Abstract
Experience-dependent critical period plasticity has been extensively studied in the visual cortex. Monocular deprivation during the critical period affects ocular dominance, limits visual performance, and contributes to the pathological etiology of amblyopia. Neuregulin-1 (NRG1) signaling through its tyrosine kinase receptor ErbB4 is essential for the normal development of the nervous system, and has been linked to neuropsychiatric disorders such as schizophrenia. We discovered recently that NRG1/ErbB4 signaling in PV neurons is critical for the initiation of critical period visual cortical plasticity by controlling excitatory synaptic inputs onto PV neurons and thus PV-cell mediated cortical inhibition that occurs following visual deprivation. Building on this discovery, we review the existing literature of neuregulin signaling in developing and adult cortex, and address the implication of NRG/ErbB4 signaling in visual cortical plasticity at the cellular and circuit levels. NRG-directed research may lead to therapeutic approaches to reactivate plasticity in the adult cortex.
Keywords: Neuregulin-1, ErbB4, PV neurons, visual cortex, adult plasticity
Graphical abstract
This image is associated with Figure 2 in Grieco et al. in this issue. It is an artistic rendition of their image data illustrating enhanced local excitatory inputs to monocularly deprived PV neurons (white circles) with bath applied NRG1. The eye-like overlay in black conveys that therapeutic intervention of NRG1/ErbB4 signaling can be developed to help treat critical period-relevant disorders such as amblyopia. The review article of Grieco et al. addresses a novel and critical role of NRG1/ErbB4 in regulation of visual cortical critical period plasticity.
Introduction
This article is in memory of Prof. Vivien A. Casagrande who was Xiangmin Xu’s graduate school mentor at Vanderbilt University during 1998 – 2004. Prof. Casagrande passed away on January 21, 2017 at the age of 74 at her home in Nashville, TN. She was a pioneer in visual neuroscience and she made tremendous contributions to our understanding of early visual area organization in primates in relation to the development and physiological interplay between parallel visual information pathways. She cared deeply about teaching and scientific training. As living memorials to Vivien, many of those she mentored continue work as successful scientists. Her legacy profoundly influenced Xiangmin Xu’s career in a lasting way. Not only did she teach Xiangmin to ask the right questions and to get the answers, she also inspired him to do his work with determination and passion. Vivien’s work established the organization principles of parallel visual pathways and functional modules in early visual areas as outlined in her published papers (Casagrande, 1994; Xu et al., 2004; Xu, Bosking, White, Fitzpatrick, & Casagrande, 2005; Xu et al., 2001). This provides an important framework towards unraveling the details of the molecular and cellular basis of functional plasticity. Following Prof. Casagrande’s scientific influence, Xiangmin Xu’s laboratory research continues a major focus on visual cortical development and functional plasticity.
Experience alters brain circuits through neural plasticity mechanisms. Cortical networks can adapt to sensory input over a range of timescales by adjusting excitatory and inhibitory synaptic strength. Neural circuitry in the brain is strongly shaped by experience during relatively short temporal postnatal windows called 'critical periods'. Experience-dependent critical period plasticity has been extensively studied in the visual cortex (Hensch, 2005; Levelt & Hubener, 2012; Tropea, Van Wart, & Sur, 2009). Monocular deprivation during this critical period affects ocular dominance, limits visual performance, and contributes to the etiology of amblyopia. Although physiological aspects of visual cortical ocular dominance plasticity (ODP) were described by the pioneering discoveries of Hubel and Wiesel more than 50 years ago, our understanding of the underlying cellular locus and molecular mechanisms has lagged (Levelt & Hubener, 2012).
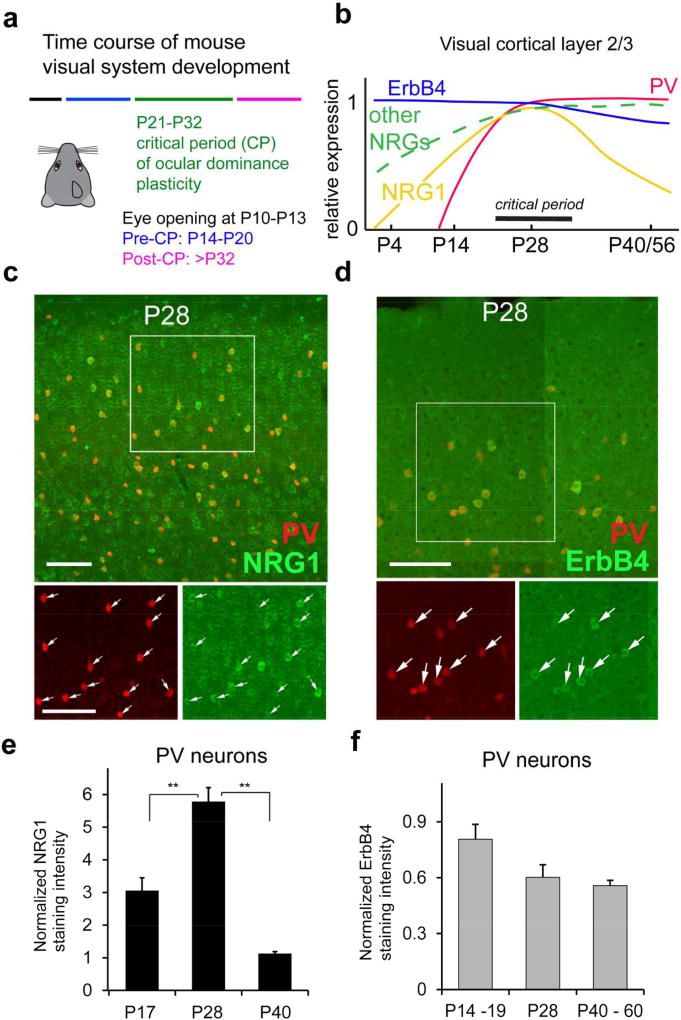
We identified parvalbumin-expressing (PV) inhibitory neurons as the key initial locus for critical period cortical plasticity (Kuhlman et al., 2013). PV neurons are rapidly inhibited by visual deprivation via monocular eyelid suture during the critical period due to a decrease in local excitatory circuit inputs onto these interneurons. This initial and transient reduction of PV cell activity establishes the conditions necessary for the experience-dependent excitatory cortical plasticity for ocular dominance. Previous studies identified neurotrophins, extracellular matrix components, and synapse formation molecules as modulators of visual cortical plasticity (Berardi, Pizzorusso, Ratto, & Maffei, 2003; Gu et al., 2013; Z. J. Huang et al., 1999; Murase, Lantz, & Quinlan, 2017; Pizzorusso et al., 2002; Stephany, Ikrar, Nguyen, Xu, & McGee, 2016; Sugiyama et al., 2008; Tropea et al., 2006). However, they do not account for the translation of brief sensory deprivation into functional changes in circuit connections. We discovered recently that neuregulin-1 (NRG1) signaling through its cognate ErbB4 receptor is critical for the initiation of critical period visual cortical plasticity by controlling excitatory synaptic inputs onto PV inhibitory interneurons (Sun et al., 2016). NRG1 is expressed widely in excitatory neurons, inhibitory interneurons and glial cells in the visual cortex (Liu et al., 2011; Sun et al., 2016). A critical clue is that NRG1 expression temporally correlates with the critical period (Fig. 1a–b) (Sun et al., 2016). ErbB4 expression is largely restricted to PV neurons and temporally extends beyond the critical period (Fazzari et al., 2010; Sun et al., 2016; Yau, Wang, Lai, & Liu, 2003). Thus ErbB4 presents a tempting molecular target for controlling NRG1 signaling during and beyond the normal window of critical period plasticity (Fig. 1b); manipulation of ErbB4 signaling in PV neurons may be sufficient to extend the ODP beyond the normal temporal boundary of the critical period.
Figure 1. NRG1 and ErbB4 expression in PV neurons is developmentally regulated.
a, The developmental times for mouse eye opening; before, during and after the critical period (CP) for ocular dominance plasticity (Smith & Trachtenberg, 2007). b, A summary diagram for NRG1, other NRGs and ErbB4 and PV expression levels in mouse visual cortex L2/3 from P0 to P56 compared with the critical period for ocular dominance plasticity. The diagram is constructed based on our immunostaining data (Sun et al., 2016), in situ gene hybridization data from the Allen Brain Atlas, and other published data (Anton et al., 2004; Liu et al., 2011; Longart, Liu, Karavanova, & Buonanno, 2004; Woo et al., 2007). c, Representative confocal images of PV expression (red) and NRG1 immunolabeling (green) in PV-Cre; Ai9 mouse sections of postnatal day (P) P28 (dual overlap in larger upper panel, detailed single label images in smaller lower panels). d, Representative confocal images of PV expression (red) and ErbB4 immunolabeling (green) in PV-Cre; Ai9 mouse sections at P28. For both (c) and (d), the arrows indicate PV cells (red) and their corresponding NRG1/ErbB4 immunolabeling (green). Scale bar = 100 µm. e, A bar graph shows that the average strength of NRG1 expression in PV cells varies across P17, P28 and P40, and peaks at P28. The NRG1 staining intensity at different age groups was normalized to the staining intensity of P40 in the staining series. PV cell measurements were obtained from 5 different mice for each age group. **, p <0.01 (Non-parametric one-way ANOVA Kruskal Wallis test, followed by Mann–Whitney U tests). f, A bar graph shows the average ErbB4 expression strength across P14–19, P28 and P40. The ErbB4 staining intensity of PV neurons at different age groups (n = 5, 3 and 3 mice, respectively) was normalized to the staining intensity of P40 in the staining series. There appears to be a trend for decreased ErbB4 expression in older ages, but does not reach statistical significance (Kruskal Wallis test, p = 0.07). The figure is modified from published data figures in Sun et al. (2016) with permission from the Cell Press.
Within this context, we provide a topical review of neuregulin-directed molecular mechanisms of cortical plasticity. In this article, we first summarize our progress in understanding cell-type specific cellular circuits underlying cortical plasticity. We combine this with the existing literature of neuregulin signaling in developing and adult cortex. We then address the implication of NRG/ErbB4 in visual cortical plasticity at the cellular and circuit levels. Lastly, we discuss outstanding questions and directions for further research that aim to establish a molecular switch role of NRG1/ErbB4 signaling for re-opening the critical period window of plasticity.
Visual cortical plasticity and specific neuron types
Critical period plasticity in the visual cortex has been well-studied (Hensch, 2005). Measurements of visual cortex responses were first made using “single unit” recordings in cats and monkeys (Hubel, 1957; Hubel & Wiesel, 1968, 1970). These seminal experiments showed that in young animals before the closure of the critical period, monocular deprivation alters ocular dominance and results in an amblyopic phenotype. This provided insight into the mechanisms of amblyopia and an animal model for the disease. Later it was discovered that various species display analogous ocular dominance critical periods, suggesting that in visual cortex this is an important and evolutionarily conserved mechanism. For example, studies in mice show that they have a critical period of ocular dominance, and that monocular deprivation during the critical period in mice recapitulates the amblyopic phenotype (Gordon & Stryker, 1996).
The excitatory-inhibitory balance of neural circuits is important for cortical plasticity (Hensch & Fagiolini, 2005). Cortical inhibition initiates and accelerates the opening of the critical period (Fagiolini & Hensch, 2000). Some of the first findings underlying this concept used benzodiazepine treatments, which increase GABAergic inhibition. This results in the early opening of the critical period (Fagiolini & Hensch, 2000; Hensch et al., 1998). However, it then appears that increased excitatory activity or decreased inhibitory activity during the critical period allowed for increased cortical ODP. Treating mice with a glutamic acid decarboxylase (GAD) inhibitor reduced cortical inhibition and increases ODP (Harauzov et al., 2010). Similarly, housing mice in darkness before monocular deprivation during the critical period results in increased ODP shifts and cortical excitation in the visual cortex (Fagiolini, Pizzorusso, Berardi, Domenici, & Maffei, 1994; Morales, Choi, & Kirkwood, 2002). Also, mice completely lacking GAD65, the enzyme that catalyzes GABA production, do not display critical period ODP (Hensch et al., 1998). But treatment of these mice with a benzodiazepine reconstitutes ODP, suggesting that cortical inhibition must first be in place to open the critical period before increased excitation/inhibition ratios promote plasticity (Fagiolini & Hensch, 2000). These findings suggest that inhibitory interneurons in the visual cortex may be important for regulating the critical period and ODP.
There is a variety of inhibitory interneurons present in the mammalian cortex that comprise about 10% of all cortical neurons (Callaway, 2016; Klausberger & Somogyi, 2008; Markram et al., 2004). The three major, non-overlapping groups of interneurons in the visual cortex express PV, somatostatin (SOM), or VIP (Xu, Roby, & Callaway, 2010); the predominant sub-group in the visual cortex is the PV type (Gonchar & Burkhalter, 1997; Xu et al., 2010). Most PV neurons are fast-spiking basket cells and are highly integrated into cortical circuits (Freund & Katona, 2007). The main function of PV interneurons involves inhibiting pyramidal neurons and maintaining the temporal window in which they integrate incoming signals (Atallah, Bruns, Carandini, & Scanziani, 2012; Kuhlman, Lu, Lazarus, & Huang, 2010). It was recently discovered that PV interneurons regulate the visual critical period, and are the initial locus for ODP (Kuhlman et al., 2013).
Determining the molecular signaling related to PV interneuron regulation of the critical period and cortical plasticity will be important for explaining the underlying processes of cortical plasticity as well as for developing novel therapeutics. Progress has been made towards identifying the signaling molecules responsible for regulating the critical period and cortical plasticity. For example, precocious critical periods may be induced by neurotrophic factor expression or by the disruption of perineural cell adhesion (Di Cristo et al., 2007; Hanover, Huang, Tonegawa, & Stryker, 1999; Z. J. Huang et al., 1999; Wang, Feng, Liu, Liu, & Cang, 2013). Critical period cortical plasticity may be regulated by BDNF and other neurotrophins, OTX2, Histone deacetylases, neuromodulators, the degradation of perineural cell adhesion nets and matrices, and by synaptic molecules (Gu et al., 2013; Hanover et al., 1999; Z. J. Huang et al., 1999; Kaneko & Stryker, 2017; Mardinly et al., 2016; Morishita, Miwa, Heintz, & Hensch, 2010; Pizzorusso et al., 2002; Pizzorusso et al., 2006; Putignano et al., 2007; Silingardi, Scali, Belluomini, & Pizzorusso, 2010; Sugiyama et al., 2008; Sur, Nagakura, Chen, & Sugihara, 2013; Tropea et al., 2006; Wang et al., 2013). The maturation of perineural nets and extracellular matrix proteins that surround PV neurons, as well as the maturation of myelination in the cortex, occurs on a similar timeline as the critical period (Berardi, Pizzorusso, & Maffei, 2004; Carulli et al., 2010; McGee, Yang, Fischer, Daw, & Strittmatter, 2005; Pizzorusso et al., 2002). While these molecular signaling components may help developmentally set the stage, recent findings show that the initiation of critical period ODP is regulated by PV interneuron NRG1-ErbB4 signaling.
The NRG/ErbB molecular family and their modulation of neural circuits
How could NRG1-ErbB4 signaling and the greater NRG-ErbB molecular families regulate cortical plasticity? NRG ligands and ErbBs receptor tyrosine kinases are widely expressed in the brain. The NRG genes encode multiple splicing isoforms, and most are expressed as transmembrane proteins. These subsequently can undergo proteolytic processing to release a ligand ectodomain containing an EGF-like structure (Kamezaki et al., 2016). The EGF-like domain is required for receptor binding and is sufficient to elicit ErbB receptor dimerization, tyrosine phosphorylation and the activation of downstream signaling pathways. There are six known NRGs (NRG1, 2, 3, 4, 5 and 6). NRG1, 2, 3 and 4 are known to be able to activate ErbB1, 2, 3 and 4. There are excellent detailed reviews on NRG/ErbB signaling available for further reading (Birchmeier, 2009; Britsch, 2007; Buonanno & Fischbach, 2001; Buonanno et al., 2008; Corfas, Roy, & Buxbaum, 2004; Esper, Pankonin, & Loeb, 2006; Falls, 2003; Fischbach, 2007; Gassmann & Lemke, 1997; Iwakura & Nawa, 2013; Mei & Nave, 2014; Mei & Xiong, 2008; Rico & Marin, 2011; Talmage, 2008).
NRG1 is the best studied NRG. It was identified first for its ability to induce acetycholine receptor (AChR) activity and synapse formation at neuromuscular junctions (Falls, Rosen, Corfas, Lane, & Fischbach, 1993; Sandrock et al., 1997). Although NRG1 can bind ErbB1, ErbB2 and ErbB3 receptors, only ErbB4 is then activated as a tyrosine-kinase following NRG1 binding. ErbB1 and ErbB2 cannot bind NRG1 alone, but do so when forming heterodimers with ErbB4 (Tzahar et al., 1996). ErbB3 can bind NRG1, but its homodimers are signaling inactive (Guy, Platko, Cantley, Cerione, & Carraway, 1994). In the brain ErbB4 is the most abundant of all ErbBs and represents the major NRG receptor (Bernstein et al., 2006; Fox & Kornblum, 2005; Gerecke, Wyss, Karavanova, Buonanno, & Carroll, 2001; Neddens & Buonanno, 2011; Steiner, Blum, Kitai, & Fedi, 1999). ErbB4 signaling activates PI3K-AKT, Ras-MAPK and JAK-SRC pathways (Iwakura & Nawa, 2013). Previous studies have focused on ErbB4’s involvement in NRG signaling in hippocampal and prefrontal cortical circuitry. ErbB4 is primarily expressed in interneurons in the brain, as in situ and immunfluorescence methods have co-localized ErbB4 expression to interneurons (Bean et al., 2014; Buonanno, 2010; Fox & Kornblum, 2005; Longart, Chatani-Hinze, Gonzalez, Vullhorst, & Buonanno, 2007; Vullhorst et al., 2009; Woo et al., 2007). ErbB4 is also highly expressed in PV interneurons (Abe, Namba, Kato, Iwakura, & Nawa, 2011; Chen et al., 2010; Fazzari et al., 2010; Neddens & Buonanno, 2010; Tan et al., 2011; Wen et al., 2010; Yau et al., 2003). At the ultrastructural level, ErbB4 co-localizes with the PSD95 synaptic marker and is expressed primarily on the dendrites of interneurons (Fazzari et al., 2010; Garcia, Vasudevan, & Buonanno, 2000; Y. Z. Huang et al., 2000; Krivosheya et al., 2008; Longart et al., 2007; L. Ma et al., 2003; Vullhorst et al., 2009). Thus, ErbB4 on post-excitatory synaptic dendrites of PV interneurons may regulate synaptic input activity of these neurons. Long-term potentiation (LTP) studies in hippocampus have helped to develop the role of NRG-ErbB4 signaling in neural circuit activity regulation. NRG1 inhibits LTP (Bjarnadottir et al., 2007; Y. Z. Huang et al., 2000; Kwon, Longart, Vullhorst, Hoffman, & Buonanno, 2005; Pitcher et al., 2011; Tamura, Kawata, Hamaguchi, Ishikawa, & Shiosaka, 2012). This effect is ErbB4 dependent (Chen et al., 2010; Y. Z. Huang et al., 2000; L. Ma et al., 2003; Pitcher, Beggs, Woo, Mei, & Salter, 2008; Shamir et al., 2012). Further, in ErbB4 KO mice, LTP is enhanced (Lu et al., 2014; Pitcher et al., 2008). However, neither acute application of NRG1 nor deleting NRG1 or ErbB4 influences basal levels of synaptic activity in hippocampus (Bjarnadottir et al., 2007; Y. Z. Huang et al., 2000; Iyengar & Mott, 2008; Kwon et al., 2005; Pitcher et al., 2008), which suggest that NRG1 signaling actions are dependent on neural activity states (i.e., LTP stimulation versus basal synaptic activity). In addition, PV interneuron ErbB4 signaling has broader implications on animal behavior. NRG1 and ErbB4 have been genetically identified in humans as schizophrenia "at-risk" genes (Banerjee, Macdonald, Borgmann-Winter, & Hahn, 2010). Ablation of ErbB4 in PV neurons leads to impairment of contextual fear conditioning, increased general locomotor activity, impairment of social behavior, and deficits in pre-pulse inhibition and working memory (Chen et al., 2010; Del Pino et al., 2013; Shamir et al., 2012; Wen et al., 2010).
NRG1/ErbB4, PV neurons and critical period plasticity
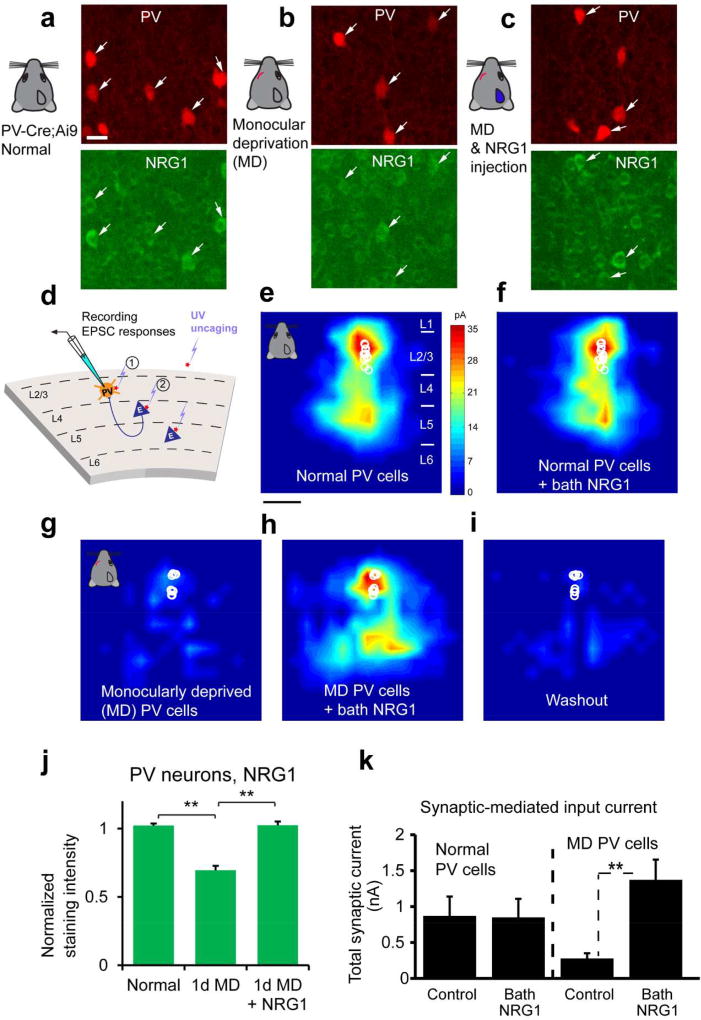
Our recently published work in Neuron (Sun et al., 2016) establishes that NRG1 signaling in PV neurons is a critical component regulating visual critical period plasticity. We examined developmental expression and experience-dependent regulation of NRG1/ErbB4 signaling in mouse visual cortex (Fig. 1, 2). PV neurons show strong ErbB4 expression during the critical period of mouse visual development. PV neurons have stronger and more concentrated NRG1/ErbB4 expression than surrounding cells as revealed by immunostaining; this distinguishes them from surrounding putative excitatory neurons (Fig. 1c). The co-expression of the ligand NRG1 and its receptor ErbB4 in PV neurons (Fig. 1c–d) may allow these neurons to regulate their synaptic plasticity in cell autonomous manner. Indeed, brief monocular deprivation during the critical period quickly decreases NRG1/ErbB4 signaling in PV neurons (Fig. 2 a–c, j), causing downregulation of excitatory inputs to PV neurons in visual cortex (Fig. 2g–i, k). We measured the connectivity strength and laminar distribution of presynaptic excitatory inputs onto L2/3 PV neurons in brain slices taken from binocular visual cortex of critical period mice (P27-P30) using laser scanning photo stimulation (LSPS) via glutamate uncaging (Xu et al., 2016)(Fig. 2d). The LSPS approach is effective for detailed local circuit mapping. Exogenous NRG1 rapidly restores excitatory inputs onto deprived PV cells through downstream PKC-dependent activation and AMPA receptor exocytosis, thus enhancing PV neuronal inhibition to excitatory neurons. Further, we show that NRG1 regulates PV neuronal activity in a state-dependent manner. In contrast to the finding that bath NRG1 rapidly increases the amplitude of excitatory synaptic input to 1–2 day deprived PV cells, NRG1 does not significantly modulate local excitatory synaptic inputs to PV neurons in binocular V1 of normal, non-lid sutured mice (Fig. 2e–f, k). This suggests that NRG1 signaling is sufficiently high to maintain synaptic input to normal PV neurons during the critical period.
Figure 2. Monocular deprivation rapidly down regulates NRG1 in PV neurons of visual cortex during the critical period and exogenous NRG1 rapidly restores excitatory synaptic input onto these deprived PV neurons.
a–c, Immunochemical analysis of NRG1 signaling. Representative images of NRG1 immunostaining are shown for (a) PV neurons in the PV-Cre; Ai9 mouse at postnatal day 28, (b) after 1 day of monocular deprivation (1d MD), and (c) after 1d MD with NRG1 injections [3 times (3×) daily, 0.5 µg NRG1 per mouse]. The image panels are contrast enhanced for the display, but not for quantitative analysis. The arrows indicate tdTomato+ PV cells (red) and their corresponding immunolabel (green). Scale bar = 20 µm. d, Schematic of laser scanning photostimulation (LSPS) mapping of local synaptic connections to individually recorded PV neurons in V1 slices. e–f, Acute bath application of NRG1 (5 nM) does not significantly modulate local excitatory synaptic inputs and glutamate mediated excitability of normal PV neurons in non-deprived binocular V1 of control mice. Group-averaged, excitatory input maps of L2/3 PV cells (n = 13 cells) are shown for before (e) and during bath NRG1 (20 minutes after NRG1 application) (F). White circles represent individual PV neurons. The color scale (e) codes integrated excitatory input strength (blue = low, red = high) and applies to all other maps. The spatial scale beneath (e) indicates 200 µm. PV neurons in mice with 1–2 days of monocular lid suture show dramatically lower excitatory synaptic inputs compared to controls (g, n = 10 cells). However, excitatory inputs to PV neurons in these deprived mice are restored to levels above that of controls by acute bath application of NRG1 (h). This restoration is eliminated by washout of bath NRG1 (i). J–K, quantitative data summaries. A bar graph in (j) shows that the average NRG1 immunostaining signal in L2/3 PV cells significantly differs following 1 d MD but does not differ between control and 1 d MD + NRG1. PV cell measurements in (j) were obtained from 4–6 different mice for each condition. **, p < 0.01 (Kruskal Wallis test, followed by Mann–Whitney U tests). On average, 1d MD PV cells express significantly less NRG1 signal (67.9%) relative to normal control; PV cells of 1d MD occluded by NRG1 injections express similar levels of NRG1 signal relative to control. k, Summary data of average total synaptic input strength measured for L2/3 PV neurons under the specified conditions (d–i). ***, p < 0.001. These data demonstrates how activity-dependent NRG1 signaling and sensory experience interacts to rapidly shape functional circuit connections in the cortex. The figure is modified from published data figures in Sun et al. (2016) with permission from the Cell Press.
We examined further if NRG1 modulates PV neuron-mediated postsynaptic responsiveness in excitatory neurons or the strength of PV inhibitory output connections to excitatory neurons by optogenetically evoking PV inhibitory inputs to pyramidal neurons. The experiments were performed in brain slices with Cre-directed channelrhodopsin-2 (ChR2) expression in PV neurons that synapse on pyramidal neurons. Direct inhibitory connections to pyramidal neurons were mapped by ChR2 photoactivation of somatic spiking of presynaptic PV inhibitory neurons. Bath NRG1 application does not modulate PV specific inhibition to L2/3 pyramidal cells. Thus the circuit basis of NRG1-enhanced cortical inhibition in deprived cortex is localized specifically to enhance excitatory drive to deprived PV neurons, rather than general modulation of PV inhibitory synaptic connections to pyramidal cells.
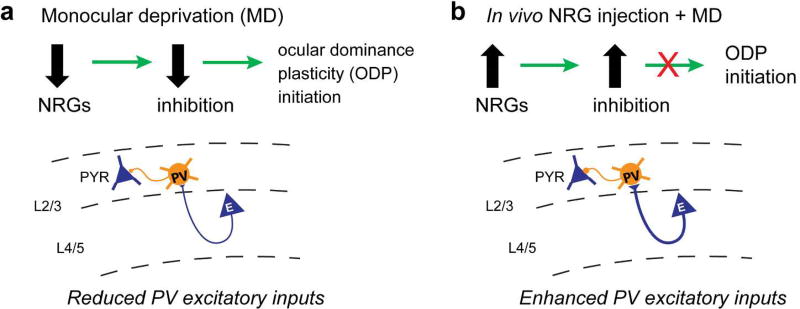
We have investigated the function of NRG1/ErbB4 signaling in ocular dominance plasticity in vivo. As illustrated in our model (Fig. 3a), we reason that down-regulation of NRG1 signaling following visual deprivation reduces excitatory inputs to PV neurons and subsequent inhibition onto excitatory neurons. Considering that enhancing inhibition prevents ocular dominance plasticity during the critical period (Kuhlman et al., 2013; W. P. Ma, Li, & Tao, 2013), we tested the hypothesis that enhancing NRG1/ErbB4 signaling in the cortex through systemic NRG1 administration suppresses ODP (Fig. 3b). To measure this plasticity, we determined the strength of eye-specific visual responses before and after four days of monocular deprivation using intrinsic signal optical imaging. In support of our hypothesis, the enhancement of NRG1 signaling strongly reduces ODP. To further test the role of ErbB4 signaling in ODP, we performed imaging experiments before and after four days of monocular deprivation (without NRG1 treatment) using the PV-Cre; ErbB4flx/flx mice, and found that critical period plasticity is impaired in these animals with PV specific ablation of ErbB4 receptors. Taken together, our study defines a novel and critical role of NRG1/ErbB4 in regulating visual critical period plasticity.
Figure 3. Schematic of our model of ocular dominance plasticity regulation by NRG1/ErbB4 signaling in PV neurons.
a, Down-regulation of NRG signaling following monocular deprivation (MD) reduces excitatory inputs onto PV neurons and subsequent inhibition onto excitatory neurons. The decrease of cortical inhibition is the necessary first step in ocular dominance plasticity (ODP) initiation. b, Elevated NRG in the cortex enhances excitatory inputs to PV neurons during MD, which occludes the MD-induced reduction of cortical inhibition and suppresses ODP during the normal critical period. While NRG1-ErbB4 signaling is predominant in juvenile critical period plasticity, other NRGs (particularly NRG3) signaling may also be implicated in cortical plasticity in adulthood. The schematic is modified from Sun et al. (2016) with permission from the Cell Press.
In a closely related but independent study, Gu et al. (2016) asked if modulation of NRG1/ErbB4 signaling in PV interneurons could allow for the regulation of ODP in adult visual cortex. They recapitulated some of the findings of Sun et al. (2016), as it was reported that during the critical period NRG1 blocks ODP and induces an early closure of the critical period. Gu et al. (2016) then used pharmacology approaches to examine the relationship of NRG1-ErbB4 signaling and adult cortical plasticity. ODP can be partially reinstated by dark exposure in adult mice (Eaton, Sheehan, & Quinlan, 2016; Kloska et al., 2007; Montey & Quinlan, 2011). In adult mice dark exposure decreases PV interneuron activity, increases pyramidal activity, and induces ODP. NRG1 treatment, however, blocks all of these effects in adult mice exposed to the dark, suggesting ErbB4 signaling controls adult visual cortical plasticity as well as during the critical period (Gu et al., 2016). The ErbB inhibitor PD158780 reduces the activity of PV interneurons and the increases the activity of pyramidal neurons in the visual cortex. This ErbB antagonism also facilitates ocular dominance plasticity in adult mice exposed to brief monocular deprivation. Altogether, these two important studies (Gu et al., 2016; Sun et al., 2016) support the implication of NRG1/ErbB4 signaling in visual cortical plasticity during the critical period and in adulthood.
Outstanding questions and future directions
Despite the progress made in understanding NRG1/ErbB4 signaling in visual cortical plasticity, outstanding questions remain. First, there are multiple cellular NRG1 sources. How NRG1 is processed and released in the central nervous system remains unclear. In the peripheral nervous system, NRG1 is mainly produced by neurons while ErbB receptors are expressed in signal-recipient cells such as Schwann cells and skeletal muscle cells; NRG1-ErbB signaling mostly mediates cell-cell interaction in a paracrine or juxtacrine manner (Kamezaki et al., 2016; Vullhorst, Ahmad, Karavanova, Keating, & Buonanno, 2017). However, the strong expression of both the ligand NRG1 and its receptor ErbB4 in PV neurons suggests potential autocrine regulation of NRG1/ErbB4 signaling in PV neurons. Our on-going studies intend to use new genetic tagging and manipulation (Kamezaki et al., 2016; Yin et al., 2013) to identify the source of PV neuron NRG1 in the context of visual cortical plasticity.
NRG1, which is present in PV interneurons as well as in excitatory neurons in visual cortex, decreases after the closure of the critical period, whereas ErbB4 expression on PV interneurons remains high even into adulthood (Sun et al., 2016). This suggests the possibility that there may be other neuregulins which signal through ErbB4 receptors to regulate PV neuron activity in adult cortex (Fig. 1b). This is supported by the finding that ErbB inhibition on PV interneurons in adult mice facilitates ODP (Gu et al., 2016). Similarly, ErbB4 forms heterodimers with other ErbBs which receive cross-talk from other neuregulins (Iwakura & Nawa, 2013; Mei & Nave, 2014). Thus, future studies should identify NRG/ErbB expression in specific cell types in visual cortex during the critical period and in adulthood to further address molecular components of the NRG directed signaling.
Considering the multiple cellular sources of NRGs, this raises interesting questions of whether and how molecular signaling with NRGs from different cellular sources might differentially regulate synaptic connections of PV inhibitory interneurons and excitatory neurons in response to short versus prolonged visual deprivation. A relatively small proportion of VIP cells are immunopositive for ErbB4, and there is virtually no co-localization between SOM and ErbB4 (Sun et al., 2016). However, transplantation of embryonic SOM+ interneurons can indeed drive cortical plasticity (Tang, Stryker, Alvarez-Buylla, & Espinosa, 2014); PV neurons can be inhibited by SOM-expressing interneurons and VIP preferentially inhibit somatostatin positive (SST) cells (Fu et al., 2014; Jiang et al., 2015; Pakan et al., 2016; Pfeffer, Xue, He, Huang, & Scanziani, 2013). These findings indicate that in addition to our understanding of PV interneuron mediated plasticity, it is important to examine whether and how NRG1 influences circuit development and function of non-PV neuron types. Further, our preliminary data indicate that compared with 1 day monocularly deprived PV neurons, 6–7 day deprived PV cells have recovered from a loss of excitatory circuit inputs. Thus, it appears that after the initial reduction of PV responses via NRG1 signaling down-regulation, this compensatory change has to be cooperatively engaged for ocular dominance plasticity to be consolidated following prolonged deprivation. This occurs likely by a cascade of signals that links excitatory drive to inhibitory neurons, inhibitory drive to excitatory neurons and eye-specific drive to synapses. NRG-ErbB signaling has properties that makes it a strong candidate for coordinating such diverse components of plasticity. In this regard, functional mapping approaches including ex vivo LSPS and in vivo multi-photon calcium imaging can be combined to test the hypothesis that NRG1/ErbB4 signaling is required for restoration of their excitatory inputs in visual cortex of prolonged deprivation. Mouse genetic approaches can be employed to selectively downregulate NRG1/ErbB4 expression in specific cell types in deprived cortex. While these are complex questions, we have the tools (Sun et al., 2016) and the scientific framework to address them.
NRG-directed research may lead to therapeutic approaches that robustly reactivate plasticity in the adult cortex. Since neurodevelopmental disorders such as schizophrenia and autism appear to result from brain developmental defects that occur during defined postnatal windows, the linkage of NRG1 signaling to critical periods may provide important new insights into the pathology of these neurodevelopmental disorders. This raises the interesting possibility that PV neuronal dysfunction, and late adolescent and early adult onset of schizophrenia may be temporally contingent on NRG1/ErbB4 signaling defects in the relevant brain regions. Further understanding of NRG signaling in shaping cortical development and therapeutic interventions targeting NRG1 may be exploited to treat amblyopia and other neurodevelopmental disorders. In summary, we dedicate this article to the memory of Prof. Vivien A. Casagrande. As an ongoing living memorial honoring Vivien, we and others in the field will continue to study molecular, cellular and circuit mechanisms that drive cortical plasticity and promote the development of novel therapeutic strategies to treat human diseases caused by critical period disorders.
Acknowledgments
This work was supported by US National Institutes of Health (NIH) grants R01 EY028212, R01 NS078434 and R01 MH105427 to X.X. TCH is supported by NIH grants R01 GM102965 and R01 GM107405.
Footnotes
Author contributions: X.X. and T.C.H. conceived this work; S.F.G and X.X. prepared illustrations; S.F.G, T.C.H. and X.X. wrote the paper.
Statement of conflict of interests
All authors disclose no conflict of interests for this work.
References
- Abe Y, Namba H, Kato T, Iwakura Y, Nawa H. Neuregulin-1 signals from the periphery regulate AMPA receptor sensitivity and expression in GABAergic interneurons in developing neocortex. J Neurosci. 2011;31(15):5699–5709. doi: 10.1523/JNEUROSCI.3477-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Lai C. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7(12):1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73(1):159–170. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Macdonald ML, Borgmann-Winter KE, Hahn CG. Neuregulin 1-erbB4 pathway in schizophrenia: From genes to an interactome. Brain Res Bull. 2010;83(3–4):132–139. doi: 10.1016/j.brainresbull.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean JC, Lin TW, Sathyamurthy A, Liu F, Yin DM, Xiong WC, Mei L. Genetic labeling reveals novel cellular targets of schizophrenia susceptibility gene: distribution of GABA and non-GABA ErbB4-positive cells in adult mouse brain. J Neurosci. 2014;34(40):13549–13566. doi: 10.1523/JNEUROSCI.2021-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Extracellular matrix and visual cortical plasticity: freeing the synapse. Neuron. 2004;44(6):905–908. doi: 10.1016/j.neuron.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26(7):369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Lendeckel U, Bertram I, Bukowska A, Kanakis D, Dobrowolny H, Bogerts B. Localization of neuregulin-1alpha (heregulin-alpha) and one of its receptors, ErbB-4 tyrosine kinase, in developing and adult human brain. Brain Res Bull. 2006;69(5):546–559. doi: 10.1016/j.brainresbull.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Birchmeier C. ErbB receptors and the development of the nervous system. Exp Cell Res. 2009;315(4):611–618. doi: 10.1016/j.yexcr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Andresson T. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci. 2007;27(17):4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res Bull. 2010;83(3–4):122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11(3):287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Kwon OB, Yan L, Gonzalez C, Longart M, Hoffman D, Vullhorst D. Neuregulins and neuronal plasticity: possible relevance in schizophrenia. Novartis Found Symp. 2008;289:165–177. doi: 10.1002/9780470751251.ch13. discussion 177-169, 193-165. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Inhibitory Cell Types, Circuits and Receptive Fields in Mouse Visual Cortex. In: Kennedy H, Van Essen DC, Christen Y, editors. Micro-, Meso- and Macro-Connectomics of the Brain. 2016. Cham (CH) [PubMed] [Google Scholar]
- Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133(Pt 8):2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- Casagrande VA. A third parallel visual pathway to primate area V1. Trends Neurosci. 1994;17(7):305–310. doi: 10.1016/0166-2236(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, Gao TM. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010;107(50):21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7(6):575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- Del Pino I, Garcia-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, Martinez de Lagran M, Rico B. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79(6):1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Chattopadhyaya B, Kuhlman SJ, Fu Y, Belanger MC, Wu CZ, Huang ZJ. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat Neurosci. 2007;10(12):1569–1577. doi: 10.1038/nn2008. [DOI] [PubMed] [Google Scholar]
- Eaton NC, Sheehan HM, Quinlan EM. Optimization of visual training for full recovery from severe amblyopia in adults. Learn Mem. 2016;23(2):99–103. doi: 10.1101/lm.040295.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper RM, Pankonin MS, Loeb JA. Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res Rev. 2006;51(2):161–175. doi: 10.1016/j.brainresrev.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404(6774):183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34(6):709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284(1):14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993;72(5):801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464(7293):1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Fischbach GD. NRG1 and synaptic function in the CNS. Neuron. 2007;54(4):495–497. doi: 10.1016/j.neuron.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Kornblum HI. Developmental profile of ErbB receptors in murine central nervous system: implications for functional interactions. J Neurosci Res. 2005;79(5):584–597. doi: 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56(1):33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Stryker MP. A cortical circuit for gain control by behavioral state. Cell. 2014;156(6):1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97(7):3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Lemke G. Neuregulins and neuregulin receptors in neural development. Curr Opin Neurobiol. 1997;7(1):87–92. doi: 10.1016/s0959-4388(97)80125-0. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433(1):86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7(4):347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16(10):3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Huang S, Chang MC, Worley P, Kirkwood A, Quinlan EM. Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron. 2013;79(2):335–346. doi: 10.1016/j.neuron.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Tran T, Murase S, Borrell A, Kirkwood A, Quinlan EM. Neuregulin-Dependent Regulation of Fast-Spiking Interneuron Excitability Controls the Timing of the Critical Period. J Neurosci. 2016;36(40):10285–10295. doi: 10.1523/JNEUROSCI.4242-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., 3rd Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci U S A. 1994;91(17):8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19(22):RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, Maffei L. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci. 2010;30(1):361–371. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period mechanisms in developing visual cortex. Curr Top Dev Biol. 2005;69:215–237. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res. 2005;147:115–124. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282(5393):1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26(2):443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH. Tungsten Microelectrode for Recording from Single Units. Science. 1957;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Nawa H. ErbB1-4-dependent EGF/neuregulin signals and their cross talk in the central nervous system: pathological implications in schizophrenia and Parkinson's disease. Front Cell Neurosci. 2013;7:4. doi: 10.3389/fncel.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar SS, Mott DD. Neuregulin blocks synaptic strengthening after epileptiform activity in the rat hippocampus. Brain Res. 2008;1208:67–73. doi: 10.1016/j.brainres.2008.02.045. [DOI] [PubMed] [Google Scholar]
- Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, Tolias AS. Principles of connectivity among morphologically defined cell types in adult neocortex. Science. 2015;350(6264):aac9462. doi: 10.1126/science.aac9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamezaki A, Sato F, Aoki K, Asakawa K, Kawakami K, Matsuzaki F, Sehara-Fujisawa A. Visualization of Neuregulin 1 ectodomain shedding reveals its local processing in vitro and in vivo. Sci Rep. 2016;6:28873. doi: 10.1038/srep28873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Stryker MP. Homeostatic plasticity mechanisms in mouse V1. Philos Trans R Soc Lond B Biol Sci. 2017;372(1715) doi: 10.1098/rstb.2016.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321(5885):53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloska SP, Fischer T, Nabavi DG, Wessling J, Dittrich R, Fischbach R, Heindel W. Comparison of different iodine concentration contrast media in perfusion computed tomography of the brain: is high iodine concentration useful? Invest Radiol. 2007;42(8):564–568. doi: 10.1097/RLI.0b013e318042b608. [DOI] [PubMed] [Google Scholar]
- Krivosheya D, Tapia L, Levinson JN, Huang K, Kang Y, Hines R, El-Husseini A. ErbB4-neuregulin signaling modulates synapse development and dendritic arborization through distinct mechanisms. J Biol Chem. 2008;283(47):32944–32956. doi: 10.1074/jbc.M800073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Lu J, Lazarus MS, Huang ZJ. Maturation of GABAergic inhibition promotes strengthening of temporally coherent inputs among convergent pathways. PLoS Comput Biol. 2010;6(6):e1000797. doi: 10.1371/journal.pcbi.1000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501(7468):543–546. doi: 10.1038/nature12485. doi:nature12485 [pii]10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25(41):9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt CN, Hubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- Liu X, Bates R, Yin DM, Shen C, Wang F, Su N, Mei L. Specific regulation of NRG1 isoform expression by neuronal activity. J Neurosci. 2011;31(23):8491–8501. doi: 10.1523/JNEUROSCI.5317-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longart M, Chatani-Hinze M, Gonzalez CM, Vullhorst D, Buonanno A. Regulation of ErbB-4 endocytosis by neuregulin in GABAergic hippocampal interneurons. Brain Res Bull. 2007;73(4–6):210–219. doi: 10.1016/j.brainresbull.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longart M, Liu Y, Karavanova I, Buonanno A. Neuregulin-2 is developmentally regulated and targeted to dendrites of central neurons. J Comp Neurol. 2004;472(2):156–172. doi: 10.1002/cne.20016. [DOI] [PubMed] [Google Scholar]
- Lu Y, Sun XD, Hou FQ, Bi LL, Yin DM, Liu F, Mei L. Maintenance of GABAergic activity by neuregulin 1-ErbB4 in amygdala for fear memory. Neuron. 2014;84(4):835–846. doi: 10.1016/j.neuron.2014.09.029. [DOI] [PubMed] [Google Scholar]
- Ma L, Huang YZ, Pitcher GM, Valtschanoff JG, Ma YH, Feng LY, Mei L. Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J Neurosci. 2003;23(8):3164–3175. doi: 10.1523/JNEUROSCI.23-08-03164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WP, Li YT, Tao HW. Downregulation of cortical inhibition mediates ocular dominance plasticity during the critical period. J Neurosci. 2013;33(27):11276–11280. doi: 10.1523/JNEUROSCI.5598-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardinly AR, Spiegel I, Patrizi A, Centofante E, Bazinet JE, Tzeng CP, Greenberg ME. Sensory experience regulates cortical inhibition by inducing IGF1 in VIP neurons. Nature. 2016;531(7594):371–375. doi: 10.1038/nature17187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309(5744):2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83(1):27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9(6):437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montey KL, Quinlan EM. Recovery from chronic monocular deprivation following reactivation of thalamocortical plasticity by dark exposure. Nat Commun. 2011;2:317. doi: 10.1038/ncomms1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22(18):8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330(6008):1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Lantz CL, Quinlan EM. Light reintroduction after dark exposure reactivates plasticity in adults via perisynaptic activation of MMP-9. Elife. 2017;6 doi: 10.7554/eLife.27345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20(6):724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Expression of the neuregulin receptor ErbB4 in the brain of the rhesus monkey (Macaca mulatta) PLoS One. 2011;6(11):e27337. doi: 10.1371/journal.pone.0027337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakan JM, Lowe SC, Dylda E, Keemink SW, Currie SP, Coutts CA, Rochefort NL. Behavioral-state modulation of inhibition is context-dependent and cell type specific in mouse visual cortex. Elife. 2016;5 doi: 10.7554/eLife.14985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16(8):1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher GM, Beggs S, Woo RS, Mei L, Salter MW. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. Neuroreport. 2008;19(2):139–143. doi: 10.1097/WNR.0b013e3282f3da10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher GM, Kalia LV, Ng D, Goodfellow NM, Yee KT, Lambe EK, Salter MW. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med. 2011;17(4):470–478. doi: 10.1038/nm.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103(22):8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L, Pizzorusso T. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53(5):747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Rico B, Marin O. Neuregulin signaling, cortical circuitry development and schizophrenia. Curr Opin Genet Dev. 2011;21(3):262–270. doi: 10.1016/j.gde.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Sandrock AW, Jr, Dryer SE, Rosen KM, Gozani SN, Kramer R, Theill LE, Fischbach GD. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science. 1997;276(5312):599–603. doi: 10.1126/science.276.5312.599. [DOI] [PubMed] [Google Scholar]
- Shamir A, Kwon OB, Karavanova I, Vullhorst D, Leiva-Salcedo E, Janssen MJ, Buonanno A. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci. 2012;32(9):2988–2997. doi: 10.1523/JNEUROSCI.1899-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silingardi D, Scali M, Belluomini G, Pizzorusso T. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci. 2010;31(12):2185–2192. doi: 10.1111/j.1460-9568.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- Smith SL, Trachtenberg JT. Experience-dependent binocular competition in the visual cortex begins at eye opening. Nat Neurosci. 2007;10(3):370–375. doi: 10.1038/nn1844. [DOI] [PubMed] [Google Scholar]
- Steiner H, Blum M, Kitai ST, Fedi P. Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Exp Neurol. 1999;159(2):494–503. doi: 10.1006/exnr.1999.7163. [DOI] [PubMed] [Google Scholar]
- Stephany CE, Ikrar T, Nguyen C, Xu X, McGee AW. Nogo Receptor 1 Confines a Disinhibitory Microcircuit to the Critical Period in Visual Cortex. J Neurosci. 2016;36(43):11006–11012. doi: 10.1523/JNEUROSCI.0935-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134(3):508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ikrar T, Davis MF, Gong N, Zheng X, Luo ZD, Xu X. Neuregulin-1/ErbB4 Signaling Regulates Visual Cortical Plasticity. Neuron. 2016;92(1):160–173. doi: 10.1016/j.neuron.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Nagakura I, Chen N, Sugihara H. Mechanisms of plasticity in the developing and adult visual cortex. Prog Brain Res. 2013;207:243–254. doi: 10.1016/B978-0-444-63327-9.00002-3. [DOI] [PubMed] [Google Scholar]
- Talmage DA. Mechanisms of neuregulin action. Novartis Found Symp. 2008;289:74–84. doi: 10.1002/9780470751251.ch6. discussion 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Kawata M, Hamaguchi S, Ishikawa Y, Shiosaka S. Processing of neuregulin-1 by neuropsin regulates GABAergic neuron to control neural plasticity of the mouse hippocampus. J Neurosci. 2012;32(37):12657–12672. doi: 10.1523/JNEUROSCI.2542-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GH, Liu YY, Hu XL, Yin DM, Mei L, Xiong ZQ. Neuregulin 1 represses limbic epileptogenesis through ErbB4 in parvalbumin-expressing interneurons. Nat Neurosci. 2011;15(2):258–266. doi: 10.1038/nn.3005. [DOI] [PubMed] [Google Scholar]
- Tang Y, Stryker MP, Alvarez-Buylla A, Espinosa JS. Cortical plasticity induced by transplantation of embryonic somatostatin or parvalbumin interneurons. Proc Natl Acad Sci U S A. 2014;111(51):18339–18344. doi: 10.1073/pnas.1421844112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Kreiman G, Lyckman A, Mukherjee S, Yu H, Horng S, Sur M. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nat Neurosci. 2006;9(5):660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- Tropea D, Van Wart A, Sur M. Molecular mechanisms of experience-dependent plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364(1515):341–355. doi: 10.1098/rstb.2008.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16(10):5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullhorst D, Ahmad T, Karavanova I, Keating C, Buonanno A. Structural Similarities between Neuregulin 1–3 Isoforms Determine Their Subcellular Distribution and Signaling Mode in Central Neurons. J Neurosci. 2017;37(21):5232–5249. doi: 10.1523/JNEUROSCI.2630-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, Buonanno A. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29(39):12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. doi:29/39/12255 [pii] 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BS, Feng L, Liu M, Liu X, Cang J. Environmental enrichment rescues binocular matching of orientation preference in mice that have a precocious critical period. Neuron. 2013;80(1):198–209. doi: 10.1016/j.neuron.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, Mei L. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107(3):1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Mei L. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54(4):599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Xu X, Bosking W, Sary G, Stefansic J, Shima D, Casagrande V. Functional organization of visual cortex in the owl monkey. J Neurosci. 2004;24(28):6237–6247. doi: 10.1523/JNEUROSCI.1144-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Bosking WH, White LE, Fitzpatrick D, Casagrande VA. Functional organization of visual cortex in the prosimian bush baby revealed by optical imaging of intrinsic signals. J Neurophysiol. 2005;94(4):2748–2762. doi: 10.1152/jn.00354.2005. [DOI] [PubMed] [Google Scholar]
- Xu X, Ichida JM, Allison JD, Boyd JD, Bonds AB, Casagrande VA. A comparison of koniocellular, magnocellular and parvocellular receptive field properties in the lateral geniculate nucleus of the owl monkey (Aotus trivirgatus) J Physiol. 2001;531(Pt 1):203–218. doi: 10.1111/j.1469-7793.2001.0203j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ikrar T, Sun Y, Santos R, Holmes TC, Francesconi W, Berton F. High-resolution and cell-type-specific photostimulation mapping shows weak excitatory vs. strong inhibitory inputs in the bed nucleus of the stria terminalis. J Neurophysiol. 2016;115(6):3204–3216. doi: 10.1152/jn.01148.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518(3):389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13(3):252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- Yin DM, Chen YJ, Lu YS, Bean JC, Sathyamurthy A, Shen C, Mei L. Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron. 2013;78(4):644–657. doi: 10.1016/j.neuron.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]