Abstract
Hepatitis C virus (HCV) infection is a global public health problem. The implementation of public health interventions (PHI) to control HCV infection could effectively interrupt HCV transmission. PHI targeting high-risk populations, e.g., people who inject drugs (PWID), are the most efficient but there is a lack of tools for prioritizing individuals within a high-risk community. Here, we present Intelligent Network DisRuption Analysis (INDRA), a targeted strategy for efficient interruption of hepatitis C transmissions. Using a large HCV transmission network among PWID in Indiana as an example, we compare effectiveness of random and targeted strategies in reducing the rate of HCV transmission in two settings: (1) long-established and (2) rapidly spreading infections (outbreak). Identification of high centrality for the network nodes co-infected with HIV or >1 HCV subtype indicates that the network structure properly represents the underlying contacts among PWID relevant to the transmission of these infections. Changes in the network’s global efficiency (GE) were used as a measure of the PHI effects. In setting 1, simulation experiments showed that a 50% GE reduction can be achieved by removing 11.2 times less nodes using targeted vs random strategies. A greater effect of targeted strategies on GE was consistently observed when networks were simulated: (1) with a varying degree of errors in node sampling and link assignment, and (2) at different levels of transmission reduction at affected nodes. In simulations considering a 10% removal of infected nodes, targeted strategies were ~2.8 times more effective than random in reducing incidence. Peer-education intervention (PEI) was modeled as a probabilistic distribution of actionable knowledge of safe injection practices from the affected node to adjacent nodes in the network. Addition of PEI to the models resulted in a 2–3 times greater reduction in incidence than from direct PHI alone. In setting 2, however, random direct PHI were ~3.2 times more effective in reducing incidence at the simulated conditions. Nevertheless, addition of PEI resulted in a ~1.7-fold greater efficiency of targeted PHI. In conclusion, targeted PHI facilitated by INDRA outperforms random strategies in decreasing circulation of long-established infections. Network-based PEI may amplify effects of PHI on incidence reduction in both settings.
Keywords: Hepatitis C virus, Transmission network, Targeted Public Health Intervention, Simulation Experiments, Computational Models
1. Introduction
Hepatitis C virus (HCV) infects nearly 2.8% of the world’s population and is a major cause of liver disease worldwide (Mohd Hanafiah et al., 2013). HCV infection is an important US public health problem, being the most common chronic blood-borne infection and the leading cause for liver transplantation (Alter, 2007; Stanaway et al., 2016). Since 2007, HCV surpassed HIV as a cause of death in the US and HCV associated mortality exceeds that from 60 other nationally notifiable infectious conditions combined (Ly et al., 2012). It is estimated that 0.9% of people in the US have viraemic infections (HCV RNA positive) (Polaris Observatory, 2017) and that more than 15,000 die each year from HCV-related disease, with mortality expected to rise in the coming years (Ward, 2013). Approximately 80% of persons who become infected with HCV develop chronic hepatitis and are at risk for advanced liver disease; 15%-30% of these persons have progression to liver fibrosis and cirrhosis and up to 5% will die from liver failure due to cirrhosis or hepatocellular carcinoma (Alter, 2007). The HCV epidemic is ongoing in the US and globally. Incidence rates remain high, especially among young people who inject drugs (PWID) in predominantly suburban and rural areas (Page et al., 2009; Page et al., 2013).
Viral hepatitis is a leading cause of death and disability worldwide (Stanaway et al., 2016). Recent availability of highly efficacious direct-acting antivirals (DAA) for treatment of hepatitis C offers a new opportunity for controlling HCV infections and eliminating hepatitis C as a public health threat worldwide (WHO, 2016) and in the US (The National Academies of Sciences, 2017). However, there are several research gaps, including investigation of social networks at risk of acquisition and dissemination of HCV infection, which prevent development of optimal and cost-effective public health interventions for interruption of HCV transmission among high-risk population groups.
People-who-inject-drugs (PWID) are at a significant risk of HCV infection and are major contributors to HCV incidence. Many PWID communities have >40% prevalence of HCV infection and are high priority for treatment (Martin et al., 2013). Although historically viewed as being difficult to treat, PWID have been recently shown to have treatment adherence and SVR rates comparable to non-drug users (Elsherif et al., 2017) as well as a low re-infection rate (1.14 per 100 person-years)(Islam et al., 2017), indicating equally effective applicability of DAA therapy to this high-risk population. Several public health interventions (PHI) are currently available for effectively controlling HCV infections among PWID if coverage is sufficient; namely, specific DAA therapy and harm reduction programs, including syringe service programs, medication substitution treatment and peer-education interventions (PEI) (Bruggmann and Grebely, 2015; Platt et al., 2016). Although each of the interventions can contribute to reduction of incidence and prevalence of HCV infection among PWID, significant cost, complex logistics and social factors confound application of the interventions, indicating need for the development of efficient implementation strategies to eliminate hepatitis C in these communities.
Approaches targeting high-risk populations are efficient but not applicable to differentiate among members of the community where all members share the same risk factor. However, the structure of such high-risk communities is not uniform; community members contribute differentially to dissemination of infection and, therefore, to force of infection, with a few members having many connections relevant to transmission of infections in the community and many members having only few such connections. Targeting interventions to the most contributing members intuitively seems to be the most efficient way to interrupt disease dissemination within the community. Indeed, among PWID, transmissions usually occur through preferential contacts, resulting in contact networks that are not randomly organized but are modular and have a skewed degree distribution (Latora et al., 2006; Leigh Brown et al., 2011; Villandre et al., 2016; Wertheim et al., 2014). However, the structure of contact or transmission networks is not always available and can be identified only through intense research, which prevents common application of the networks to public health interventions (PHI). Several strategies, like an acquaintance approach, were developed to approximate such networks without completely mapping its structure for HCV simulation studies (Hellard et al., 2014) and implementation of PHI in rural villages in Uganda (Chami et al., 2017). The lack of effective strategies to direct PHI hinders the development of precision public health, the strategy of using data to guide interventions that benefit populations most efficiently (Arnett and Claas, 2016; Bayer and Galea, 2015; Khoury et al., 2016).
Here, we take advantage of a complex HCV transmission network, which was recently identified among PWID in Indiana (Conrad et al., 2015; Peters et al., 2016; Ramachandran et al., 2016) using the Global Hepatitis Outbreak and Surveillance Technology (GHOST) (Campo et al., 2015; Longmire et al., 2017; Rytsareva et al., 2017), to devise and evaluate a new targeted, network-based strategy for reducing circulation and dissemination of HCV infection in 2 epidemiological settings: (1) long-established HCV infection as was observed in a PWID community during investigation in Indiana (Peters et al., 2016) and (2) a hypothetical rapid spread of a single HCV strain as observed during an outbreak.
2. Material and methods
2.1. Transmission Network
The Indiana State Department of Health recorded a cluster of 11 HIV infections in a small rural community in Scott County, which led to detection of 181 HIV-positive patients from November 2014 to November 2015 linked to injection use of oxymorphone (Conrad et al., 2015; Peters et al., 2016; Ramachandran et al., 2016). Serological and molecular analyses showed that 92.3% of the HIV patients were co-infected with many HCV strains, which belonged to three genotypes(Ramachandran et al., 2016). Genetic analyses further revealed a long-standing and continued HCV transmission within this affected community, and a dense and dynamic network of HCV transmission among PWID that enabled an explosive HIV transmission. Please refer to (Peters et al., 2016; Ramachandran et al., 2016; Ramachandran et al., 2018) for complete demographic and clinical data.
Intra-host HCV HVR1 variants were sampled from 281 persons using next-generation sequencing. GHOST (Campo et al., 2015; Longmire et al., 2017; Rytsareva et al., 2017) was used to genetically characterize HCV strains and detect a transmission network. GHOST generates networks where 2 nodes representing infected individuals are linked by transmission if the minimal Hamming distance between any pair of HCV HVR1 sequences obtained from these individuals is below a relatedness threshold of 3.77% (Campo et al., 2015). We used this HCV transmission network (ITN) as a case study (Fig. 2A). In ITN, 23.5% of nodes are HIV co-infected and 34.5% are infected with >1 HCV subtype (Ramachandran et al., 2016).
Figure 2.
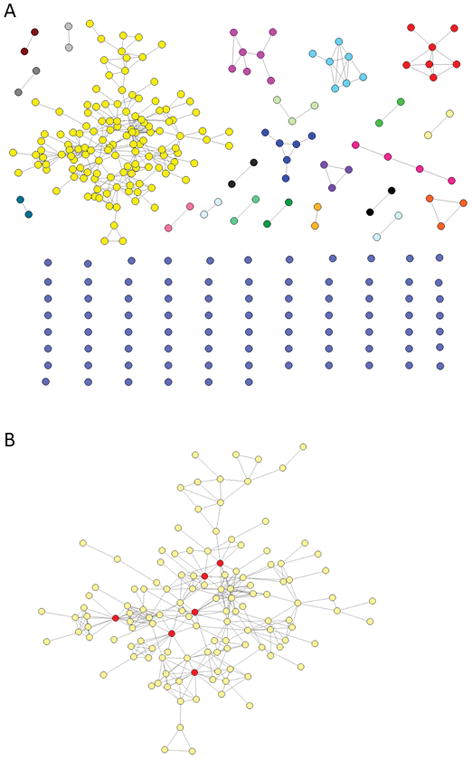
A) ITN. Each node corresponds to one HCV-infected individual and two nodes have an edge if they share HCV strain (linkage by transmission). B) Major component of the ITN, with nodes in red belonging to the GE50 group (if removed, the efficiency drops to 44.8%).
In this paper, we compare effectiveness of random and targeted strategies in reducing the rate of HCV transmission in two epidemiological settings: (1) long-established infection with many HCV strains as was observed in a PWID community during investigation in Indiana (Peters et al., 2016; Ramachandran et al., 2016) and (2) a hypothetical rapid spread of a single HCV strain as observed during an outbreak.
2.2. Global efficiency (GE)
GE is a measure of how efficiently information can be exchanged in a network that can also be used to determine cost-effective structures in weighted and unweighted networks (Latora and Marchiori, 2001). GE, is calculated as:
Where n is the number of nodes and d(i, j) is the path distance between nodes i and j. For an example, see figure 1A. Here, GE was used to evaluate efficacy of intervention on a network.
Figure 1.
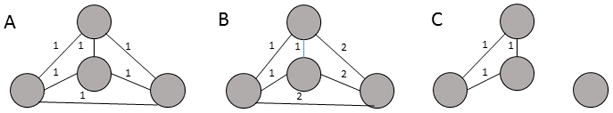
GE of networks. A) All possible links are present and equal, GE = 1.0; B) All possible links are present but some links are weaker (distance between nodes = 2), GE = 0.75; C) One node is disconnected, GE = 0.5.
2.3. Network disruption
Contribution of individual nodes to GE was calculated as change in GE of a network before and after a node (x) experienced a transmission reduction (d) modeled as modification of link weights (0%< d≤100%, with d=100% indicating a complete node removal):
For the random-based strategy, the nodes were selected at random until all nodes were affected. This procedure was repeated 10,000 times, and the global efficiency change was averaged over all runs.
For the network-based strategy, nodes were selected according their network importance, using different measures of centrality such as degree, closeness, betweenness and Eigen vector centrality (Newman, 2010). A fifth measure was devised that we call Glocal efficiency, a greedy measure where the node which would cause the highest GE change was identified and removed, a procedure that is repeated iteratively in the remaining network until all nodes have been removed. Finally a removal rank is calculated for each node.
To compare different strategies, we used HR50 (Harm Reduction 50%), which is calculated as the number of nodes to be disrupted for 50% reduction in GE.
2.4. Robustness of network to error
Considering that actual transmission networks can be (1) incompletely sampled (missing nodes) and (2) modeled with inaccurate links among nodes, we explored contribution of both these errors on GE estimates using 1000 simulated networks. The observed network is used for choosing the target nodes, but its effect on GE is measured over the simulated network. Two types of simulated networks were built in the following way:
For the first type of error (links), observed networks were simulated by rewiring links among nodes; e.g. links A-B and C-D were reassigned as A-D and B-C. This procedure continued until the required fraction of links has been rewired. The total number of nodes, links and degree distribution remain constant in all rewiring experiments.
For the second type of error (missing nodes), we first calculate the edge probability that any two nodes are linked in the original network. Then we create a new node and check it against every other node in the network, creating a link with a probability equal to the original edge probability. We continue until the required fraction of nodes has been added.
2.5. Incidence reduction
To study the effect of disruption strategies on reducing incidence, we devised the following in silico sampling experiment:
Exclude a fraction of nodes (test set) from the original network, the remaining nodes compose the sampled network;
On the sampled network, disrupt a fraction of nodes, either with the random- or network-based strategy. The nodes that were not disrupted are labelled as infected;
For each node in the test set, count the number of infection links, defined as links to infected nodes in the original network;
Calculate incidence reduction, , where t is the number of nodes in the test set and c is the number of test nodes that have ≥1 paths to any infected node;
Steps i to iv are repeated 1000 times and an average is calculated over all runs.
To study the effect of PEI on incidence reduction, the procedure described above was repeated, with the difference that each neighboring node of the disrupted node has a probability of being disrupted itself. This probability was set to 0.76 according to (Garfein et al., 2007), who showed a 76% decline in overall injection risk 6 months after PEI.
2.6. Agent-based simulation
We performed an agent-based simulation of a single HCV strain spread across a network of susceptible individuals as observed during a hypothetical outbreak (setting 2). The ITN major component (n=130) (Fig. 2B) was used in simulations. All links were considered to be bi-directional. Thousand repetitions starting from a randomly defined node were performed. We used the following estimates: (a) each individual uses drugs on average 11 times per week (Scott et al., 2015); (2) a probability of transmission from an infected node to a single linked susceptible node is 0.05 per a drug use incident (Wicker et al., 2008); and (3) after 180 days, an infected node has a probability of 0.25 of clearing infection but may become infected again with the same probability of a naïve node (Micallef et al., 2006).
3. Results
3.1. ITN features
Distribution of links in ITN is different from a random network constructed using same number of nodes and links (Fig. 3A). ITN contains many nodes with few links and a few nodes with many links.
Figure 3.
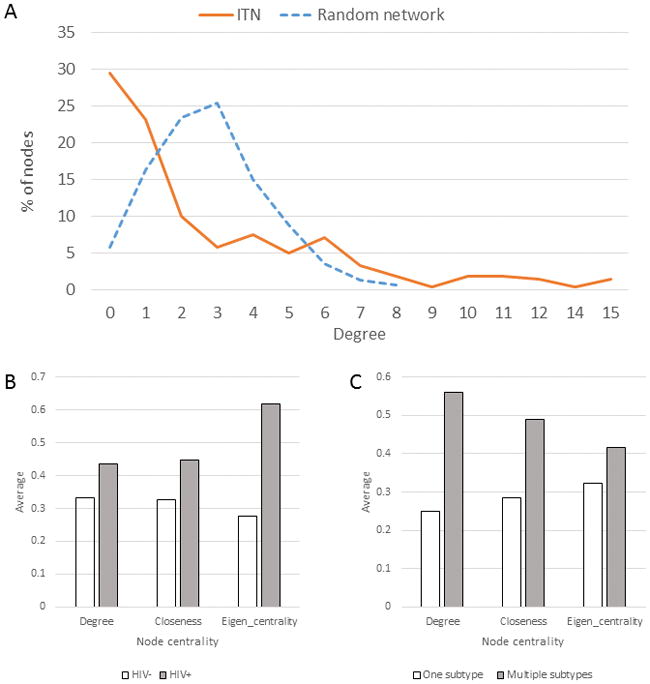
ITN features. A) Degree distribution. The dashed line shows the degree distribution of the Erdos-Renyi network of the same number of nodes, links and edge probability; B) Node centrality and its HIV status; C) Node centrality and its coinfection with >1 HCV subtype.
In ITN, 23.5% of nodes are HIV co-infected and 34.5% are infected with >1 HCV subtype (Ramachandran et al., 2016). The HIV co-infected nodes have significantly greater degree (Mann Whitney test, p = 0.047), closeness (p = 0.032) and Eigen-vector centrality (p=0.021) than nodes without HIV (Fig. 3B). Likewise, nodes with multiple HCV subtypes have a significantly greater degree (p = 0.001), closeness (p = 0.001) and Eigen-vector centrality (p=0.001) than nodes with a single subtype (Fig. 3C). In ITN, Eigen-vector and degree centralities have a modest correlation (r = 0.5568); among the 20 top nodes with high-degree centrality, only 8 are in the top 20 nodes with high Eigen-vector centrality. Both measures showed different patterns between nodes co-infected with HIV and infected with >1 HCV subtype, indicating differences in ITN exploration by HCV and HIV.
3.2. Random vs network-based disruption
Fig. 4 shows effects of the random vs network-based node removal on ITN GE. All targeted strategies based on using different centrality measures for disrupting networks have a significant effect on GE, with removal of only ~10% of all nodes resulting in ~90% reduction in GE. HR50 for the random removal (n=55.7, 19.82% of all nodes) is 11.2 times greater than HR50 for the targeted removal (n=5, 1.78% of all nodes). Figure 2B shows the major component of the ITN, with nodes in red belonging to the GE50 group: when removed, the GE of the network drops to 44.8%.
Figure 4.
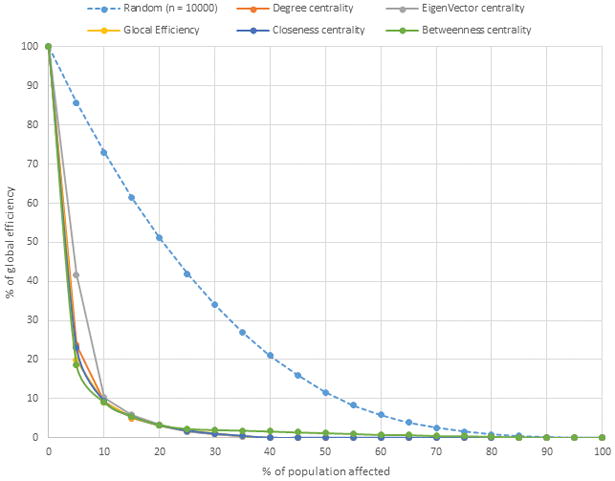
Effect of targeted vs. random intervention on the ITN GE. X axis shows the percent of nodes removed from ITN according to the targeted or random strategies. Y axis shows the percent of remaining GE of ITN after node exclusions. Each line represents results of implementation of random or targeted strategies. Targeted strategies were based on various network centrality measures for removal of nodes.
3.3. Robustness to errors
Observed networks may not accurately represent actual networks, owing to incorrect link assignments (error 1) and under-sampling resulting in missing nodes (error 2). Such misrepresentation may change the network structure to such an extent that interventions designed using a sampled network would not be equally efficient for the actual network. We simulated networks with different levels of these two error types and used these networks to measure the ratio between HR50 for targeted and random strategies. As expected, the performance of the network-based approach decreases when the error increases for both error types, especially for error type 2. However, the advantage of network-based over random is quite robust to both types of error, as can be seen by the ratio of the network-based HR50 to random-based HR50 being >1 up to very high levels of error (Fig. 5).
Figure 5.

Robustness of targeted interventions to errors in estimating the network structure. Error 1 – inaccurate assignment of links among nodes. Error 2 – incomplete sampling of nodes. X axis shows the percent of erroneous links (error 1) or nodes (error 2) in simulated networks; Y axis shows the ratio of the targeted HR50 over the random-based HR50. The observed network is used for choosing the target nodes, but its effect on GE is measured over the simulated network.
3.4. Partial transmission reduction
ITN used in this study involves PWID. Therefore, a node removed from ITN (e.g., Fig. 1C) indicates that the corresponding person stopped participating in HCV transmissions, for example, by completely adopting safe injection practices. However, such scenario is not always achievable and persons affected by interventions may continue unsafe injections but at a reduced rate. The last scenario is shown in Fig. 1B depicting a network, where a 2-fold increase in weight of links results in the 50% decline in the rate of transmission involving the affected node. To investigate the effect of the incomplete transmission reduction of the nodes affected by interventions based on targeted or random strategies, we simulated interventions under different levels of transmission reduction (Fig. 6A and B).
Figure 6.

Effect of partial transmission reduction. A) GE after a random intervention with different combinations of transmission reduction and population affected, from 0 to 100 in increments of 5%. Side bar shows the GE scale. B) GE after a targeted intervention. C) Ratio of the random to targeted HR50.
The simulation experiments show that the random exclusion of a small set of nodes (e.g. 10%) results in a very limited effect on GE, with an average of 5.77% GE reduction over all levels of transmission reduction (ranging from 0.13% to 10.63%). The targeted exclusion of the same number of nodes yields an average of 43.15% GE reduction over all levels of transmission reduction (ranging from 3.90% to 88.69. Both random and targeted strategies become very similar in terms of GE reduction when transmission reduction is ≤30%. In general, analyses show that a higher transmission reduction is associated with a greater improvement in efficacy of targeted interventions (Fig. 6C). A modest 35% reduction in the level of transmission yields a 3.3-fold greater HR50 for the random vs targeted strategy, demonstrating a much greater efficacy of a network-based approach, especially when only limited resources are available for PHI. Please note that all subsequent analyses are performed assuming a total transmission reduction (100%).
3.5. Effect on incidence in setting 1
The results so far indicate superiority of targeted over random interventions in reduction of the network GE, which can be interpreted as a reduction in circulation of infection within the network remaining after exclusion of nodes. Intuitively, it suggests that the remaining HCV strains cannot be as efficiently transmitted among members of the residual network. However, this effect does not measure directly the contribution of the GE reduction to incidence or the rate of new infections among naïve members of the community because, until now, we considered a transmission network where all nodes were infected. To study the effect of targeted and random strategies on incidence in the epidemiological setting 1 (long-standing infection), we devised an in silico sampling experiment, in which 90% of nodes from ITN (n=252) were randomly sampled to represent infected individuals, with the remaining 10% being considered naïve and used as a test set (n=29). Among infected nodes, 10% were removed, assuming the 100% transmission reduction, using random or targeted approaches. The immediate reduction in incidence was calculated as a fraction on test nodes that have lost direct links to the infected part of the network because of the node removal procedure. After averaging the data from 100 trials, the targeted approach was found to result in the 18.65% incidence reduction, which is a 2.8–fold improvement over the random approach (Fig. 7A). When a similar experiment was conducted using only nodes (n=130) from the major component of ITN (Fig. 2), the targeted approach was shown to be 1.8 times more efficient in reducing the immediate incidence than the random approach (Fig. 7B). Both approaches performed 2–3 times more efficiently in the major component than in the entire ITN, suggesting a greater effect of targeted interventions on incidence reduction in tightly linked transmission networks that are usually found in high-risk populations.
Figure 7.
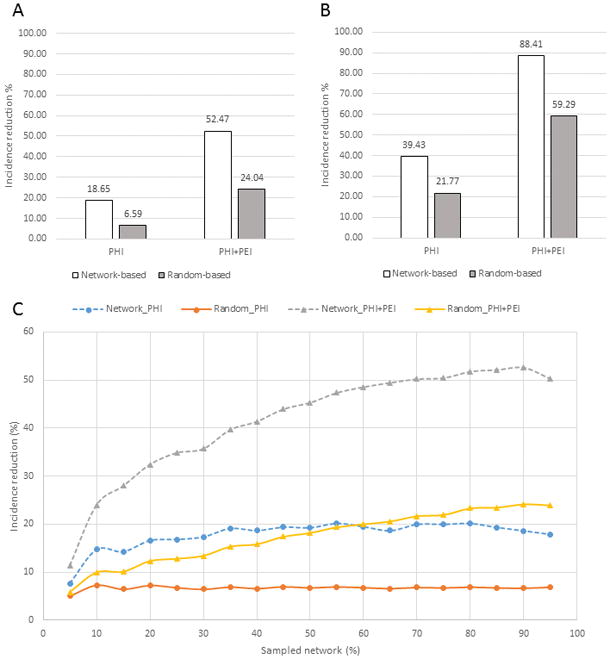
Immediate incidence reduction by random and targeted PHI and PHI+PEI with the in silico sampling experiment. A) 90% of the ITN nodes infected; 10% of infected node disrupted by direct interventions; B) 90% of the major ITN component infected; 10% of infected nodes disrupted by direct interventions. C) Incidence reduction with different levels of infected ITN nodes (sampled network percentage); 10% of infected nodes disrupted by direct interventions. Contribution of PEI to overall disruption varies based on the network topology around a node affected by direct interventions.
3.6. PEI and incidence in setting 1
PEI may spread actionable knowledge of safe injection practices among members of PWID community. To model additional effects of PEI on incidence, the experiments described above were repeated to include a probability of disrupting nodes neighboring to a node affected by PHI+PEI. Under these conditions, the random PHI+PEI applied to the entire ITN yielded a 24.04% incidence reduction, which is 3.6-times greater than a random PHI alone (Fig. 7A). The targeted PHI+PEI, however, resulted in the 52.47% incidence reduction, which is 2.2-times greater than reduction achieved by the random PHI+PEI, 8-times greater than by a random PHI alone and 2.8-times greater than by a targeted PHI alone (Fig. 7A). The incidence reduction of both strategies is greater when applied to the major ITN component (Fig. 7B), with the targeted PHI+PEI resulting in the 88.41% incidence reduction.
Modeling of random and targeted PHI and PHI+PEI directly affecting 10% of infected nodes at different levels of the network infection, from 5% – 95% of all ITN nodes, showed a much greater effect of targeted strategies on the immediate incidence reduction in overall (Fig. 7C). While PHI alone has a stable effect on incidence at almost all levels of ITN infection in both strategies, PHI+PEI has a greater effect on the incidence reduction with the increase of a fraction of infected nodes. This observation indicates the important contribution of PEI in stopping dissemination of infections in high-risk communities where actionable knowledge of safe practices, as related to infections, can be efficiently spread among members of a contact network.
3.7. PHI and PEI in setting 2
All aforementioned analyses were conducted using the epidemiological setting (setting 1) found in Indiana during investigation of HCV infection among PWID. This PWID community was infected for several years with many HCV strains of different genotypes and subtypes (Peters et al., 2016; Ramachandran et al., 2016). To model an outbreak setting (setting 2), which is characterized by a rapid spread of a single viral strain across a contact network, we performed an agent-based simulation using the major connected ITN component containing 130 nodes. At each repetition of simulation (n=1000), the simulated spread was stopped when 90% of the nodes were infected, after which we calculated the effect of disruption strategies (random or targeted) on reducing incidence among the remaining 10% of nodes. In contrast to setting 1, these simulations showed that random direct PHI were ~3.2 times more effective in reducing incidence. However, addition of PEI resulted in a ~1.7-fold greater efficiency of targeted PHI (Fig. 8).
Figure 8.
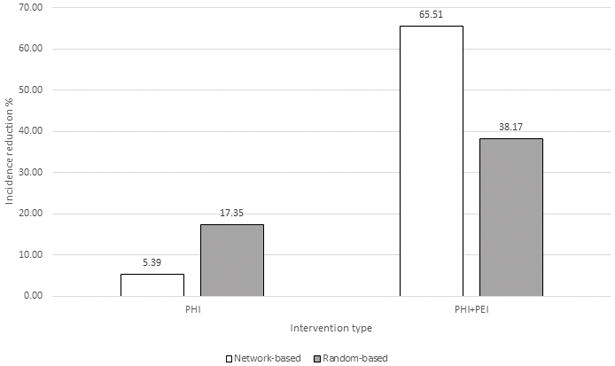
Incidence reduction by random and targeted PHI and PHI+PEI with an agent-based simulation of HCV spreading in a susceptible network. 90% of the major ITN component infected; 10% of infected nodes disrupted by direct interventions.
4. Discussion
The effective control of HCV infections depends on availability of powerful strategies to interrupt HCV transmission. HCV spread among high-risk populations such as PWID and men-who-have-sex-with-men (MSM) is facilitated by tight contact networks (Rolls et al., 2013), which can be modeled as transmission networks identified using genetic approaches (Wertheim et al., 2017). However, transmission networks are not frequently available to guide PHI, prompting the use of other approaches like an acquaintance network (Chami et al., 2017; Hellard et al., 2014) to estimate indirectly the underlying contact network structure. Availability of transmission networks enables the development of targeted strategies for elimination of HCV infections in high-risk communities. ITN used in this study (Ramachandran et al., 2016) was generated by GHOST, a new computational and molecular technology for automatic detection of HCV transmission networks from next-generation sequence data (Campo et al., 2015; Longmire et al., 2017). Here, we used this network for the development and evaluation of targeted approaches for preventing prevention of new HCV infections in two epidemiological settings: (1) long-established infection with many HCV strains as was observed in a PWID community during investigation in Indiana and (2) a hypothetical rapid spread of a single HCV strain as observed during an outbreak.
4.1. High centrality nodes
Identification of a high centrality for the network nodes co-infected with HIV or >1 HCV subtype indicates that the ITN topology properly represents the underlying contact network in this community and is suitable for modeling of PHI strategies The observation of more frequent HIV infections among nodes with high centrality is consistent with the recent introduction of HIV to this community in Indiana (Conrad et al., 2015; Peters et al., 2016; Ramachandran et al., 2016). As only a fraction of nodes has been infected with HIV, more central nodes have a higher probability of getting infected. This is in agreement with theoretical studies that showed that high Eigen-vector centrality nodes tend to be visited more often by agents that move randomly over a network (Rocha and Masuda, 2014). In contrast to HIV, nodes co-infected with >1 HCV subtype have a greater degree than Eigen-vector centrality when compared with mono-infected nodes. The Indiana PWID community was infected with many HCV strains (Peters et al., 2016; Ramachandran et al., 2016). Thus, it is conceivable that members of the high-eigenvector centrality part of the network were frequently exposed to different HCV strains. It has been reported that HCV re-infection clears more frequently than initial HCV infections (Sacks-Davis et al., 2013). Based on these results, (Sacks-Davis et al., 2013) suggested the existence of, at least partial, immunity against subsequent infections with a different HCV strain. Thus, nodes in the high Eigen-vector centrality space could accumulate some immunity, which may reduce presentation of HCV co-infections despite frequent opportunities for transmission. This process may be further facilitated by competition among HCV strains co-infecting a single individual, resulting in infection with one dominant strain (Laskus et al., 2001a; Laskus et al., 2001b; Ramirez et al., 2010). However, peripheral high-degree nodes outside of the high Eigen-vector centrality space may experience less frequent infections, resulting in lower immunity against HCV and in a more frequent presentation of HCV co-infections in these nodes. Such distinction between nodes is seemingly concordant with a long-term HCV infection in the community. These observations further suggest the interesting possibility that measures of network centrality may be used to aid in the discrimination of recent vs long-term introductions of infections in high-risk communities.
4.2. Advantages and disadvantages of GE
We explored several global network measures for guiding and monitoring the PHI implementation before choosing GE, which measures efficiency of information exchange across a network (Latora and Marchiori, 2001). GE has been used in many applications of network science (Crucitti et al., 2006; Latora and Marchiori, 2001; Latora and Marchiori, 2005; Tieri et al., 2005) and when compared with other topological measures has several important advantages for the objectives of this study:
It is applicable to weighted or unweighted networks. Contact frequency usually varies among members of community, which results in variable weights associated with different links in the transmission network. Here, we used this GE property in experiments simulating a different degree of transmission reduction upon the PHI application in the setting where affected nodes, for example, do not adhere completely to safe injection practices.
It is applicable to connected or disconnected networks. Owing to incomplete sampling or significant modularity of contact networks, transmission networks identified among persons at high-risk of infection are frequently organized into several components and unlinked nodes (Wertheim et al., 2017; Wertheim et al., 2014), similar to the ITN used in this study (Fig. 2). Such topological structure complicates agent-based simulations of viral transmission, as it is difficult to ascertain the transmission “jump” probability among components.
The network structure solely defines GE. Thus, GE can be readily used for comparing different networks and provides a new effective measure for monitoring progress of PHI implementations.
GE was used in this study as a measure of the network capacity to sustain HCV infection. In simulation experiments, PHI was modeled as a node removal from ITN. Targeted strategies should be especially efficient on networks having a skewed degree distribution because of a differential contribution of nodes to force of infection, with a few highly linked nodes spreading infection to many adjacent nodes and many peripheral nodes infecting one or only few nodes (Latora et al., 2006; Leigh Brown et al., 2011; Villandre et al., 2016; Wertheim et al., 2014). Thus, high-degree nodes will be removed only with low probability at random, which will have a little effect on reduction of force infection, while targeting high-degree nodes will have a significant impact on force of infection. Indeed, our simulation experiments showed that elimination of only 1.78% of all nodes (n=5) by a targeted intervention reduces GE by more than 50%, while a similar effect on GE will require removal of 19.82% of all nodes (n=56) by a random intervention. Targeted removal of 40% of nodes completely disables the network capacity to support HCV circulation (Fig. 4).
Although GE is directly associated with the rate of dissemination of messages across a network or, in epidemiological terms, with force of infection, its greatest disadvantage is that it is a purely topological measure. In dynamic settings, the importance of GE may greatly depend on many factors beyond the known network topology such as duration of the outbreak and rates of exposure, transmission, and the network growth, which can be assessed through epidemiological observations. If we consider a very high prevalence of infection among PWID (Degenhardt et al., 2017) in setting 1 of well-established infection and assume an equal rate of exposure at each link between members of the network, GE can be used to accurately approximate force of infection on a network. Decline in GE indicates reduction in a network capacity to support a rapid spread of infections. Although such decline may not have a strong effect on the overall infection prevalence beyond removal of the targeted few nodes, nevertheless, targeting may have a strong effect on force of infection on the network or on capacity to infect new members of the community. On a random network, force of infection is evenly distributed among infected nodes, while on a network with a skewed degree distribution, high-centrality nodes have a much greater contribution to force of infection than peripheral nodes. Contribution of a node to GE is the most direct measure of its contribution to force of infection and, thus, to the potential of establishing new infections or incidence, which is one of the most important measures of effectiveness of public health interventions to curb infection in the targeted community.
GE defines more accurately the rate of transmission of new infections in high-risk communities than a simple count of currently infected cases. Intuitively, a 90% GE reduction suggests that spread of new infections, for example, HIV among HCV infected PWID, across the entire network will be 10 times more difficult than in the unreduced network. This reduction can be achieved by removal of fewer nodes using targeted vs random strategies, indicating non-uniform distribution of capacity to cause new infections among members of high-risk communities. To evaluate this capacity, we conducted simulation experiments to measure incidence. Difference in the number of uninfected nodes losing their direct connection to infected nodes after the targeted or random node removal was used to measure the incidence reduction resulted from PHI. In each simulation experiment, infected nodes were randomly assigned and comprised 5%-95% of ITN. Again, the targeted strategies were found to be ~2.8 times more effective than random in reducing the number of new infections in communities with 10%-95% prevalence of infection.
4.3. Caveats of a targeted approach
If resources permit, all nodes in the transmission network must be treated. However, application of targeted PHI allows for achieving a significant reduction in incidence using limited resources or faster infection elimination using sufficient resources. Thus, targeted PHI benefits the treated community by rapidly reducing opportunity for efficient transmission of new infections, like HIV to HCV infected PWID or HCV to naïve members of the community.
Significant dependence of targeted strategies on the network structure indicates that the effective PHI implementation may be affected by accuracy of the network estimation. Simulation experiments conducted here to evaluate the contribution of errors related to node sampling and link assignments showed a consistently greater effect of targeted over random strategies on GE at almost all levels of errors. The difference between both strategies becomes undetectable when errors exceed 80%. This observation indicates that targeted strategies applied to inaccurately or incompletely estimated networks never perform less efficiently than random; however, their efficiency may decline to approximate the effect of random strategies when the network topology is identified with a very low accuracy.
Another factor that may have a significant effect on success of PHI and may diminish contribution of targeted vs random strategies is incomplete reduction of transmission at the level of individual nodes in the network, which may be caused by, for example, poor adherence to safe injection practices among members of PWID community. Indeed, transmission reduction at the level of <30% results in both strategies being inefficient in controlling HCV infections (Fig. 6). Nevertheless, the targeted node removal outperforms the random strategy at all levels of transmission reduction above 35% (Fig. 6C). Removal of a small set of nodes at random results in a very limited, if any, effect on GE even at the 100% transmission reduction, while the targeted removal of the same number of nodes may have a profound effect on GE. These observations indicate that PHI with significant limitations in resources, which do not allow to access >10% of the community, may be very ineffective when applied at random. Also, ineffective PHI implementation, which does not produce a >35% transmission reduction among affected individuals, may result in an almost undetectable effect on GE and infection dissemination.
It is important to note that ITN did not contain information on directionality. However, many, if not all, edges in ITN are likely bidirectional. Although previous studies have found that highly connected nodes did not necessarily have a high number of outgoing edges (Bartlett et al., 2017)in outbreak settings, ITN does not reflect a rapid spread of HCV in the community (as observed during an outbreak) but rather is a result of a long-going epidemic. Although directionality must be extremely important and likely fixed when a new strain is introduced and spreads across a network within a short period of time, in the case of a well-established epidemic lasting for years, directionality of each edge is not necessarily constant. It is difficult to expect for transmission directionality to be accurately assessed or even to be of significance for each HCV strain circulating in the community if we consider: (i) a long standing of HCV infection, which suggests that all members of the community may participate in HCV transmission as both sources and recipients, (ii) a potential for differential acquisition of partial immunity depending on position in the network, (iii) a dynamic competition among co-infecting HCV strains (Laskus et al., 2001a; Laskus et al., 2001b; Ramirez et al., 2010), (iv) a variation in the exposure rates at different edges, and (v) a low probability of complete sampling of all members of transmission chains.
4.4. PEI
PEI applied at the level of PWID communities through randomly selected peer-educators was shown to be efficient, with 76% of PWID having continued to follow safe injection practices at least for 6 months after PEI (Garfein et al., 2007; Mackesy-Amiti et al., 2013). Given that this does not necessarily equate with a 76% effectiveness at preventing HCV transmission, this value was considered here solely as an upper bound estimate. Within communities, in difference to other PHI, PEI appears to act as “infection” that transmits with some probability from the affected node to adjacent nodes in the network, spreading, for example, actionable knowledge of safe injection practices among members of a PWID community. Therefore, we may assume that nodes with many links in the transmission network have a strong effect on dissemination of such actionable messages, owing to the substantial standing of the corresponding individuals in the community (e.g., greater financial resources to purchase drugs or seniority in social relationships, etc.)(De et al., 2007). Accordingly, knowledge and adoption of safe practices preventing transmission of infections by these individuals should be more readily accepted by their contacts. In our study, addition of PEI resulted in a 2–3 times greater reduction in incidence than from PHI alone, with the reduction reaching 88.4% on the tightly linked major component of ITN. These observations from simulation experiments show that PEI implemented using nodes maximally contributing to GE, and thus taking into consideration the topological structure of transmission networks, greatly amplifies effects of PHI on incidence reduction. To our knowledge, this is the first application of quantitative models to study PEI effects on incidence using actual transmission networks.
4.5. Different effect of targeted intervention in the two settings
Considering a significant HCV prevalence among PWID (Degenhardt et al., 2017), the epidemiological setting 1 of well-established HCV infection should be frequently observed among PWID communities. Indeed, the recent investigation in Indiana showed infection of a large rural PWID community with many HCV strains of different genotypes(Peters et al., 2016; Ramachandran et al., 2016), indicating a long-term standing of the infection and numerous introductions of different strains over time. However, this investigation was prompted by outbreak of HIV infection, which shares the same mode of transmission with HCV, indicating another setting of a rapid spread of infection with a single strain across the PWID contact network(Campbell et al., 2017; Peters et al., 2016). Here, we modeled both settings. Setting 1 of the well-established HCV infection is relatively stable. It was modeled using random sampling of infected nodes, assuming that the remaining nodes represent recent new members of the network. Setting 2 is very dynamic. The rapid growth of the network of infected nodes was modeled using agent-based simulations. Taking into consideration a dynamic nature of this setting and a short duration of the process, the remaining uninfected nodes were assumed to represent peripheral members of the network. Application of targeted PHI has different outcomes in these two settings. In difference to setting 1, targeted PHI were found to be less efficient than random in setting 2, although addition of PEI is equally beneficial in both settings. These findings are in concert with other studies on modeling PHI in outbreak settings (Bartlett et al., 2017; Zelenev et al., 2018). The difference can be explained by topological features of uninfected nodes. In setting 1, which is expected to be of some duration, uninfected nodes join the network by a preferential attachment, linking to the existing nodes with a probability proportional to their degree (Latora et al., 2006; Leigh Brown et al., 2011; Villandre et al., 2016; Wertheim et al., 2014). Thus, removal of high-centrality nodes results in a greater effect on attachment of new nodes, significantly reducing probability of the newly joining members to become infected. However, in setting 2, which is rapid, modeling of the network exploration as dispersion results in a very high probability of infection of high-centrality nodes and their immediate neighbors, leaving uninfected mainly peripheral nodes that have edges to low-degree nodes. In this case, the targeted removal of high-centrality nodes should have a very limited effect on separation of uninfected nodes from the network than removal of nodes at random.
It should be noted that effect of PHI on GE reduction can be accurately estimated only on the entire contact network, which frequently remains unknown. Lack of such knowledge limits application of agent-based or any other approaches to modeling effects of interventions. Genetic analyses applied to dynamic outbreak settings likely do not expose the entire network of contacts relevant to the infection dissemination because the process of infection in the affected community is not complete. In addition, accuracy of simulations in dynamic settings may greatly depend on many factors beyond the known network topology such as duration of the outbreak and rates of exposure, transmission, and the network growth, which can be assessed through epidemiological observations. Development of effective PHI in dynamic settings of outbreaks is complex and warrants further research.
4.6. INDRA: a potential tool for public health institutions
INDRA is a versatile method that provides a clear prioritization strategy of the available PHI when only a transmission network has been inferred. When applied to a transmission network, INDRA provides a report on: (i) GE50 estimate; (ii) a probability to achieve a similar reduction in GE by a random removal of the same number of nodes; and (iii) a rank of network nodes by their contribution to GE, suggesting targeting PHI towards the high-ranked nodes to maximize incidence reduction. Although INDRA can analyze a network enhanced or solely created using epidemiological information, a limiting factor is the availability and accuracy of such data for network construction. Genetic data are more objective than personal recollections of contacts for inferring transmission networks. A low cost and broad availability of next-generation sequencing coupled with bioinformatics tools freely available to users (Longmire et al., 2017) make genetic analyses for the detection of transmission networks accessible and provide real opportunities for field applications of INDRA. Nevertheless, epidemiological variables such as an estimated size of community, duration of drug use, drug itself, rate of injection as well as gender, age, and ethnicity of network members are very useful for accurate assessment of network topology and application of specific PHI and PEI. Application of genetic networks adjusted using these variables should further improve effectiveness of INDRA.
5. Conclusions
In high-risk communities, transmission networks may significantly facilitate HCV elimination efforts. In setting of a long-established infection in the community, targeted strategies based on maximizing the GE reduction of the transmission networks are most efficient in controlling HCV infections, indicating a differential contribution of infected members of the community to force of infection. GE is an effective measure for guiding and monitoring PHI in this setting. Targeted PHI facilitated by INDRA outperforms random strategies in decreasing circulation of long-established infections. However, targeted PHI in the dynamic outbreak setting are less efficient than random, suggesting a significant dependence on completeness of the known network and/or additional factors beyond the network topology. Nevertheless, network-based PEI may amplify effects of PHI on incidence reduction in both settings.
Acknowledgments
We are deeply grateful to all researchers who tirelessly worked on establishing ITN, including Sumathi Ramachandran, Gilberto Vaughan, Joseph C. Forbi, Hong Thai, Lilia Ganova-Raeva, Romeo Regi Galang, Lili Punkova, Yulin Lin, Inna Rytsareva, Amanda Sue, Zoya Dimitrova, Pavel Skums, Seth Sims, Atkinson G. Longmire, Mahder Teka, Guo-liang Xia, Pamela Pontones, Jessica Gentry, Sara J. Blosser, Judy Lovchik, Ellsworth Campbell, William M. Switzer, Eyasu Teshale, Philip Peters and John Ward.
Funding.
This work was supported by the Division of Viral Hepatitis, Centers for Disease Control and Prevention.
Abbreviations
- HR50
Harm reduction 50%
- PHI
Public Health Intervention
- PEI
Peer-education intervention
- GE
Global efficiency
- ITN
Indiana Transmission Network
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Alter M. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett DK, Claas SA. Precision Medicine, Genomics, and Public Health. Diabetes Care. 2016;39:1870–1873. doi: 10.2337/dc16-1763. [DOI] [PubMed] [Google Scholar]
- Bartlett SR, Wertheim JO, Bull RA, Matthews GV, Lamoury FM, Scheffler K, Hellard M, Maher L, Dore GJ, Lloyd AR, Applegate TL, Grebely J. A molecular transmission network of recent hepatitis C infection in people with and without HIV: Implications for targeted treatment strategies. J Viral Hepat. 2017;24:404–411. doi: 10.1111/jvh.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer R, Galea S. Public Health in the Precision-Medicine Era. N Engl J Med. 2015;373:499–501. doi: 10.1056/NEJMp1506241. [DOI] [PubMed] [Google Scholar]
- Bruggmann P, Grebely J. Prevention, treatment and care of hepatitis C virus infection among people who inject drugs. Int J Drug Policy. 2015;26(Suppl 1):S22–26. doi: 10.1016/j.drugpo.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Jia H, Shankar A, Hanson D, Luo W, Masciotra S, Owen SM, Oster AM, Galang RR, Spiller MW, Blosser SJ, Chapman E, Roseberry JC, Gentry J, Pontones P, Duwve J, Peyrani P, Kagan RM, Whitcomb JM, Peters PJ, Heneine W, Brooks JT, Switzer WM. Detailed Transmission Network Analysis of a Large Opiate-Driven Outbreak of HIV Infection in the United States. J Infect Dis. 2017;216:1053–1062. doi: 10.1093/infdis/jix307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo D, Xia G, Dimitrova Z, Lin Y, Ganova-Raeva L, Punkova L, Ramachandran S, Thai H, Sims S, Rytsareva I, Skums P, Vaughan G, Roh H, Purdy M, Sue A, Roh H, Khudyakov Y. Accurate genetic detection of hepatitis C virus transmissions in outbreak settings. Journal of Infectious Diseases. 2015;213:957–965. doi: 10.1093/infdis/jiv542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chami GF, Ahnert SE, Kabatereine NB, Tukahebwa EM. Social network fragmentation and community health. Proc Natl Acad Sci U S A. 2017;114:E7425–E7431. doi: 10.1073/pnas.1700166114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C, Bradley HM, Broz D, Buddha S, Chapman EL, Galang RR, Hillman D, Hon J, Hoover KW, Patel MR, Perez A, Peters PJ, Pontones P, Roseberry JC, Sandoval M, Shields J, Walthall J, Waterhouse D, Weidle PJ, Wu H, Duwve JM Centers for Disease C, Prevention. Community Outbreak of HIV Infection Linked to Injection Drug Use of Oxymorphone--Indiana, 2015. MMWR Morbidity and mortality weekly report. 2015;64:443–444. [PMC free article] [PubMed] [Google Scholar]
- Crucitti P, Latora V, Porta S. Centrality measures in spatial networks of urban streets. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;73:036125. doi: 10.1103/PhysRevE.73.036125. [DOI] [PubMed] [Google Scholar]
- De P, Cox J, Boivin JF, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007;102:1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, Stone J, Cunningham EB, Trickey A, Dumchev K, Lynskey M, Griffiths P, Mattick RP, Hickman M, Larney S. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5:e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsherif O, Bannan C, Keating S, McKiernan S, Bergin C, Norris S. Outcomes from a large 10 year hepatitis C treatment programme in people who inject drugs: No effect of recent or former injecting drug use on treatment adherence or therapeutic response. PLoS One. 2017;12:e0178398. doi: 10.1371/journal.pone.0178398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfein RS, Golub ET, Greenberg AE, Hagan H, Hanson DL, Hudson SM, Kapadia F, Latka MH, Ouellet LJ, Purcell DW, Strathdee SA, Thiede H, Team DS. A peer-education intervention to reduce injection risk behaviors for HIV and hepatitis C virus infection in young injection drug users. Aids. 2007;21:1923–1932. doi: 10.1097/QAD.0b013e32823f9066. [DOI] [PubMed] [Google Scholar]
- Hellard M, Rolls DA, Sacks-Davis R, Robins G, Pattison P, Higgs P, Aitken C, McBryde E. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60:1861–1870. doi: 10.1002/hep.27403. [DOI] [PubMed] [Google Scholar]
- Islam N, Krajden M, Shoveller J, Gustafson P, Gilbert M, Buxton JA, Wong J, Tyndall MW, Janjua NZ British Columbia Hepatitis Testers Cohort t. Incidence, risk factors, and prevention of hepatitis C reinfection: a population-based cohort study. Lancet Gastroenterol Hepatol. 2017;2:200–210. doi: 10.1016/S2468-1253(16)30182-0. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Iademarco MF, Riley WT. Precision Public Health for the Era of Precision Medicine. Am J Prev Med. 2016;50:398–401. doi: 10.1016/j.amepre.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskus T, Wang LF, Radkowski M, Nowicki M, Wilkinson J, Rakela J. Exposure of hepatitis C virus-negative recipients to > or =2 infected blood donors. J Infect Dis. 2001a;183:666–669. doi: 10.1086/318531. [DOI] [PubMed] [Google Scholar]
- Laskus T, Wang LF, Radkowski M, Vargas H, Nowicki M, Wilkinson J, Rakela J. Exposure of hepatitis C virus (HCV) RNA-positive recipients to HCV RNA-positive blood donors results in rapid predominance of a single donor strain and exclusion and/or suppression of the recipient strain. J Virol. 2001b;75:2059–2066. doi: 10.1128/JVI.75.5.2059-2066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Physics Review Letters. 2001;87:1–14. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Vulnerability and protection of infrastructure networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:015103. doi: 10.1103/PhysRevE.71.015103. [DOI] [PubMed] [Google Scholar]
- Latora V, Nyamba A, Simpore J, Sylvette B, Diane S, Sylvere B, Musumeci S. Network of sexual contacts and sexually transmitted HIV infection in Burkina Faso. J Med Virol. 2006;78:724–729. doi: 10.1002/jmv.20614. [DOI] [PubMed] [Google Scholar]
- Leigh Brown AJ, Lycett SJ, Weinert L, Hughes GJ, Fearnhill E, Dunn DT, Collaboration UHDR. Transmission network parameters estimated from HIV sequences for a nationwide epidemic. J Infect Dis. 2011;204:1463–1469. doi: 10.1093/infdis/jir550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmire A, Sims S, Rytsareva I, Campo D, Skums P, Dimitrova Z, Ramachandran S, Medrzycki M, Thai H, Ganova-Raeva L, Lin Y, Punkova L, Sue A, Mirabito M, Wang S, Tracy R, Bolet V, Sukalac T, Lynberg C, Khudyakov Y. GHOST: Global Hepatitis Outbreak and Surveillance Technology. BMC genomics. 2017;18:916. doi: 10.1186/s12864-017-4268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Annals of internal medicine. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- Mackesy-Amiti ME, Finnegan L, Ouellet LJ, Golub ET, Hagan H, Hudson SM, Latka MH, Garfein RS. Peer-education intervention to reduce injection risk behaviors benefits high-risk young injection drug users: a latent transition analysis of the CIDUS 3/DUIT study. AIDS Behav. 2013;17:2075–2083. doi: 10.1007/s10461-012-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57(Suppl 2):S39–45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- Newman MEJ. Networks: an introduction. Oxford University Press; Oxford; New York: 2010. [Google Scholar]
- Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, Tobler L, Andrews W, Avanesyan L, Cooper S, Busch MP. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis. 2013;57(Suppl 2):S32–38. doi: 10.1093/cid/cit300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Pontones P, Hoover KW, Patel MR, Galang RR, Shields J, Blosser SJ, Spiller MW, Combs B, Switzer WM, Conrad C, Gentry J, Khudyakov Y, Waterhouse D, Owen SM, Chapman E, Roseberry JC, McCants V, Weidle PJ, Broz D, Samandari T, Mermin J, Walthall J, Brooks JT, Duwve JM, Indiana HIVOIT. HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014–2015. N Engl J Med. 2016;375:229–239. doi: 10.1056/NEJMoa1515195. [DOI] [PubMed] [Google Scholar]
- Platt L, Reed J, Minozzi S, Vickerman P, Hagan H, French C, Jordan A, Degenhardt L, Hope V, Hutchinson S, Maher L, Palmateer N, Taylor A, Hickman M. Effectiveness of needle/syringe programmes and opiate substitution therapy in preventing HCV transmission among people who inject drugs. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Teshale E, Switzer W, Peters P, Galang R, Pontones P, Gentry J, Blosser S, Ward J, Khudyakov Y. Networks of HCV Transmissions Among Persons Who Inject Drugs: Indiana, 2015. Conference on Retroviruses and Opportunistic Infections (CROI 2016); Boston, MA, USA. 2016. [Google Scholar]
- Ramachandran S, Thai H, Forbi J, Regi Galang R, Dimitrova Z, Xia G, Lin Y, Punkova L, Ganova-Raeva L, Sue A, Vaughan G, Roh H, Campo D, Skums P, Rytsareva I, Sims S, Khudyakov Y. A vast HCV transmission network enabled a large HIV outbreak in Indiana, 2014–15. 2018 doi: 10.1016/j.ebiom.2018.10.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S, Perez-del-Pulgar S, Carrion JA, Coto-Llerena M, Mensa L, Dragun J, Garcia-Valdecasas JC, Navasa M, Forns X. Hepatitis C virus superinfection of liver grafts: a detailed analysis of early exclusion of non-dominant virus strains. J Gen Virol. 2010;91:1183–1188. doi: 10.1099/vir.0.018929-0. [DOI] [PubMed] [Google Scholar]
- Rocha L, Masuda N. Random walk centrality for temporal networks. New J Phys. 2014:16. [Google Scholar]
- Rolls DA, Sacks-Davis R, Jenkinson R, McBryde E, Pattison P, Robins G, Hellard M. Hepatitis C transmission and treatment in contact networks of people who inject drugs. PLoS One. 2013;8:e78286. doi: 10.1371/journal.pone.0078286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytsareva I, Campo DS, Zheng Y, Sims S, Thankachan SV, Tetik C, Chirag J, Chockalingam SP, Sue A, Aluru S, Khudyakov Y. Efficient detection of viral transmissions with Next-Generation Sequencing data. BMC genomics. 2017;18:372. doi: 10.1186/s12864-017-3732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks-Davis R, Aitken CK, Higgs P, Spelman T, Pedrana AE, Bowden S, Bharadwaj M, Nivarthi UK, Suppiah V, George J, Grebely J, Drummer HE, Hellard M. High rates of hepatitis C virus reinfection and spontaneous clearance of reinfection in people who inject drugs: a prospective cohort study. PLoS One. 2013;8:e80216. doi: 10.1371/journal.pone.0080216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott N, Caulkins J, Ritter A, Dietze P. How patterns of injecting drug use evolve in a cohort of people who inject drugs. Trends & issues in crime and criminal justice Canberra: Australian Institute of Criminology. 2015:502. [Google Scholar]
- Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, Forouzanfour MH, Groeger J, Hanafiah KM, Jacobsen KH, James SL, MacLachlan J, Malekzadeh R, Martin NK, Mokdad AA, Mokdad AH, Murray CJL, Plass D, Rana S, Rein DB, Richardus JH, Sanabria J, Saylan M, Shahraz S, So S, Vlassov VV, Weiderpass E, Wiersma ST, Younis M, Yu C, El Sayed Zaki M, Cooke GS. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Academies of Sciences, E.a.M. A National Strategy for the Elimination of Hepatitis B and C. 2017. [PubMed] [Google Scholar]
- Tieri P, Valensin S, Latora V, Castellani GC, Marchiori M, Remondini D, Franceschi C. Quantifying the relevance of different mediators in the human immune cell network. Bioinformatics. 2005;21:1639–1643. doi: 10.1093/bioinformatics/bti239. [DOI] [PubMed] [Google Scholar]
- Villandre L, Stephens DA, Labbe A, Gunthard HF, Kouyos R, Stadler T, Swiss HIVCS. Assessment of Overlap of Phylogenetic Transmission Clusters and Communities in Simple Sexual Contact Networks: Applications to HIV-1. PLoS One. 2016;11:e0148459. doi: 10.1371/journal.pone.0148459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JW. The hidden epidemic of hepatitis C virus infection in the United States: occult transmission and burden of disease. Topics in antiviral medicine. 2013;21:15–19. [PMC free article] [PubMed] [Google Scholar]
- Wertheim JO, Kosakovsky Pond SL, Forgione LA, Mehta SR, Murrell B, Shah S, Smith DM, Scheffler K, Torian LV. Social and Genetic Networks of HIV-1 Transmission in New York City. PLoS Pathog. 2017;13:e1006000. doi: 10.1371/journal.ppat.1006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim JO, Leigh Brown AJ, Hepler NL, Mehta SR, Richman DD, Smith DM, Kosakovsky Pond SL. The global transmission network of HIV-1. J Infect Dis. 2014;209:304–313. doi: 10.1093/infdis/jit524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO; WHO, editor Combating hepatitis B and C to reach elimination by 2030. 2016. [Google Scholar]
- Wicker S, Cinatl J, Berger A, Doerr HW, Gottschalk R, Rabenau HF. Determination of risk of infection with blood-borne pathogens following a needlestick injury in hospital workers. Ann Occup Hyg. 2008;52:615–622. doi: 10.1093/annhyg/men044. [DOI] [PubMed] [Google Scholar]
- Zelenev A, Li J, Mazhnaya A, Basu S, Altice FL. Hepatitis C virus treatment as prevention in an extended network of people who inject drugs in the USA: a modelling study. Lancet Infect Dis. 2018;18:215–224. doi: 10.1016/S1473-3099(17)30676-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


