Abstract
A fasting mimetic diet blunts inflammation and intermittent fasting has shown ameliorative effects in obese asthmatics. To examine whether canonical inflammatory pathways linked with asthma are modulated by fasting we designed a pilot study in mild asthmatic subjects to assess the effect of fasting on: the NLRP3 inflammasome; Th2 cell activation and airway epithelial cell cytokine production. Subjects with documented reversible airway obstruction and stable mild asthma were recruited into this study where pulmonary function testing (PFT) and peripheral blood mononuclear cells (PBMCs) extraction was performed 24 hours after fasting, with repeated PFT-testing and blood draw 2.5 hours after refeeding. PFT’s were not changed by a prolonged fast. However, steroid-naïve mild asthmatics showed fasting-dependent blunting of the NLRP3 inflammasome. Furthermore, PBMCs from these fasted asthmatics co-cultured with human epithelial cells resulted in blunting of house dust mite-induced epithelial cell cytokine production, and reduced CD4+ T cell Th2 activation compared to refed samples. This pilot study shows that prolonged fasting blunts the NLRP3 inflammasome and Th2 cell activation in steroid-naïve asthmatics, as well as diminishes airway epithelial cell cytokine production. This identifies a potential role for nutrient-level dependent regulation of inflammation in asthma. Our findings support the evaluation of this concept in a larger study, as well as the potential development of caloric restriction interventions for the treatment of asthma.
Keywords: Steroid-naïve asthma, NLRP3 inflammasome, Fasting, Refeeding, Airway Epithelial Cell Inflammation
Caloric excess and obesity increase the risk of the development of asthma, and obese asthmatics show distinct immune modulatory effects relative to asthma in non-obese individuals (1). In contrast, studies exploring weight reduction diets and intermittent caloric restriction improve asthma symptoms and lung function (2, 3). Interestingly, asthmatic subjects on an intermittent caloric restriction diet showed evidence of blunting of immune activation as measured by serum levels of brain-derived neurotropic factor and of tumor necrosis factor α (TNFα) after approximately four weeks of alternate day caloric restriction (3). These data and other caloric restriction and fasting studies (4–7), suggest that dietary manipulation may have ameliorative effects due to modulation of immune function.
The immune pathways linked to asthma have been extensively explored and include a role for environmental triggers, signaling from airway epithelial cells and activation of innate and adaptive immune cells. The major signaling from airway epithelial cells involves the secretion of cytokines thymic stromal lymphopoietin (TSLP), interleukin (IL)-25 and IL-33. The predominant immune cells involved in triggering inflammation and smooth muscle cell mediated bronchoconstriction include: myeloid-derived macrophages, dendritic cells, eosinophils and neutrophils; lymphoid CD4+ helper Th2 and Th17 cells; and innate type 2 lymphocytes (iLC2). Recent evidence also supports a regulatory role of the intracellular Nod-Like Receptor Pyrin domain 3 (NLRP3) inflammasome in the pathophysiology of asthma (8, 9). Interestingly, this inflammasome is activated by nutrient excess (10) and blunted by prolonged fasting (7, 11). Furthermore, as a component of innate immunity, the NLRP3 inflammasome is linked to the activation of pulmonary iLC2 cells (12) and to the upregulation of Th2 signaling (13). Furthermore, the NLRP3 inflammasome is linked to an experimental model of obesity-associated airway hyperreactivity (12).
Despite the initial studies on the effects of caloric restriction or fasting on disease pathophysiology (4, 6, 14, 15), the exploration of the effects of these nutrient restriction interventions on the control of inflammatory or immune pathways has not been well characterized. This has recently begun to be addressed in two studies where normal volunteers with various risk factors, including obesity, were given a fasting mimetic diet for the first 5 days of each month followed by their routine diet for the rest of the month. After an assessment, three months after this monthly restricted caloric intervention, numerous disease risk factors were blunted including a reduction in the levels of the inflammatory acute phase reactant C-reactive protein (CRP) (5, 16). Furthermore, a single 24-hour fasting period has also been found to blunt the NLRP3 inflammasome in circulating peripheral blood mononuclear cells (PBMCs) and in monocytes isolated from healthy subjects (7).
Taken together, these data suggest that the link between caloric load and asthma may, in part be due to nutrient-dependent immune regulation. In this context, we reasoned that fasting may blunt canonical asthma immune regulatory programs. To explore this, we undertook a pilot study in mild asthmatics and assayed the effect of a 24-hour fast on the NLRP3 inflammasome, on Th2 activation and on the cytokine secretory capacity of lung epithelial cells.
Materials and Methods
Study Design and Subjects
This pilot study was performed at the NIH Clinical Center on 18 mild-asthmatic subjects with documented reversible bronchial obstruction by pulmonary function testing and this study was registered in ClinicalTrials.gov with the registration number NCT02471300 and approved by the National Heart and Lung Institute IRB. Subjects were screened in the ambulatory clinic and signed consent for the protocol prior to undertaking the study. Eighteen stable mild-asthmatic subjects were consented for this protocol. Subjects had to be stable on their current medical regimen which could either consist of inhaled β-agonist therapy alone or the combination of inhaled corticosteroids and β-agonists and were considered sufficiently stable that they could withhold their asthma medication for the 27-hour duration of the study. Subjects initiated the study with an early morning fixed caloric meal followed by fasting, except for unrestricted water intake, for 24 hours prior to undergoing the fasting blood draw and initial PTF testing. This was followed by a fixed 500 calorie meal and a post-prandial blood draw 2.5 hours later followed by repeat PFT testing. Subjects had a choice between 3 isocaloric breakfasts: option 1) vegetable omelet, toast with butter and jelly, and orange juice; option 2) oatmeal with walnuts, brown sugar, dried cranberries and milk; and option 3) turkey bacon, egg and cheese breakfast sandwich with orange slices and apple juice. The schematic of the blood draw protocol is shown in Figure 1A and subjects’ sera insulin, glucose, and growth hormone levels at the end of the 24-hour fast and 2.5-hour following the fixed caloric meal are shown in Fig 1B-D. The schematic of the blood draw protocol is shown in Figure 1. Given our previous data that prolonged fasting could blunt the NLRP3 inflammasome in PBMCs, the initial 13 subjects were screened for their fasting and refeeding NLRP3 response. Given that differing responses in asthmatics exposed to, or not exposed to inhaled corticosteroids, the enrollment of the final 5 individuals in the study were restricted to asthmatic subjects who did not require corticosteroid-therapy and effects of the NLRP3 inflammasome, Th2 endotype and the effects on lung epithelial cell cytokine secretion were studied in these individuals. The schematic of immune function studies in the subjects is shown as Figure 2.
FIGURE 1.
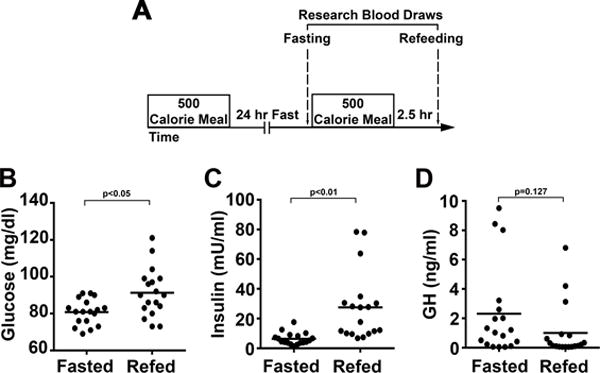
Clinical protocol and intervention characteristics. (A) Schematic of the protocol, showing intervals between the fixed caloric meals and temporal drawing of research blood. Asthmatic subjects were given an early morning fixed calorie meal followed by a 24 hr fast. The next morning, fasting blood was drawn and subjects were given another fixed calorie meal. Blood was drawn 2.5 hr after the fixed calorie meal. (B-D) Data points and means (horizontal bars) of subjects’ sera glucose (B), insulin (C), and growth hormone (D) levels at the end of 24 hr fast and 2.5 hr following the fixed caloric meal (n=18). Statistical analysis using paired student t-test with p values shown in each panel.
FIGURE 2.
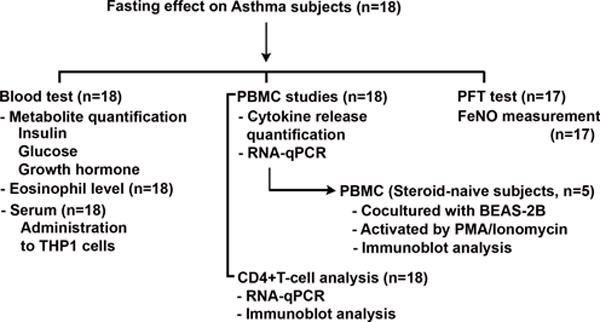
Fasting/refeeding study flow diagram of assays performed on the study subjects. PFT - pulmonary functional testing; FeNO - fractional exhaled nitric oxide – a biomarker of bronchial inflammation.
Blood biochemical assays and Complete blood count (CBC) with differential testing
Blood was collected after fasting and refeeding for standard laboratory testing in the NIH Clinical Center Clinical Laboratory. Standard laboratory tests included the measurement of glucose, insulin and growth hormone levels and the assessment of the complete blood count with differential cell analysis.
Pulmonary function testing
Pulmonary function testing and the fraction of exhaled nitric oxide (FeNO) measurements were performed by respiratory therapists at the NIH Clinical Center Pulmonary Function Laboratory using the Vmax® Encore PFT System (CareFusion, Yorba Linda, CA) and the Sievers 280i Nitric Oxide Analyzer (GE Analytical Instruments, Boulder, CO), respectively. The forced expiratory volume in 1 second (FEV1) was used as the standard measure for the degree of bronchoconstriction in asthma and FeNO reflects airway inflammation.
Cell culture protocols
Primary PBMCs were isolated from human blood by density centrifugation using Lymphocyte Separation Medium (MP Biomedicals). CD4+ T cells were negatively selected from PBMCs using the CD4+ T Cell Isolation Kit (Miltenyi Biotec). THP-1 human monocytes obtained from ATCC were cultured in RPMI 1640 media supplemented with 25 mM HEPES, 10% heat-inactivated FBS, and Penicillin/Streptomycin. They were differentiated into macrophages by incubation with 5 ng/ml PMA for 48 hours. BEAS-2B human epithelial cells were cultured in LHC-8 medium.
Cell Stimulation and Cytokine Assays
PBMCs were incubated at 2×106 cells/ml in 96-well plates with 3mM ATP (Sigma-Aldrich) for 30 mins to induce IL-1β and TNFα secretion. THP-1 macrophages were cultured in media supplemented with 10% fasted or refed serum from the asthmatic subjects for 24 hours. These cells were then incubated with 10 ng/mL LPS (Ultrapure Salmonella Minnesota R595; Enzo Life Sciences) for 4 hours with supplementation with 5mM ATP for the last 30 mins of incubation. To stimulate T cell cytokine release, PBMCs were incubated at 5×106 cells/ml in 96-well plates with or without 50ng/ml PMA and 1μg/ml Ionomycin (Sigma-Aldrich) for 4 hours. BEAS-2B epithelial cells (2×106 cells/ml) were co-cultured with PBMCs in 96-well plates with 100μg/ml HDM protein (D. pteronyssinus; Stallergenes Greer) in media supplemented with 10% fasted or refed serum from the asthmatic subjects for 24 hours. Supernatants were collected, centrifuged to remove cells and debris, and stored at −80°C. The levels of cytokines, including IL-1β, TNFα, IL-4, IL-17E, and TSLP were measured by ELISA (R&D Systems and PeproTech for IL-17E). Results were normalized to cell number using the CyQuant cell proliferation assay (Invitrogen).
Quantitative PCR Analysis
Total RNAs was extracted from PBMCs isolated in the fasted or fed state following the incubation of these cells with 1 ng/mL LPS for 4 hours. RNA from naïve CD4+ T cells were isolated at the fasted and refed time points, using Tripure (Roche) and cDNA was produced using a first-strand synthesis kit (Invitrogen). Quantitative real-time PCR was performed using SYBR green PCR master mix (Roche) and run on Light cycler 96 systems (Roche). Transcript levels of IL-1β, TNFα, NLRP3, IL-18, GATA3, CD3, CD14, and CD19 were measured using validated gene-specific primers (Qiagen). Relative gene expression was quantified by normalizing Ct values with 18S and EF1α (only for GATA3 expression) using the ΔΔCt method.
Immunoblot Analysis
Total proteins were extracted using RIPA buffer (50 mM Tris-HCl, pH 8.0, 0.5% deoxycholic acid, 1% NP-40, 0.1% sodium dodecyl sulfate and 0.5 M NaCl) supplemented with protease inhibitor cocktails and phosphatase inhibitors (Sigma). The lysates were separated by Tris-Glycine gel (Invitrogen) and transferred to nitrocellulose membranes. Antibodies were purchased from Cell signaling (pNFκB, NFκB, pIκB, and IκB), Adipogen (NLRP3), Santa Cruz Biotechnology (ASC), Abcam (IL-1β), and Millipore (Actin). Immunoblots were scanned in Odyssey Clx imaging system (Li-Cor Biosciences). Protein band intensity was measured the Odyssey imaging software and normalized to actin.
Statistical Analysis
Statistical analyses were performed using PRISM version 7 (GraphPad Software). Parametric data were described as means ± SD for the indicated number of observations. Statistical significance between groups was determined using two-tailed Student’s test when analyzing the response between groups. P value < 0.05 was considered statistically significant.
RESULTS
Study subjects and baseline characteristics
Eighteen subjects (7 men; 11 women), with a median age of 38 (range 26-55) years and body mass index of 26 (range 19-48) kg/m2, were recruited to participate in this study. The steroid-naïve asthmatics showed lower eosinophil levels compared to the steroid-dependent asthmatics whereas baseline FeNO levels were not different (Fig 3A-B). Additionally, the fasting and refeeding protocol had no effects on FEV1 or FeNO (Table I). As this was a pilot study and as isolation of primary cells were analyzed in real time, it was clearly noted after the recruitment of the first 13 subjects that the immunological responses were different in the steroid-naïve compared to inhaled corticosteroid treated subjects. To exclude the effects of steroids, the final 5 subjects recruited into this study were restricted to steroid-naïve asthmatics.
FIGURE 3.
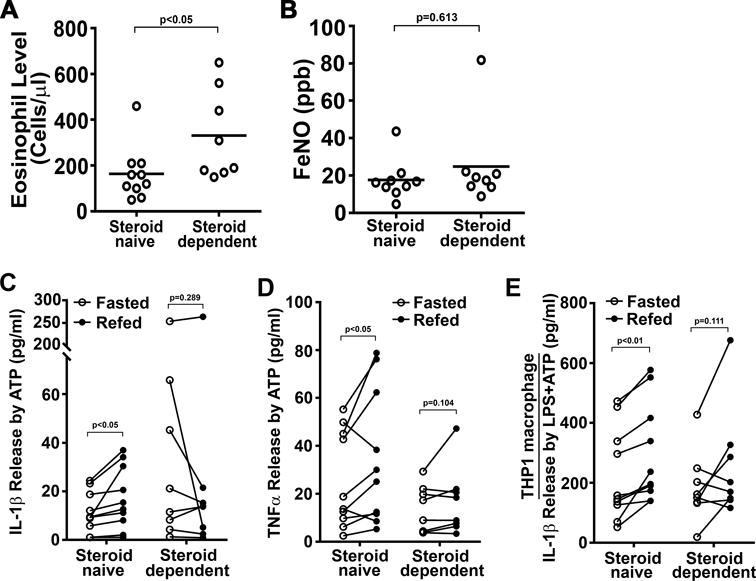
IL-1β and TNFα release in PBMCs isolated from asthmatics and serum effects on THP-1 macrophages. (A) Baseline eosinophil levels comparing steroid-naïve (n=10) and steroid-dependent (n=8) asthmatics. (B) Baseline fractional exhaled nitric oxide comparing steroid-naïve and steroid-dependent asthmatics. (C-D) IL-1β and TNFα release in primary PBMCs isolated from asthmatics (n=18). PBMCs were treated with 3 mM ATP, an NLRP3 activator, for 30 minutes. Cytokine release was determined by ELISA and was significantly increased in steroid-naïve cells extracted after refeeding compared to fasting. (E) IL-1β release in THP-1 macrophages supplemented with fasted or refed subject serum. Macrophages was primed with 10 ng/ml LPS and the stimulated with 5 mM ATP prior to ELISA assays. Statistical analysis using paired student t-test with p values shown in each panel.
Table I.
Research subjects’ characteristics and laboratory results
| Characteristics | Steroid-naive Means (ranges) |
Steroid-dependent Means (ranges) |
|
|---|---|---|---|
| Subjects (n) | 10 | 8 | |
| Sex: males/females | 5/5 | 2/6 | |
| Age (y) | 36.8 (26 - 54) | 41.5 (30 - 55) | |
| P value (naive vs. dependent) | 0.32 | ||
| Body mass index (kg/m2) | 29.8 (21.9 - 39.6) | 27.8 (19.1 - 47.8) | |
| P value (naive vs. dependent) | 0.62 | ||
| Blood Eosinophils (cells/μl) | 150 (50 - 460) | 330 (150 - 650) | |
| P value (naive vs. dependent) | 0.03 | ||
| FeNO (ppb) | Fasted state | 17.7 (4.7 - 43.6) | 24.8 (8.9 - 81.8) |
| Refed state | 19.4 (5.6 - 42.5) | 24.9 (6.0 - 82.7) | |
| P value (refed vs. fasted) | 0.75 | 0.99 | |
| FEV1 (% predicted) | Fasted state | 76.9 (68 - 100) | 85.8 (76 - 109) |
| Refed state | 87.0 (67 - 100) | 90.1 (79 - 112) | |
| P value (refed vs. fasted) | 0.29 | 0.42 | |
| Glucose (mg/dl) | Fasted state | 79.6 (69 - 91) | 82.4 (76 - 89) |
| Refed state | 94.6 (73 - 121) | 87.3 (73 - 104) | |
| P value (refed vs. fasted) | 0.01 | 0.20 | |
| Insulin (mU/ml) | Fasted state | 7.0 (3.5 - 12.5) | 5.5 (1.4 - 17.6) |
| Refed state | 31.9 (6.8 - 78.4) | 22.1 (7.4 - 77.9) | |
| P value (refed vs. fasted) | 0.003 | 0.07 | |
| Growth Hormone (ng/ml) | Fasted state | 1.4 | 3.5 |
| Refed state | 1.0 | 1.1 | |
| P value (refed vs. fasted) | 0.55 | 0.17 | |
The p values were calculated using paired student t-test analysis. The level for significance was defined as p< 0.05 and significant p value are indicated in boldface. FeNO, Fractional exhaled nitric oxide; FEV1, Forced expiratory volume in 1 second.
Fasting blunts the NLRP3 inflammasome in steroid-naïve asthmatics
As we had previously shown that fasting blunts the NLRP3 inflammasome in healthy volunteers (7), we evaluated whether this same intervention was operational in asthmatic subjects. PBMCs from the fasting and refed state were exposed to 3mM of ATP for 30 minutes and the release of interleukin-1β (IL-1β) was measured. Interestingly, steroid-naïve asthmatics treated only with short-acting β2-agonists showed a significant increase in ATP-stimulated IL-1β release in the refed state versus the fasted state, whereas subjects treated with inhaled steroids did not (Fig 3C). This dissociation between steroid-naïve and steroid-dependent asthmatics was also evident in the release of the inflammasome-independent cytokine, TNFα (Fig 3D). As these studies were performed in PBMC’s rather than in a population of NLRP3-enriched monocytes, a potential confounding factor could be a differential change in monocyte numbers in the steroid-naïve versus steroid-dependent asthmatic subjects in response to fasting and refeeding. However, blood differential counts showed no difference in lymphocyte, monocyte or neutrophil numbers between the groups (Supplemental Table 1) or in the transcript levels of genes encoding canonical cell surface receptors on monocytes, T cells or B cells (Supplemental Fig 1). It should also be noted in this pilot study, that the refeeding induced 1L-1β release by steroid-naïve asthmatics was independent of meal selection (data not shown).
Previously, we demonstrated that human THP-1 macrophages incubated with serum extracted from healthy volunteers following a 24-hour fast and refeeding paralleled the primary PBMC data and blunted the fasting NLRP3 inflammasome compared to refed serum (7). As this suggested a serum-paracrine mediator of this regulation, we next incubated THP-1 macrophages with serum from steroid-naïve asthmatics and showed a recapitulation of the primary PBMCs results with the significant induction of IL-1β release in cells incubated with refed serum (Fig 3E). Furthermore, the steroid-treated subject serum did not show these nutrient-level dependent changes (Fig 3E). To determine whether steroids per se modulate nutrient-level dependent activation of the inflammasome, we supplemented THP-1 cultured cells with steroid-naïve subjects sera with dexamethasone or cortisol prior to inflammasome induction with LPS and ATP. As may have been expected, both steroids blunted IL-1β release and ameliorated the difference in cytokine production between the fasted and refed states (Supplemental Fig 2A). This blunting of the inflammasome was also evident if dexamethasone or cortisol was introduced following LPS administration but prior to the ATP trigger of IL-1β release (Supplemental Fig 2B).
Refeeding primes the NLRP3 inflammasome in steroid-naïve asthmatics
To explore the potential regulatory control of the inflammasome comparing the steroid-naïve to dependent asthmatic subjects we assayed PBMC’s in the two nutritional states. The transcript levels of genes encoding NLRP3, the canonical inflammasome cytokines IL-1β and IL-18, and TNFα were upregulated in parallel with the increased cytokine levels following refeeding in the steroid-naïve asthmatic PBMCs, but not in the steroid-dependent asthmatic cells (Fig 4A-B). In parallel we quantified steady-state protein levels of NLRP3 components and IL-1β and, the phosphorylation level of the canonical transcriptional mediators of the inflammasome, NFκB and IκB, in steroid-native asthmatic subjects. We show that the levels of NLRP3 and of phospho-NFκB are increased in the refed compared to the fasting state (Fig 4C-D). These findings support our previous data suggesting that fasting and feeding have distinct inflammasome blunting and activating effects in healthy volunteers, respectively (7). Furthermore, the data from this study suggest that inhaled steroids ameliorate nutrient level regulation of the NLRP3 inflammasome. Given this immunomodulatory effect of the steroids subsequent studies were performed using only PBMCs and serum from the steroid-naïve subgroup.
FIGURE 4.
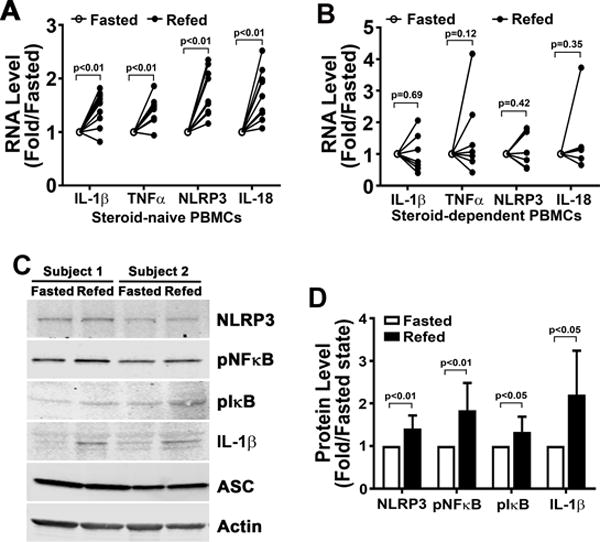
Expression level of genes encoded for inflammatory and NLRP3 components in PBMCs. (A-B) mRNA expression in primary PBMCs isolated from steroid-naïve (n=10) and steroid-dependent (n=8) asthmatics. mRNA expression in primary PBMCs primed with 1 ng/ml LPS showed increased transcript levels in the refed state. (C-D) Representative protein levels and relative quantitative changes of signaling molecules activating the NLRP3 inflammasome in PBMCs isolated from steroid-naïve asthmatics (n=8). Statistical analysis using paired student t-test with p values shown in each panel.
Fasting and refeeding differentially modulate airway epithelial cell inflammation and Th2 activation
Due to the absence of the fasting-refeeding effects on the NLRP3 inflammasome in asthmatic subjects exposed to inhaled corticosteroids in the analysis of the initial 13 subjects, the enrollment of the final 5 subjects were restricted to steroid-naïve asthmatic subjects. As the NLRP3 inflammasome is postulated to modulate lung epithelial cell inflammation, PBMCs, from these 5 steroid-naïve enrolled subjects on this pilot study, were co-cultured with human BEAS-2B lung epithelial cells in the presence of 100 μg/ml of house dust mite extract (HDM). In parallel with the induction of the inflammasome with refeeding, the release of IL-1β was similarly induced to a significantly greater degree in response to HDM following co-culturing with refed PBMCs (Fig 5A). Additionally, the epithelial cell specific cytokines IL-17E and thymic stromal lymphopoietin (TSLP) were secreted in significantly greater amounts, in response to HDM in the presence of PBMCs extracted from refed versus fasted steroid-naïve asthmatics (Fig 5B-C). To validate which cells secreted specific cytokines, IL-1β release was assayed in isolated BEAS-2B cells and IL-17E and TSLP cytokine release was measured in isolated PBMCs in the presence of HDM. IL-1β was not detectable in BEAS-2B cells and the levels of IL-17E and TSLP secreted from PBMCs was markedly lower than the levels released in response to HDM in coculture conditions (data not shown).
FIGURE 5.
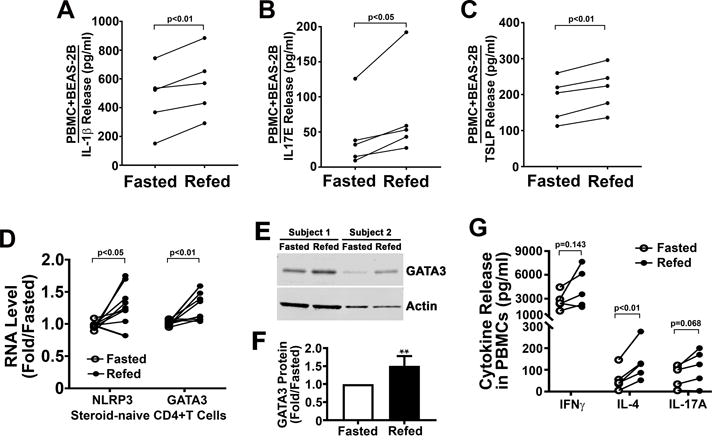
Assessment of the fasting and refeeding effects on epithelial airway cell inflammation and the Th2 endotype. (A) IL-1β release from fasted or refed steroid-naïve PBMCs co-cultured with BEAS-2B lung epithelial cells incubated in their respective serum in the presence of HDM extract (n=5). (B-C) IL-17E and TSLP release from BEAS-2B epithelial cells co-cultured with PBMCs (n=5). (D) mRNA expression of genes encoding NLRP3 and GATA3 from negatively selected CD4+ T-cells from steroid-naïve PBMCs (E, n=10). (E) Representative protein levels of GATA3 in CD4+ T-cells. (F) Quantification of GATA3 protein levels comparing fasted to refed states in steroid-naïve subjects (n=8). (G) IFNγ, IL-4, and IL-17A cytokine release as measured by ELISA following the stimulation of fasted and refed PBMCs with PMA and ionomycin (n=5) Statistical analysis using paired student t-test with p values shown in each panel.
In addition, given the emerging evidence supporting NLRP3 inflammasome induction of Th2 cell polarity (13), we assayed the transcript levels of NLRP3 and of the canonical Th2 transactivator, GATA3, in CD4+ T cells. Transcript levels of both NLRP3 and GATA3 were significantly higher in the refed versus fasted CD4+ T cells (Fig 5D). Furthermore, the steady-state levels of GATA3 were also significantly induced in CD4+ T cells isolated from the steroid-naïve subjects in the refed compared to fasted state (Fig 5E-F). To validate this T cell response to fasting and refeeding, we concurrently measured the secretion of the canonical CD4+ T cell cytokines including interferon gamma, IL-4 and IL-17A from PBMC’s isolated form these subjects and exposed to PMA and ionomycin. In parallel with the gene and protein regulatory effects in CD4+ T cells, only the canonical Th2 cytokine, i.e. IL4 secretion was statistically significantly higher in the refed compared to the fasting state (Fig 5G).
DISCUSSION
In this pilot study, we show that fasting and refeeding modulate numerous aspects of immune regulation in asthma. These include evidence that fasting blunts the activation of the NLRP3 inflammasome, suppresses the expression of genes governing CD4+ Th2 cell activation and reduces lung epithelial cell cytokine production in response to house dust mite. These immunomodulatory effects were evaluated relative to their activation following refeeding. These nutrient regulatory effects appear to be operational in myeloid, lymphoid and epithelial cellular components of the asthmatic immune axis and suggest that nutrient-level sensing immunomodulatory programs may contribute to the pathophysiology of asthma.
The mechanisms underpinning caloric-load effects on immune activation are beginning to be explored and appear to be regulated at multiple levels. These include regulation at the level of changes in metabolic intermediates, such as where levels of glucose and ketones modulate neuronal signaling response to either bacterial or viral infections (17) and β-hydroxybutyrate-levels block NLRP3 inflammasome activation (18). Interestingly, the loss of mitochondrial integrity facilitates NLRP3 inflammasome activation (19) and fasting has been shown to blunt this inflammasome via the activation of the Sirt3 regulatory proteins that protect mitochondrial fidelity (7, 11). Conversely, in experimental models, the Western diet has been found promote myeloid immunological reprograming with evidence of hyper-responsiveness to inflammatory triggers (20). Interestingly, the Western diet-mediated immune hyper-responsiveness is dependent on NLRP3 signaling (20). Whether and how the complex array of immunological pathways operational in asthma are regulated by nutrient levels has not been well characterized, however, in experimental studies the NLRP3 inflammasome was required for high-fat diet induced airway hyperreactivity (12) and the adipokine leptin, which is elevated in obesity, induced Th2 and iLC2 responsiveness in experimental allergic airway disease (21). To explore the ameliorative effects of fasting, a larger asthmatic population study is required to both validate the findings from this pilot study, and to enable the exploration of potential regulatory pathways underpinning these immune-modulatory effects.
An additional interesting aspect of this pilot study is that the inhaled corticosteroid-dependent asthmatic subjects did not show a blunted inflammasome response to fasting. Interestingly, glucocorticoids, acting through the glucocorticoid receptor, have been shown to upregulate NLRP3 at the transcriptional level with the subsequent sensitization of macrophages to inflammasome-induction with the excess secretion of IL-1β and other pro- and anti-inflammatory cytokines (22). Our findings did not replicate this prior study, as we found that IL-1β release from THP-1 macrophages exposure was blunted by prior exposure to dexamethasone or cortisol. Taken together, our data suggest the inhaled corticosteroids may have indirect systemic effects on the NLRP3 inflammasome. This concept is interesting given that a large population study suggested that inhaled corticosteroids can increase cardiometabolic risk and metabolic syndrome, disease-states that are linked to activation of the NLRP3 inflammasome (23). Given that inhaled corticosteroids are very effective in the control of mild-moderate asthma, it would be useful to explore in future studies whether caloric restriction interventions could still modulate adaptive and epithelial cell immunity in asthmatics who require corticosteroid therapy.
Our study did not show an effect of fasting on FeNO. This is in contrast to a prior study, where the contrary intervention i.e. acute high fat feeding, induced FeNO two hours after the dietary intervention (24). It should be noted that this high-fat feeding intervention was performed in healthy volunteers and not in subjects with preexisting pulmonary inflammation. We also did not see any improvement in PFT measurements. This is in contrast to the alternate day caloric restriction study where asthmatics subjected to the consumption of 20% of their usual daily caloric intake on alternate days, showed a significant improvement of their peak expiratory flow by three weeks into the study with maintenance of this improvement for the duration of the 8 week study (3). Given this, we would postulate that a single day of fasting was probably too acute a duration of caloric restriction to effect pulmonary function directly.
In conclusion, given the effects of obesity on asthma and the emerging demonstration of health benefits of fasting mimetic diets (5, 16), our findings, in this pilot study, support the concept that nutrient intervention studies may benefit asthmatics. In addition, the emerging role of the NLRP3 inflammasome in asthma and the development of pharmacologic antagonists have suggested that the NLRP3 inflammasome might be targeted as a new treatment approach (8, 25). Thus, our findings suggest that caloric restriction might be considered as a strategy to attenuate activation of the NLRP3 inflammasome and thereby ameliorate nutrient level effects on systemic and pulmonary inflammation in asthma.
Supplementary Material
Acknowledgments
The authors are grateful to the asthmatic patients who agreed to participate in this study. We acknowledge the support of Dr. Myron A. Waclawiw of the NHLBI Office of Biostatistics Research in the design of the protocol.
Funding: Funding was supplied by the Division of Intramural Research to the National Heart, Lung and Blood Institute to Dr. Michael Sack. Grant number: HL005199-09 and Dr. Stewart Levine, Grant numbers: HL006197-02 and HL006166-04.
Supported by funding from the NHLBI Division of Intramural Research.
ABBREVIATIONS
- CRP
C-reactive protein
- FeNO
fractional exhaled nitric oxide
- HDM
house dust mite extract
- iLC2
innate type 2 lymphocytes
- IL
interleukin
- NFκB
nuclear factor kappa B
- NLRP3
Nod-Like Receptor Pyrin domain 3
- PBMCs
peripheral blood mononuclear cells
- PFT
pulmonary function testing
- TNFα
tumor necrosis factor alpha
- TSLP
thymic stromal lymphopoietin
Footnotes
Disclosures of potential conflicts of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Carpaij OA, van den Berge M. The asthma-obesity relationship: underlying mechanisms and treatment implications. Curr Opin Pulm Med. 2018;24:42–49. doi: 10.1097/MCP.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 2.Jensen ME, Gibson PG, Collins CE, Hilton JM, Wood LG. Diet-induced weight loss in obese children with asthma: a randomized controlled trial. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2013;43:775–784. doi: 10.1111/cea.12115. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free radical biology & medicine. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell metabolism. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, Suarez J, Michalsen A, Cross AH, Morgan TE, Wei M, Paul F, Bock M, Longo VD. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell reports. 2016;15:2136–2146. doi: 10.1016/j.celrep.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traba J, Kwarteng-Siaw M, Okoli TC, Li J, Huffstutler RD, Bray A, Waclawiw MA, Han K, Pelletier M, Sauve AA, Siegel RM, Sack MN. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. The Journal of clinical investigation. 2015;125:4592–4600. doi: 10.1172/JCI83260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim RY, Pinkerton JW, Essilfie AT, Robertson AAB, Baines KJ, Brown AC, Mayall JR, Ali MK, Starkey MR, Hansbro NG, Hirota JA, Wood LG, Simpson JL, Knight DA, Wark PA, Gibson PG, O’Neill LAJ, Cooper MA, Horvat JC, Hansbro PM. Role for NLRP3 Inflammasome-mediated, IL-1beta-Dependent Responses in Severe, Steroid-Resistant Asthma. Am J Respir Crit Care Med. 2017;196:283–297. doi: 10.1164/rccm.201609-1830OC. [DOI] [PubMed] [Google Scholar]
- 9.Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014;43:1067–1076. doi: 10.1183/09031936.00105013. [DOI] [PubMed] [Google Scholar]
- 10.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature immunology. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Traba J, Geiger SS, Siaw M Kwarteng, Han K, Ra OH, Siegel RM, Gius D, Sack MN. Prolonged fasting suppresses mitochondrial NLRP3 inflammasome assembly and activation via SIRT3 mediated activation of Superoxide Dismutase 2. The Journal of biological chemistry. 2017 doi: 10.1074/jbc.M117.791715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, DeKruyff RH, Umetsu DT. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nature medicine. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruchard M, Rebe C, Derangere V, Togbe D, Ryffel B, Boidot R, Humblin E, Hamman A, Chalmin F, Berger H, Chevriaux A, Limagne E, Apetoh L, Vegran F, Ghiringhelli F. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nature immunology. 2015;16:859–870. doi: 10.1038/ni.3202. [DOI] [PubMed] [Google Scholar]
- 14.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E, Team CP Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS medicine. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser DA, Thoen J, Djoseland O, Forre O, Kjeldsen-Kragh J. Serum levels of interleukin-6 and dehydroepiandrosterone sulphate in response to either fasting or a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000;18:357–362. [PubMed] [Google Scholar]
- 16.Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, Groshen S, Mack WJ, Guen E, Di Biase S, Cohen P, Morgan TE, Dorff T, Hong K, Michalsen A, Laviano A, Longo VD. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, Medzhitov R. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell. 2016;166:1512–1525 e1512. doi: 10.1016/j.cell.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nature medicine. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traba J, Sack MN. The role of caloric load and mitochondrial homeostasis in the regulation of the NLRP3 inflammasome. Cellular and molecular life sciences : CMLS. 2017;74:1777–1791. doi: 10.1007/s00018-016-2431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christ A, Gunther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Bassler K, Klee K, Schulte-Schrepping J, Ulas T, Moorlag S, Kumar V, Park MH, Joosten LAB, Groh LA, Riksen NP, Espevik T, Schlitzer A, Li Y, Fitzgerald ML, Netea MG, Schultze JL, Latz E. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell. 2018;172:162–175 e114. doi: 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng H, Zhang X, Castillo EF, Luo Y, Liu M, Yang XO. Leptin Enhances TH2 and ILC2 Responses in Allergic Airway Disease. The Journal of biological chemistry. 2016;291:22043–22052. doi: 10.1074/jbc.M116.743187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. The Journal of biological chemistry. 2011;286:38703–38713. doi: 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savas M, Muka T, Wester VL, van den Akker ELT, Visser JA, Braunstahl GJ, Slagter SN, Wolffenbuttel BHR, Franco OH, van Rossum EFC. Associations Between Systemic and Local Corticosteroid Use With Metabolic Syndrome and Body Mass Index. The Journal of clinical endocrinology and metabolism. 2017;102:3765–3774. doi: 10.1210/jc.2017-01133. [DOI] [PubMed] [Google Scholar]
- 24.Rosenkranz SK, Townsend DK, Steffens SE, Harms CA. Effects of a high-fat meal on pulmonary function in healthy subjects. European journal of applied physiology. 2010;109:499–506. doi: 10.1007/s00421-010-1390-1. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Zhao J, Wang H, Liang Y, Yang N, Huang Y. Blockage of P2×7 attenuates acute lung injury in mice by inhibiting NLRP3 inflammasome. Int Immunopharmacol. 2015;27:38–45. doi: 10.1016/j.intimp.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


