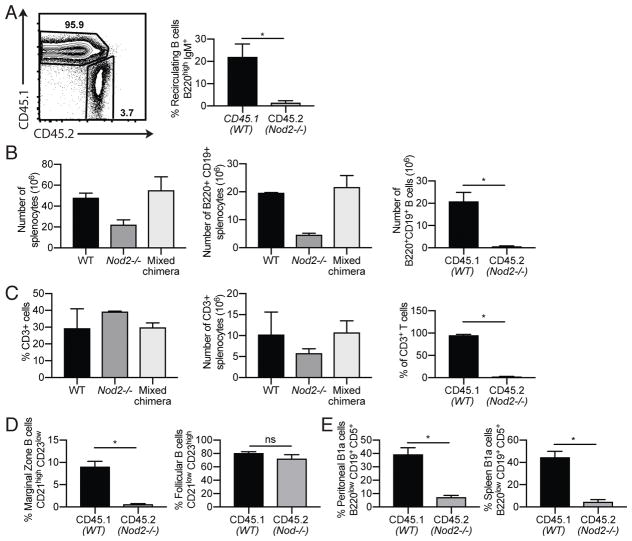
The lymphocyte-intrinsic function of NOD2 is controversial (17–22). To ask whether B cell defects are due to cell-intrinsic changes in lymphocytes and lymphocyte progenitor cells or induced by extrinsic factors, we reconstituted Rag1−/− mice with mixed bone marrow from Nod2−/− mice (CD45.2+) and congenic CD45.1+ WT mice. We observed that the proportion of CD45.2+ cells in recipients were decreased when compared with CD45.1+ cells (Fig. 2A), suggesting a general defect in the development or maintenance of Nod2−/− leukocytes when forced to compete with WT cells. Analysis of splenocytes further support this observation. Total splenocyte and splenic B cell numbers in chimeric mice were comparable to WT, and the majority of splenic B cells were of WT origin (Fig. 2B). The percentage and number of CD3+ T cells were similarly affected (Fig. 2C), suggesting a possible defect in progenitor populations. For subsequent analyses, the proportion of WT and Nod2−/− B cell populations were normalized to total B cells (B220+ cells) of the respective genotypes. The proportion of Nod2−/−-derived CD45.2+ recirculating B cells was reduced compared with WT-derived CD45.1+ cells (Fig. 2A). Analysis of spleens and peritoneal cells from the mixed bone marrow chimera mice revealed fewer Nod2−/−-derived MZ B cells and B1a cells (Fig. 2D, E). In contrast, although the total number of follicular B cells in the spleen were decreased due to the reduced reconstitution of the B cell compartment by Nod2−/−-derived cells, the relative proportion of Nod2−/− follicular B cells was similar to their WT-derived counterparts (Fig. 2D). Together, these data suggest that extrinsic factors do not explain the loss of recirculating, MZ, and B1a B cells in the Nod2−/− mice.
Figure 2.
Proportions of Nod2−/− compared with wild-type B cells are decreased in mixed bone marrow chimera mice. Rag1−/− mice were reconstituted with mixed bone marrow from Nod2−/− (CD45.2+) and congenic CD45.1+ WT mice. (A) Representative plot of CD45.1+ and CD45.2+ mature B cells in the bone marrow. Single cells were gated on CD45.1 or CD45.2 followed by IgM versus B220. The percentages of CD45.1 or CD45.2 recirculating B cells are shown. (B) Number of total live cells and B220+ CD19+ B cells in the spleen. The percentages of B cells that are CD45.1 or CD45.2 are shown. (C) Percentage and numbers of CD3+ T cells in the spleen. The percentages of T cells that are CD45.1 or CD45.2 are shown. (D) Percentages of marginal zone (CD21 high CD23 low) and follicular (CD21 low CD23 high) B cells among CD45.1+ or CD45.2 positive cells in the spleen. (E) Percentages of B1a cells (B220 low, CD19+ CD5+) from peritoneal lavage and spleen. CD19+ CD3- cells were gated into CD45.1 and CD45.2 populations followed by B1 gate (CD19+ B220 low) from which B1a cells were gated (CD5+ B220 low). Data is representative of two independent experiments from a total of WT (n = 4), Nod2−/− (n = 8), and mixed bone marrow chimera Rag1−/− (n = 4) mice. ns p > 0.05 and *p ≤ 0.05 by two-tailed Mann-Whitney.
RIP2 deficiency does not recapitulate B cell defects observed in Nod2−/− mice
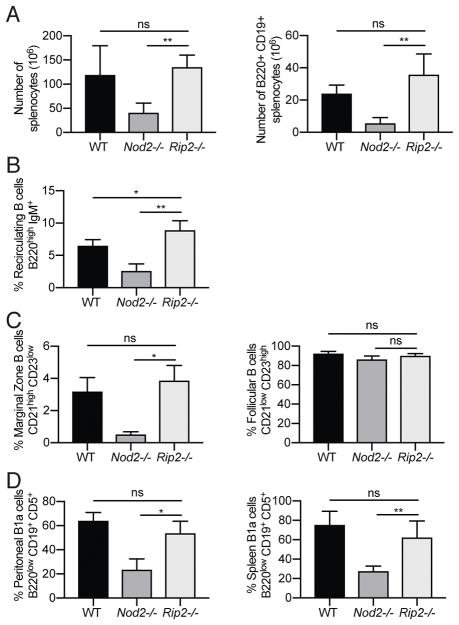
In the presence of the peptidoglycan derivative muramyl dipeptide (MDP), NOD2 engages receptor interacting protein 2 (RIP2) to initiate downstream NF-κB and MAPK signaling. In our previous study, we demonstrated that Rip2−/− mice phenocopied intestinal pathologies and susceptibility to alterations in the microbiota observed in Nod2−/− mice (24). We therefore asked whether disruption of RIP2 function would recapitulate the B cell defects seen in the Nod2−/− mice. Unexpectedly, total splenocyte and splenic B cell numbers were normal in Rip2−/− mice, and recirculating B cells in the bone marrow and MZ B cells in the spleen of Rip2−/− mice were present in proportions similar to WT mice (Fig. 3A–C). Similarly, we did not observe a decrease in splenic or peritoneal B1a B cells in Rip2−/− mice (Fig. 3D). Thus, the B cell defects in the Nod2−/− mice are not observed in mice deficient in the signaling adaptor protein RIP2.
Figure 3.
Rip2−/− mice have normal recirculating B cell, marginal zone B cell, and B1a cell compartments. (A) Number of total live cells and B220+ CD19+ B cells in the spleen. (B) Percentage of bone marrow recirculating B cells (B220high IgM+). (C) Percentages of marginal zone (CD21 high CD23 low) and follicular (CD21 low CD23 high) B cells in the spleen. (D) Percentages of B1a cells (B220 low, CD19+ CD5+) from peritoneal lavage andspleen. Data is representative of three independent experiments from a total of WT (n = 4), Nod2−/− (n = 5), and Rip2−/− (n = 6) mice. ns p > 0.05, *p ≤ 0.05, and **p ≤ 0.01 by two-tailed Mann-Whitney.
Commercially-available Nod2−/− mice do not show B cell defects
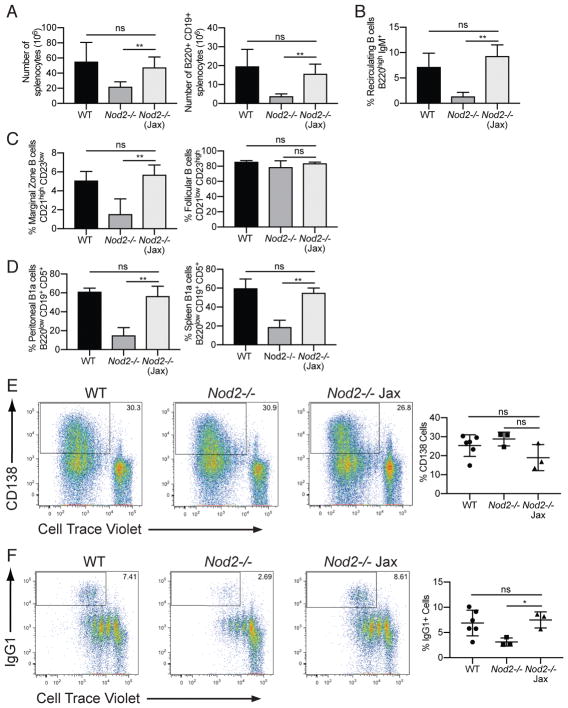
The observation that NOD2 may be functioning in a manner independent of RIP2 was unexpected. Thus, we sought to verify our findings by obtaining Nod2−/− mice from another source. Nod2−/− mice available for purchase from Jackson Laboratory were initially generated from the same gene targeted founders as the Nod2−/− mice maintained in our facility, but were backcrossed to the C57BL/6J background independently. These mice (Nod2−/−Jax) should be genetically identical to our Nod2−/− mice at the Nod2 locus. However, whereas our Nod2−/− mice have been backcrossed using an unspecified C57BL/6 substrain for 12 generations and have passed through multiple institutions (see Methods), the Nod2−/−Jax mice were deposited after six generations of backcrossing and backcrossed for one additional generation to C57BL/6J mice; the Jackson Laboratory reports that single nucleotide polymorphism (SNP) analysis shows that these mice are on a mixed C57BL/6J and C57BL/6N background. Analyses of bone marrow, spleen, and peritoneal cells showed that the Nod2−/−Jax mice did not harbor any of the B cell defects found in the Nod2−/− mice (Fig. 4A–D). Thus, commercially available Nod2−/−Jax mice do not reproduce the reduction in B cell populations observed in our Nod2−/− colony.
Figure 4.
Nod2−/− Jax mice have normal recirculating B cell, marginal zone B cell, and B1a cell compartments, and display normal proliferation and class switch recombination (CSR) in vitro. (A) Number of of total live cells and B220+ CD19+ B cells in the spleen. (B) Percentage of bone marrow recirculating B cells (B220high IgM+). (C) Percentages of marginal zone (CD21 high CD23 low) and follicular (CD21 low CD23 high) B cells in the spleen. Population are gated on B220+ CD19+ AA4.1- mature B cells. (D) Percentages of B1a cells (B220 low, CD19+ CD5+) from peritoneal lavage and spleen. (E) Representative plots and percentages of proliferating CD138+ plasma cells after in vitro stimulation of mature B cells with LPS for 4 days. (F) Representative plots and percentages of proliferating IgG1+ B cells after in vitro stimulation of mature B cells with Il-4 and anti-CD40 for 4 days. For A–D, data is representative of three independent experiments from a total of WT (n = 3), Nod2−/− (n = 5), and Nod2−/− Jax (n = 6) mice. For E–F, data is representative of two independent experiments from a total of WT (n = 7), Nod2−/− (n = 3), and Nod2−/− Jax (n = 4) mice. ns p > 0.05 and **p ≤ 0.01 by two-tailed Mann-Whitney.
To examine the impact of loss of Nod2 on mature B cells, we examined differences in terminal B cell differentiation in lymphocytes isolated from our Nod2−/− mice and Nod2−/−Jax mice using in vitro differentiation assays. We stimulated mature B cells isolated from WT, Nod2−/−, and Nod2−/−Jax mice with LPS or IL-4 with anti-CD40 for four days and subsequently analyzed the cells for proliferation and class switch recombination. While cells from all three strains exhibited a similar pattern of proliferation (Fig. 4E), Nod2−/− B cells showed a defect in class switching to IgG1 that was not observed in Nod2−/−Jax mice (Fig. 4F). These data collectively indicate that our Nod2−/− mice have a B cell-intrinsic defect that is not present in the commercially available line.
B cell defects in Nod2−/− mice are associated with a mutation in Dock2
The disparity between the two Nod2−/− lines we observed could be due to an unknown genetic factor or a difference in an environmental variable, such as the microbiota. In our previous study, we demonstrated that antibiotics-treatment of Nod2−/− lines reversed intestinal pathologies (24). However, administration of antibiotics did not alter the number or proportion of B cell subsets in Nod2−/− mice maintained at our institution, and co-housing the two Nod2−/− lines did not transfer the phenotype in either direction (data not shown). Because the Nod2−/− animals in our colony used in the above experiments were maintained as homozygous mice, we sought to eliminate the effect of the microbiota and avoid artifacts due to housing conditions by examining animals derived from an intercross between Nod2+/− mice. To generate littermate WT (Nod2+/+) and Nod2−/− mice for comparison, Nod2−/− mice from our colony were crossed to WT C57BL/6J mice, and F1 Nod2+/− mice were bred to each other to generate an F2 generation (Fig. 5A). Suprisingly, one of eight Nod2+/− mice from the first F2 litter showed B cell defects similar to the F0 Nod2−/− mice (Fig. 5B). None of the other mice from that litter, including those with a Nod2−/− genotype, harbored the B cell defects. Although it is theoretically possible that a microbial factor that blocks the B cell defect was transferred from WT to Nod2−/− mice during these crosses, it is difficult to reconcile this possibility with our finding that one of the Nod2+/− mice displayed the B cell defect. Instead, these results suggested the presence of an additional genetic alteration in the Nod2−/− mice that is causing B cell abnormalities.
Figure 5.
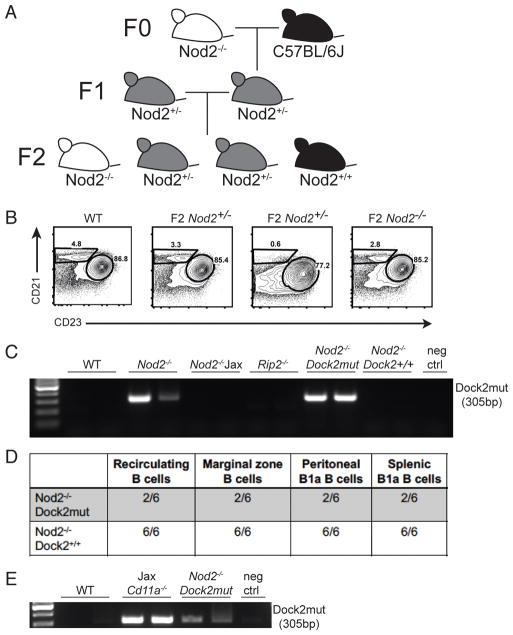
B cell defects in Nod2−/− mice are due to a mutation in Dock2 and independent of Nod2 deficiency. (A) Breeding scheme to generate WT and Nod2−/− littermate controls. (B) Representative plot showing percentages of marginal zone (CD21 high CD23 low) B cells in F2 generation littermates. (C) PCR for presence of Dock2mut in WT, Nod2−/−, Nod2−/− Jax, Rip2−/−, and F2 Nod2−/− mice. (D) Summary table showing number of mice with normal B cell populations as indicated/total number of mice tested. (E) PCR for presence of Dock2mut in WT, Jax Cd11a−/− mice, and Nod2−/−Dock2mut mice. Data is representative of three independent experiments.
The splenocytopenia and MZ B cell deficiency we observed in our Nod2+/− mice are reminiscent of similar defects observed in Siae−/− mice, which were shown to be due to a duplication event involving exons 28 and 29 in Dock2 (9). The Dock2 mutation (Dock2mut) was shown to be present in the C57BL/6 Hsd substrain and may have been introduced into gene-targeted mice during backcrossing (9). PCR analysis revealed the presence of the Dock2mut allele in our Nod2−/− mice, but not WT, Nod2−/−Jax, or Rip2−/− mice (Fig. 5C), suggesting that the presence of the Dock2 exon duplication is responsible for the B cell defects we identified.
We then genotyped the F2 mice from the aforementioned F1 Nod2+/− cross (Fig. 5A) for the presence of Dock2mut. The Dock2 genotyping PCR, as previously described, only reveals the presence or absence of the duplication (9, 30) and does not indicate heterozygosity or homozygosity for the mutation. Thus, we relied on the analysis of the characteristic loss of MZ B cells (previously described to be a consequence of Dock2 mutation (9)) to reveal which mice were homozygous for Dock2mut. We were able to separate the Nod2 knockout allele from Dock2mut by analyzing F2 pups that were Nod2−/− with and without the presence of a Dock2mut allele. All six Nod2−/− Dock2+/+ mice tested had normal B cell compartments (Fig. 5D). In contrast, four Nod2−/− mice that presumably harbor two copies of the Dock2mut allele based on the loss of MZ B cells displayed reductions in recirculating B cells in the bone marrow and loss of peritoneal and splenic B1a cells (Fig. 5D). There were also two F2 Nod2−/− mice that were positive for the Dock2 mutation by PCR but did not show the B cell defects, likely because these animals carried one intact, WT copy of the Dock2 allele.
The mutation in Dock2 has been traced to C57Bl/6 Hsd mice from Harlan Sprague Dawley, which were previously commercially available. Cd11a−/− mice from The Jackson Laboratory are known to have been backcrossed to this line. Genotyping of these mice revealed the presence of the Dock2 mutation (Fig. 5E). Thus, in addition to the previously reported Irf5−/− and Siae−/− mice, this mutation is present in at least two other genetically-modified mouse strains, highlighting the potential prevalence of this mutation in inbred strains.
Dock2−/− mice show B cell defects
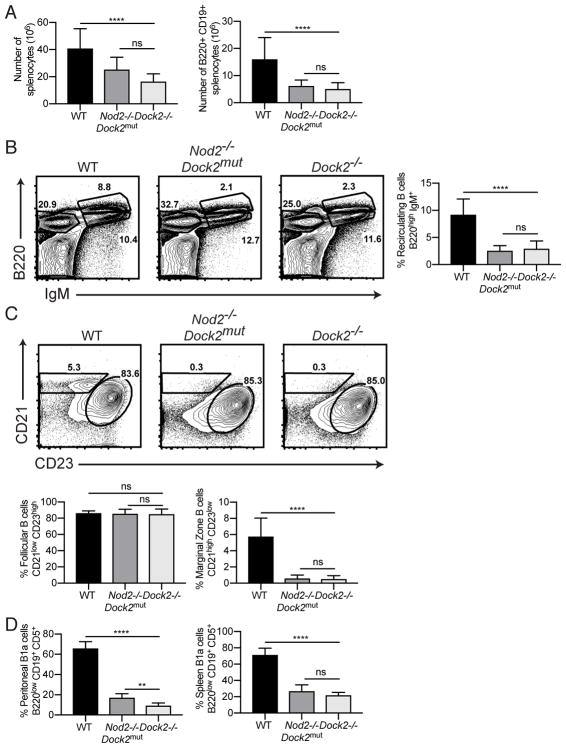
The duplication in exons 28 and 29 lead to decreased expression of Dock2 (8). Although the splenocytopenia and loss of MZ B cells have been described in other mouse lines that harbor the Dock2mut allele, we sought to determine whether loss of DOCK2 function is sufficient to cause the collection of B cell defects we observed. Therefore, we evaluated B cell subsets in Dock2−/− mice generated by traditional gene-targeting (3). We found that Dock2−/− mice reproduced reductions in recirculating B cells in the bone marrow, MZ B cells in the spleen, and B1a B cells in the spleen and peritoneum observed in Nod2−/− mice harboring Dock2mut (Fig. 6). Therefore, DOCK2 function is essential for maintaining these key B cell populations.
Figure 6.
Dock2−/− mice have defects in recirculating B cells, marginal zone B cells, B1a cells. Comparison of B cell populations in WT (n = 12), Nod2−/−Dock2mut (labelled as Nod2−/− in previous figures) (n = 6), and Dock2−/− (n = 11) mice. (A) Number of of total live cells and B220+ CD19+ B cells in the spleen. (B) Representative plot and percentage of bone marrow recirculating B cells (B220high IgM+). (C) Representative plot and percentages of marginal zone (CD21 high CD23 low) and follicular (CD21 low CD23 high) B cells in the spleen. (D) Representative plot and percentages of B1a cells (B220 low, CD19+ CD5+) from peritoneal lavage and spleen. Data is representative of three independent experiments. ns p > 0.05, **p ≤ 0.01, ****p ≤ 0.0001 by two-tailed Mann-Whitney.
DISCUSSION
Mutation of NOD2 is one of the strongest risk factors for Crohn’s disease (37–39). NOD2 is well characterized as a bacterial sensor, but linking this molecular function with specific pathological outcomes has been challenging. We were eager to examine the role of NOD2 in B cell development and function in light of the critical role of B cells, and antibodies they produce, in intestinal homeostasis. Furthermore, analyses of immunoglobulin (Ig) titers in IBD patients revealed a significant reduction in IgA (40), changes in IgA subclass distribution (41), and an increase in mucosal IgG directed against bacteria compared with healthy individuals (42). A central role for B cells in intestinal disease is further supported by the observation that individuals with common variable immunodeficiency, a primary immune deficiency that leads to decreased antibody production, develop an IBD-like disorder that includes villi blunting and intestinal inflammation (43–47).
The profound defect in lymphocytes we observed in Nod2 deficient animals was rewarding, but the sheer magnitude of the difference in multiple B cell subsets, in the absence of any immunogen, was, nonetheless, surprising given that Nod2−/− mice that share a common origin have been used by many laboratories around the world in the context of IBD studies and beyond (14, 20, 23, 31, 48–60). Unexpected phenotypes in analysis of gene targeted animal models can be a consequence of differences in the microbiota, genetic drift, or experimental assiduousness. We previously attributed goblet cell abnormalities in Nod2−/− mice to the presence of a specific member of the microbiota and were able to validate the results in mice deficient for RIP2, the downstream signal tranducer of NOD2 (24). The finding that the B cell deficiencies were not reproduced in Rip2−/− prompted us to examine commercially-available Nod2−/− mice. The animals we obtained from Jackson Laboratories were derived from the same gene targeted founders as the animals within our colony, yet lacked the B cell defects we had observed. Numerous studies have established a direct role of microbial triggers in immune development (61). Microbial composition within strains and individual mice can vary greatly within the same facility (62). A prior analysis of TLR deficient mice (63) highlighted the importance of microbial history of the mice as the authors found that familial transmission of microbiota, rather than genetic loss of the pattern recognition receptors, directed the observed immune phenotypes. For this reason we undertook cross-fostering and extended cohousing experiments to control for changes in microbiota within our strains as a consequence of animal husbandry history. Ultimately, analysis of heterozygote matings to generate Nod2−/− revealed that a genetic factor was at play.
Finally, our findings with Dock2−/− mice confirmed that mutation in Dock2 is sufficient to explain the B cell deficiencies we initially identified in Nod2−/− mice and also demonstrated that these defects were due to a loss of DOCK2 function. In addition to maintaining MZ B cells in the spleen, we have revealed additional roles for DOCK2 in the B cell compartment. All together, our findings indicate that DOCK2 is required for the maintenance of proper numbers of recirculating bone marrow cells, MZ B cells and B1a cells in the spleen, and peritoneal B1a cells. Although the near complete absence of these cells precludes biochemical analyses of molecular mechanism, previous studies have indicated that DOCK2 functions as a Rho guanine nucleotide exchange factor (GEF) involved in immune signaling and cell migration (3, 7, 64). Specifically, DOCK2 functions as a GEF for RAC1 and RAC2, which are highly expressed in leukocytes and known to be necessary for transduction of signals downstream of the B cell antigen receptor and thus critical for induction of proliferation and survival (65, 66). Failure to respond to mitogenic signals likely explains the importance of DOCK2 for maintenance of dynamic B cell populations. Given the association between DOCK2 mutations and immune-deficiency in humans, an important future direction will be to carefully examine the B cell compartment in patients for defects similar to those we have identified. Coincidentally, DOCK2 was recently identified as a key driver of gene expression patterns associated with IBD, and Dock2−/− mice are susceptible to intestinal injury (67). Therefore, a B cell specific function of DOCK2 in IBD pathogenesis may require consideration.
Acknowledgments
This work was supported by US National Institute of Health (NIH) grants R01 HL123340 (K.C.), R01 DK093668 (K.C.), R01 DK103788 (K.C.), R01 AI121244 (K.C.), R01 AI130945 (K.C.), R01 HL125816 (S.B.K.), R21 AI124129 (S.B.K.), R21 AI110830 (S.B.K.), and P30 CA016087 (NYUSoM Flow Cytometry and Cell Sorting Center); Faculty Scholar grant from the Howard Hughes Medical Institute, Stony Wold-Herbert Fund, pilot funding from the Colton Center for Autoimmunity, and philanthropy from Bernard Levine (K.C.); K.C. is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases; Feinberg Lymphoma Grant (S.B.K.), Irma T. Hirschl Career Scientist Award (S.B.K.), Colton Center for Autoimmunity Award (S.B.K.), and Beckman Foundation Award (S.B.K.); S.Y.W. is a Ruth and Gerald Dickler Faculty Scholar in Inflammatory Bowel Diseases; American Gastroenterological Association Clinical Research Pilot Award (S.Y.W.) and philanthropy from Paul and Jenna Segal (S.Y.W.); MSTP T32GM007308 (MJH).
We would like to thank Dr. Susan Schwab of NYU School of Medicine for the Cd11a−/− mice and Dr. Yoshinori Fukui of Kyushu University in Japan for the Dock2−/− mice.
Abbreviations used in this article
- ASC
Apoptosis-associated speck-like protein containing a CARD
- DOCK2
dedicator of cytokinesis 2
- GEF
guanine nucleotide exchange factor
- MZ
marginal zone
- IBD
inflammatory bowel disease
- NOD2
nucleotide-binding oligomerization domain-containing protein 2
- pDC
plasmacytoid dendritic cell
- RIP2
receptor interacting protein kinase 2
- SIAE
sialic acid acetyl esterase
- WT
wild-type
References
- 1.Justice MJ, Dhillon P. Using the mouse to model human disease: increasing validity and reproducibility. Dis Model Mech. 2016;9:101–103. doi: 10.1242/dmm.024547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zurita E, Chagoyen M, Cantero M, Alonso R, Gonzalez-Neira A, Lopez-Jimenez A, Lopez-Moreno JA, Landel CP, Benitez J, Pazos F, Montoliu L. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 2011;20:481–489. doi: 10.1007/s11248-010-9403-8. [DOI] [PubMed] [Google Scholar]
- 3.Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, Inayoshi A, Noda M, Oike M, Shirai T, Sasazuki T. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 4.Dobbs K, Dominguez Conde C, Zhang SY, Parolini S, Audry M, Chou J, Haapaniemi E, Keles S, Bilic I, Okada S, Massaad MJ, Rounioja S, Alwahadneh AM, Serwas NK, Capuder K, Ciftci E, Felgentreff K, Ohsumi TK, Pedergnana V, Boisson B, Haskologlu S, Ensari A, Schuster M, Moretta A, Itan Y, Patrizi O, Rozenberg F, Lebon P, Saarela J, Knip M, Petrovski S, Goldstein DB, Parrott RE, Savas B, Schambach A, Tabellini G, Bock C, Chatila TA, Comeau AM, Geha RS, Abel L, Buckley RH, Akinciogullari I, Al-Herz W, Helminen M, Dogu F, Casanova JL, Boztug K, Notarangelo LD. Inherited DOCK2 Deficiency in Patients with Early-Onset Invasive Infections. N Engl J Med. 2015;372:2409–2422. doi: 10.1056/NEJMoa1413462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nombela-Arrieta C, Mempel TR, Soriano SF, Mazo I, Wymann MP, Hirsch E, Martinez AC, Fukui Y, von Andrian UH, Stein JV. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med. 2007;204:497–510. doi: 10.1084/jem.20061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoh K, Tanaka Y, Nishikimi A, Inayoshi A, Enjoji M, Takayanagi R, Sasazuki T, Fukui Y. Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood. 2008;111:2973–2976. doi: 10.1182/blood-2007-09-112169. [DOI] [PubMed] [Google Scholar]
- 7.Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, Watanabe K, Sanematsu F, Sasazuki T, Sasaki T, Fukui Y. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. The Journal of cell biology. 2006;174:647–652. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purtha WE, Swiecki M, Colonna M, Diamond MS, Bhattacharya D. Spontaneous mutation of the Dock2 gene in Irf5−/− mice complicates interpretation of type I interferon production and antibody responses. Proc Natl Acad Sci U S A. 2012;109:E898–904. doi: 10.1073/pnas.1118155109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahajan VS, Demissie E, Mattoo H, Viswanadham V, Varki A, Morris R, Pillai S. Striking Immune Phenotypes in Gene-Targeted Mice Are Driven by a Copy-Number Variant Originating from a Commercially Available C57BL/6 Strain. Cell reports. 2016;15:1901–1909. doi: 10.1016/j.celrep.2016.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuda K, Nundel K, Watkins AA, Dhawan T, Bonegio RG, Ubellacker JM, Marshak-Rothstein A, Rifkin IR. Phenotype and function of B cells and dendritic cells from interferon regulatory factor 5-deficient mice with and without a mutation in DOCK2. Int Immunol. 2013;25:295–306. doi: 10.1093/intimm/dxs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ippagunta SK, Malireddi RK, Shaw PJ, Neale GA, Vande Walle L, Fukui Y, Green DR, Lamkanfi M, Kanneganti TD. Addendum: defective Dock2 expression in a subset of ASC-deficient mouse lines. Nat Immunol. 2012;13:701–702. doi: 10.1038/ni.2389. [DOI] [PubMed] [Google Scholar]
- 12.Al Nabhani Z, Dietrich G, Hugot JP, Barreau F. Nod2: The intestinal gate keeper. PLoS Pathog. 2017;13:e1006177. doi: 10.1371/journal.ppat.1006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidiq T, Yoshihama S, Downs I, Kobayashi KS. Nod2: A Critical Regulator of Ileal Microbiota and Crohn’s Disease. Front Immunol. 2016;7:367. doi: 10.3389/fimmu.2016.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 15.Ramanan D, Cadwell K. Intrinsic Defense Mechanisms of the Intestinal Epithelium. Cell Host Microbe. 2016;19:434–441. doi: 10.1016/j.chom.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanahan MT, I, Carroll M, Grossniklaus E, White A, von Furstenberg RJ, Barner R, Fodor AA, Henning SJ, Sartor RB, Gulati AS. Mouse Paneth cell antimicrobial function is independent of Nod2. Gut. 2014;63:903–910. doi: 10.1136/gutjnl-2012-304190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 18.Shaw MH, Reimer T, Sanchez-Valdepenas C, Warner N, Kim YG, Fresno M, Nunez G. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat Immunol. 2009;10:1267–1274. doi: 10.1038/ni.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YG, Shaw MH, Warner N, Park JH, Chen F, Ogura Y, Nunez G. Cutting edge: Crohn’s disease-associated Nod2 mutation limits production of proinflammatory cytokines to protect the host from Enterococcus faecalis-induced lethality. Journal of immunology. 2011;187:2849–2852. doi: 10.4049/jimmunol.1001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caetano BC, Biswas A, Lima DS, Jr, Benevides L, Mineo TW, Horta CV, Lee KH, Silva JS, Gazzinelli RT, Zamboni DS, Kobayashi KS. Intrinsic expression of Nod2 in CD4+ T lymphocytes is not necessary for the development of cell-mediated immunity and host resistance to Toxoplasma gondii. European journal of immunology. 2011;41:3627–3631. doi: 10.1002/eji.201141876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanello G, Goethel A, Forster K, Geddes K, Philpott DJ, Croitoru K. Nod2 Activates NF-kB in CD4(+) T Cells but Its Expression Is Dispensable for T Cell-Induced Colitis. PLoS One. 2013;8:e82623. doi: 10.1371/journal.pone.0082623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin GH, Wortzman ME, Girardin SE, Philpott DJ, Watts TH. T cell intrinsic NOD2 is dispensable for CD8 T cell immunity. PLoS One. 2013;8:e56014. doi: 10.1371/journal.pone.0056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biswas A, Liu YJ, Hao L, Mizoguchi A, Salzman NH, Bevins CL, Kobayashi KS. Induction and rescue of Nod2-dependent Th1-driven granulomatous inflammation of the ileum. Proc Natl Acad Sci U S A. 2010;107:14739–14744. doi: 10.1073/pnas.1003363107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41:311–324. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson SJ, Zhou JY, Geddes K, Rubino SJ, Cho JH, Girardin SE, Philpott DJ. Nod1 and Nod2 signaling does not alter the composition of intestinal bacterial communities at homeostasis. Gut microbes. 2013;4:222–231. doi: 10.4161/gmic.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A, Gaillot O, Schreiber S, Lemoine Y, Ryffel B, Hot D, Nunez G, Chen G, Rosenstiel P, Chamaillard M. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. The Journal of clinical investigation. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, Honda K, Gause WC, Blaser MJ, Bonneau RA, Lim YA, Loke P, Cadwell K. Helminth infection promotes colonization resistance via type 2 immunity. Science. 2016;352:608–612. doi: 10.1126/science.aaf3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Marchiando AM, Ramanan D, Ding Y, Gomez LE, Hubbard-Lucey VM, Maurer K, Wang C, Ziel JW, van Rooijen N, Nunez G, Finlay BB, Mysorekar IU, Cadwell K. A deficiency in the autophagy gene Atg16L1 enhances resistance to enteric bacterial infection. Cell Host Microbe. 2013;14:216–224. doi: 10.1016/j.chom.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasuda K, Watkins AA, Kochar GS, Wilson GE, Laskow B, Richez C, Bonegio RG, Rifkin IR. Interferon regulatory factor-5 deficiency ameliorates disease severity in the MRL/lpr mouse model of lupus in the absence of a mutation in DOCK2. PLoS One. 2014;9:e103478. doi: 10.1371/journal.pone.0103478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, Nunez G. The Nod2 Sensor Promotes Intestinal Pathogen Eradication via the Chemokine CCL2-Dependent Recruitment of Inflammatory Monocytes. Immunity. 2011;34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 34.Wang JH, Avitahl N, Cariappa A, Friedrich C, Ikeda T, Renold A, Andrikopoulos K, Liang L, Pillai S, Morgan BA, Georgopoulos K. Aiolos regulates B cell activation and maturation to effector state. Immunity. 1998;9:543–553. doi: 10.1016/s1074-7613(00)80637-8. [DOI] [PubMed] [Google Scholar]
- 35.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunological reviews. 2000;175:70–79. [PubMed] [Google Scholar]
- 36.Kenny JJ, Derby EG, Yoder JA, Hill SA, Fischer RT, Tucker PW, Claflin JL, Longo DL. Positive and negative selection of antigen-specific B cells in transgenic mice expressing variant forms of the V(H)1 (T15) heavy chain. Int Immunol. 2000;12:873–885. doi: 10.1093/intimm/12.6.873. [DOI] [PubMed] [Google Scholar]
- 37.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 38.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 39.Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, Frenzel H, King K, Hasselmeyer A, MacPherson AJ, Bridger S, van Deventer S, Forbes A, Nikolaus S, Lennard-Jones JE, Foelsch UR, Krawczak M, Lewis C, Schreiber S, Mathew CG. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet. 2001;357:1925–1928. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- 40.Engstrom JF, Arvanitakis C, Sagawa A, Abdou NI. Secretory immunoglobulin deficiency in a family with inflammatory bowel disease. Gastroenterology. 1978;74:747–751. [PubMed] [Google Scholar]
- 41.MacDermott RP, Nash GS, Bertovich MJ, Mohrman RF, Kodner IJ, Delacroix DL, Vaerman JP. Altered patterns of secretion of monomeric IgA and IgA subclass 1 by intestinal mononuclear cells in inflammatory bowel disease. Gastroenterology. 1986;91:379–385. doi: 10.1016/0016-5085(86)90572-x. [DOI] [PubMed] [Google Scholar]
- 42.Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–375. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Washington K, Stenzel TT, Buckley RH, Gottfried MR. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. The American journal of surgical pathology. 1996;20:1240–1252. doi: 10.1097/00000478-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Kalha I, Sellin JH. Common variable immunodeficiency and the gastrointestinal tract. Current gastroenterology reports. 2004;6:377–383. doi: 10.1007/s11894-004-0053-y. [DOI] [PubMed] [Google Scholar]
- 45.Hermans PE, Diaz-Buxo JA, Stobo JD. Idiopathic late-onset immunoglobulin deficiency. Clinical observations in 50 patients. Am J Med. 1976;61:221–237. doi: 10.1016/0002-9343(76)90173-x. [DOI] [PubMed] [Google Scholar]
- 46.Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. The Quarterly journal of medicine. 1993;86:31–42. [PubMed] [Google Scholar]
- 47.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clinical immunology. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 48.Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011;35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis KM, Nakamura S, Weiser JN. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Invest. 2011;121:3666–3676. doi: 10.1172/JCI57761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, Kobayashi KS, Flavell RA, Gros P, Behr MA. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. Journal of immunology. 2008;181:7157–7165. doi: 10.4049/jimmunol.181.10.7157. [DOI] [PubMed] [Google Scholar]
- 52.Jamontt J, Petit S, Clark N, Parkinson SJ, Smith P. Nucleotide-binding oligomerization domain 2 signaling promotes hyperresponsive macrophages and colitis in IL-10-deficient mice. J Immunol. 2013;190:2948–2958. doi: 10.4049/jimmunol.1201332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Penack O, Smith OM, Cunningham-Bussel A, Liu X, Rao U, Yim N, Na IK, Holland AM, Ghosh A, Lu SX, Jenq RR, Liu C, Murphy GF, Brandl K, van den Brink MR. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J Exp Med. 2009;206:2101–2110. doi: 10.1084/jem.20090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh SJ, Kim JH, Chung DH. NOD2-mediated suppression of CD55 on neutrophils enhances C5a generation during polymicrobial sepsis. PLoS Pathog. 2013;9:e1003351. doi: 10.1371/journal.ppat.1003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan Q, Mathison J, Fearns C, Kravchenko VV, Da Silva Correia J, Hoffman HM, Kobayashi KS, Bertin J, Grant EP, Coyle AJ, Sutterwala FS, Ogura Y, Flavell RA, Ulevitch RJ. MDP-induced interleukin-1beta processing requires Nod2 and CIAS1/NALP3. J Leukoc Biol. 2007;82:177–183. doi: 10.1189/jlb.1006627. [DOI] [PubMed] [Google Scholar]
- 57.Rehman A, Sina C, Gavrilova O, Hasler R, Ott S, Baines JF, Schreiber S, Rosenstiel P. Nod2 is essential for temporal development of intestinal microbial communities. Gut. 2011;60:1354–1362. doi: 10.1136/gut.2010.216259. [DOI] [PubMed] [Google Scholar]
- 58.Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saha S, Qi J, Wang S, Wang M, Li X, Kim YG, Nunez G, Gupta D, Dziarski R. PGLYRP-2 and Nod2 are both required for peptidoglycan-induced arthritis and local inflammation. Cell Host Microbe. 2009;5:137–150. doi: 10.1016/j.chom.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J Biol Chem. 2007;282:36223–36229. doi: 10.1074/jbc.M703079200. [DOI] [PubMed] [Google Scholar]
- 61.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoy YE, Bik EM, Lawley TD, Holmes SP, Monack DM, Theriot JA, Relman DA. Variation in Taxonomic Composition of the Fecal Microbiota in an Inbred Mouse Strain across Individuals and Time. PLoS One. 2015;10:e0142825. doi: 10.1371/journal.pone.0142825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, Equinda M, Khanin R, Pamer EG. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med. 2012;209:1445–1456. doi: 10.1084/jem.20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, Tanaka Y, Shibasaki M, Kanaho Y, Sasaki T, Frohman MA, Fukui Y. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science. 2009;324:384–387. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, Mathiot A, Vanes L, Williams DA, Cancro MP, Tybulewicz VL. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 66.Croker BA, Tarlinton DM, Cluse LA, Tuxen AJ, Light A, Yang FC, Williams DA, Roberts AW. The Rac2 guanosine triphosphatase regulates B lymphocyte antigen receptor responses and chemotaxis and is required for establishment of B-1a and marginal zone B lymphocytes. J Immunol. 2002;168:3376–3386. doi: 10.4049/jimmunol.168.7.3376. [DOI] [PubMed] [Google Scholar]
- 67.Peters LA, Perrigoue J, Mortha A, Iuga A, Song WM, Neiman EM, Llewellyn SR, Di Narzo A, Kidd BA, Telesco SE, Zhao Y, Stojmirovic A, Sendecki J, Shameer K, Miotto R, Losic B, Shah H, Lee E, Wang M, Faith JJ, Kasarskis A, Brodmerkel C, Curran M, Das A, Friedman JR, Fukui Y, Humphrey MB, Iritani BM, Sibinga N, Tarrant TK, Argmann C, Hao K, Roussos P, Zhu J, Zhang B, Dobrin R, Mayer LF, Schadt EE. A functional genomics predictive network model identifies regulators of inflammatory bowel disease. Nat Genet. 2017;49:1437–1449. doi: 10.1038/ng.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]