Abstract
Urinary bladder cancer (UBC) has a high incidence rates in many southern and eastern European countries, in parts of Africa and the Middle East, and in North America. It exhibits a wide variety of histological types that goes from less aggressive to rapid-growing ones. In order to compare the different presentations, etiologies, and prognoses among racial groups, including NHW (non-Hispanic white), HW (Hispanic white), blacks, and API (Asian and Pacific Islander), we analyzed the UBC patients diagnosed between 1973 and 2014 using SEER (Surveillance, Epidemiology, and End Results) database. Patient characteristics, age-adjusted incidence rates, and survival were compared across races. There are significant racial differences in patients’ characteristics, including gender, marital status, age at diagnosis, treatment strategies, grade, stage, survival time, and so on. Overall, non-Hispanic whites have the highest incidence rate, followed by blacks, Hispanic whites, and APIs. In the analysis of survival, significant racial differences exist when stratified by gender, age group, histological type, stage, location and treatment strategies. Racial differences exist among UBC patients in the United States in terms of characteristics, incidence, and survival. Future studies may collect and analyze more data for comprehensive description and interpretation of the racial differences.
Introduction
Study on racial differences among cancer patients have attracted extensive attention1–3. It can assist diagnosis, implementation of tailored treatment strategies, and elimination of racial disparity. However, the related research on urinary bladder cancer (UBC) remains limited. Urinary bladder cancer is a type of cancer that arises from the epithelial lining of the urinary bladder. UBC has the fifth highest incidence among all cancers in the United States; an estimated 76,960 new cases of UBC and 16,390 deaths from UBC occurred in 20164. Incidence rates of UBC are high in many southern and eastern European countries, in parts of Africa and the Middle East, and in North America5. UBC has many histological types. Transitional Cell Carcinoma (TCC) is the most common histological type of primary UBC, which comprises more than 90% of all bladder tumors6. Except for TCC, this article also considers two other histological types which are squamous cell carcinoma (SCC) and Carcinoma. TCC, the most common bladder cancer, arises from the transitional epithelium and the pattern of growth of TCC can be papillary, sessile, or carcinoma-in-situ (CIS)7. So, TCC can be divided into two types: PTCC (Papillary Transitional Cell Carcinoma) and NPTCC (Non-Papillary Transitional Cell Carcinoma). In the type of TCC, PTCC accounts for a large proportion, and it’s nearly 70%. PTCC are often multifocal and only occasionally progress8. Compared with NPTCC, PTCC has a higher differentiated degree, so its malignant degree is lower and the survival rate of patients is usually higher. SCC (Squamous Cell Carcinoma) is an epithelial malignant tumor originating from the bladder mucosa8. The incidence rate of bladder squamous cell carcinoma in bladder malignancies worldwide varies widely. It is usually associated with the chronic urinary infection caused by schistosoma haematobium (also known as schistosomiasis or bilharzia). In regions where this infection is endemic, like Egypt, SCC was for years the dominant histological type of UBC9. Carcinoma Malignant epithelial tumor is another type of malignant tumor derived from bladder epithelial tissue. It is a rare histological type too and with a very scarce literature to pay attention to it. Urinary bladder cancer is the second most common genitourinary malignant disease in the United States and the 13th most common cause of death worldwide. In western countries, the incidence rate of UBC continues to rise in recent years5. There are a lot of risk factors for UBC. Smoking is recognized as the most important risk factor for UBC and is estimated to account for 50% of tumors. Occupational exposure to carcinogens— namely, aromatic amines (benzidine, 4-aminobiphenyl, 2-naphthylamine, 4-chloro-o-toluidine), polycyclic aromatic hydrocarbons, and chlorinated hydrocarbons—is viewed as the second most important risk factor10. Some other risk factors just like coffee drinking, artificial sweeteners, and nutrition intake were discussed by some other researchers11,12]. Treatment options for bladder cancer depend on a number of factors, including the type of cancer, the grade, and the stage and so on. The most commonly used treatment strategy is transurethral resection of the bladder tumor. Other treatments include the cystectomy, the intravesical chemotherapy, the systemic chemotherapy, the radiation therapy, and the immunotherapy13. Cystectomy has the best treatment effect. It can prevent recurrence to a large extent. Transurethral resection of the bladder tumor followed by combined chemotherapy and radiation therapy also can achieve as effective as cystectomy14. Although the five-year survival rate of UBC is good (62–88%), the recurrence rate is also high15. There are a lot of prognostic factors for recurrence of UBC, including the number of tumors present at diagnosis, the recurrence rate in the previous period, the size of tumor, and the grade of tumor16,17.
This study aims to comprehensively describe the racial differences among UBC patients in the United States in terms of patients’ characteristics, clinical-pathologic features, incidence rates, and survival rates by analyzing the SEER database. Recently, researches on racial differences among cancer patients have attracted extensive attention1–3, but the discussion on UBC remains scarce. The available UBC studies may have limitations by either the numbered racial groups or the specified outcomes. For instance, Scosyrev and others18 only compared blacks and whites. They found that African American patients present with less favorable tumor characteristics compared with white patients, accounting for a much higher UBC mortality experienced by these groups. Burger and others10 (2013) mainly studied on risk factors of urothelial bladder cancer. Although the racial difference of UBC are mentioned in his article, the discussion only focuses on survival outcome as well as the discussion about racial difference of UBC is not deep enough. Yee and others19 also just focus on survival outcome for different racial groups in UBC. They found that ethnic disparities in bladder cancer survival persist between whites and blacks, whereas survival in other ethnic minority groups appears similar to that of whites. However, our study on UBC are more comprehensive than previous study. We compare a wider spectrum of racial groups, including NHW (non-Hispanic white), HW (Hispanic white), blacks, and API (Asian and Pacific Islander), and analyze multiple aspects, including patients’ characteristics, clinical-pathological features, incidence rate, and survival rate.
Methods
Source population
The Surveillance, Epidemiology, and End Results (SEER, http://seer.cancer.gov) database of the National Cancer Institute is utilized to provide population-based sample data in this study. This program collects information on cancer statistics in an effort to reduce the cancer burden among the U.S. population. Approximately 9.5%, 14%, and 28% of the U.S. population are analyzed by covering registry 9, 13, and 18 respectively, and the International Classification of Disease for Oncology (ICD-O-3) site code C670- C679 are referred to identify UBC cases in those population. Tumor sites and histological types are coded based on WHO specified criteria in ICD-O-3. And histological types are also grouped by ICD-O-3. Patients with PTCC, NPTCC, Carcinoma, and SCC are identified by the histology codes 8130–8131, 8120–8124, 8010–8015, and 8070–8078, respectively. The division of treatment variable is classified comprehensively in this study. There are three main treatment variables in the SEER database, including surgery, radiotherapy, and chemotherapy. We consider a variety of combinations of these three methods including surgery, chemotherapy, radiation, SR (surgery and radiation), SC (surgery and chemotherapy), RC (radiation and chemotherapy), SRC (surgery, radiation and chemotherapy) and no treatment.
Different SEER registry groupings are used to maximize the sample size. In the analysis of patients’ characteristics and clinical-pathological features, SEER 9 data is applied and the following information is included: cancers diagnosed between 1973 and 2014, gender, marital status, age at diagnosis, age group (0–39 years, 40–64 years, 65–84 years, 85+ years), survival time, histological types, grade, stage (II–IV), treatment, tumor size, lymph node site (nodal, extra-nodal), and location. SEER 13 data containing detailed race and incidence information for cancers diagnosed between 1992 and 2014, is used in the analysis of incidence rate. Cancer diagnosed between 1973 and 2008 with follow-ups up to the end of 2013 from SEER 18 data is analyzed in the survival analysis.
Statistical analysis
This study uses Chi-square tests and ANOVA analysis in order to compare the patients’ characteristics and clinical pathologic features across racial groups by SAS version 9.4. The age-standardized incidence rates and five-year relative survival rates are calculated by SEER* Stat software using US 2000 Census data. Multivariate Cox regressions are also performed when adjusting for gender, age, histological type, stage, location and treatment strategy.
Data availability
The data is available on the Surveillance, Epidemiology, and End Results (SEER, http://seer.cancer.gov) database.
Result
Patients’ characteristics and clinical-pathological features
Results are presented in Table 1. There are overall more male patients. The gender distributions are different across races (p-value<0.001), with NHWs having the most male patients (75.5%) and blacks having the least (66.5%). Most UBC patients are married (65.0%). Different racial groups have significantly different marital status (p-value<0.001). Among API patients, 70.2% are married. In contrast, only 45.8% of blacks are married. The age at diagnosis are also significantly different across races (p-value <0.001). Non-Hispanic whites have the highest age at diagnosis (70.9 years) and Hispanic whites have the lowest age at diagnosis (66.6 years).The median survival time for all patients is 50.0 months. Non-Hispanic whites have the longest survival time with a median of 51 months, and blacks have the shortest survival time with a median of 33 months. The distribution of histological types has racial differences (p-value <0.001) too. Non-Hispanic whites have most PTCC (66.3%), whereas blacks have most NPTCC (27.6%), CARCINOMA (1.6%), and SCC (4.1%). The tumor grade is different across different races (p-value<0.001). APIs (27.9%) have more grade IV patients, blacks (31.2%) have more grade III patients, whereas NHWs (34.5%) and HWs (33.3%) have more grade II patients. Most UBCs are diagnosed at an early stage. In our analysis, 76.4% tumors are stage II. Non-Hispanic whites have most tumors at stage II (77.2%), blacks have most stage III (26.6%) and stage IV (7.8%). For all patients, the average tumor size is 41.1mm. Blacks have the largest tumor size (46.3mm) while APIs have the smallest tumor size (38.5mm). NHWs (92.5%) have the highest rates of all nodes negative while blacks have the lowest (90.1%). This racial difference is significant. Further, there is a significant racial difference in tumor location distribution (p-value <0.001). The most common tumor location is the lateral wall of bladder. Non-Hispanic whites have the highest rates of lateral wall of bladder, trigone of bladder, ureteric orifice (20.0%, 6.0%, 4.8%, respectively), blacks have the highest rates of overlapping lesion of bladder, dome of bladder, anterior wall of bladder (12.0%, 4.7%, 2.3%, respectively) and APIs have the highest rates of posterior wall of bladder, bladder neck, urachus (9.4%, 3.8%, 0.4%, respectively). Different races also have different treatment strategies (p-value <0.001). For all patients, the surgery and SC account for more than 80%. Non-Hispanic whites have the highest rates of surgery (77.6%), APIs have the highest rates of surgery and chemotherapy (14.1%), whereas blacks have the highest rates of no treatment (9.7%), SR (4.6%), SRC (3.1%), chemotherapy (1.0%), radiation (1.0%) and RC (0.3%), respectively.
Table 1.
UBC patients’ characteristics and clinical-pathologic features for the whole cohort and different racial groups.
| Total (n = 377400) | NHW (n = 325612) | HW (n = 17613) | Black (n = 19646) | API (n = 14147) | P | |
|---|---|---|---|---|---|---|
| Gender | <0.001 | |||||
| Male | 282714 (74.9) | 245806 (75.5) | 12953 (73.5) | 13073 (66.5) | 10586 (74.8) | |
| Female | 94686 (25.1) | 79806 (24.5) | 4660 (26.5) | 6573 (33.5) | 3561 (25.2) | |
| Marital Status | <0.001 | |||||
| Single | 34171 (9.6) | 26861 (8.7) | 2237 (13.4) | 3759 (20.4) | 1258 (9.4) | |
| Married | 231638 (65.0) | 203205 (66.1) | 10354 (62.1) | 8452 (45.8) | 9409 (70.2) | |
| Separated/d/w | 90451 (25.4) | 77329 (25.2) | 4078 (24.5) | 6251 (33.9) | 2736 (20.4) | |
| Age group | ||||||
| 0–39 | 5124 (1.4) | 3970 (1.2) | 521 (3.0) | 364 (1.9) | 246 (1.7) | <0.001 |
| 40–64 | 103273 (27.4) | 87161 (26.8) | 5533 (31.4) | 6550 (33.3) | 3883 (27.5) | |
| 65–84 | 223595 (59.3) | 194596 (59.8) | 9714 (55.2) | 10850 (55.2) | 8248 (58.3) | |
| 85+ | 45398 (12.0) | 39881 (12.3) | 1844 (9.6) | 1880 (9.6) | 1767 (12.5) | |
| Age at diagnosis | 70.7 ± 14.9 | 70.9 ± 12.4 | 66.6±13.7 | 68.6 ± 12.9 | 70.7 ± 18.6 | <0.001 |
| Survival time (month) | 50.0 ± 93.0 | 51.0 ± 95.0 | 43.0 ± 85.0 | 33.0 ± 77.0 | 48.0 ± 94.0 | <0.001 |
| Histological types | <0.001 | |||||
| PTCC | 246724 (65.4) | 215800 (66.3) | 11130 (63.2) | 10786 (54.9) | 8721 (61.7) | |
| NPTCC | 102611 (27.2) | 87054 (26.7) | 4862 (27.6) | 6362 (32.4) | 4271 (30.2) | |
| CARCINOMA | 6364 (1.7) | 5303 (1.6) | 311 (1.8) | 476 (2.4) | 270 (1.9) | |
| SCC | 5194 (1.4) | 4091 (1.3) | 303 (1.7) | 630 (3.2) | 166 (1.2) | |
| Others | 16507 (4.4) | 13364 (4.1) | 1007 (5.7) | 1392 (7.1) | 719 (5.1) | |
| Grade | <0.001 | |||||
| Grade I | 53673 (16.9) | 47393 (17.3) | 2344 (16.1) | 2344 (14.7) | 1541 (13.1) | |
| Grade II | 107825 (34.0) | 94567 (34.5) | 4851 (33.3) | 4537 (28.4) | 3769 (31.9) | |
| Grade III | 84665 (26.7) | 72609 (26.5) | 3806 (26.1) | 4975 (31.2) | 3206 (27.1) | |
| Grade IV | 70885 (22.4) | 59857 (21.8) | 3575 (24.5) | 4101 (25.7) | 3295 (27.9) | |
| Stage | <0.001 | |||||
| stage II | 275889 (76.4) | 241037 (77.2) | 12403 (74.0) | 12073 (65.6) | 10075 (74.6) | |
| stage III | 70845 (19.6) | 59712 (19.1) | 3414 (20.4) | 4885 (26.6) | 2791 (20.7) | |
| stage IV | 14422 (4.0) | 11401 (3.7) | 936 (5.6) | 1430 (7.8) | 645 (4.8) | |
| Tumor size (mm) | 41.1 ± 49.2 | 40.9 ± 47.2 | 41.3 ± 61.1 | 46.3 ± 58.9 | 38.5 ± 58.2 | <0.001 |
| Regional nodes | <0.001 | |||||
| All nodes negative | 54839 (92.2) | 47552 (92.5) | 2518 (90.7) | 2763 (90.1) | 1978 (90.7) | |
| ≥1 nodes positive | 4642 (7.8) | 3873 (7.5) | 259 (9.3) | 305 (10.0) | 204 (9.4) | |
| Location | <0.001 | |||||
| NOS | 156540 (41.5) | 134286 (41.2) | 7325 (41.6) | 8882 (45.2) | 5861 (41.4) | |
| Lateral wall of bladder | 74042 (19.6) | 65004 (20.0) | 3266 (18.5) | 3061 (15.6) | 2640 (18.7) | |
| Overlapping lesion of bladder | 41538 (11.0) | 35805 (11.0) | 1936 (11.0) | 2361 (12.0) | 1409 (10.0) | |
| Posterior wall of bladder | 32095 (8.5) | 27599 (8.5) | 1632 (9.2) | 1515 (7.7) | 1328 (9.4) | |
| Trigone of bladder | 22677 (6.0) | 19673 (6.0) | 1059 (6.0) | 1100 (5.6) | 830 (5.9) | |
| Ureteric orifice | 17362 (4.6) | 15538 (4.8) | 685 (3.9) | 560 (2.8) | 555 (3.9) | |
| Dome of bladder | 13755 (3.6) | 11483 (3.5) | 720 (4.1) | 930 (4.7) | 611 (4.3) | |
| Bladder neck | 11734 (3.1) | 9878 (3.0) | 573 (3.3) | 729 (3.7) | 543 (3.8) | |
| Anterior wall of bladder | 7116 (1.9) | 5978 (1.8) | 366 (2.1) | 453 (2.3) | 313 (2.2) | |
| Urachus | 541 (0.1) | 368 (0.1) | 60 (0.3) | 55 (0.3) | 57 (0.4) | |
| Treatment | <0.001 | |||||
| Surgery | 285607 (76.9) | 248900 (77.6) | 13244 (76.1) | 13248 (69.0) | 10215 (73.2) | |
| SC | 41615 (11.2) | 35417 (11.0) | 1929 (11.0) | 2308 (12.0) | 1961 (14.1) | |
| No treatment | 20298 (5.5) | 16480 (5.1) | 1241 (7.1) | 1741 (9.7) | 836 (6.0) | |
| SR | 11555 (3.1) | 9822 (3.1) | 405 (2.3) | 883 (4.6) | 445 (3.2) | |
| SRC | 7905 (2.1) | 6651 (2.1) | 349 (2.0) | 589 (3.1) | 316 (2.3) | |
| Chemotherapy | 2017 (0.5) | 1619 (0.5) | 137 (0.8) | 186 (1.0) | 75 (0.5) | |
| Radiation | 1706 (0.5) | 1380 (0.4) | 65 (0.4) | 183 (1.0) | 78 (0.6) | |
| RC | 562 (0.2) | 428 (0.1) | 34 (0.2) | 66 (0.3) | 34 (0.2) |
Cancers diagnosed 1973–2014 in the SEER 9 database. For a survival time, median ± interquartile range, for age at diagnosis and tumor size, mean ± standard deviation and for a categorical variable, count (percentage).
Incidence rates
The overall age-adjusted incidence rate is 19.53 per 100,000 person-years (Table 2). Incidence rates increase with age. The four age groups have incidence rates 0.43, 15.36, 110.28, 154.23, respectively. Overall, non-Hispanic whites have the highest incidence rate, and the incidence rate is far greater than those of the other races. For the four age groups, non-Hispanic whites have the highest incidence rate, followed by blacks. APIs have the lowest rate. Overall, male have much higher incidence rate (34.46) than female (8.62). For both male and female, NHWs have the highest incidence rate, followed by blacks and then HWs. For the four major histological types, PTCC has the highest incidence rate, followed by NPTCC and then carcinoma. For PTCC and NPTCC, NHWs have the highest incidence rate (16.14 and 5.59, respectively), followed by blacks. While for Carcinoma, non-Hispanic whites and blacks have the same highest incidence rate (0.32). For SCC, blacks have the highest incidence rate (0.33), followed by NHWs (0.25). For all the four major histological types, APIs have the lowest incidence rate.
Table 2.
Age-adjusted UBC incidence rates per 100,000 person-years for the whole cohort and different racial groups, stratified by gender, age and histological type.
| Total | NHW | HW | Black | API | |
|---|---|---|---|---|---|
| All ages | 19.53 (19.44–19.62) | 23.09 (22.97–23.21) | 11.10 (10.86–11.34) | 12.60 (12.33–12.87) | 9.51 (9.31–9.71) |
| 0–39 | 0.43 (0.41–0.44) | 0.57 (0.55–0.60) | 0.25 (0.22–0.28) | 0.27 (0.23–0.31) | 0.18 (0.15–0.22) |
| 40–64 | 15.36 (15.22–15.50) | 19.11 (18.92–19.31) | 7.87 (7.59–8.17) | 10.22 (9.85–10.59) | 6.48 (6.21–6.75) |
| 65–84 | 110.28 (109.60–110.97) | 129.22 (128.34–130.10) | 62.72 (60.97–64.52) | 69.70 (67.74–71.70) | 53.64 (52.19–55.12) |
| ≥85 | 154.23 (152.15–156.34) | 169.34 (166.86–171.85) | 103.75 (97.18–110.66) | 104.18 (97.49–111.20) | 95.93 (90.51–101.59) |
| Gender | |||||
| Male | 34.46 (34.26–34.65) | 40.58 (40.33–40.83) | 19.41 (18.90–19.92) | 20.78 (20.22–21.35) | 16.57 (16.16–16.98) |
| Female | 8.62 (8.53–8.70) | 10.00 (9.90–10.11) | 5.25 (5.03–5.46) | 7.25 (6.99–7.52) | 4.24 (4.06–4.42) |
| Histological types | |||||
| PTCC | 13.37 (13.29–13.45) | 16.14 (16.04–16.24) | 7.08 (6.89–7.27) | 7.23 (7.03–7.44) | 6.06 (5.90–6.22) |
| NPTCC | 4.91 (4.86–4.96) | 5.59 (5.53–5.65) | 3.07 (2.95–3.20) | 3.97 (3.82–4.12) | 2.82 (2.71–2.93) |
| CARCINOMA | 0.23 (0.22–0.24) | 0.32 (0.31–0.34) | 0.21 (0.18–0.24) | 0.32 (0.28–0.36) | 0.17 (0.14–0.20) |
| SCC | 0.30 (0.28–0.31) | 0.25 (0.24–0.26) | 0.19 (0.16–0.22) | 0.33 (0.29–0.38) | 0.07 (0.06–0.09) |
Diagnoses in the period of 1992–2014 in the SEER 13 database. In each cell, estimated rate (95% confidence interval). Rates were age-adjusted using the U.S. 2000 Census population.
Survival rates
Figure 1 shows the unadjusted survival rate for five years. Non-Hispanic whites have better survival rates at all times, whereas the survival curves of other racial groups may cross. Blacks have a much lower survival rate than other racial groups. The five-year relative survival rates, stratified by gender, age group, histological types, stage, location, and treatment strategies are shown in Table 3. When stratified by gender, for male or female, non-Hispanic whites have the best survival rate and blacks have the worst. The racial differences are significant in multivariate Cox regression. When stratified by age, for the <40, 65–84 and 85+ years group, non-Hispanic whites all have the best survival rate and blacks all have the worst. For 40–64 years’ group, both non-Hispanic whites and APIs have the best survival rate and blacks still have the worst. The racial differences are significant in multivariate analysis. When stratified by histological type, for PTCC, both non-Hispanic whites and Hispanic whites have the best five-year survival rate (89.0%), followed by APIs (86.2%) and blacks (80.7%). For NPTCC, APIs have the best survival rate (61.3%), followed by NHWs (58.1%), HWs (56.1%) and blacks (42.8%). For Carcinoma, Hispanic whites have the best survival rate (50.2%), followed by NHWs (48.0%), APIs (37.9%) and blacks (35.1%). For SCC, APIs have the best survival rate (32.2%), followed by non-Hispanic white (28.0%), blacks (20.2%) and Hispanic whites (18.5%). For these histological types, the multivariate Cox regression suggests significant racial differences. When stratified by stage, for stage II, Hispanic whites have the best five-year survival rate (91.1%), followed by non-Hispanic whites (90.9%), APIs (89.0%) and blacks (83.6%). For stage III, APIs have the best survival rate (47.0%), followed by Hispanic whites (44.4%), non-Hispanic whites (43.4%) and blacks (33.8%). For stage IV, Hispanic whites have the best survival rate (7.0%), followed by non-Hispanic whites (5.9%), blacks (5.6%) and APIs (5.1%). For all the three tumor stages, the racial differences are significant in multivariate Cox regression. When stratified by tumor location, for trigone of the bladder, both non-Hispanic whites and Hispanic whites have the best survival rate (78.2%) and blacks have the worst (62.6%). For lateral wall of bladder, anterior wall of bladder, bladder neck, Ureteric orifice, and NOS, non-Hispanic whites have the best survival rate (85.9%, 72.3%, 75.3%, 56.3%, 76.2%, respectively) and blacks have the worst (72.9%, 56.7%, 56.2%, 39.6%, 59.2%, respectively). For dome of bladder and posterior wall of bladder, Hispanic whites have the best survival rate, and blacks still have the worst (64.1%, 70.8%). For ureteric orifice, non-Hispanic whites have the best survival rate (90.4%), and APIs have the worst (84.6%). For overlapping lesion of bladder, APIs have the highest five-year survival rate (65.8%) and blacks have the worst (52.2%). The racial differences are significant in a multivariate Cox regression for all locations except urachus. When stratified for treatment strategies, for surgery, non-Hispanic whites have the best survival rate (39.2%) while blacks have the worst (25.4%). For the SRC and SR, APIs have the best survival rate (40.2%, 36.8%, respectively) and blacks have the worst (26.9%, 22.3%, respectively). For these three treatment strategies, the racial differences are significant in a multivariate Cox regression. For other treatment strategies, there is no significant racial difference from the results of multivariate Cox regression.
Figure 1.
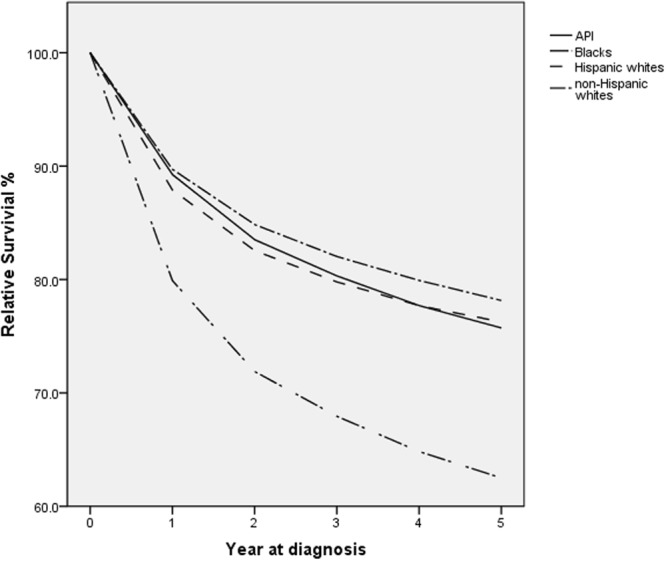
Relative survival up to five years for different racial groups. Cancers diagnosed in the period of 1973–2008, and followed up to 12/31/2013.
Table 3.
Survival rates of UBC for different racial groups, stratified by gender, age, histologic type, stage at diagnosis, location and treatment.
| Survival rates | Total | NHW | HW | Black | API | P |
|---|---|---|---|---|---|---|
| Gender | 77.4 (77.1–77.6) | 78.2 (77.9–78.4) | 76.3 (75.1–77.3) | 62.5 (61.4–63.6) | 75.7 (74.5–76.9) | |
| Male | 79.0 (78.7–79.3) | 79.5 (79.2–79.8) | 78.9 (77.5–80.1) | 67.5 (66.1–68.9) | 77.5 (76.2–78.8) | <0.001 |
| Female | 72.5 (72.0–73.0) | 74.1 (73.6–74.6) | 69.01 (66.9–71.0) | 53.1 (51.2–54.9) | 70.2 (67.8–72.5) | <0.001 |
| Age group | ||||||
| 0–39 | 92.7 (91.9–93.5) | 94.0 (93.1–94.8) | 89.3 (85.6–92.1) | 83.0 (78.1–86.9) | 89.4 (83.5–93.2) | 0.001 |
| 40–64 | 84.1 (83.8–84.4) | 85.1 (84.8–85.4) | 81.4 (79.9–82.7) | 69.0 (67.4–70.5) | 85.1 (83.4–86.5) | <0.001 |
| 64–84 | 75.5 (75.2–75.8) | 76.3 (76.0–76.7) | 73.9 (72.2–75.4) | 59.3 (57.6–60.9) | 74.3 (72.6–75.8) | <0.001 |
| 85+ | 60.7 (59.3–62.0) | 61.5 (60.0–63.0) | 60.5 (53.8–66.6) | 45.0 (39.4–50.5) | 52.0 (46.4–57.3) | <0.001 |
| Histologic type | ||||||
| PTCC | 88.7 (88.4–89.0) | 89.0 (88.7–89.3) | 89.0 (87.6–90.1) | 80.7 (79.2–82.1) | 86.2 (84.8–87.5) | <0.001 |
| NPTCC | 57.4 (56.9–57.9) | 58.1 (57.6–58.7) | 56.1 (53.9–58.3) | 42.8 (41.0–44.6) | 61.3 (59.0–63.5) | <0.001 |
| Carcinoma | 47.0 (45.0–48.9) | 48.0 (45.8–50.1) | 50.2 (41.5–58.3) | 35.1 (287–41.6) | 37.9 (29.4–46.3) | 0.005 |
| SCC | 26.6 (25.0–28.3) | 28.0 (26.1–29.9) | 18.5 (12.9–25.1) | 20.2 (16.1–24.7) | 32.2 (22.1–42.7) | 0.003 |
| Stage at diagnosis | ||||||
| Stage II | 90.7 (90.4–90.9) | 90.9 (90.6–91.2) | 91.1 (89.9–92.2) | 83.6 (82.2–84.9) | 89.0 (87.7–90.2) | <0.001 |
| Stage III | 42.9 (42.4–43.5) | 43.4 (42.8–44.0) | 44.4 (41.9–46.9) | 33.8 (31.9–35.7) | 47.0 (44.3–49.7) | <0.001 |
| Stage IV | 5.9 (5.4–6.5) | 5.9 (5.3–6.5) | 7.0 (4.9–9.6) | 5.6 (4.1–7.4) | 5.1 (3.0–8.0) | 0.024 |
| Location | ||||||
| Trigone of bladder | 77.6 (76.6–78.5) | 78.2 (77.2–79.3) | 78.2 (73.5–82.1) | 62.6 (57.8-67.0) | 77.6 (72.6–81.8) | <0.001 |
| Dome of bladder | 71.6 (70.2–72.9) | 71.6 (70.1–73.0) | 77.3 (71.107–82.4) | 64.1 (58.8–68.9) | 75.1 (69.0–80.2) | <0.001 |
| Lateral wall of bladder | 85.4 (84.9–85.9) | 85.9 (85.4–86.4) | 85.6 (83.1–87.7) | 72.9 (70.1–75.5) | 84.5 (81.8–86.8) | <0.001 |
| Anterior wall of bladder | 71.0 (69.1–72.9) | 72.3 (70.2–74.3) | 63.4 (53.8–71.5) | 56.7 (48.8–63.8) | 71.5 (62.1-78.9) | <0.001 |
| Posterior wall of bladder | 83.0 (82.2–83.8) | 83.4 (82.4–84.2) | 85.8 (82.0–88. 8) | 70.8 (66.5–74.6) | 83.0 (78.9–86.3) | <0.001 |
| Bladder neck | 74.1 (72.6–75.5) | 75.3 (73.7–76.9) | 73.7 (67.0–79.2) | 56.2 (50.0–61.8) | 73.8 (67.2–79.3) | <0.001 |
| Ureteric orifice | 90.2 (89.2–91.0) | 90.40 (89.4–91.3) | 88.7 (83.6–92.2) | 87.4 (81.5–91.6) | 84.6 (78.7–89.0) | <0.001 |
| Urachus | 53.0 (47.2–58.4) | 56.3 (49.2–62.8) | 52.1 (33.1–68.2) | 39.6 (23.0–55.7) | 44.9 (27.4–61.0) | 0.192 |
| Overlapping lesion of bladder | 64.6 (63.9–65.4) | 65.4 (64.6–66.2) | 62.9 (59.4–66.1) | 52.2 (49.1–55.1) | 65.8 (61.9–69.3) | <0.001 |
| Bladder, NOS | 75.2 (74.8–75.6) | 76.2 (75.8–76.6) | 72.3 (70.5–74.0) | 59.2 (57.5–60.9) | 71.8 (69.9–73.7) | <0.001 |
| Treatment | ||||||
| SC | 68.7 (67.8–69.6) | 69.6 (68.6–70.6) | 66.7 (62.5–70.6) | 56.2 (52.2–60.0) | 73.9 (69.7–77.7) | 0.132 |
| Chemotherapy | 62.0 (60.4–63.4) | 63.6 (61.9–65.2) | 50.2 (42.2–57.6) | 42.2 (36.2–48.0) | 65.8 (58.7–72.1) | 0.288 |
| SRC | 33.4 (31.6–35.2) | 33.3 (31.3–35.3) | 37.7 (29.6–45.8) | 26.9 (21.1–33.0) | 40.2 (31.1–49.0) | <0.001 |
| Surgery | 37.9 (36.6–39.2) | 39.2 (37.8–40.6) | 33.4 (26.3–40.6) | 25.4 (21.3–29.8) | 35.5 (28.7–42.3) | <0.001 |
| SR | 33.4 (32.2–34.6) | 34.2 (32.9–35.5) | 33.9 (27.3–40.5) | 22.3 (18.7–26.2) | 36.8 (30.8–42.8) | <0.001 |
| Radiation | 26.8 (25.1–28.5) | 27.8 (26.0–29.7) | 34.3 (24.4–44.5) | 12.5 (8.5–17.0) | 30.8 (22.4–39.5) | 0.087 |
| RC | 19.2 (15.3–23.4) | 18.9 (14.5–23.7) | 26.5 (8.5–48.9) | 18.6 (9.6–29.9) | 13.1 (3.27–29.8) | 0.184 |
Cancers diagnosed in the period of 1973–2008, and followed up to 12/31/2013 in the SEER 18 database. In each cell, estimated rate (95% confidence interval).
Discussion
Bladder cancer is the most common malignancy of the urinary system. It is also one of the top ten common tumors of the body. Current studies investigated racial differences for a small number of racial groups, most of them focus on blacks and whites only20,21. Some other studies just cared about a small number of outcome variables22. In this study, UBC patients in the United States among non-Hispanic whites, Hispanic whites, blacks and APIs are compared comprehensively on the basis of SEER data. Significant racial differences are found in terms of patients’ characteristics, clinical-pathologic features, incidence and survival. Such results can be informative for cancer epidemiologists, clinicians, and policy-makers.
The observed gender, age, and tumor grade distributions are similar to those in the literature18,21. The statistical analyses suggest the existing of racial differences on the distributions of gender, marital status, age, age at diagnosis, survival time, histological types, grade, stage, treatment, tumor size, regional nodes negative, and tumor location. These findings are mostly consistent with those in the literature23,24. It is possible that the complex interactions of genetic makeup, living habits, occupational exposures, and environmental exposures cause the racial differences among UBC patients25.
Racial differences in incidence have been studied in a few publications6,20. Chen and others limited their study to blacks and whites, analyzed SEER data from 2010 to 2013, and found that blacks have lower incidence rates than whites6. Our analysis includes much wider racial distributions and generates more comprehensive results. Non-Hispanic whites have higher incidence rates of UBC than blacks while Hispanic whites have lower incidence rates than blacks and APIs have lower incidence rates than other races. Multiple factors may have contributions to the racial differences in incidence. One possible risk factor is smoking. As some studies reported that smoking significantly elevated bladder cancer risk (odds ratio = 2.4) and there are some known bladder carcinogens just like 2-napthylamine in tobaccos26–28. Non-Hispanic whites have been found that have a higher proportion of smokers compared with Hispanic whites, blacks, and Asian American28. The racial difference in UBC can also be attributed to occupational exposures. Numerous studies have demonstrated that workers in high-risk occupations involving exposure to dyes, rubber, leather, ink, or paints have an increased risk of bladder cancer. Three of the chemicals used in these industries (2-naphthylamine, benzidine, and 4-aminobiphenyl) are classified as human carcinogens by the International Agency for Research on Cancer (IARC)29,30. Some researchers already found the racial differences between non-Hispanic whites, Hispanic whites and blacks in exposure to occupational hazards31. Coffee drinking has been studied extensively as a potential risk factor, but the inconsistency of the observed association suggests that the relationship is quite weak11. In our study, we also found that when stratified by histological types, for SCC and Carcinoma, blacks have the highest incidence rate. Kantor and others also found that blacks have the highest incidence rate of SCC32.The high incidence of SCC appears to be related to a high incidence of endemic schistosomiasis in blacks33. Eradication of schistosomiasis can effectively reduce the incidence of SCC in blacks. It’s not clear why blacks have the highest incidence of Carcinoma: further studies are needed.
The racial difference for survival outcome of UBC patients has been studied in some publications. Scosyrev and others compared the mortality rates of blacks and whites adjusted for grade, stage, and cell type in 2009, and found that for a time less than three years since diagnosis, blacks have higher mortality rates than whites18. Hollenbeck and others used SEER data for the years from 1992 through 2002 and found that blacks have an approximately 70% greater risk of cancer-related death compared with whites21. In our analysis, the survival advantage of whites is observed and blacks have the worst survival rate. The results are the same as those mentioned above. When stratified by treatment strategies, racial differences still exist for SR, SRC, and surgery, with blacks having the lowest survival rate. So we think that treatment may not be the main reason for the racial difference in survival rate. Hollenbeck’s research already showed that the use of aggressive therapies (cystectomy, systemic chemotherapy, radiation) was found to be similar among black patients and white patients. After adjusting for the difference in treatment intensity and provider effects, the hazard ratios for blacks and whites just decrease 0.0121. Racial differences could also be observed from our study after stratified by stage and histological types, with blacks still have the worst survival rate. These findings support a recent SEER study that reported an excess hazard of death from bladder cancer among blacks despite adjusting for age, stage and grade18. Delays in diagnosis resulting in higher stage disease and different histological types may not be the reasons for racial difference in survival. Walker et al. have also show that incidence of more advanced tumors is similar among blacks and whites20. Genetic differences or tumors with poor prognostic molecular markers may correlate with more aggressive bladder cancer in blacks. But now, we don’t have clear evidence. Additionally, different lifestyles could also be the reason of the racial difference of UBC. Parkin et al. already show that survival is better in the developed areas than in developing areas34 because people are more likely to have unhealthy lifestyles such as smoking, physical inactivity, and consumption of calorie-dense food in less developed areas35. Racial differences in survival may also be the end result of disparities in the healthcare provided to different races. Blacks may receive a lower quality of care relatively, which can reflect differential treatment by race.
In this study, we also found that APIs have the highest survival rate instead of whites in some cases. For histological types NPTCC and SCC, APIs have the highest survival rate. The possible reason may be different treatment strategies for some specific histological type in different racial groups. Additionally, different genetic makeup and lifestyle behaviors may contribute to this result. For treatment strategies SC, Chemotherapy, SRC, and SR, APIs have the highest survival rate than other racial groups. It reminds us that APIs may be more suitable for these treatment strategies mentioned above than other racial groups. This result can provide a reference for choosing the appropriate treatment strategies for different racial groups. The survival advantage of APIs is observed for stage II and overlapping lesion of bladder. Reasons for that need further researches. We can also learn from our study that tumor location in urachus and overlapping lesion of bladder has a lower survival rate than other locations. Tumor locations of UBC have rarely been studied before. It can provide a reference for prevention and treatment of bladder cancer.
Limitations
The SEER database is analyzed in this study because it is the current largest cancer registry in the United States. However, it still has many limitations. Firstly, errors may arise in tumor classification and staging in multiple sites. But we do not expect a series of systematic errors correlated with ethnicity. Secondly, data collected in SEER may not be comprehensive enough. For instance, there are no data of genetic factors or lifestyle that may contribute to UBC. Furthermore, SEER does not collect data regarding insurance type, educational level, socioeconomic status, availability to healthcare and other factors that affect one’s treatment, and as a consequence, the survival outcome. Additionally, the details about treatments are not recorded in SEER, such as surgical methods, chemotherapy dosage and so on. All above unmeasured patient differences may confound correlations between race and outcomes. Also, the study on SEER data is focus on patients from the United States only. It is not clear whether the results hold for other nations. In addition, although the sample size is at the same level as in related studies18,36, it would possible be over powered.
Conclusion
This study shows that there exist significant racial differences in patient characteristics, incidence and survivals among UBC patients in the U.S. The exact cause of such difference is not revealed. The identified racial difference could be informative and leveraged by UBC epidemiologists and clinicians despite the limitations.
Acknowledgements
We thank Wenjing Kong and Fan Wang for their suggestions on writing. National Natural Science Foundation of China (71771211, 81774146), MOE Project of Key Research Institute of Humanities and Social Sciences at Universities (16JJD910002).
Author Contributions
Y.L. and Y.W. designed the study. Y.W. and Q.C. analyzed data. Y.W., Q.C. and Y.L. wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang Y, Ma S. Racial differences in mantle cell lymphoma in the United States. BMC Cancer. 2014;14:764. doi: 10.1186/1471-2407-14-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer. 2016;16:691. doi: 10.1186/s12885-016-2747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Wang Y, Ma S. Racial differences in four Leukemia subtypes: comprehensive descriptive epidemiology. Scientific reports. 2018;8:548. doi: 10.1038/s41598-017-19081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA: a cancer journal for clinicians. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM. The global burden of urinary bladder cancer. Scandinavian journal of urology and nephrology. 2008;42:12–20. doi: 10.1080/03008880802285032. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, et al. The prognostic value of histological subtype in patients with metastatic bladder cancer. Oncotarget. 2017;8:28408–28417. doi: 10.18632/oncotarget.16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svatek RS, Lee D, Lotan Y. Correlation of office-based cystoscopy and cytology with histologic diagnosis: how good is the reference standard. Urology. 2005;66:65–68. doi: 10.1016/j.urology.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Spruck CH, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer research. 1994;54:784–788. [PubMed] [Google Scholar]
- 9.Felix AS, et al. The changing patterns of bladder cancer in Egypt over the past 26 years. Cancer Causes & Control. 2008;19:421–429. doi: 10.1007/s10552-007-9104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burger M, et al. Epidemiology and risk factors of urothelial bladder cancer. European Urology. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Silverman DT, et al. Epidemiology of bladder cancer. Hematology/oncology clinics of North America. 1992;6:1–30. doi: 10.1016/S0889-8588(18)30360-5. [DOI] [PubMed] [Google Scholar]
- 12.Mteelin C, Graham S. Dietary risk factors in human bladder cancer. American journal of epidemiology. 1979;110:255–263. doi: 10.1093/oxfordjournals.aje.a112810. [DOI] [PubMed] [Google Scholar]
- 13.Traxer O, et al. Technique and complications of transurethral surgery for bladder tumours. BJU international. 2004;94:492–496. doi: 10.1111/j.1464-410X.2004.04990.x. [DOI] [PubMed] [Google Scholar]
- 14.Miller KD, et al. Cancer treatment and survivorship statistics. CA: a cancer journal for clinicians. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 15.Soloway MS. The management of superficial bladder cancer. Cancer. 1980;45:1856–1865. doi: 10.1002/cncr.1980.45.s7.1856. [DOI] [PubMed] [Google Scholar]
- 16.Kurth KH, et al. Factors affecting recurrence and progression in superficial bladder tumours. European Journal of Cancer. 1995;31:1840–1846. doi: 10.1016/0959-8049(95)00287-S. [DOI] [PubMed] [Google Scholar]
- 17.Millan-Rodriguez F, et al. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. The Journal of Urology. 2000;163:73–78. doi: 10.1016/S0022-5347(05)67975-X. [DOI] [PubMed] [Google Scholar]
- 18.Scosyrev E, et al. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 19.Yee DS, et al. Ethnic differences in bladder cancer survival. Urology. 2011;78:544–549. doi: 10.1016/j.urology.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker B, Figgs LW, Zahm SH. Differences in cancer incidence, mortality, and survival between African Americans and whites. Environmental health perspectives. 1995;103:275. doi: 10.1289/ehp.95103s8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollenbeck BK, et al. Racial differences in treatment and outcomes among patients with early stage bladder cancer. Cancer. 2010;116:50–56. doi: 10.1002/cncr.25310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Underwood W, et al. Gender and geographic influence on the racial disparity in bladder cancer mortality in the US. Journal of the American College of Surgeons. 2006;202:284–290. doi: 10.1016/j.jamcollsurg.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Madeb R, Messing EM. Urologic Oncology: Seminars and Original Investigations. Elsevier. 2004. Gender, racial and age differences in bladder cancer incidence and mortality; pp. 86–92. [DOI] [PubMed] [Google Scholar]
- 24.Mayer WJ, McWhorter WP. Black/White differences in non-treatment of bladder cancer patients and implications for survival. American journal of public health. 1989;79:772–775. doi: 10.2105/AJPH.79.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negri, E. & Vecchia, C. Epidemiology and prevention of bladder cancer. Invasive Bladder Cancer, 1–14 (2007).
- 26.World Health Organization, International Agency for Research on Cancer. Tobacco smoke and involuntary smoking. World Health Organization (2004).
- 27.Cancer epidemiology and prevention. Oxford University Press (2006).
- 28.Trinidad DR, et al. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. American Journal of Public Health. 2011;101:699–706. doi: 10.2105/AJPH.2010.191668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns PB, Swanson GM. Risk of urinary bladder cancer among Blacks and whites: the role of cigarette use and occupation. Cancer Causes and Control. 1991;2:371–379. doi: 10.1007/BF00054297. [DOI] [PubMed] [Google Scholar]
- 30.Swanson GM. Cancer prevention in the workplace and natural environment. Cancer. 1988;62:1725–1746. doi: 10.1002/1097-0142(19881015)62:1+<1725::AID-CNCR2820621310>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 31.Robinson JC. Exposure to occupational hazards among Hispanics, Blacks and non-Hispanic whites in California. American Journal of Public Health. 1989;79:629–630. doi: 10.2105/AJPH.79.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantor AF, et al. Epidemiological characteristics of squamous cell carcinoma and adenocarcinoma of the bladder. Cancer research. 1988;48:3853–3855. [PubMed] [Google Scholar]
- 33.Groeneveld AE, Marszalek WW, Heyns CF. Bladder cancer in various population groups in the greater Durban area of KwaZulu‐Natal. South Africa. 1996;78:205–208. doi: 10.1046/j.1464-410x.1996.09310.x. [DOI] [PubMed] [Google Scholar]
- 34.Parkin DM, et al. Global cancer statistics. CA: a cancer journal for clinicians. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 35.Jemal A, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiology and Prevention Biomarkers. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 36.David SY, et al. Ethnic differences in bladder cancer survival. Urology. 2011;78:544–549. doi: 10.1016/j.urology.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available on the Surveillance, Epidemiology, and End Results (SEER, http://seer.cancer.gov) database.


