Abstract
Summary
The study showed that in African American men with type 2 diabetes mellitus (T2D) vertebral volumetric bone mineral density (vBMD) predicts all-cause mortality, independent of other risk factors for death.
Introduction
Compared to European Americans, African Americans have lower rates of osteoporosis and higher rates of T2D. The relationships between BMD and fractures with mortality are unknown in this population. The aim of this study was to determine relationships between vertebral fractures and vertebral vBMD and mortality in African Americans with T2D.
Methods
Associations between vertebral fractures and vBMD with all-cause mortality were examined in 675 participants with T2D (391 women and 284 men) in the African American-Diabetes Heart Study (AA-DHS). Lumbar and thoracic vBMD were measured using quantitative computed tomography (QCT). Vertebral fractures were assessed on sagittal CT images. Associations of vertebral fractures and vBMD with all-cause mortality were determined in sex-stratified analyses and in the full sample. Covariates in a minimally-adjusted model included age, sex, BMI, smoking, and alcohol use; the full model was adjusted for those variables plus cardiovascular disease, hypertension, coronary artery calcified plaque, hormone replacement therapy (women), African ancestry proportion, and eGFR.
Results
After mean 7.6 ±1.8 year follow-up, 59 (15.1%) of women and 58 (20.4%) of men died. In men, vBMD was inversely associated with mortality in the fully-adjusted model: lumbar hazard ratio (HR) per standard deviation (SD) = 0.70 (95% CI 0.52–0.95, p=0.02); thoracic HR per SD = 0.71 (95% CI 0.54–0.92, p=0.01). Only trends toward association between vBMD and mortality were observed in the combined sample of men and women, as significant associations were absent in women. Vertebral fractures were not associated with mortality in either sex.
Conclusions
Lower vBMD was associated with increased all-cause mortality in African American men with T2D, independent of other risk factors for mortality including subclinical atherosclerosis.
Keywords: African American, gender, mortality, vertebral fracture, bone mineral density, quantitative computed tomography, type 2 diabetes
Introduction
Osteoporosis and type 2 diabetes mellitus (T2D) have high morbidity and mortality. These disorders are increasing in incidence and often coexist. Patients with T2D are at higher risk of suffering an osteoporotic fracture [1–2]. Secondary analysis of three prospective observational studies, including 1199 men and 770 women with T2D, reported that individuals with diabetes had a higher risk of hip and non-spine fractures compared to age- and bone mineral density (BMD)-matched controls [2]. Mortality rates following hip fracture are also higher in patients with T2D, compared to non-diabetics [3–5].
The relative contribution of BMD to increased mortality in patients with T2D is difficult to determine. Many confounding factors are present, including those relating to the measurement of areal BMD (aBMD), and the complex interactions that exist between bone, body weight, and metabolism in T2D. A cohort study including 222 men and 189 women with T2D reported an inverse association between lumbar spine and femoral neck aBMD measured by Dual X-ray Absorptiometry (DXA) and all-cause mortality, and a positive association of severe vertebral fractures and mortality [6]. These observations require confirmation in larger cohorts and using more specific measures including volumetric BMD (vBMD) measured with Quantitative Computed Tomography (QCT).
Compared to European Americans, African Americans have lower rates of osteoporosis and higher rates of T2D [7,8]. The relationships between BMD and fractures with mortality are unknown in this population. The present study assessed the relationships between thoracic and lumbar vBMD and all-cause mortality in African American men and women with T2D in the African American-Diabetes Heart Study (AA-DHS), an intensively phenotyped cohort for indices influencing bone and cardiovascular health [9].
Methods
Study Participants
The AA-DHS includes all African Americans with T2D recruited in two Wake Forest School of Medicine (WFSM) studies: the family-based Diabetes Heart Study (DHS) and unrelated individuals in the AA-DHS. DHS is a cross-sectional study of European American and African American sibling pairs concordant for T2D. AA-DHS was initiated after DHS and enrolled unrelated African Americans using identical inclusion criteria. T2D was diagnosed in all participants after the age of 30 years in the absence of diabetic ketoacidosis and defined as fasting blood glucose (FBG) ≥126 mg/dL or a random glucose ≥200 mg/dL, history of physician diagnosis of diabetes, or use of insulin or an oral hypoglycemic agent.
Sibling pairs with type 2 diabetes were recruited between April 1998 and August 2006 and unrelated African Americans with type 2 diabetes were recruited from May 2007 to August 2010. Participants in both samples met identical inclusion/exclusion criteria and were identified at internal medicine clinics at the WFSM or with community advertising [10]. No participants received SGLT2 inhibitors or a GLP-1 receptor agonist and 13.7% were taking a TZD. The study was approved by the WFSM Institutional Review Board and all participants provided written informed consent.
CT Assessments
CT scans of the chest and abdomen were obtained at the initial study visit on General Electric systems (Discovery CT750 HD and LightSpeed VCT; GE Healthcare, Waukesha, WI) using a protocol validated for volumetric measurement of BMD in the spine [11, 12]. Trabecular volumetric BMD (mg/cm3) was measured in the thoracic (T8–T11) and lumbar vertebrae (T12–L3) using QCT-5000 software (Image Analysis, Columbia, KY, USA) with an external calibration phantom. The vBMD measured by QCT is highly precise with coefficients of variation <1% [12].
Sagittal CT reconstructions were used for the detection and classification of vertebral fractures using Genant semi-quantitative method: Grade 1, 20–25% height loss, Grade 2, 25–40% height loss, grade 3 >40% height loss [13].
The amount of coronary artery calcified atherosclerotic plaque (CAC) was measured using a modified Agatston method with the traditiona130 Hounsfield Unit (HU) threshold and a minimal lesion definition of 0.52 mm2 [14].
Vital Status
Vital status was assessed through Dec 31, 2015 using the National Death Index. We observed 117 deaths; however, cause of death was reported for only 54 participants. Cause of death was classified based on the primary factor reported on death certificates; 16 (23.5%) of the 54 deaths were attributed to CVD, 27.9% to cancer, 7.4% to infection, 7.4% to type 2 diabetes and 33.8% to other causes. Since causes of death could not be adjudicated, all-cause mortality was selected as the primary outcome.
Statistical Analyses
Demographic and laboratory characteristics of AA-DHS participants were contrasted by sex using Wilcoxon two-sample tests for the continuous variables of vBMD and using Chi-square (where appropriate) and Fisher’s exact for categorical variables. Analyses were performed in the full sample and after stratification by sex. The primary outcome was time to death, determined by the interval between the date of study enrollment and death. Study participants who were known to be alive as of December 31, 2015 were censored.
Cox proportional hazard models were subsequently fitted. Covariates were selected to limit confounding effects and ensure that reported effects were not due to other measured variables not accounted for in the model. Association results are presented for a minimally-adjusted model accounting for age, sex, BMI, smoking, and alcohol consumption, as well as a fully-adjusted model with all covariates in the minimally-adjusted model plus hormone replacement therapy (women), hypertension, prior cardiovascular disease, CAC score, eGFR, and African ancestry proportion.
Participants on osteoporosis medications (n=7) and glucocorticoids (n=33) were excluded. There was no adjustment for diabetes medications because their use varied during the course of the study. There were no data collected on fragility fractures other than vertebral fractures.
Kaplan-Meier survival curves were constructed to examine patterns of mortality for increasing tertiles of vertebral vBMD.
Results
The AA-DHS cohort of 721 participants was followed for 7.6 ± 1.8 years. Table 1 presents demographic characteristics, vertebral fracture, and vertebral vBMD data. Compared to women, men had a significantly lower BMI. The higher BMI in women versus men mirrors observations in the general African American population and supports generalizability of our results in this cohort with type 2 diabetes [15]. Compared to women, men had a significantly higher proportion of smokers and higher CAC score denoting more severe subclinical cardiovascular disease. Although men had higher prevalence of vertebral fractures, there was no significant difference in vertebral vBMD in men and women.
Table 1.
Demographic Characteristics of the AA-DHS cohort
| Variable | All | vBMD<150.3 | 150.3<vBMD<191.8 | vBMD>191.8 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Q2 (Q1, Q3) | N | Q2 (Q1, Q3) | N | Q2 (Q1, Q3) | N | Q2 (Q1, Q3) | ||
| Age, years | 675 | 56.0 (50.0, 63.0) | 210 | 62.0 (57.0, 68.0) | 209 | 55.0 (50.0, 61.0) | 210 | 51.0 (45.0, 57.0) | 5.0×10−28 |
| Female, % | 675 | 57.9% | 210 | 59.0% | 209 | 53.6% | 210 | 57.6% | 0.51 |
| Age at diagnosis, years | 674 | 46.0 (39.0, 53.0) | 210 | 50.0 (43.0, 57.0) | 208 | 45.0 (40.0, 52.0) | 210 | 42.0 (36.0, 49.0) | 1.0 ×10−12 |
| African ancestry proportion, % | 672 | 77.4 (65.5, 86.5) | 210 | 73.9 (60.4, 83.9) | 207 | 79.0 (68.3, 87.2) | 209 | 79.9 (69.1, 87.8) | 2.0 ×10−4 |
| Death, % | 675 | 17.3% | 210 | 23.3% | 209 | 14.8% | 210 | 10.5% | 0.001 |
| Follow-up time, years | 674 | 7.1 (5.9, 8.2) | 210 | 7.0 (5.7, 8.2) | 209 | 6.9 (5.9, 7.9) | 209 | 7.1 (6.1, 8.0) | 0.81 |
| Prior CVD, % | 675 | 28.7% | 210 | 33.3% | 209 | 31.1% | 210 | 25.2% | 0.17 |
| Body mass index, kg/m2 | 674 | 33.4 (28.9, 38.8) | 210 | 31.3 (27.5, 36.6) | 209 | 34.0 (29.5, 39.2) | 209 | 34.5 (29.8, 40.3) | 4.0×10−5 |
| Current or former smoker | 672 | 59.5% | 210 | 67.1% | 208 | 65.4% | 209 | 48.8% | 1.0×10−4 |
| Systolic blood pressure, mmHg | 640 | 133.8 (122.0, 147.0) | 201 | 137.0 (124.0, 150.0) | 198 | 134.0 (121.1, 147.9) | 198 | 129.5 (119.0, 143.8) | 0.003 |
| Diastolic blood pressure, mmHg | 640 | 78.0 (70.0, 84.5) | 201 | 75.5 (68.0, 82.0) | 198 | 78.5 (70.0, 85.0) | 198 | 78.0 (71.0, 85.0) | 0.02 |
| Creatinine, mg/dl | 669 | 1.0 (0.8, 1.2) | 209 | 1.0 (0.8, 1.2) | 206 | 1.0 (0.8, 1.2) | 208 | 1.0 (0.8, 1.1) | 0.24 |
| CKD EPI eGFR, ml/min/1.73m2 | 669 | 85.0 (67.6, 103.0) | 209 | 80.4 (65.4, 95.6) | 206 | 85.0 (68.9, 105.1) | 208 | 90.1 (72.9, 111.5) | 1.0×10−4 |
| GFR < 60 ml/min/1.73m2, % | 669 | 15.1% | 209 | 18.2% | 206 | 15.5% | 208 | 9.6% | 0.04 |
| ACR, mg/g | 659 | 14.4 (5.0, 54.5) | 206 | 16.6 (5.7, 67.3) | 202 | 13.2 (5.2, 44.3) | 205 | 15.3 (5.0, 52.9) | 0.36 |
| ACR > 300 mg/g, % | 659 | 9.1% | 206 | 10.2% | 202 | 5.9% | 205 | 9.8% | 0.25 |
| CAC plaque, HU | 661 | 49.5 (2.0, 617.5) | 205 | 215.0 (9.0, 1551.5) | 206 | 39.5 (1.0, 497.4) | 209 | 18.0 (1.0, 138.5) | 2.0×10−8 |
| Hemoglobin A1c, % | 659 | 7.7 (6.7, 9.2) | 204 | 7.7 (6.7, 8.9) | 205 | 7.7 (6.7, 9.1) | 206 | 7.8 (6.7, 9.6) | 0.84 |
| C-reactive protein, mg/dl | 572 | 0.4 (0.2, 1.0) | 181 | 0.3 (0.2, 0.8) | 182 | 0.5 (0.2, 1.1) | 187 | 0.6 (0.2, 1.1) | 0.003 |
| Use of statins, % | 673 | 44.6% | 209 | 52.2% | 209 | 40.7% | 209 | 44.5% | 0.06 |
| High density lipoprotein cholesterol, mg/dl | 662 | 46.0 (39.0, 54.0) | 206 | 47.5 (40.0, 56.0) | 203 | 45.0 (37.5, 52.0) | 207 | 44.0 (38.5, 54.0) | 0.02 |
| Low density lipoprotein cholesterol, mg/dl | 648 | 106.0 (84.0, 131.2) | 204 | 97.5 (76.8, 123.0) | 197 | 110.0 (88.0, 135.0) | 201 | 108.0 (86.0, 132.0) | 0.009 |
| Triglycerides, mg/dl | 662 | 104.0 (78.0, 147.0) | 206 | 104.0 (76.0, 145.8) | 203 | 110.0 (80.5, 156.0) | 207 | 102.0 (79.5, 141.5) | 0.4 |
| Lumbar vBMD, mg/cm3 | 629 | 170.6 (140.3, 205.3) | 210 | 129.3 (113.2, 140.2) | 209 | 170.6 (159.6, 181.3) | 210 | 220.1 (205.3, 239.9) | NA |
| Thoracic vBMD, mg/cm3 | 666 | 194.2 (160.4, 226.3) | 210 | 149.9 (130.0, 166.1) | 209 | 194.7 (179.4, 207.7) | 208 | 245.3 (224.1, 273.3) | 1.0 ×10−122 |
| Vertebral fractures, % | 675 | 11.0% | 176 | 13.8% | 173 | 11.5% | 190 | 9.0% | 0.31 |
CVD=Cardiovascular Disease, CAC=Coronary Artery Calcification, HU=Hounsfield Units, vBMD=Volumetric BMD, N=sample size.
Over a mean 7.6 ± 1.8 years of follow-up, 59 (15.1%) of the women and 58 (20.4%) of the men had died. Relationships between vertebral fractures, vertebral vBMD, and all-cause mortality are shown in Table 2. Across the full cohort of men and women, vertebral fractures were not associated with mortality. In men alone, vertebral vBMD was inversely associated with all-cause mortality in the fully-adjusted model: lumbar vBMD HR per SD = 0.70 (95% CI 0.52–0.95, p=0.02); thoracic vBMD HR per SD = 0.71 (95% CI 0.54–0.92, p=0.01). In women, the association of vBMD with mortality was not significant: lumbar vBMD HR per SD = 1.1 (95% CI 0.7–1.72, p=0.68); thoracic vBMD HR per SD = 1.1 (95% CI 0.74–1.47, p=0.79).
Table 2.
Relationships between vertebral fracture, vertebral vBMD, and all-cause mortality
| CT Measure | Sample | Model | HR per SD | 95% CI | P-value |
|---|---|---|---|---|---|
| Vertebral Fracture (N=74) | Full | 1 | 0.95 | (0.51, 1.76) | 0.86 |
| 2 | 1.00 | (0.59, 1.69) | 0.99 | ||
| 3 | 0.95 | (0.56, 1.62) | 0.86 | ||
| Men | 1 | 1.13 | (0.55, 2.32) | 0.74 | |
| 2 | 1.11 | (0.61, 2.05) | 0.73 | ||
| 3 | 1.09 | (0.60, 1.98) | 0.78 | ||
| Women | 1 | 0.60 | (0.14, 2.52) | 0.49 | |
| 2 | 0.54 | (0.14, 2.07) | 0.37 | ||
| 3 | 0.56 | (0.14, 2.18) | 0.40 | ||
| Lumbar vBMD (mg/cm3) | Full | 1 | 0.70 | (0.53, 0.93) | 0.01 |
| 2 | 0.84 | (0.65, 1.09) | 0.19 | ||
| 3 | 0.82 | (0.63, 1.06) | 0.13 | ||
| Men | 1 | 0.61 | (0.40, 0.94) | 0.02 | |
| 2 | 0.72 | (0.53, 0.97) | 0.03 | ||
| 3 | 0.70 | (0.52, 0.95) | 0.02 | ||
| Women | 1 | 0.80 | (0.55, 1.15) | 0.23 | |
| 2 | 1.15 | (0.73, 1.81) | 0.54 | ||
| 3 | 1.10 | (0.70, 1.72) | 0.68 | ||
| Thoracic vBMD (mg/cm3) | Full | 1 | 0.75 | (0.58, 0.96) | 0.02 |
| 2 | 0.83 | (0.66, 1.06) | 0.13 | ||
| 3 | 0.84 | (0.66, 1.06) | 0.14 | ||
| Men | 1 | 0.66 | (0.46, 0.94) | 0.02 | |
| 2 | 0.72 | (0.55, 0.94) | 0.01 | ||
| 3 | 0.71 | (0.54, 0.92) | 0.01 | ||
| Women | 1 | 0.80 | (0.57, 1.13) | 0.21 | |
| 2 | 1.01 | (0.68, 1.49) | 0.97 | ||
| 3 | 1.05 | (0.74, 1.47) | 0.79 |
Model 1: Age, sex, BMI, smoking, and alcohol consumption
Model 2: Model 1 plus hypertension, prior cardiovascular disease, coronary artery calcified plaque score, hormone replacement therapy (women), and African ancestry proportion
Model 3: Model 2 plus eGFR
HR=Hazard Ratio; SD=Standard Deviation; CI=Confidence Interval
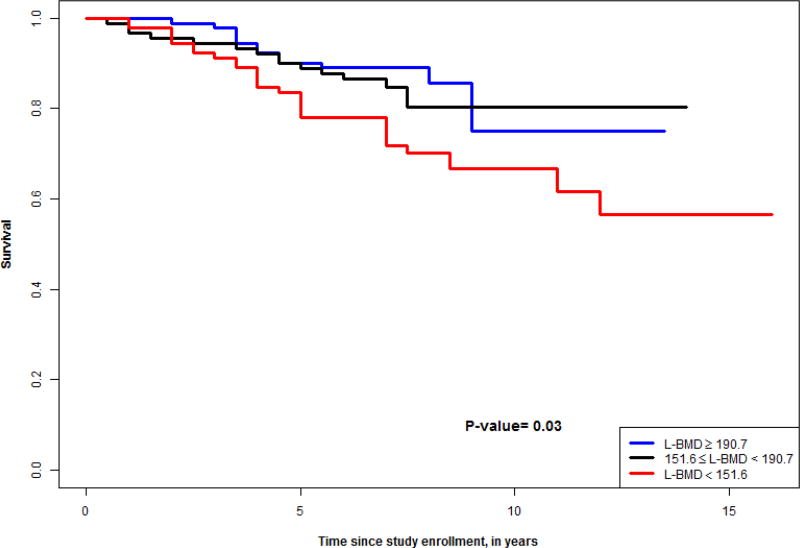
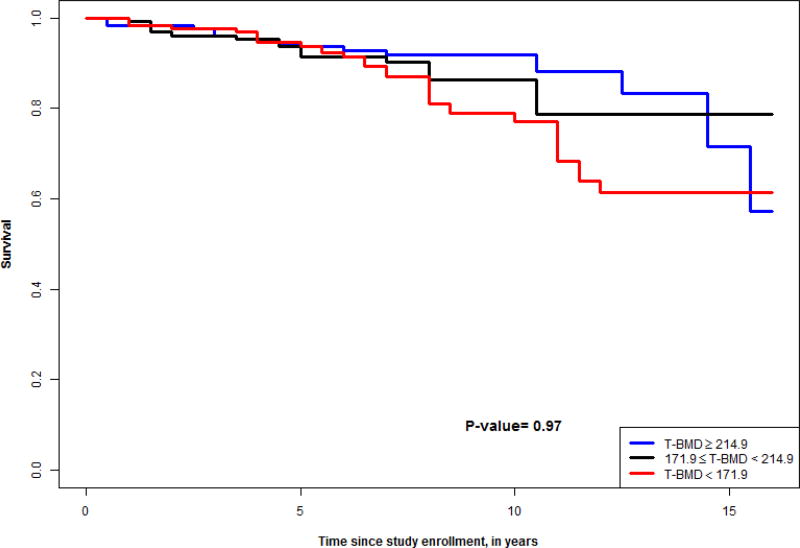
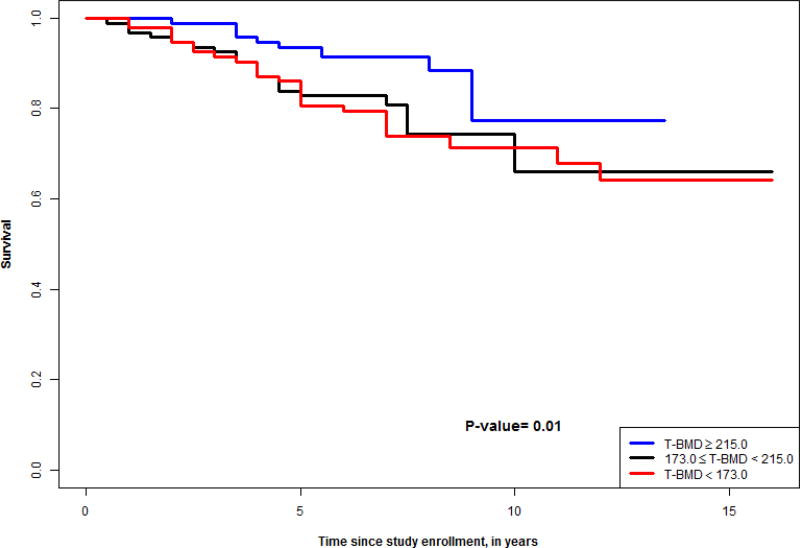
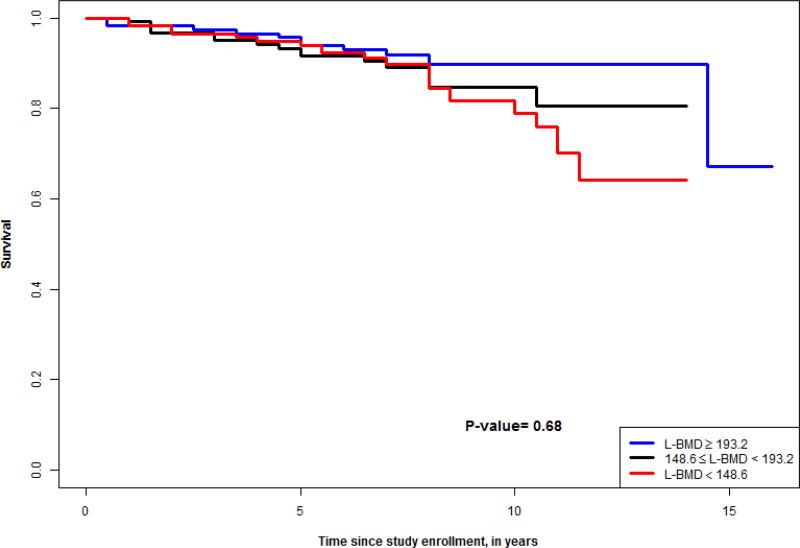
Figures 1–4 show survival curves based on tertiles of lumbar and thoracic vBMD in men and women. Men in the higher 2 tertiles of lumbar vBMD had lower mortality than men in the lowest tertile of lumbar vBMD. In women, trabecular vBMD of lumbar and thoracic vertebra were not predictive of mortality over the time course evaluated.
Figure 1.
Survival curve for tertiles of lumbar vBMD in men
Figure 4.
Survival curve for tertiles of thoracic vBMD in women
Discussion
The novel finding in the current study of African Americans with T2D is that lower lumbar and thoracic vBMD measured by QCT was associated with higher mortality over a 7 year period in men, but not women. Importantly, the association persisted even after adjustment for other determinants of mortality in men with T2D including, age, BMI, smoking, alcohol use, hypertension, cardiovascular disease, coronary artery calcification, and kidney function. The apparent sexual dimorphism in vBMD relationships with mortality could relate to differential contribution of covariates to causes of mortality, or to other physical, metabolic, or hormonal differences in the sexes.
In individuals without diabetes, low BMD is predictive of all-cause mortality [16–23]. A meta-analysis of 10 studies containing 46,182 participants and 3991 deaths, reported a 1.17-fold increase in mortality per standard deviation (SD) decrease in DXA-measured aBMD [23]. In a lung screening trial, Buckens et al. [24] reported an inverse relationship between CT-measured bone attenuation and mortality. However, the study did not use a calibration phantom and reported bone attenuation in Hounsfield Units (HU), rather than mg/cm3. Recently, we used phantom-based CT measurements to determine the association of vBMD with all-cause mortality in a study of predominantly European Americans with T2D [25]. Vertebral trabecular vBMD was not associated with mortality after adjusting for covariates including CAC, which has shown an age-independent inverse relationship with BMD [25].
Individuals with T2D have higher aBMD than non-diabetics [26]. However, the higher aBMD in patients with T2D does not result in lower fracture rates. In men with T2D, a meta-analysis of 16 studies reported a 2.6-fold increase hip fracture risk [2]. The BMD measurement method itself may be a confounder in these studies. Most studies of T2D use DXA, where vertebral aBMD measurements may be falsely elevated due to degenerative diseases, diffuse idiopathic skeletal hyperostosis (DISH), or aortic calcifications. Because individuals with T2D have an increased incidence of vascular calcifications and DISH, vertebral aBMD measurements are less accurate in this group. To minimize the effects of potential confounders, AA-DHS measured spinal trabecular vBMD, instead of aBMD.
This is the first study to evaluate the relationship between BMD and mortality in African Americans. African Americans have higher BMD than European Americans [27] and higher proportions of recent African ancestry (e.g., lower proportions of European ancestry) are also associated with higher BMD [28]. In this study of African Americans with T2D, vertebral vBMD was predictive of all-cause mortality and the association was stronger than what we previously reported in European Americans [25]. Importantly, in the current study, the association with mortality was robust to adjustment for African ancestry proportion.
The clinical utility of DXA testing in patients with T2D has been subject to controversy due to the increased likelihood of fracture despite similar or higher BMD. Bone fragility in T2D is likely due to declines in bone quality, which is not measured by DXA, so some caution in the use of aBMD measurement is warranted. Assessment of trabecular vBMD instead of aBMD measurements in patients with T2D avoids many of the confounders associated with aBMD. Focusing on all-cause mortality, the present data shows that vBMD is useful prognostic biomarker. If future studies confirm our findings, vBMD measurements may become more widely used in the clinical setting for determining prognosis and the need for pharmacologic intervention in patients with T2D.
This study has some limitations. Although we excluded participants on bisphosphonates and steroids, we did not account for the prevalent use of statins and anti-diabetes drugs in the cohort, since the use of these agents varied substantially during the course of the study. Prior studies in individuals with T2D have shown that more severe disease, evidenced by insulin use and higher HbA1c is associated with increased fracture risk. In addition, over the follow-up period, there was approximately 15% mortality in women and over 20% mortality in men. The relatively low number of deaths among women may have limited our ability to detect mortality relationships with vBMD, which may appear with time.
Our study also has several important strengths. Adjustment was performed for overall African ancestry proportion using genome-wide single nucleotide polymorphism (SNP) data on the Illumina 5 million SNP platform [29]. This measure is often absent from reports of bone health in African Americans. We also obtained QCT measurements of vBMD as well as CAC scores for subclinical cardiovascular disease. CAC is highly predictive of all-cause mortality in patients with T2D [30, 31]. We found that vBMD was predictive of subsequent mortality, even after adjusting for CAC, which has a strong, age-independent inversely relationship with BMD in African-American men and women [32].
The AA-DHS is the largest observational study of African Americans with T2D with vBMD phenotyping and genotyping to confirm recent African ancestry. These analyses evaluated the understudied African American population and showed that in African American men vertebral vBMD predicts all-cause mortality, independent of other risk factors for death.
Figure 2.
Survival curve for tertiles of thoracic vBMD in men
Figure 3.
Survival curve for tertiles of lumbar vBMD in women
Acknowledgments
This study was supported by NIH Grants: 2R01 DK071891 (BIF), and HL67348 (DWB).
Footnotes
Conflicts of Interest
No author declares a conflict of interest related to this work.
References
- 1.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz AV, Vittinghoff E, Bauer DC, et al. Study of Osteoporotic Fractures (SOF) Research Group. Osteoporotic Fractures in Men (MrOS) Research Group. Health, Aging, and Body Composition (Health ABC) Research Group. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305(21):2184–92. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey A, Aharonoff GB, Zuckerman JD, Koval KJ. The effects of diabetes on outcome after hip fracture. Bull Hosp Jt Dis. 2000;59(2):94–98. [PubMed] [Google Scholar]
- 4.Huang YF, Shyu YI, Liang J, Chen MC, Cheng HS, Wu CC. Diabetes and health outcomes among older Taiwanese with hip fracture. Rejuvenation Res. 2012;15(5):476–482. doi: 10.1089/rej.2011.1308. [DOI] [PubMed] [Google Scholar]
- 5.Muraki S, Yamamoto S, Ishibashi H, Nakamura K. Factors associated with mortality following hip fracture in Japan. J Bone Miner Metab. 2006;24(2):100–104. doi: 10.1007/s00774-005-0654-z. [DOI] [PubMed] [Google Scholar]
- 6.Miyake H, Kanazawa I, Sugimoto T. Association of Bone Mineral Density, Bone Turnover Markers, and Vertebral Fractures with All-Cause Mortality in Type 2 Diabetes Mellitus. Calcif Tissue Int. 2018;102(1):1–13. doi: 10.1007/s00223-017-0324-x. [DOI] [PubMed] [Google Scholar]
- 7.Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891–9. doi: 10.1007/s11999-011-1863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 9.Freedman BI, Divers J, Russell GB, Palmer ND, Wagenknecht LE, Smith SC, Xu J, Carr JJ, Bowden DW, Register TC. Vitamin D Associations With Renal, Bone, and Cardiovascular Phenotypes: African American-Diabetes Heart Study. J Clin Endocrinol Metab. 2015;100(10):3693–3701. doi: 10.1210/jc.2015-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman BI, Langefeld CD, Lu L, et al. APOL1 associations with nephropathy, atherosclerosis, and all-cause mortality in African Americans with type 2 diabetes. Kidney Int. 2015;87(1):176–81. doi: 10.1038/ki.2014.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Register TC, Lenchik L, Hsu FC, Lohman KK, Freedman BI, Bowden DW, Carr JJ. Type 2 diabetes is not independently associated with spinal trabecular volumetric bone mineral density measured by QCT in the Diabetes Heart Study. Bone. 2006;39(3):628–633. doi: 10.1016/j.bone.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Lenchik L, Shi R, Register TC, Beck SR, Langefeld CD, Carr JJ. Measurement of trabecular bone mineral density in the thoracic spine using cardiac gated quantitative computed tomography. J Comput Assist Tomogr. 2004;28(1):134–9. doi: 10.1097/00004728-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal S, Cox AJ, Herrington DM, et al. Coronary calcium score predicts cardiovascular mortality in diabetes: diabetes heart study. Diabetes Care. 2013;36(4):972–7. doi: 10.2337/dc12-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishna A, Razak F, Lebel A, et al. Trends in group inequalities and interindividual inequalities in BMI in the United States, 1993–2012. Am J Clin Nutr. 2015;101(3):598–605. doi: 10.3945/ajcn.114.100073. [DOI] [PubMed] [Google Scholar]
- 16.Mussolino ME, Gillum RF. Low bone mineral density and mortality in men and women: the Third National Health and Nutrition Examination Survey linked mortality file. Ann Epidemiol. 2008;18(11):847–50. doi: 10.1016/j.annepidem.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bliuc D, Alarkawi D, Nguyen TV, et al. Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 2015;30(4):637–46. doi: 10.1002/jbmr.2393. [DOI] [PubMed] [Google Scholar]
- 18.Campos-Obando N, Castano-Betancourt MC, Oei L, et al. Bone mineral density and chronic lung disease mortality: the Rotterdam study. J Clin Endocrinol Metab. 2014;99(5):1834–42. doi: 10.1210/jc.2013-3819. [DOI] [PubMed] [Google Scholar]
- 19.Johansson H, Odén A, Kanis J, McCloskey E, Lorentzon M, Ljunggren Ö, Karlsson MK, Orwoll E, Tivesten Å, Ohlsson C, Mellström D. Low bone mineral density is associated with increased mortality in elderly men: MrOS Sweden. Osteoporos Int. 2011;22(5):1411–8. doi: 10.1007/s00198-010-1331-1. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki T, Yoshida H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporos Int. 2010;21(1):71–9. doi: 10.1007/s00198-009-0970-6. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Klift M, Pols HA, Geleijnse JM, et al. Bone mineral density and mortality in elderly men and women: the Rotterdam Study. Bone. 2002;30(4):643–8. doi: 10.1016/s8756-3282(02)00670-1. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi DP, Khaw KT. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos Int. 2001;12(4):259–65. doi: 10.1007/s001980170114. [DOI] [PubMed] [Google Scholar]
- 23.Qu X, Huang X, Jin F, et al. Bone mineral density and all-cause, cardiovascular and stroke mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;166(2):385–93. doi: 10.1016/j.ijcard.2011.10.114. [DOI] [PubMed] [Google Scholar]
- 24.Buckens CF, van der Graaf Y, Verkooijen HM, et al. Osteoporosis markers on low-dose lung cancer screening chest computed tomography scans predict all-cause mortality. Eur Radiol. 2015;25(1):132–9. doi: 10.1007/s00330-014-3361-0. [DOI] [PubMed] [Google Scholar]
- 25.Lenchik L, Register TC, Hsu FC, Xu J, Smith SC, Carr JJ, Freedman BI, Bowden DW. Bone Mineral Density of the Radius Predicts All-Cause Mortality in Patients With Type 2 Diabetes: Diabetes Heart Study. J Clin Densitom. 2017 Dec 1; doi: 10.1016/j.jocd.2017.11.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–32. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz AV, Sellmeyer DE, Strotmeyer ES, et al. Diabetes and bone loss at the hip in older black and white adults. J Bone Miner Res. 2005;20(4):596–603. doi: 10.1359/JBMR.041219. [DOI] [PubMed] [Google Scholar]
- 28.Ochs-Balcom HM, Preus L, Wactawski-Wende J, et al. Association of DXA-derived bone mineral density and fat mass with African ancestry. J Clin Endocrinol Metab. 2013;98(4):E713–717. doi: 10.1210/jc.2012-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Divers J, Palmer ND, Langefeld CD, et al. Genome-wide association study of coronary artery calcified atherosclerotic plaque in African Americans with type 2 diabetes. BMC Genet. 2017;18(1):105. doi: 10.1186/s12863-017-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raffield LM, Hsu FC, Cox AJ, et al. Predictors of all-cause and cardiovascular disease mortality in type 2 diabetes: Diabetes Heart Study. Diabetol Metab Syndr. 2015;7:58. doi: 10.1186/s13098-015-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox AJ, Hsu FC, Agarwal S, et al. Prediction of mortality using a multi-bed vascular calcification score in the Diabetes Heart Study. Cardiovasc Diabetol. 2014;13:160. doi: 10.1186/s12933-014-0160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Divers J, Register TC, Langefeld CD, et al. Relationships between calcified atherosclerotic plaque and bone mineral density in African Americans with type 2 diabetes. J Bone Min Res. 2011;26:1554–60. doi: 10.1002/jbmr.389. [DOI] [PMC free article] [PubMed] [Google Scholar]