Abstract
Stromal cell-derived factor 1-alpha (SDF) is a potent bone marrow chemokine capable of recruiting circulating progenitor populations to injured tissue. SDF has known angiogenic capabilities, however, bone marrow-derived cellular contributions to tissue regeneration remain controversial. Bone marrow from DsRed transgenic donors was transplanted into recipients to lineage trace circulating cells after myocardial infarction (MI). SDF was delivered post-MI and hearts were evaluated for recruitment and plasticity of bone marrow-derived populations. SDF treatment improved ventricular function, border zone vessel density, and CD31+ cell frequency post-MI. Bone marrow-derived endothelial cells were observed; these cells arose through both cell fusion and transdifferentiation. Circulating cells also adopted cardiomyocyte fates, but such events were exceedingly rare and almost exclusively resulted from cell fusion. SDF did not significantly alter the proportion of circulating cells that adopted non-hematopoietic fates. Mechanistic insight into the governance of circulating cells is essential to realizing the full potential of cytokine therapies.
Keywords: angiogenesis, bone marrow, cell fusion, myocardial infarction, regeneration
INTRODUCTION
The capacity of the adult mammalian heart to reconstitute lost cardiomyocytes, smooth muscle, and endothelial cells after myocardial infarction (MI) is limited. Yet, cardiac and other progenitor cells are increasingly identified and hold promising translational potential[1–4]. Chemokines that attenuate ischemic damage to the heart via their angiogenic or regenerative effects are also attractive candidates for clinical translation[5, 6]. In fact, stromal cell-derived factor 1-alpha’s (SDF) potent angiogenic and anti-apoptotic effects have led to testing in humans.
SDF – a crucial component of the hematopoietic niche – recruits endothelial progenitor cells as well as cells from the hematopoietic lineage from the bone marrow to the site of organ injury [5, 6]. However, significant controversy remains regarding the role that bone marrow-derived circulating cells play in post-MI myocardial repair, particularly whether they adopt non-hematopoietic fates [7–11]. Many scrutinize the proposed multipotency and existence of hematopoietic cell transdifferentiation into cardiomyocytes and vascular cells [7, 9, 12]. Conversely, others support the plasticity of bone marrow-derived cells [1, 8, 10, 11, 13]. Despite enduring disagreement regarding the existence, functionality, and durability of bone marrow-derived cardiomyocytes, physicians continue to test bone marrow as a cellular therapy for the treatment of patients with acute MI and chronic ischemic cardiomyopathy [14–20]. Unfortunately, such studies often yield statistically significant – but clinically insignificant – results.
We investigated the fate of bone marrow cells recruited to injured myocardium by SDF. We lineage traced the bone marrow and used florescent activated cell sorting (FACS) and histology to evaluate: 1) if bone marrow-derived cells contribute directly to the myocardium, and 2) if SDF augments endogenous repair mechanisms via altering hematopoietic plasticity. Because of the demonstrated therapeutic efficacy of SDF, it is essential to analyze how SDF regulates circulating cell populations in order to pave the way for future therapies to leverage responsive circulating populations.
METHODS
Bone marrow lineage tracing mouse model
We used mice transgenic for DsRed (driven by the chicken β-actin promoter) [21] as bone marrow donors. C57Bl/6 mice or mice transgenic for eGFP (driven by the chicken β-actin promoter) on a C57Bl/6 background were used as recipients. All mice were given sterile food, autoclaved acidified water, and housed under pathogen free conditions. All experiments were approved by the Administrative Panel on Laboratory Animal Care at Stanford University (protocol 28921) and conform to the NIH guidelines. Two mouse strains were used as bone marrow recipients in this study: C57Bl/6 recipients and C57Bl/6-eGFP recipients. C57Bl/6 recipients were transplanted with C57Bl/6-DsRed bone marrow and randomized to one of three groups: Sham surgery (N=3), Myocardial infarction with intramyocardial injection of PBS (phosphate buffered saline, N=7), or Myocardial infarction with intramyocardial injection of SDF and intraperitoneal injection of GM-CSF (N=7). C57Bl/6-eGFP recipients were transplanted with C57Bl/6-DsRed bone marrow and were randomized into two groups: Myocardial infarction with intramyocardial injection of PBS (N=5), or myocardial infarction with intramyocardial injection of SDF and IP injection of GM-CSF (N=5). At experimental endpoints, cardiac tissue was analyzed through histological sections or flow cytometry analysis.
Generation of bone marrow chimeric mice
Recipient C57Bl/6 or eGFP transgenic mice were lethally irradiated with 10 Gy split-dose radiation in a cabinet x-ray irradiator (5 Gy the evening before transplantation and 5 Gy the morning of transplantation). After lysing erythrocytes, whole bone marrow cells (5×106) from DsRed transgenic mice were retro-orbitally injected into recipient mice four hours after the final dose of radiation.
Analysis of chimerism
Hematopoietic reconstitution was evaluated in peripheral blood two to four weeks after transplantation, prior to ligation of the left anterior descending coronary artery. 100 μL of peripheral blood was collected from the tail vein and red blood cells were lysed. Peripheral blood mononuclear cells were stained with fluorochrome-conjugated antibodies against CD3, CD19, Mac-1, Gr-1. Granulocytes were defined as CD3-, CD19-, Mac-1+, Gr-1+ (Supplementary Figure 1). Long-term hematopoietic stem cells were also assessed in transplanted recipients and phenotypically described as Lineage-, ckit+, Sca-1+, Slam+, Flt3−, CD34− (Supplementary Figure 1). Flow cytometry was performed using a LSR II machine (BD Biosciences, San Jose, CA, USA) and FlowJo software (Flowjo LLC, Ashland, OR, USA) was used for data analysis.
Culture of endothelial progenitor cells
Bone marrow mononuclear cells were isolated from the long bones of adult male mice by density centrifugation with Histopaque 1083 (Sigma-Aldrich, St. Louis, MO, USA), plated on vitronectin-coated dishes, and cultured in endothelial basal medium-2 supplemented with EGM-2 SingleQuot (Lonza, Basel, Switzerland) containing human epidermal growth factor, fetal bovine serum, vascular endothelial growth factor, basic human fibroblast growth factor, recombinant human long R3 insulin-like growth factor-1, ascorbic acid, heparin, gentamicin, and amphotericin-B. Media was changed 24 hours after plating to facilitate removal of non-adherent bone marrow mononuclear cells. Subsequent media changes were performed every 48 hours for a total of 7 days to select for the endothelial progenitor cell phenotype. This culture method selects for the early outgrowth population of endothelial progenitor cells.
Model of acute myocardial infarction
Myocardial infarction was induced using an established and highly reproducible model [22]. Briefly, mice were anesthetized in a 2 L induction chamber (VetEquip, CA, USA) and 3% isoflurane was continuously delivered. Mice were intubated endotracheally with a 20-gauge angiocatheter and subsequently connected to a mechanical ventilator (Hallowell EMC, Pittsfeild, MA USA); 2% isoflurane was maintained throughout the procedure. Buprenorphine (0.5 mg/kg) and carprofen (5 mg/kg) were administered for pain control. The heart was exposed through the left fourth intercostal space, and an 8-0 polypropylene suture was tied around the left anterior descending coronary artery mid-way between the left atrium and ventricular apex. Mice randomized to the sham control group (n=3) underwent thoracotomy without coronary ligation. The animals were randomized to two experimental groups (n=13/group), and received three 20 μL intramyocardial injections within the border zone myocardium of either PBS (60 μL total) or SDF (6 μg/kg in 60 μL total, Anaspec, Fremont, CA, USA). Mice randomized to the SDF group also received one subcutaneous injection of recombinant mouse granulocyte/macrophage colony-stimulating factor (40 μg/kg) (Genzyme, Cambridge, MA, USA). Our experimental design was based on previous work demonstrating a synergistic effect of GM-CSF and SDF therapy in treating ischemic injury from myocardial infarction [23]. Of note, this publication demonstrated no difference between the control groups of PBS alone and PBS with GMCSF, indicating that GM-CSF only has a benefit when paired with SDF and has no angiogenic properties when used alone. For this reason, we did not include it as a negative control.
Echocardiography assessment
Left ventricular geometry and function (n=20) were evaluated 2 weeks after MI with a high-resolution (30 MHz) Vevo 2100 transthoracic echocardiography system (VisualSonics, Toronto, Canada). Images were obtained through a parasternal short-axis view with M-mode ultrasound at the level of the papillary muscle and mid-way between the papillary muscle and apex. Ejection fraction, fractional shortening, and internal ventricular dimensions were computed with the Vevo 770 Standard Measurement Package. All analyses were performed by a single investigator blinded to the treatment assignment.
Langendorff dissociation
Mice were sedated in an isoflurane induction chamber and their hearts were excised under inhaled anesthesia. The aorta was cannulated for anterograde coronary artery perfusion through the Langendorff system. The Adult Rat/Mouse Cardiomyocyte Isolation Kit (Cat# AC-7031 from Cellutron Life Technologies, Baltimore, MD, USA) was used to digest the heart. The heart was perfused with wash buffer for 4 minutes and then with collagenase for 10 minutes at a constant flow of 3 mL/min. The heart was subsequently minced and shaken for 5 minutes in 7 mL of collagenase solution. The resulting solution was then centrifuged for 1 minute at 500 RPM to separate the cardiomyocytes and supernatant. Cardiomyocytes were resuspended using a cell suspension buffer.
Fluorescent activated cell sorting and analysis
The Langendorff procedure separates the cardiomyocytes (in the pellet) from the cardiac associated small cells (in the supernatant). Both samples were stained with CD45-APC-cy7, CD31-Pecy7, and troponin-APC. Extracellular antigens were stained first. Cells were then treated with transcription factor buffer set (BD) and stained for intracellular troponin. Flow cytometry was performed with an Aria machine (BD). The supernatant was sorted with FSC/SSC voltages appropriate for hematopoietic cells whereas the pellet was sorted with FSC/SSC voltages appropriate for cardiomyocytes. FlowJo software was used for data analysis.
Immunohistochemistry and histology analysis
At the study endpoint, a subset of animals was anesthetized and their hearts were explanted. Hearts were flushed with PBS, filled retrograde with Tissue-Plus optimal cutting temperature compound (Fisher Scientific, Hampton, NH, USA), frozen at −80°C, and sectioned onto Superfrost Plus microscope slides (Fisher Scientific) using a Cryostar NX70 cyrostat (Thermo Scientific, Waltham, MA, USA) at 10μm thickness. Next, the samples were fixed with 4% paraformaldehyde and blocked with 10% fetal bovine serum. Separate sections were stained for cardiac troponin (ab47003, Abcam, Cambridge, UK) and Von Willebrand factor (Abcam ab11713). The antibodies were diluted at 1:200 in PBS and incubated for 2 hours at 37°C. The sections were washed in PBS, and the appropriate secondary antibodies (Abcam) were applied at a dilution of 1:200 for 1 hour incubation at 37°C.
The slides were washed in PBS and counterstained for nuclei with NucBlue DAPI (4′,6-diamidino-2-phenylindole; ThermoFisher Scientific) following the manufacturer’s protocol. Stained sections were imaged with a LSM 780 confocal microscope (Zeiss, Oberkochen, Germany). Vessel density was calculated using ImageJ software (v1.49, NIH).
Statistical analysis
Continuous variables are presented as means with corresponding standard errors and categorical variables are presented as proportions. Differences between groups were assessed with the Student t test for independent samples. All tests were two-tailed, and a P value less than 0.05 was considered statistically significant. Statistical analyses were performed with Prism 6.0 (GraphPad, La Jolla, CA, USA).
RESULTS
SDF enhances post-infarction ventricular function and vessel density
Consistent with previous reports, we observed that SDF significantly enhanced ventricular function after MI when delivered at the time of injury as compared to PBS. Three weeks after treatment, echocardiographic assessment demonstrated a significantly improved left ventricular ejection fraction (57.6±5.0% vs. 41.9±3.7%, P=0.018), fractional shortening (31.2±3.5% vs. 21.1±2.1%, P=0.02), and a strong trend toward a reduction in left ventricular end systolic diameter (2.44±0.21 mm vs. 2.96±0.19 mm, P=0.076) (Figure 1A–B). Histological evaluation of Von Willibrand Factor (VWF) demonstrated increased vessel density in the border zone region of SDF treated recipients compared with that of PBS treated recipients (Figure 1D–E). Quantification of VWF signal confirmed this observed increase was statistically significant (P=0.0002).
Figure 1.
Exogenous SDF enhances post-infarction myocardial function and increases border zone vessel density. (A) Left ventricular ejection fraction (B) and fractional shortening (FS) measured by transthoracic echocardiography and stratified by treatment group. (C) Comparison of relative Von Willebrand factor expression in the infarct border zone between SDF-treated and PBS-treated groups. (D, E) Representative images comparing infarct border zone capillary density between SDF-treated and PBS-treated groups. Scale bar, 100 μm.
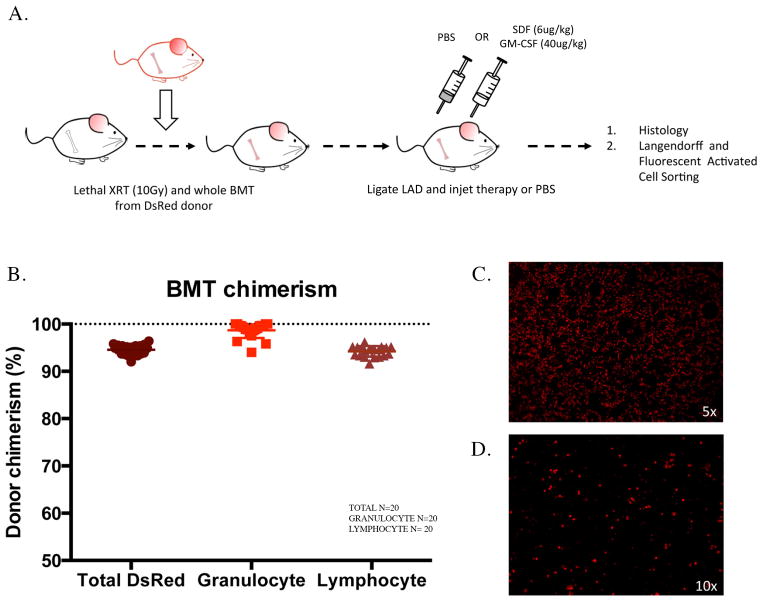
Successful bone marrow transplantation results in near 100% multi-lineage donor chimerism
To study whether circulating bone marrow cells contribute to myocardial repair and investigate how SDF treatment attenuates myocardial injury, we established a lineage-tracing model whereby recipient bone marrow was replaced with bone marrow from DsRed transgenic mice (Figure 2A). All mice survived the procedure, and 4 weeks after transplant the total peripheral blood chimerism was 95±1%. Granulocyte chimerism – a proxy for hematopoietic stem cell (HSC) chimerism [24] – was 99±2% (Figure 2B), and strongly correlated with long-term HSC chimerism in the bone marrow (Supplemental Figure 1A). To confirm successful engraftment of non-HSC lineages, endothelial progenitor cells were isolated and cultured from the mouse bone marrow after transplant. Fluorescent microscopy of bone marrow smears and isolated endothelial progenitor cells demonstrated diffuse expression of DsRed (Figure 2C–D). The near 100% chimerism obtained with this model permits multi-lineage fate tracking of bone marrow cells with high sensitivity.
Figure 2.
Lineage-tracing model for tracking the fate of circulating bone marrow cells within the heart. (A) Schematic of experimental model for fate tracking. (B) Percentages of recipient-donor chimerism after bone marrow transplant stratified by hematopoietic cell type at 6 weeks. (C) Representative bone marrow smear of transplanted recipient. (D) Endothelial progenitor cells from transplanted recipients.
Circulating bone marrow cells contribute to coronary vessel endothelium post MI
We then used the aforementioned model to trace the fate of circulating bone marrow cells before and after MI to investigate the endogenous repair mechanisms after ischemic injury. Because the plasticity of circulating BM cells remains controversial, we thought it necessary to evaluate the baseline recruitment and repair mechanisms post MI using this transplantation model. With flow cytometry, we categorized cells as hematopoietic (CD45+), endothelial (CD31+), or cardiomyocytes (troponin+); cells that additionally expressed DsRed originated from the bone marrow (Figure 3A, 4A). After MI, the vast majority of bone marrow-derived cells found within the heart were hematopoietic (94.1±0.3%) (Supplemental Figure 1B). The large influx of hematopoietic cells localized primarily to area of myocardial injury (the anterolateral left ventricular wall), and decreased over time (Supplemental Figure 1C).
Figure 3.
Bone marrow cells adopt endothelial cell fates within the heart. (A) Flow cytometry analysis of cardiac associated cells demonstrating the DsRed+/CD31+ and DsRed+/CD45+ populations. (B) Percentage of CD31+ cells in MI injured and sham surgery hearts. (C) Percentage of bone marrow-derived CD31+ cells in MI injured and sham surgery hearts. (D) Percentage of CD31+ cells due to fusion (GFP+/DsRed+) or de novo formation (GFP-/DsRed+). (E, F) Representative images of bone marrow-derived endothelial cells that co-express Von Willebrand factor and DsRed in boarder zone and remote regions. Scale bar, 100 μm.
Figure 4.
Bone marrow cells adopt cardiomyocyte fates within the heart. (A) Flow cytometry analysis demonstrating the DsRed+/troponin+ population. (B) Percentage of troponin+ cells in MI injured and sham surgery hearts. (C) Percentage of bone marrow-derived troponin+ cells in MI injured and sham surgery hearts. (D) Percentage of troponin+ cells due to fusion (GFP+/DsRed+) or de novo formation (GFP−/DsRed+). (E) Bone marrow-derived troponin+ cells outside of infarct area that co-express DsRed and troponin. Scale bar, 25 μm. (F) Sorted cardiomyocytes. The superior cell expresses troponin, DsRed and GFP and likely arose from cell fusion. The inferior cell only expresses troponin and DsRed and likely arose from de novo generation. Scale bar, 50 μm.
The number of endothelial cells significantly decreased after MI (Figure 3B). Notably, DsRed+/CD31+ endothelial cells were observed in very small quantities in uninjured hearts. Myocardial injury, however, stimulated an increase in the proportion of bone marrow-derived endothelial cells (1.0±0.2% vs. 0.2±0.03%, P=0.028) (Figure 3C). This increase in bone marrow-derived endothelial cells was spatially confined to the area of injury and infarct border zone (Figure 3E). Negligible numbers of bone marrow-derived endothelial cells were observed in remote myocardium (Figure 3F). These data suggest myocardial ischemia stimulates bone marrow-derived vasculogenesis in the compromised area. However, the bone marrow minimally contributes to endothelial maintenance in the absence of injury.
Circulating bone marrow cells adopt cardiomyocyte fates post MI
We then characterized the cardiomyocyte population in injured and uninjured hearts with flow cytometry. The plasticity of circulating bone marrow-derived populations remains controversial, however FACS analysis allows for high throughput analysis of cardiomyocytes (troponin+), providing more accuracy to rare occurring events that may be inaccurately accounted for with manual assessment. A small population of troponin+/DsRed+ cells were identified (0.046±0.023% of troponin+ cells), thereby suggesting that bone marrow cells may adopt a cardiomyocyte phenotype (Figure 4A). Immunohistochemistry and confocal microscopy of cardiac tissue confirmed the rare presence of troponin+/DsRed+ cells, but true sarcomeric structures were not clearly identifiable (Figure 4E). These bone marrow-derived cardiomyocytes were observed outside the area of injury and the number of these rare cells was not altered by myocardial injury (Figure 4C).
Bone marrow-derived endothelial cells and cardiomyocytes primarily result from cell fusion
We modified our lineage-tracing model to examine whether bone marrow-derived cardiomyocytes arise from cell fusion or transdifferentiation. By transplanting bone marrow from C57Bl/6-DsRed transgenic mice into recipient C57Bl/6 mice transgenic for GFP, we could identify GFP+/DsRed+ cells as having arisen from cell fusion, and GFP-/DsRed+ cells as having arisen from de novo formation of bone marrow cells.
The vast majority of DsRed+/troponin+ cells co-expressed GFP (95.2±1.0%) (Figure 4D). Therefore, bone marrow-derived cardiomyocytes are primarily generated from fusion with existing GFP+ cardiomyocytes. A very small population of GFP-/DsRed+ cells was also identified (0.0029±0.0014% of troponin+ cells), and does suggest the potential – though rare – for circulating bone marrow cells to form de novo cardiomyocytes. Histologic examination of sorted DsRed+ and GFP+DsRed+ cells confirmed the existence of troponin+ fusion and de novo formation cell types (Figure 4F). Whereas de novo formation was exceedingly rare among bone marrow-derived cardiomyocytes, this lineage-tracing analysis demonstrated bone marrow-derived vascular endothelial cells do arise from de novo generation. However, rates of cell fusion remained greater than de novo generation among the bone marrow-derived endothelial population (Figure 3D).
SDF improves capillary density without increasing the direct contribution from bone marrow cells
To assess if the baseline contribution of circulating cells is augmented by SDF treatment, we performed the same FACS and histological analyses on animals treated with SDF at the time of ischemic injury. A significant increase in the frequency of CD31+ cells was noted in SDF-treated hearts compared to that of PBS-treated hearts (Figure 5A). The increase in CD31+ cells correlated with an increase in vessel density in the infarct border zone (Figure 1C–D). These observations confirm the pro-angiogenic properties of SDF. However, lineage tracing demonstrated that SDF did not alter the proportion of endothelial cells that were derived from the bone marrow (Figure 5B), indicating that SDF likely affects angiogenesis through sprouting of pre-existing endothelium rather than through bone marrow-mediated vasculogenesis.
Figure 5.
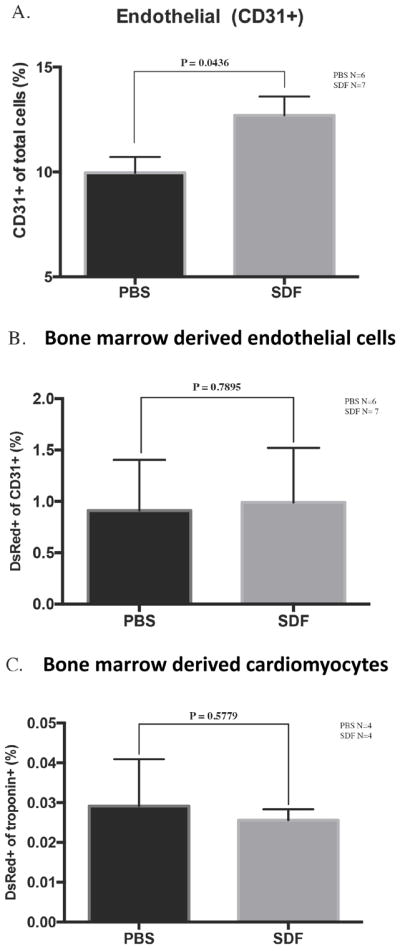
The pro-angiogenic effect of SDF is not due to an alteration in the direct contribution of circulating bone marrow cells. (A) The percent of CD31+ cells stratified by treatment assignment. (B) Comparison of the percent of CD31+ cells that are derived from bone marrow cells between SDF-treated and PBS-treated groups. (C) Comparison of the percent of troponin+ cells that are derived from bone marrow cells between SDF-treated and PBS-treated groups.
Functional benefits of SDF do not result from expansion of the bone marrow-derived cardiomyocyte population
After MI, SDF administration did not alter the proportion of bone marrow-derived (DsRed+) cardiomyocytes (Figure 5C).
DISCUSSION
The delivery of cytokines to augment endogenous repair processes are increasingly studied as potential therapeutics for ischemic heart disease. SDF, a molecule expressed in response to ischemia and with known pro-angiogenic properties, is a potent chemokine for endothelial progenitor cells. Similar to previous reports [21, 23, 25–27], exogenous administration of SDF significantly improved left ventricular function after MI, and increased vessel density in the infarct border zone.
By combining bone marrow transplantation and FACS we have evaluated the ability of bone marrow cells to reconstitute vascular endothelium and ventricular myocardium and examined the influence of exogenous SDF treatment post MI. Researchers may miss identifying rare contributions from the bone marrow if models with ≤50% chimerism, such as parabiosis, are utilized [7, 11]. Additionally, manual quantification of an event that may occur at a frequency as low as 0.001% is prone to error or miscalculation. FACS analysis allowed a new resolution of rare occurring events in the endogenous repair mechanisms post MI and with the treatment of SDF. We chose three cell subtypes to evaluate and were described by their phenotypic expression of CD31, CD45, and Troponin. These are markers of mature populations and do not delineate tissue resident or circulating stem cells such as the endothelial progenitor cell. In addition, there are a variety of cell subtypes in the bone marrow such as fibroblasts and mesenchymal cells that we haven’t evaluated in this model. However, we believe our data captured some of the crucial cellular components of SDF repair and laid the foundation for further FACS and analysis of mature and progenitor populations to elucidate mechanisms of bone marrow recruitment and repair.
In agreement with other groups [9, 11, 28], we observed that the clear majority of circulating cells that engraft into injured myocardium retain hematopoietic markers and phenotypes. This influx and engraftment of bone marrow-derived circulating cells into injured myocardium is transient, and is consistent with the post-infarction inflammatory response [9], However, our studies also confirmed the ability of bone marrow-derived circulating cells to adopt non-hematopoietic fates within mouse hearts subjected to a MI, albeit at low frequencies.
A small population of vascular endothelial cells originated from the bone marrow in the present investigation. This observation is in accord with prior evidence that bone marrow-derived cells form satellite reservoirs in the stroma of distant organs, or home to remote areas of injury to reconstitute vascular endothelium [11, 13, 29]. Myocardial injury stimulated the repopulation of endothelial cells by bone marrow-derived cells. After MI, bone marrow-derived endothelial cells primarily localized to the infarct border zone and area of injury. Additionally, lineage tracing demonstrated that a large proportion of bone marrow-derived endothelial cells arose from de novo formation. These findings indicate that postnatal neovascularization does not rely exclusively on sprouting from preexisting blood vessels [29]. Rather, circulating cells from the bone marrow also incorporate into, and thus contribute to, postnatal reparative vasculogenesis [11, 13, 29].
Treatment with SDF increased the total number of bone marrow-derived endothelial cells in the heart. However, it did not increase the percent of endothelial cells that were derived from the bone marrow. Thus, SDF recruits bone marrow derived cells to the heart after MI, but it does not increase the frequency at which these circulating bone marrow cells adopt non-hematopoietic fates. The observed increase in vessel density after treatment with SDF may be due to recruitment of progenitor cells from the bone marrow which promote endogenous growth of preexisting vessels via paracrine signaling rather than direct contribution to the endothelium. Alternatively, we may have not observed a significant population of bone marrow-derived endothelial cells if bone marrow transplant failed to engraft late outgrowth endothelial progenitor cells; in our study we only confirmed the presence of the early outgrowth population.
Unexpectedly, we observe a CD31+ endothelial population derived from fusion events of circulating bone marrow (DsRed+) and endogenous cardiac tissue (GFP+). Although the mechanism of endothelial fusion events is not fully described, it has been observed that dendritic cells as well as monocytes can fuse with endothelial cells [30, 31]. We believe our study could be an excellent lineage tracing model to further investigate this phenomenon in vivo.
We also observed that bone marrow-derived cells may also assume a cardiomyocyte fate within the heart, although to an even lesser extent than the aforementioned endothelial cell fate. Furthermore, the bone marrow-derived cardiomyocytes identified in tissue sections did not appear to have a mature sarcomeric structure. Interestingly, this rare event was not stimulated by myocardial injury; the same frequency of bone marrow-derived cardiomyocytes was observed in both infarcted and non-infarcted hearts. The rare bone marrow-derived cardiomyocytes were also observed as isolated cells, without the appearance of clonal expansion. Unlike reparative vasculogenesis, the endogenous contribution of the bone marrow to cardiomyocyte reconstitution may therefore be limited to cell maintenance rather than injury response [32–34].
The apparent plasticity of bone marrow cells to form cardiomyocytes was almost entirely attributable to cell fusion rather than transdifferentiation. Importantly, the experiments performed in the present investigation utilized whole bone marrow transplant rather than purified hematopoietic stem cell transplant to generate chimeric recipients. Therefore, we hypothesize it is not the hematopoietic stem cell and rather a different progenitor population within the heterogeneous bone marrow pool that is responsible for de novo generation of cardiomyocytes. In addition, the functionality and true sarcomeric structure of these rare occurring populations require further investigation. The limited contribution of the bone marrow to cardiomyocyte reconstitution underscores the inadequate improvements in ventricular function reported in clinical trials of intramyocardial or intracoronary bone marrow cell administration [14–19].
Treatment with exogenous SDF failed to increase the percentage of bone marrow derived cardiomyocytes. Because we used an acute MI model, it is possible that additional benefits of SDF in preserving ventricular function are due to its direct anti-apoptotic signaling [25]. In models of chronic ischemic cardiomyopathy, SDF improves vessel density and decreases scar size [35, 36]. Because SDF did not augment the formation of bone marrow-derived cardiomyocytes in acute ischemia, its mechanism of repair in chronic heart failure requires further study, but is unlikely mediated via stimulation of bone marrow-derived de novo vasculogenesis and cardiomyogenesis.
Here, we demonstrate a potent chemokine of progenitor cells within the bone marrow, SDF, improved myocardial function but did not alter the plasticity of circulating bone marrow cells. Rather, the recruited bone marrow cells likely attenuate myocardial injury via paracrine signaling [37]. Also, the direct anti-apoptopic effect of SDF is well described, and likely limits cardiomyocyte loss [25]. Identification of the signaling pathway that regulates bone marrow cell transdifferentiation and fusion, as well as the specific cell that undergoes such transformation, is essential to understand how bone marrow cells and therapeutic chemokines may be most efficiently utilized for myocardial repair.
Supplementary Material
Supplemental Figure 1. (A) Long-term hematopoietic stem cell and granulocyte chimerism after bone marrow transplant. (B) The percentage of hematopoietic cells within the myocardium significantly increases after myocardial infarction. (C) After myocardial infarction, there is a transient influx of hematopoietic cells that decreases over time.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the National Institutes of Health [R01 HL089315-01 to Y.J.W., S10OD010344-01A1 to Stanford Center for In Vivo Imaging]; and the American Heart Association [14POST20380744 to A.B.G.]
ABBREVIATIONS
- MI
myocardial infarction
- SDF
stromal cell-derived factor 1-alpha
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS:
HUMAN SUBJECTS/ANIMAL SUBJECTS STATEMENT:
No human studies were carried out by the authors for this article. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
CONFLICT OF INTEREST:
Andrew B. Goldstone, MD, PhD, Jeffery E. Cohen, MD, and Y. Joseph Woo, MD are coauthors on US patent application number 15/136,612. The remaining authors have no conflicts of interest.
References
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, … Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, … Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433(7026):647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, … Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(21):12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smart N, Bollini S, Dubé KN, Vieira JM, Zhou B, Davidson S, … Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474(7353):640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macarthur JW, Cohen JE, McGarvey JR, Shudo Y, Patel JB, Trubelja A, … Woo YJ. Preclinical evaluation of the engineered stem cell chemokine stromal cell-derived factor 1α analog in a translational ovine myocardial infarction model. Circulation Research. 2014;114(4):650–659. doi: 10.1161/CIRCRESAHA.114.302884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung ES, Miller L, Patel AN, Anderson RD, Mendelsohn FO, Traverse J, … Penn MS. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized Phase II trial. European Heart Journal. 2015;36(33):2228–2238. doi: 10.1093/eurheartj/ehv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 8.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, … Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 9.Nygren JM, Jovinge S, Breitbach M, Säwén P, Röll W, Hescheler J, … Jacobsen SEW. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nature Medicine. 2004;10(5):494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 10.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, … Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circulation Research. 2005;97(11):1090–1092. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 11.Wu JMF, Hsueh YC, Ch’ang HJ, Luo CY, Wu LW, Nakauchi H, Hsieh PCH. Circulating cells contribute to cardiomyocyte regeneration after injury. Circulation Research. 2015;116(4):633–641. doi: 10.1161/CIRCRESAHA.116.304564. [DOI] [PubMed] [Google Scholar]
- 12.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297(5590):2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 13.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, … Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. The Journal of Clinical Investigation. 2001;107(11):1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Ramshorst J, Bax JJ, Beeres SLMA, Dibbets-Schneider P, Roes SD, Stokkel MPM, … Atsma DE. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. The Journal of the American Medical Association. 2009;301(19):1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 15.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DXM … Cardiovascular Cell Therapy ResearchNetwork. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. The Journal of the American Medical Association. 2011;306(19):2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, … Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106(15):1913–1918. doi: 10.1161/01.CIR.0000034046.87607.1C. [DOI] [PubMed] [Google Scholar]
- 17.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H … REPAIR-AMI Investigators. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. The New England Journal of Medicine. 2006;355(12):1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 18.Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W … REGENT Investigators. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. European Heart Journal. 2009;30(11):1313–1321. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 19.Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, De Bondt P, … Wijns W. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112(9 Suppl):I178–83. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 20.Ascheim DD, Gelijns AC, Goldstein D, Moye LA, Smedira N, Lee S, … Woo YJ. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation. 2014;129(22):2287–2296. doi: 10.1161/CIRCULATIONAHA.113.007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vintersten K, Monetti C, Gertsenstein M, Zhang P, Laszlo L, Biechele S, Nagy A. Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004;40(4):241–246. doi: 10.1002/gene.20095. [DOI] [PubMed] [Google Scholar]
- 22.Hiesinger W, Brukman MJ, McCormick RC, Fitzpatrick JR, Frederick JR, Yang EC, … Woo YJ. Myocardial tissue elastic properties determined by atomic force microscopy after stromal cell-derived factor 1α angiogenic therapy for acute myocardial infarction in a murine model. The Journal of Thoracic and Cardiovascular Surgery. 2012;143(4):962–966. doi: 10.1016/j.jtcvs.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo YJ, Grand TJ, Berry MF, Atluri P, Moise MA, Hsu VM, … Sweeney HL. Stromal cell-derived factor and granulocyte-monocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. The Journal of Thoracic and Cardiovascular Surgery. 2005;130(2):321–329. doi: 10.1016/j.jtcvs.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 24.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318(5854):1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxena A, Fish JE, White MD, Yu S, Smyth JWP, Shaw RM, … Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117(17):2224–2231. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiesinger W, Perez-Aguilar JM, Atluri P, Marotta NA, Frederick JR, Fitzpatrick JR, … Woo YJ. Computational protein design to reengineer stromal cell-derived factor-1α generates an effective and translatable angiogenic polypeptide analog. Circulation. 2011;124(11 Suppl):S18–26. doi: 10.1161/CIRCULATIONAHA.110.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacArthur JW, Purcell BP, Shudo Y, Cohen JE, Fairman A, Trubelja A, … Woo YJ. Sustained release of engineered stromal cell-derived factor 1-α from injectable hydrogels effectively recruits endothelial progenitor cells and preserves ventricular function after myocardial infarction. Circulation. 2013;128(11 Suppl 1):S79–86. doi: 10.1161/CIRCULATIONAHA.112.000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, … Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 29.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, … Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation Research. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Mao Q, He J, Su J, Peng Y, Liang W, … Zhao Y. Fusions of Tumor-derived Endothelial Cells with Dendritic Cells Induces Antitumor Immunity. Scientific reports. 2017;7:46544. doi: 10.1038/srep46544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, … Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, … Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425(6961):968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 33.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, … Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, … Sadek HA. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature. 2015;523(7559):226–230. doi: 10.1038/nature14582. [DOI] [PubMed] [Google Scholar]
- 35.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, … Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. The Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 36.Sundararaman S, Miller TJ, Pastore JM, Kiedrowski M, Aras R, Penn MS. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Therapy. 2011;18(9):867–873. doi: 10.1038/gt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ascione R, Rowlinson J, Avolio E, Katare R, Meloni M, Spencer HL, … Madeddu P. Migration towards SDF-1 selects angiogenin-expressing bone marrow monocytes endowed with cardiac reparative activity in patients with previous myocardial infarction. Stem Cell Research & Therapy. 2015;6:53. doi: 10.1186/s13287-015-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. (A) Long-term hematopoietic stem cell and granulocyte chimerism after bone marrow transplant. (B) The percentage of hematopoietic cells within the myocardium significantly increases after myocardial infarction. (C) After myocardial infarction, there is a transient influx of hematopoietic cells that decreases over time.