Abstract
Oligodendrocyte progenitor cells (OPC) undergo asymmetric cell division (ACD) to generate one OPC and one differentiating oligodendrocyte (OL) progeny. Loss of pro-mitotic proteoglycan and OPC marker NG2 in the OL progeny is the earliest immunophenotypic change of unknown mechanism that indicates differentiation commitment. Here, we report that expression of the mouse homolog of Drosophila tumor suppressor Lethal giant larvae 1 (Lgl1) is induced during OL differentiation. Lgl1 conditional knockout OPC progeny retain NG2 and show reduced OL differentiation, while undergoing more symmetric self-renewing divisions at the expense of asymmetric divisions. Moreover, Lgl1 and hemizygous Ink4a/Arf knockouts in OPC synergistically induce gliomagenesis. Time lapse and total internal reflection microscopy reveals a critical role for Lgl1 in NG2 endocytic routing and links aberrant NG2 recycling to failed differentiation. These data establish Lgl1 as a suppressor of gliomagenesis and positive regulator of asymmetric division and differentiation in the healthy and demyelinated murine brain.
Oligodendrocyte progenitor cells (OPCs) undergo asymmetric cell division, and disruption of such mechanism can generate oligodendroglioma precursors. Here, Daynac and colleagues show that Lgl1 regulates asymmetric division and differentiation of OPCs by interfering with the endocytosis pathway, and that Lgl1 knockout can lead to gliomagenesis.
Introduction
Adult oligodendrocyte progenitor cells (OPC) expressing the proteoglycan NG2 (CSPG4) continuously divide and generate differentiating oligodendrocytes (OL) throughout adulthood1. OPC divisions are frequently asymmetric and generate progeny of distinct fate, where only one of the daughter cells preserves NG2 expression and the opposite daughter cell downregulates NG22,3. Thus far, asymmetric distribution of NG2 protein is the earliest immunophenotypic change amongst daughter cell pairs generated by asymmetric cell division (ACD). NG2-positive daughter cells proliferate at higher rates than NG2-negative cells, showing that the early phenotypic asymmetry correlates with distinct short-term fate2. NG2 contributes to establishing this cell fate bias within newly generated cell pairs after ACD. NG2 binds platelet-derived growth factor-AA (PDGF-AA) and PDGF receptor alpha (PDGFRα) and thereby enhances PDGFRα signaling and promotes timely OPC proliferation4–7. Moreover, long-term fate tracking of the ACD progeny showed that early NG2 asymmetry permanently affects cell fate. The NG2-positive progeny of ACD retain OPC characteristics while the NG2-negative progeny upregulate CC1, a marker for commitment to the OL fate, and eventually differentiate2,8,9. Importantly, chemical-induced demyelination increases the rates of NG2 asymmetry9. Collectively, the data underline that asymmetric distribution of NG2 marks and also actively generates different OPC progeny. Furthermore, ACD balances OPC proliferation with differentiation in the normal brain and generates OL in demyelinated lesions to contribute to remyelination2,3,9,10. It is unclear how the downregulation of NG2 protein is achieved in the differentiating oligodendrocyte. This lack of mechanistic insights into ACD and early differentiation limits our understanding of brain homeostasis.
OPC give rise to glioma in genetically engineered mouse models11–14. When undergoing neoplastic transformation, OPC show higher rates of symmetric self-renewing divisions at the expense of ACD2. These data suggest that downregulation of NG2 in OPC progeny is critical for ACD, differentiation, and attenuation of tumorigenesis2.
Surface levels of the membrane-spanning NG2 protein are regulated by clathrin-mediated endocytosis in mouse embryonic fibroblasts15. Clathrin-mediated or receptor-mediated endocytosis is a multi-step process, whereby membrane-localized proteins are engulfed as cargo in clathrin-coated pits, which then bud off the membrane to form the early endosome. Cargo proteins are then sorted into either the recycling endosome and re-integrated into the membrane or targeted to the late endosome and subsequently the lysosome for degradation16. It is not known whether NG2 trafficking by the endocytic pathway is important for NG2 downregulation and OL differentiation.
The WD40 repeat-containing protein lethal giant larvae (Lgl) was initially characterized as a tumor suppressor gene, in Drosophila melanogaster17, and it was subsequently shown to mediate ACD of Drosophila neuroblasts18. Lgl is an evolutionary conserved protein that initiates cell polarity by recruiting proteins to membrane subdomains (for review, see ref. 19). As one of two mammalian Lgl genes20, Lgl1 is highly expressed in the brain21. Lgl1 knockout studies during mouse embryogenesis have revealed a function for Lgl1 in polarity and adherens junction integrity in neuroepithelial cells22, and in suppressing proliferation of dorsal telencephalon radial glial progenitors at early postnatal stages23. A bona-fide tumor suppressor role for Lgl1 in gliomagenesis is supported by studies showing that loss of tumor suppressor PTEN, a frequent and early driver mutation in glioblastoma24, inhibits Lgl1 activity25. Constitutively active Lgl1 inhibits invasion and induces differentiation of primary human glioblastoma cells26. Nevertheless, a tumor suppressive role for Lgl1 in OPC has not been investigated yet.
Here, we report that Lgl1 expression is upregulated during OL differentiation and that Lgl1 protein is detected in committed OL in the adult murine brain. Lgl1 conditional knockout (cKO) OPC show a differentiation defect characterized by an aberrant co-expression of NG2 with OL commitment markers. Moreover, in Lgl1 cKO OPC rates of ACD are reduced while rates of symmetric, self-renewing divisions and proliferation are increased, in both intact and chemically demyelinated corpus callosum (CC). Lgl1 knockout synergizes with hemizygous Ink4a/Arf knockout in OPC to induce gliomagenesis. Time lapse imaging of surface-labeled, endocytosed NG2 shows decreased co-localization with the lysosome in Lgl1 cKO OPC. Additionally, total internal reflection microscopy reveals that in Lgl1 cKO OPC NG2 bypasses degradation and is rather recycled to the membrane. Aberrant NG2 recycling is linked to defective OPC differentiation. Thus, we establish Lgl1 as a positive regulator of differentiation and ACD and suppressor of gliomagenesis. We suggest that Lgl1 promotes differentiation by supporting NG2 routing to the lysosome.
Results
Lgl1 expression is upregulated in OL differentiation
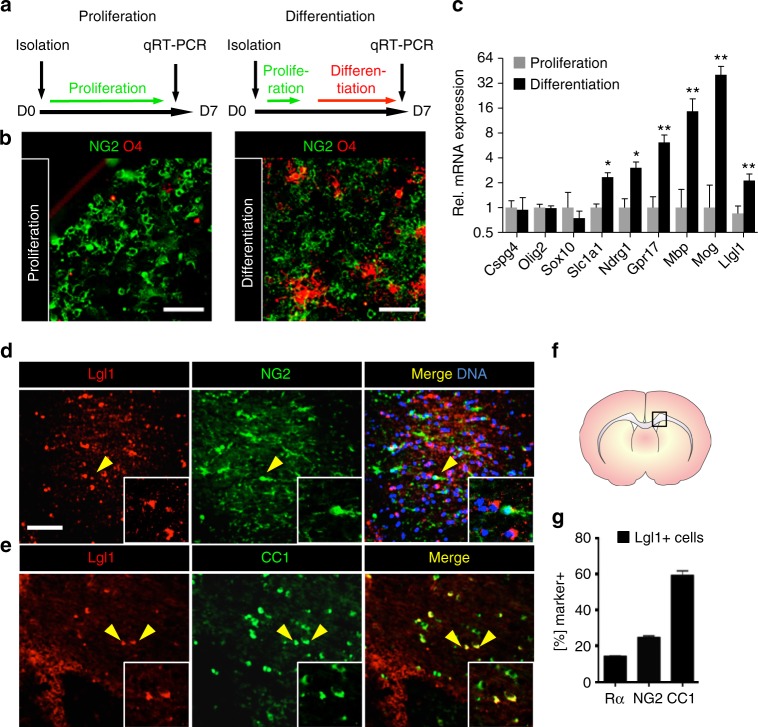
To unravel the mechanism for NG2 down-regulation, we searched the RNA-Seq transcriptome database27 for conserved ACD regulator genes with altered expression in OL versus OPC. We noticed a four-fold increase of Lgl1 (Llgl1) transcript in newly formed OL versus OPC, suggesting that Llgl1 expression is increased upon OL differentiation. To directly test this notion, OPC were isolated from the white matter area of the CC of postnatal days 30–60 (P30–P60) mice and cultured in parallel under proliferation and differentiation conditions (Fig. 1a). We performed immunofluorescence staining for OPC marker NG2 and mature OL marker O4 to show differentiation. As expected, we found O4-expressing cells displaying a branched OL morphology only under differentiation but not under proliferation conditions (Fig. 1b). We used quantitative real-time PCR (qrt-PCR) to assess changes in Llgl1 transcript levels with differentiation and aging. Analyses reveal a significant increase in Llgl1 transcript under differentiation conditions (2.5-fold, p < 0.01) (Fig. 1c), along with differentiation markers Mbp, Slc1a1, Ndrg1, GPR17, and Mog. We noted that OPC markers, such as Cspg4 (NG2), Olig2, and Sox10 are unchanged. Llgl1 transcript levels increase with age, being higher at P60 in OPC, and reaching a peak at P180 in OL (Supplementary Figs. 1d, e).
Fig. 1.
Lgl1 is expressed in oligodendrocyte progenitor cells (OPC) and its expression increases with differentiation into oligodendrocytes. a Scheme of OPC treatment before qRT-PCR. b Immunofluorescence for OPC marker NG2 and oligodendrocyte differentiation marker O4 of corpus callosum-derived OPC under proliferating or differentiating conditions. Differentiation increases O4-positive cell frequency. Scale bar = 100 µm. c Graph representing mRNA expression level changes with differentiation. qRT-PCR results are displayed on a log2 scale and normalized to proliferative conditions. Data represented as mean ± s.e.m. of five independent experiments (n = 5, *p < 0.05, **p < 0.01, Mann–Whitney test). d, e Co-immunostaining of P35 wildtype mouse corpus callosum for Lgl1 and OPC marker NG2 (top panels) or differentiation marker CC1 (bottom panels). Insets are high magnification panels of Lgl1 positive cells (yellow arrows). Scale bar: 100 μm. f Schematic of coronal section of a P60 mouse showing the corpus callosum where cells were counted. g Quantification of PDGFRα-positive, NG2-positive, and CC1-positive cells per total Lgl1-positive cells. 14% were PDGFRα-positive, 22% were NG2-positive, and 59% were CC1-positive. Data are represented as mean ± s.e.m. of four independent experiments
To test whether Lgl1 protein expression is detectable in differentiated OL in situ, as suggested by our transcript analyses, we co-immunostained P35 wildtype mouse brain sections for Lgl1, OPC markers PDGFRα, and NG2 (Fig. 1d, Supplementary Fig. 1a), and committed OL marker CC1 (Fig. 1e)8. A quantification of all detectable Lgl1-positive cells in the CC revealed that more Lgl1-expressing cells are CC1 positive (59%) than PDGFRα (14%) and NG2 (22%) positive, respectively (Fig. 1f, g). Expression of Lgl1 is undetectable in NG2-positive OPC in the subventricular zone (Supplementary Figs. 1b, c).
Hence, Lgl1 expression increases with differentiation and overlaps largely with differentiating OL, suggesting therefore its potential role in a differentiation mechanism.
Lgl1 knockout in OPC blocks OL differentiation
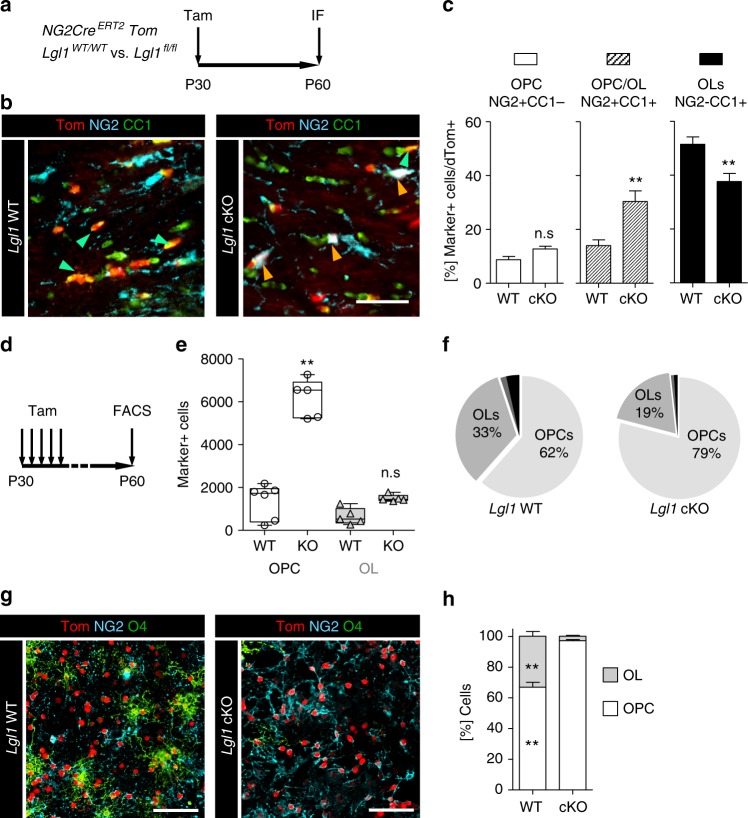
The expression pattern suggests that Lgl1 positively regulates OL differentiation. In order to investigate a putative role for Lgl1 in OPC differentiation, we used a mouse model where Lgl1 knockout is targeted to NG2 cells28. We bred NG2-CreERT2 driver mice9 with red-fluorescence protein Cre-reporter (Ai14-Tom)29 and Lgl1 floxed (Lgl1fl/fl)21,23 mice, as well as Lgl1 wildtype (Lgl1wt/wt) mice as controls. Compound transgenic mice were injected with tamoxifen once at P30 and recombined cells were identified by immunofluorescence staining for red fluorescent protein dTomato (aka Tom + cells) (Fig. 2a). NG2 and CC1 co-staining (Fig. 2b) revealed that amongst Tom+ cells, NG2 + CC1+ cells were increased (WT = 14.7% vs. cKO = 30.3%; p < 0.01), CC1+ cells were decreased (WT = 51.8% vs. cKO = 37.6%; p < 0.01) and NG2+ cells were not significantly changed in Lgl1 cKO vs. Lgl1 WT CC (Fig. 2c). These data show that Lgl1 is not required for CC1 expression in OPC progeny. Lgl1 is rather required for NG2 protein down-regulation and positively regulates OL differentiation.
Fig. 2.
Lgl1 knockout blocks OPC to OL differentiation. a Schematic of approach for clonal labeling and NG2 Cre-conditional deletion of Lgl1 in OPC. b Representative triple immunostaining of corpus callosum of Lgl1 WT and Lgl1 cKO from mice treated as in (a) for red fluorescent protein (Tom), OPC marker NG2 and differentiation marker CC1. Green arrows point to Tom+CC1+ oligodendrocytes. Orange arrows in the Lgl1 cKO panel point to Tom+NG2+CC1+ cells. Scale bar: 50 µm. c Quantification of NG2+, CC1+, and NG2+CC1+ cells ratios amongst Tom+ cells. Data are represented as mean ± s.e.m. from the analyses of 4 Lgl1 WT vs. 4 Lgl1 cKO treated mice (**p < 0.01, Mann–Whitney test). d Schematic of broad deletion of Lgl1 in P30 mice followed by FACS at P60. e Plot showing quantification of OPC (white) and OL (gray) in mice treated following scheme in (d). Data are represented as box & whiskers ± s.e.m. from the analyses of 6 Lgl1 WT vs. 5 Lgl1 cKO treated mice (**p < 0.01, Mann–Whitney test). f Pie charts of quantification for NG2+, O4+, astrocyte marker GLAST+ and neuroblast marker CD24+ cells amongst Tom+ cells, after Lgl1 knockout at P60 (see also Supplementary Fig. 2b). Data are represented as percentages from the analyses of 6 Lgl1 WT vs. 5 Lgl1 cKO mice (OPC p < 0.01; OL p < 0.01). g Representative immunostaining for NG2 and O4 of OPC and OL isolated from GFAP-Cre, Tom, Lgl1wt/wt and, GFAP-CreERT2, Tom, Lgl1fl/fl mice. Scale bars: 100 µm. h Quantification of NG2 and O4 cells amongst all red fluorescent protein (Tom) positive cells show that Lgl1 knockout strongly blocks the differentiation of OPC into O4+OL. Data are represented as mean ± s.e.m. from the independent analyses of 4 Lgl1 WT vs. 4 Lgl1 cKO mice (**p < 0.01, Mann–Whitney test)
To provide further evidence that Lgl1 positively regulates OL differentiation, we analyzed ex vivo isolated Lgl1 cKO cells (Fig. 2d) for expression of OL maturation marker O4 and OPC marker PDGFRα at P60 (Figs. 2e, f, Supplementary Figs. 2a, b) and at P120 (Supplementary Figs. 2c, d). Indeed, we find that the total number of OPC increased to a greater extent than OL (OPC = 4.5 fold cKO vs. WT; p = 0.002; OL = 2.3 fold cKO vs. WT; p = 0.06) at P60 (Fig. 2e). Accordingly, the frequency of OPC amongst Tom +, recombined cells increased in Lgl1 cKO, while the frequency of OL decreased amongst Tom+ cells (OPC/WT = 62% vs. OPC/cKO = 79%; OL/WT = 33% vs. OL/cKO = 19%; Fig. 2f). At P120, the frequency of OPC was still higher amongst Lgl1 cKO Tom+ cells than amongst Lgl1 WT Tom+ cells (OPC/cKO = 41.3% vs. OPC/WT = 23.4%; p = 0.02) and the frequency of OL was still lower (cKO = 39.4% vs. WT = 51.7%; p = 0.02; Supplementary Fig. 2d).
Taken together, these data show that upon Lgl1 knockout, OL aberrantly retain NG2 protein expression and expand as OPC marker positive cells, while failing to further differentiate and mature.
In contrast to postnatal and adult OPC, embryonic OPC are not committed to the oligodendrocyte lineage and reportedly also generate astrocytes or neurons9,30,31. To test if Lgl1 deletion affects the lineage commitment in young adult OPC, we quantified expression of neuroblast and astrocyte markers CD24 and GLAST, respectively, by flow cytometry (Supplementary Figs. 2a, b). As expected, only a small fraction of recombined cells expressed neuroblast and astrocyte markers (<2%) and the frequency of neuroblasts and astrocytes did not change significantly following Lgl1 deletion (Supplementary Fig. 2b). We concluded that Lgl1 loss does not alter the potential of young adult OPC to generate astrocytes and neuroblasts, suggesting a specific role of Lgl1 in OL differentiation.
Demyelinating injury increases the rate of OL proliferation and differentiation when compared with non-lesioned murine brain32,33. To test if Lgl1 is also required for OL differentiation in demyelinating lesions, we quantified the frequency of mature OL marker Plp in lysolecithin-induced lesions in NG2-CreERT2 Ai14-TdTom Lgl1fl/fl and Lgl1wt/wt mice injected with tamoxifen at P52 (Supplementary Figs. 2e, f). The frequency of Plp-positive cells amongst recombined cells was significantly decreased upon Lgl1 deletion (WT = 73.3% vs. cKO = 33.0%; p < 0.01; Supplementary Fig. 2g), showing that Lgl1 is critical for OL differentiation in demyelinating lesions.
Lastly, we tested if the requirement for Lgl1 in differentiation can be recapitulated ex vivo. To this end, we isolated OPC from the corpus callosum of GFAP-Cre Lgl1 cKO and GFAP-Cre Lgl1 WT control mice and cultured them under OL differentiation conditions in vitro34 and stained cells for NG2 and O4. Quantifications revealed a significant increase of NG2+ cells and a decrease of O4+ cells amongst all Tom+ cells, upon Lgl1 knockout (Figs. 2g, h).
Taken together, we conclude that Lgl1 is required for the downregulation of NG2 and OL differentiation.
Lgl1 is required for asymmetric OPC divisions
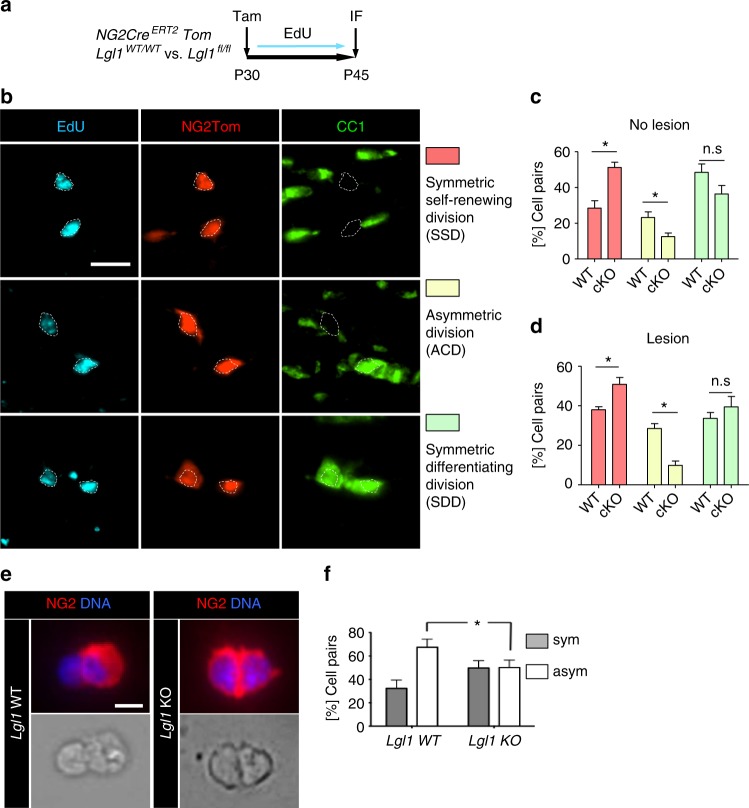
Postnatal and adult OPC divide asymmetrically to generate at a one-to-one ratio an NG2+ OPC and a CC1+ OL2,3,10. Given that Lgl1 knockout reduces the number of CC1+ cells (Fig. 2c), we hypothesize that Lgl1 regulates ACD. To test this hypothesis, we visualized and quantified ACD in Lgl1 NG2-CreERT2 Ai14-TdTom Lgl1fl/fl and Lgl1wt/wt mice that were injected with tamoxifen once at postnatal day 30 (P30) for clonal recombination35. To monitor proliferation, mice were treated with EdU for 14 days (Fig. 3a), and cell pairs were identified by co-immunostaining for Tom, CC1, and EdU2,3 (Fig. 3b). Cell pairs in the corpus callosum were scored as (i) symmetric, self-renewing (both cells = CC1 negative), (ii) asymmetric (one CC1-positive and one CC1-negative cell) and as (iii) symmetric differentiating (both cells = CC1 positive; Fig. 3b). The average cell cycle length of OPC increases from ~3 days in P20 to 9 days in P60 animals1. Assuming a linear increase in cell cycle lengths between P30 and P45, recombined OPC should have divided two to three times during this period. Indeed, we were able to detect recombined cell pairs at P45. Amongst these, ACD rates were reduced in the Lgl1 cKO (WT = 23.2% vs. cKO = 12.5%; p = 0.023). Vice versa, symmetric, self-renewing divisions were increased in the Lgl1 cKO (WT = 28.4% vs. cKO = 51.2%; p = 0.028). Noteworthy, symmetric differentiated (CC1+) cell pair rates were not significantly changed upon Lgl1 cKO (WT = 48.4% vs. cKO = 36%; p = 0.2) (Fig. 3c and Supplementary Table 1).
Fig. 3.
Lgl1 regulates asymmetric cell divisions of OPC. a Treatment schematic for in vivo pair assay in P30 NG2-CreERT2 Tom Lgl1 WT vs. Lgl1 cKO mice, which were given EdU for 14 days in drinking water. b Left panels: Representative cell pairs in the corpus callosum of treated mice and co-immunostained for red fluorescent protein (Tom) to label NG2+ cells and progeny, proliferation marker EdU and CC1. Symmetric, self-renewing divisions = two Tom+EdU+CC1− progeny, asymmetric cell division = one Tom+EdU+CC1− and one Tom+EdU+CC1+ progeny and symmetric, differentiating division = two Tom+EdU+CC1+ progeny. Scale bar: 10 µm. c Graph depicting the frequency of each cell division mode and showing a significant decrease of asymmetric divisions (yellow bars) and an increase of symmetric, self-renewing divisions (red bars) in Lgl1 knockout (cKO) mice when compared with wildtype (WT) mice. Data are represented as mean ± s.e.m. from the analyses of 4 Lgl1 WT vs. 4 Lgl1 cKO treated mice (n = 4, *p < 0.05, Mann–Whitney test). d Graph depicting the percentage of three division modes during remyelination in Lgl1 WT and Lgl1 cKO corpus callosum, and showing a significant decrease of asymmetric divisions (yellow bars) and increase of symmetric, self-renewing divisions (red bars). Data are represented as mean ± s.e.m. from the analyses of 4 Lgl1 WT vs. 4 Lgl1 cKO treated mice (n = 4, *p < 0.05, Mann–Whitney test). e Representative NG2+ OPC pairs stained for NG2 (red) and DNA (blue) showing an asymmetric division in Lgl1 WT (left panel) and symmetric, self-renewing division in Lgl1 cKO (right panel) cells. Scale bar = 10 μM. f Graph depicting percentage of cell pairs and showing that Lgl1 loss increases symmetric, self-renewing divisions at the expense of asymmetric divisions in vitro (n = 6, *p < 0.05, Mann–Whitney test)
Similarly, cell pairs in lysolecithin-induced demyelinating lesions in the corpus callosum and spinal cord, showed increased rates of symmetric self-renewing divisions at the expense of ACD upon Lgl1 knockout (Fig. 3d and Supplementary Figs. 3a, b and Supplementary Table 1).
To test for a disruption of ACD ex vivo, we generated Lgl1 KO and Lgl1 WT control OPC and plated them for pair assays, as described (Fig. 3e)2. We found that the frequency of ACD was reduced in Lgl1 KO OPC (WT = 72% vs. cKO = 49%) (Fig. 3f; p = 0.05).
These data show that Lgl1 does not prevent CC1 expression, but rather prevents downregulation of OPC marker NG2, thereby lowering rates of asymmetric distribution of NG2. Thus, Lgl1 is required for ACD and restricts symmetric, self-renewing divisions in the healthy and injured brain and in the injured spinal cord.
Lgl1 knockout increases OPC proliferation and promotes gliomagenesis
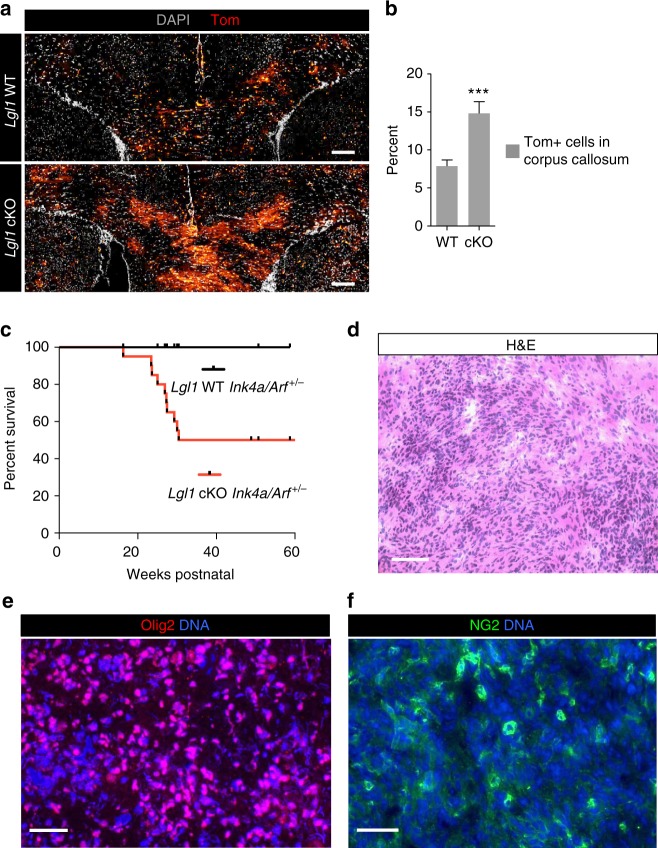
Given that Lgl1 knockout cells fail to restrict expression of pro-mitotic NG2, we hypothesize that their proliferation rates might be increased. We therefore assessed proliferation in NG2-CreERT2 Ai14-TdTom Lgl1fl/fl and Lgl1wt/wt mice injected with tamoxifen at P30 and exposed to EdU at P45 (Supplementary Figs. 4a–c) and at P60 (Figs. 4a, b and Supplementary Figs. 4d–f). Quantifications revealed that the overall percentage of Tom+ cells at P60 was significantly increased in the Lgl1 cKO (14.8%) vs. the Lgl1 WT corpus callosum (7.9%; p = 0.0005; Fig. 4a, b). Moreover, we chemically labeled EdU-positive (Edu+) cells. Edu+ cells were 2.3-fold more frequent amongst the Tom+ cells in Lgl1 cKO vs. the Lgl1 WT corpus callosum (p = 0.014; Supplementary Figs. 4b, c) at P45. A similar increase was observed in demyelinated corpus callosum of P55 mice (1.8-fold higher EdU+ cells amongst Tom+ Lgl1 cKO vs. Lgl1 WT; p = 0.007) (Supplementary Figs. 4d–f). These data indicate that Lgl1 restricts proliferation of OPC in the healthy and demyelinated CC.
Fig. 4.
Lgl1 knockout increases proliferation and promotes gliomagenesis. a Representative immunostaining of corpus callosum of Lgl1 WT mouse (top panel) and Lgl1 cKO mouse (bottom panel) for Tom (red) and co-staining for DNA (gray) after broad deletion of Lgl1 in NG2-CreERT2 Tom Lgl1 WT (top panel) vs. Lgl1 cKO P30 mice followed by analyses at P60 by FACS and IF. Scale bar: 200 µm. b Quantification of Tom+ cells by FACS from Lgl1 WT and Lgl1 cKO corpus callosum, showing a significant increase following Lgl1 knockout. Data are represented as mean ± s.e.m. from the analyses of 5 Lgl1 WT vs. 5 Lgl1 cKO mice (***p < 0.001, Mann–Whitney test). c Kaplan– Meier survival curve for Lgl1 WT (Lgl1wt/wt; NG2Cre; Ink4a/Arf+/-) and NG2Cre-driven homozygous Lgl1 cKO (Lgl1fl/fl; NG2Cre; Ink4a/Arf+/-) mice. Six animals were analyzed for each genotype. d Hematoxylin and Eosin (H&E) staining of endogenous tumors in Lgl1 cKO mice, showing infiltrative growth. Scale bar: 100 μm. e, f Immunostaining of endogenous tumors in Lgl1 NG2-Cre mice for Olig2 (blue, e) and NG2 (green, f). Scale bar: 50 μm
Increased proliferation, defective differentiation, and decreased rates of ACD are associated with gliomagenesis2. We therefore tested whether Lgl1 knockout promotes tumor formation. Immunostainings of normal adult mouse brain revealed that OL express the tumor suppressor p19Arf (Supplementary Fig. 5a)36. We therefore bred Lgl1 floxed mice21 with a NG2-Cre driver line37, and mice heterozygous for the Ink4a/Arf deletion (Ink4a/Arf+/−)38. Mice were monitored for neurological signs of tumor formation and survival. We observed that 100% of Lgl1 WT Ink4a/Arf+/− mice were still alive at 60 weeks postnatal age, whereas Lgl1 cKO Ink4a/Arf+/− mice had a significantly shorter median survival of 45.79 weeks (Fig. 4c; p = 0.0108). Histopathologic analyses of Lgl1 cKO Ink4a/Arf+/− brains showed infiltrative tumors consistent with astrocytoma morphology (Fig. 4d). Expression analysis by qrt-PCR in tumor cells isolates showed a strong reduction of p19Arf mRNA versus Lgl1 cKO Ink4a/Arf WT and Ink4a/Arf+/− cells, suggesting that the WT allele of p19Arf is not expressed in the tumor (Supplementary Fig. 5b). Fluorescent immunostainings of brain sections from tumor-bearing mice showed that tumors express the oligodendrocyte lineage and glioma markers Olig2 and NG2. Results are indicative of a mixed astrocytic and oligodendroglial high-grade glioma (Figs. 4d–f)39.
To determine whether our animal model findings were clinically relevant, we mined expression data from 99 patients from The Cancer Genome Atlas (TCGA) for a potential association of Lgl1 mRNA expression levels and glioblastoma patient survival. We found that low levels of Lgl1 mRNA expression correlated with poor patient survival (Supplementary Fig. 5c)40. In further support of a tumor suppressive function, Lgl1 protein expression was absent in six out of seven human glioblastoma xenografts as shown by immunoblotting (Supplementary Fig. 5d). Collectively, these data showed that Lgl1 attenuates OPC proliferation and synergizes with tumor suppressor Ink4a/Arf to suppress gliomagenesis.
Lgl1 cKO OPC show a defect in NG2 routing to the lysosome
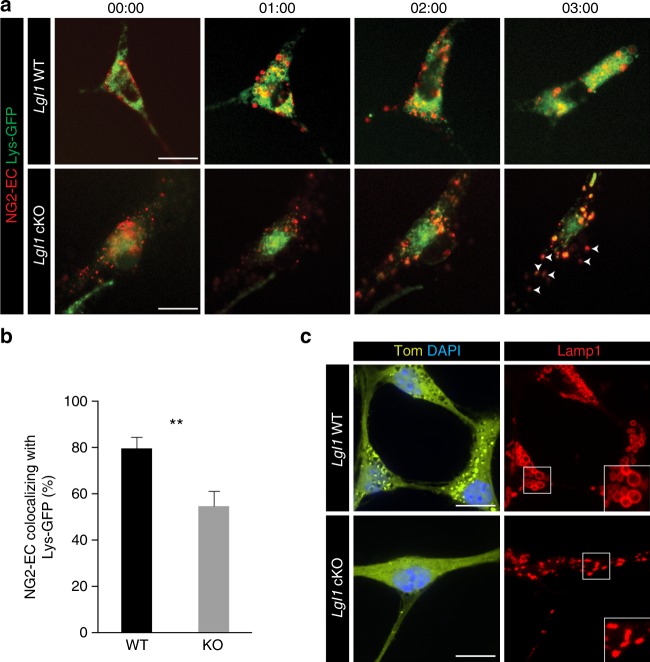
A recent study showed that levels of surface NG2 are regulated by endocytosis15. We hypothesize that Lgl1 cKO OPC fail to route NG2 to the lysosome and thereby sustain aberrantly high NG2 levels. In order to test this, we transfected Lgl1 cKO and WT cells with lysotracker GFP (Lys-GFP) and labeled cell surface NG2 with an antibody specific for its extracellular domain (NG2-EC) and a fluorescent secondary antibody. Cells were consecutively monitored by fluorescent time-lapse microscopy for up to three hours under differentiation conditions. In both Lgl1 cKO and WT OPC, NG2-EC started to reach the lysosome one hour post imaging (Fig. 5a). Co-localization of NG2-EC with Lys-GFP peaked in Lgl1 WT cells three hours after starting the imaging. Interestingly, we observed a significantly higher colocalization of NG2 with lysosomes in WT OPC compared to Lgl1 cKO OPC (WT = 79.6%; cKO = 54.7%, p = 0.0047) (Fig. 5a) suggesting that in differentiating OPC, Lgl1 acts as a positive regulator of NG2 trafficking to the lysosome. These results prompted us to stain for the lysosome associated membrane protein 1 (Lamp1) to investigate whether the lysosome is formed properly in Lgl1 knockout cells. We find that lysosomes are present in Lgl1 cKO OPC, based on Lamp1 positivity and subcellular localization. Noteworthy, lysosome shape appears to be altered due to Lgl1 knockout (Fig. 5c). Collectively, these results show that Lgl1 positively regulates NG2 trafficking to the lysosome.
Fig. 5.
Lgl1 cKO OPC show defect in NG2 routing to the lysosome. a Time-lapse fluorescent pictures of Lgl1 WT and Lgl1 cKO OPC labeled for NG2-EC (red) and lysosome (Lys-GFP, green) under differentiation conditions. Pictures are acquired every hour for 3 hours. White arrows indicate NG2-EC that did not reach the lysosome. Time indicated as hours:minutes. Scale bar: 25 μm. b Quantitative graph representing the ratio of labeled NG2 puncta in the lysosomes in WT (black) and Lgl1 cKO cells (gray). Data are represented as mean ± s.e.m., n = 10 cells each genotype, three independent experiments (**p < 0.01, Student’s t-test). c Immunostaining of NG2dTom cells (yellow) with lysosomal protein Lamp1 (red) showing the difference in lysosome shape between Lgl1 WT OPC and cKO OPC. Scale bar: 25 μm
Non-degraded NG2 in Lgl1 cKO OPC is recycled to the membrane
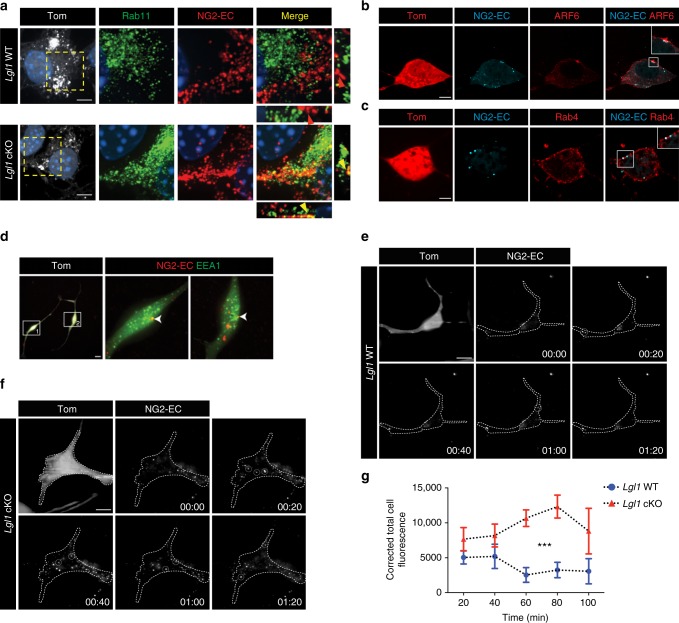
Given that NG2 is a transmembrane proteoglycan41, we suggest that it might be recycled to the membrane by the recycling endosome, a tubular structure continuous with the early endosome. To test this, we incubated Lgl1 cKO cells in differentiating conditions and live labeled NG2-EC. Cells were fixed at different time points after surface NG2 labeling and stained for recycling protein markers. At 45 min, we observed a higher colocalization of NG2-EC with the recycling regulator Rab11 in Lgl1 cKO compared to WT cells (Fig. 6a). At 24 h, NG2-EC colocalizes with ARF6, a GTPase critical for endocytic recycling, supporting the notion that NG2 is recycled to the membrane in Lgl1 cKO OPC (Fig. 6b). Additionally, NG2-EC overlaps with Rab4, a GTPase marker for fast endocytic recycling, hinting that NG2 recycling may not be restricted to the slow recycling pathway (Fig. 6c). We also observed co-localization of NG2-EC with the early endosome marker EEA1 (Fig. 6d), suggesting that NG2 is constantly uptaken and shuffled back to the membrane. Noteworthy, we observed a coexpression of Lgl1 and the early endosome-specific protein Rab10 both at the membrane and around the nucleus, further associating Lgl1 role with the endocytic machinery42 (Supplementary Fig. 6).
Fig. 6.
Non-degraded NG2 in Lgl1 cKO OPC is recycled to the membrane. a Representative co-immunostaining for NG2-EC (red) and Rab11 (green) 45 min after incubation in differentiation medium. Note the higher colocalization of NG2-EC with Rab11 in Lgl1 cKO OPC (yellow arrowheads) compared to Lgl1 WT cells (red arrowhead). Scale bar: 5 μm. b–d Fluorescent immunostaining pictures illustrating colocalization of remaining NG2 (blue) with (b) ARF6 (red), (c) Rab4 (red), and (d) EEA1 (green) in Lgl1 cKO cells 24 hours after incubation in differentiation conditions. Scale bars: 10 μm. e, f Frames illustrating TIRF time lapse of Lgl1 cKO and WT cells 4 hours after incubation in differentiation conditions. Pictures were taken each 20 min. Examples of recycling labeled NG2 puncta (white) are highlighted in dashed circle. NG2 recycling persists in Lgl1 cKO cells up to time point 01:20 whereas it is insignificant in WT cells. Time indicated as hours:minutes. Scale bar: 10 μm. g Graph representing quantification of the corrected total cell fluorescence reflecting NG2 recycling in Lgl1 cKO cells (blue circles) and in Lgl1 WT cells (red lozenges). Data are depicted as mean ± s.e.m., n = 8 cells for each genotype, three independent experiments (***p < 0.01, two-way ANOVA)
In order to better investigate a potential NG2 recycling in Lgl1 cKO cells, we used total internal reflection fluorescence (TIRF) to monitor live-labeled NG2-EC docking to the membrane. Cells were plated in differentiation medium for four hours and then monitored. By this time, the majority of NG2 is degraded in Lgl1 WT cells and no significant NG2 recycling was observed (Fig. 6e,). To the contrary, NG2-EC was more abundant and continuously re-introduced to the membrane in Lgl1 cKO OPC (Figs. 6f, g; Supplementary Movie 1 see Methods).
Collectively, these data proof that in Lgl1 cKO cells, NG2 is mostly recycled to the membrane rather than degraded.
Lgl1 cKO OPC show premature and accelerated endocytosis recycling of NG2
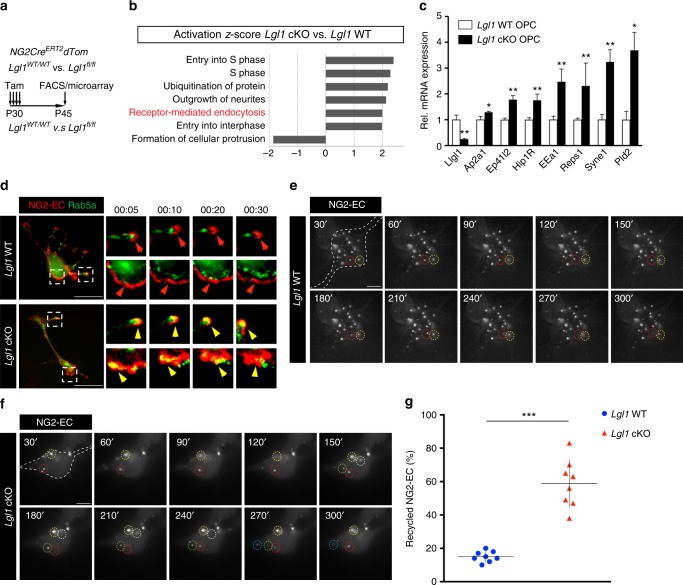
To better characterize the deregulation of endocytosis in Lgl1 knockout cells, we acutely isolated Tom+ cells from Lgl1 NG2-CreERT2 Ai14-TdTom Lgl1fl/fl and Lgl1wt/wt control mice injected with tamoxifen at P30. Fifteen days after starting Lgl1 knockout, gene expression was analyzed using high-sensitivity, whole-transcript arrays (Fig. 7a; see also Methods). By analyzing the 318 genes differentially expressed between Lgl1 WT and Lgl1 cKO OPC (p < 0.05), ingenuity pathway analysis identified several pathways including the receptor-mediated endocytosis pathway to be upregulated in Lgl1 cKO cells (Fig. 7b; activation z-score = 2, p = 0.018). Upregulation of early endocytosis genes Ap2a1, Ep41I2, Hip1R, EEa1, Reps1, Syne1, and Pdl1 was further validated by qrt-PCR in Lgl1 cKO OPC vs. Lgl1 WT OPC (Fig. 7c). These data suggest that Lgl1 knockout deregulates endocytic uptake in addition to endocytic recycling.
Fig. 7.
Lgl1 negatively regulates internalization and recycling of NG2 in OPC. a Schematic illustration of the experimental approach assessing global changes in gene expression by microarray in Lgl1 WT vs. Lgl1 cKO OPC. FACS sorted red fluorescent PDGFRα+O4− cells. b Bar graph of ingenuity pathway analyses (IPA) of Lgl1 WT vs. cKO OPC. Receptor-mediated endocytosis pathway is amongst the upregulated pathways in Lgl1 cKO cells. c Validation by q-RT-PCR for mRNA encoding receptor-mediated endocytosis regulators that were differentially expressed in Lgl1 cKO cells as determined by microarray analyses. OPC were derived from the corpus callosum of GFAP-Cre, Tom, Lgl1wt/wt and, GFAP-Cre, Tom, Lgl1fl/fl mice. Data represented as mean ±s.e.m. n = 6 Lgl1 WT and 6 Lgl1 cKO animals (*p < 0.05, **p < 0.01, Mann–Whitney test). d Steady-state frames of time lapse (1 frame/ 10 min) of NG2-EC (red) and early-endosome marker Rab5a (green) in Lgl1 cKO and WT OPC. Note that NG2 stays mostly at the membrane in Lgl1 WT cells (red arrows), whereas it co-localizes with the early endosome in the cytoplasm of Lgl1 cKO OPC (yellow arrows). Scale bar: 20 µm. e, f Frames illustrating time lapse experiment under TIRF microscopy. Pictures are acquired each 30 seconds as soon as cells were exposed to differentiation conditions. Each white puncta in focus represents labeled NG2-EC docked at the membrane. Individual recycled puncta are highlighted with dashed circles of different colors. Note that in Lgl1 cKO cells, the majority of the labeled NG2 is internalized and recycled to the membrane whereas in WT cells, the majority of NG2 puncta retains a stable localization at the membrane. Scale bars: 10 μm. g Graph representing the quantification of NG2 recycling at the membrane throughout the first 5 min of differentiation conditions. Data represented as mean ± s.e.m. n = 3 cells per genotype, three independent experiments (***p < 0.005, Student's t-test)
In order to investigate if early endocytosis is affected in Lgl1 cKO cells, we labeled surface NG2 with the NG2-EC antibody, and marked the early endosome by expression of Rab5a-GFP. Time-lapse imaging of cells for 30 min in differentiation conditions showed a prominent colocalization of NG2 with Rab5a only in mutant cells, suggesting that Lgl1 negatively regulates NG2 uptake (Fig. 7d). We corroborated these results by quantifying the endocytosis of transferrin using FACS, and found a ten-fold increase in transferrin incorporation in Lgl1 cKO OPC as compared to Lgl1 WT OPC (Supplementary Fig. 7).
Next, we tested whether NG2 is only recycled after failing to be degraded or also when prematurely internalized in Lgl1 cKO OPC. We used TIRF to monitor labeled NG2 docking to the membrane over the first 5 min of differentiation conditions. We observed that a significantly higher ratio of labeled NG2 was endocytosed and recycled to the membrane in Lgl1 cKO cells compared to WT cells (WT = 15%; cKO = 58%, p < 0.0001) (Figs. 7e–g). Taken together, these data show that Lgl1 negatively regulates NG2 internalization and recycling and establish Lgl1 as a regulator of NG2 trafficking by the endocytic pathway.
Differentiation defect in Lgl1 cKO OPC is linked to aberrant NG2 recycling
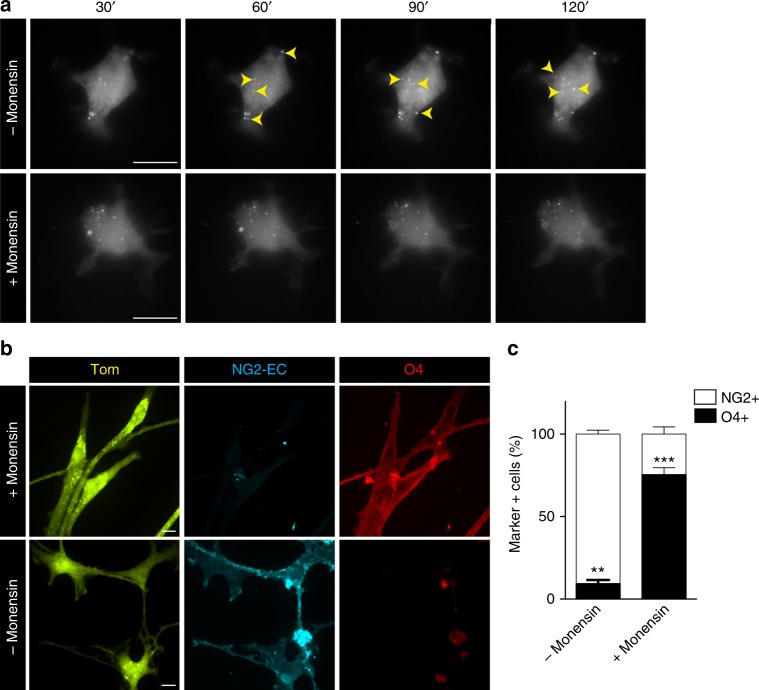
Our studies above suggest that Lgl1 knockout enhances NG2 internalization and recycling to the membrane, thereby preventing NG2 degradation and blocking OL differentiation. In order to dissect whether abnormal NG2 recycling has an effect on OL differentiation, Lgl1 cKO cells were incubated for 4 hours with monensin (10 μM), a drug widely used to interrupt protein recycling43. We tested if the drug has a specific effect on NG2 recycling in Lgl1 cKO cells by monitoring Tom+ cells under TIRF for up to one hour after monensin treatment. We observed that monensin treatment blocked NG2 recycling, validating the reported drug effect on recycling (Fig. 8a)43. Lastly, we assessed differentiation in treated and non-treated Lgl1 cKO cells kept in differentiation conditions for 5 days by O4 and NG2 co-immunostaining. A quantification of marker-positive cells showed that monensin treatment significantly increased the ratio of O4+ cells at the expense of NG2+ cells (treated/O4+ = 75% and NG2+ = 24%; non-treated/O4+ = 9% and NG2+ = 90%; p < 0.0001) (Figs. 8b, c).
Fig. 8.
Aberrant NG2 recycling is linked to differentiation defects. a Steady-state images of two single Lgl1 cKO cells monitored for NG2-EC (white dots) by TIRF, treated (bottom panel) or not treated (upper panel) with monensin. Pictures are acquired each 30 seconds. Note that NG2 is recycled to the membrane in non-treated cells (yellow arrow), whereas no recycling is observed in treated cells. Scale bar: 10 μm. b Fluorescent pictures illustrating Lgl1 cKO cells (Tom) monensin -treated or untreated and labeled for NG2 (blue) and O4 (red) after 5 days in differentiation conditions. Scale bar: 10 μm. c Graph representing quantification of O4+ and NG2+ cells in monensin-treated or untreated Lgl1 cKO cells. Untreated cells are mostly NG2+ and O4−, whereas treated cells show a reverse phenotype with a higher number of cells expressing O4. Data are depicted as mean ± s.e.m., n = 20 cells per genotype, three independent experiments (**p < 0.01, Student's t-test)
These results link abnormal recycling of surface NG2 and defective OL differentiation.
Discussion
Adult OPC differentiate in multiple steps, beginning by committing to OL fate and ending by maturing and producing myelin. There is a wealth of studies on the later steps of OL differentiation and maturation34,44. Little is known, however, about the earliest decisions between OPC and OL fate. Thus far, the down-regulation of proteoglycan NG2 is the earliest immunophenotypic change that distinguishes the two daughter cells within a newly formed OPC pair upon ACD. NG2 marks the OPC daughter, while the NG2-negative daughter acquires CC1 expression, which marks OL differentiation2,3,9. NG2 knockout OPC show delayed proliferation, which indicates that NG2 controls the timing of OPC proliferation7 (reviewed by Sakry et al.45). The NG2 knockout does not fully block OPC proliferation and differentiation and its effect is only temporary7. Thus, downregulation of NG2 is an important but not the only step for OL commitment. Indeed, PDGFRα is also asymmetrically distributed amongst newly formed OPC pairs3, further suggesting that cell fate determinants in addition to NG2 link ACD to differentiation46.
Due to the reported functional, morphologic, and neuroanatomical heterogeneity of OPC47, and its potential influence on cell division mode, cell cycle lengths, and differentiation1,9,48,49, we focused our studies on OPC in the corpus callosum. We analyzed OPC in mice between the ages P30 and P60 with the exception of tumor-bearing mice, which were older. The rationale for analyzing OPC at these stages was that the analyses of mice older than P60 would be technically very difficult due to a long OPC cell cycle time1. We expect to detect the maximum number of ACD between P30 and P60, because the rates of asymmetric OPC divisions are already high at postnatal stages (P20; ACD = ~35%) and peak at P60 (ACD = ~45%) before decreasing again with age (P120; ACD = ~35%)3. Moreover, the rate of differentiation remains stable at 40% between P30 and P609, which makes is a suitable range to identify potential effects on differentiation of a single gene knockout.
Since ACD generate differentiated cells from stem and progenitor cells across species, it was attractive to speculate that conserved regulators of ACD, such as Lgl1, regulate early steps in OL differentiation. Previous studies showed that Lgl1 localizes to the lateral membranes and cytoplasm of embryonic neural progenitor cells21,22. Expression of Lgl1 protein in rat neocortex homogenates persists into adulthood42. Functional analyses in the adult brain, however, were limited to its role in SVZ neural stem cells, neurons, and astrocytes21–23,42.
Here, we show that Lgl1 cKO cells fail to lose NG2 expression and exhibit differentiation defects. Interestingly, we find that Lgl1 knockout in OPC increases the rates of cells in a transitional state and expressing both OPC marker NG2 and OL marker CC1, at the expense of mature OL. These data indicated that Lgl1 loss does not prevent the expression of CC1 but rather prevents downregulation of NG2, and thereby permanently attenuates differentiation.
Whereas normal OPC populate the brain sparsely and show little overlap in the areas that two adjacent OPCs occupy50, they are denser in demyelinated lesions, such as those typically found in chronic inflammation models in mice and in multiple sclerosis patients32,33. We found that Lgl1 is a critical regulator of OL differentiation during remyelination following chemical-induced demyelination. OPC are considered a critical source for remyelination and failed OPC differentiation are thought to be one important underlying cause for failed remyelination in chronically demyelinated lesions51. It is interesting to speculate that Lgl1 functions at an early step in the complex regulatory process of remyelination and thus may be a viable target for approaches aimed at promoting proper differentiation of OPCs in demyelinated lesions.
Our analyses of cell division modes support the hypothesis that Lgl1 restricts NG2 during ACD as part of the mechanism to turn on OL differentiation in the NG2-negative progeny. Moreover, Lgl1 does not disrupt symmetric, differentiating divisions, which suggests that it specifically regulates differentiation in the ACD progeny. To date, only a few conserved ACD regulators have a known function in OL differentiation in addition to Lgl1. These include the polarity regulator partitioning-defective 3 (Par3) protein, which promotes myelination in the peripheral nervous system52, and the transcriptional regulator Prox1, which is required for OL lineage in the mouse. Par3, Prox1, and Lgl1 are in the same protein network that controls ACD in Drosophila neuroblasts46. A role for Par3 and Prox1 in ACD of OPC has yet to be determined.
Multiple studies have shown that OPC13,14 and OL36 are a cellular origin of glioma in genetically engineered mouse models. The neoplastic transformation of OPC and OL and formation of gliomas are associated with increased proliferation, decreased differentiation, changes in the tumor microenvironment53, and a decrease in ACD2. Lgl1 knockout during embryonic neurogenesis disrupts the asymmetric division of neuroepithelial cells and generates rosette-like structures reminiscent of PNETs but perinatal death prevented the analyses of tumor formation21. Lgl1 knockout in cell types known to only undergo symmetric but not asymmetric divisions, such as astrocytes, did not generate premalignant lesions23. Taken together with our data, these findings suggest that defective ACD might be an underlying cause for proliferation and differentiation defects in glioma cells. Our data support the notion previously observed in epithelial cells54 that loss of polarity—albeit by itself not sufficient to induce carcinogenesis—contributes to tumor formation. We show that Lgl1 loss synergizes with Ink4a/Arf loss, which is a frequent and early event in glioma24, to induce gliomagenesis (Fig. 4). There is strong precedence for Ink4a/Arf heterozygous mice to be as susceptible as Ink4a/Arf nullizygous mice to gliomagenesis55. A likely explanation for this is, that the remaining Ink4a/Arf allele in tumors arising from Ink4a/Arf+/− mice is inactivated at high frequency, which is supported by our findings that p19Arf mRNA expression is greatly reduced in Lgl1 KO tumors. Although 19Arf and p16Ink transcripts are reportedly low in OPC and OL, we find overlapping expression of the p19Arf protein and OL marker CC1 (Supplementary Fig. 5). This is interesting, because p19Arf but not p16Ink is an important tumor suppressor in a glioma mouse model study36. Collectively, the data suggest that tumor formation in NG2-Cre Lgl1 KO Ink4a/Arf+/− mice required inactivation of p19Arf expression, hence the long latency (Supplementary Fig. 5). It is also plausible that Lgl1 suppresses tumor formation by restricting NG2 function as a co-receptor for receptor tyrosine kinases, since deregulated PDGFRα and EGFR signaling activity contributes to gliomagenesis40,56 (for review see ref. 57).
Endocytosis has a range of functions including regulating the cell surface expression and subcellular localization of proteins, remodeling the membrane and controlling signaling pathways16. Studies in Drosophila epithelia cells support a link of polarized protein localization and endocytosis by showing that loss-of-function mutants of the critical endosome adaptor AP2 leads to defects in polarity and differentiation similar to those observed in Lgl loss-of-function experiments58. Our study proposes an unprecedented link between defects in NG2 endocytic trafficking, ACD, and differentiation. Indeed, in Lgl1 cKO mutant cells, we find that NG2 is not properly routed to the lysosome and instead is recycled to the membrane. These observations show that endocytic pathway defects in Lgl1 cKO OPC disturb the regular intracellular itinerary of NG2 during differentiation and lead to its recycling rather than degradation. NG2 levels are permanently suppressed in OL most likely as a result of downregulation of the NG2 promoter in the OL daughter after ACD3.
Taken together, these data suggest that the unequal distribution of NG2 in the newly formed progeny of ACD is achieved by NG2 degradation in the lysosome and is maintained by silencing the NG2 promoter in the OL daughter.
We can only speculate as to whether Lgl1 regulates NG2 trafficking directly or, indirectly, by regulating the endocytic machinery. Although not previously implicated as a negative regulator of endocytosis in mammalian cells, Lgl1 activates endocytosis protein and GTPase Rab10 by releasing the GDP dissociation inhibitor (GDI) in rat hippocampus to induce polarized axon growth42. In cells undergoing ACD, such as Drosophila neuroblasts, Lgl1 establishes cell polarity by mechanisms that regulate myosin II activity, directed vesicle transport and the subcellular localization of proteins (for review see ref. 46). Nonmuscle myosin II (NMII) is a critical regulator of clathrin-mediated endocytosis59 and is a negative regulator of oligodendrocyte morphological differentiation60. Lgl1 associates with NMII and inhibits filament formation61 and reduces the association of NMII with actin. Whether Lgl1 regulates endocytosis by affecting motor protein dynamics or through other mechanisms, such as regulating the subcellular localization of the GTPases62,63, remains to be determined. In further support of an indirect effect of Lgl1 on endocytic trafficking, Drosophila Lgl1 regulates vesicle acidification in the lysosome64,65. Noteworthy, our data indicate that lysosomes in Lgl1 cKO OPC have a tubular shape rather than the vesicular shape seen in WT OPC, hinting that lysosome maturation might be affected by Lgl1 knockout (Fig. 5).
Future studies should investigate further the potential link of defects in endocytosis and gliomagenesis.
Methods
Mice
All mouse experiments were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of California, San Francisco. Lgl1lox/lox mice were courtesy of Dr. Vasioukhin21 and were bred with NG2-Cre (Jax strain 008533)37 and GFAP-Cre (Jax strain 004600)66, NG2-CreERTM (Jax strain 008583)9 and Ai14 Cre reporter mice (Jax strain 007908)29 mice or Ink4a/Arf hemizygous knockout mice38. Ascl1GFP67 and NG2DsRed37 mice from Jackson Laboratories were bred together to obtain Ascl1GFP-NG2DsRed double transgenic mice. FVB/N mice were obtained from Simonsen Laboratories, Gilroy. For survival studies, Lgl1 cre-conditional deleted mice were kept under observation until reaching the major endpoint, which was animal neurological symptom-free survival.
Induction of Lgl1 knockout and demyelination
Tamoxifen (Sigma-Aldrich) was dissolved in corn oil (Sigma-Aldrich) at 20 mg/mL and injected intraperitoneally (i.p.) at 1 mg to induce Cre activity Lα-lysophosphatidylcholine (lysolecithin = LPC, Sigma-Aldrich) was dissolved at 1% in physiological serum and 0.5 μl were injected to induce demyelination. For clonal labeling of NG2 cells P30-P52 NG2-CreERT2, TdTom, Lgl1fl/fl and NG2-CreERT2, TdTom, Lgl1wt/wt mice were injected with 1 mg tamoxifen twice within 24 h35. To assess proliferation rates and cell division modes of OPC, mice were subsequently treated with 0.1 mg/mL EdU in drinking water for 14 days and resected brains were processed for immunofluorescence. For differentiation NG2-CreERT2, TdTom, Lgl1fl/fl and NG2-CreERT2, TdTom, Lgl1wt/wt mice were injected with tamoxifen either once or twice daily for 4 consecutive days. Mice were analyzed 30 days post injection.
For broader deletion of Lgl1, P30-P52 NG2-CreERT2, TdTom, Lgl1fl/fl and NG2-CreERT2, TdTom, Lgl1wt/wt mice were injected with tamoxifen twice daily for 4 consecutive days. FACS isolated OPC were subjected at P45 to microarray analyses. To assess proliferation and cell division modes in demyelinated lesions, demyelination was induced by a single LPC injection into the CC or the spinal cord of mice previously injected with tamoxifen. For differentiation, demyelination was induced by LPC injection after the last tamoxifen injection and mice were sacrificed 10 days after to evaluate the effect on OPC differentiation. For spinal cord white matter demyelination, mice were anesthetized with isofluorane and demyelination was induced by LPC into the ventral or dorsal white matter funiculus at the level of T1268,69. For CC demyelination, LPC was injected in the right or the left hemisphere (AP+1 mm, L ± 1 mm, DV-2.1 mm)70. For EdU labeling, mice were injected i.p. with 10 mg/mL EdU twice a day, or EdU was mixed with drinking water at a low concentration (0.1 mg/mL) for 5–14 days. For long-term tracking of Lgl1 deletion effects, tamoxifen was injected at P30 for 5 consecutive days, and tissue analyzed by FACS at P120.
Cell culture
Cells were isolated from the CC, which includes the subcallosal zone of P30–P60 mice, and cultured as non-adherent spheres in proliferative medium with NT3, CNTF, PDGFA, and FGF2 (Peprotech)34. Differentiation was induced by plating cells on poly-l-Lysine coated glass-bottom 96-well plates (CORNING) at 5000 cells/mL in medium containing CNTF, FGF2, and T32. Cells were incubated with inhibitor of endocytosis Pitstop1 at 25 μM. To induce Lgl1 deletion in vitro, cells were incubated with adenovirus-cre-GFP (Vector BioLabs) and adenovirus-GFP2. Viability assay was performed using the Alamar Blue reagents71.
Pair assay
Cells were subjected to a pair assay by plating dissociated single cells at clonal density for 12–36 h. Cell pairs formed were fixed and subjected to immunostaining with primary antibody and fluorescence-coupled secondary antibody2. Nuclei were visualized using DAPI (Sigma). Unless indicated otherwise, for pair assay a minimum of 50 and up to 200 cell pairs per cell type and condition, respectively, were analyzed, using a fluorescent microscope.
Immunofluorescence and in vivo cell pair assays
NG2-CreERT2,TdTom, Lgl1fl/fl and NG2-CreERT2,TdTom, Lgl1wt/wt mice injected with tamoxifen were PFA perfused, brains were resected and frozen, sectioned and post-fixed for 10 min with 4% PFA, blocked with PBS 5% normal goat serum (NGS) or normal donkey serum (NDS) and 0.1% Triton X-100 for 1 h. For cell pair assays, sections were stained for anti-red fluorescence antibody (to detect the NG2+ cells and progeny with Lgl1 deletion) and anti-CC1 antibody overnight, washed and stained with fluorescence-couple secondary antibodies. Cell pairs were identified as two red fluorescence-positive (NG2Tom) and EdU-positive cells spaced less than 20 µm2,3. The entire CC was counted as three or more distinct brain sections per animal in 4–5 individual animals per group. Cell pairs with one CC1-positive cell were scored as an ACD.
The following antibodies were used at the given concentration:
α-Rb NG2 (Millipore 1:200), αMs-IgM O4 (R&D, 1:400), α-Ms CC1 (Calbiochem, 1:100), α-Gt Dcx (Santa Cruz Biotechnology, 1:300), α-Gt Hugl-1 (Lgl1, Santa Cruz biotechnology, 1:200), α-Rb Hugl-1 (Lgl1, Santa Cruz Biotechnology, 1:200), α-Ms Nestin (Chemicon international, 1:400), α-Rb PlP (abcam105784, 1:1000), α-Rat MBP (abcam7349, 1:400), α-Rb-beta galactosidase (ab616, 1:200), gt-α-Olig2 (EMD Millipore; 1:200), α-Rb-Rab11 (Life Technologies Corporation, 1:200); α-Rb-p19Arf (Santa Cruz; 1:100); α-Gt-Lamp1 (R and D systems AF4320, 1:100); α-ARF 6 (Santa Cruz sc-7971; 1:200), α-Ms EEA1(Santa Cruz sc-365652, 1:150); α-Rb-Rab4 (Millipore 07-655; 1:200); α-Ms-Rab10 (Life Technologies Corporation, 1:100).
For O4 staining, cells were stained live in vitro: O4 was incubated 1 h (1:400) at 37 °C in the corresponding culture medium (OPC proliferation or OL differentiation) before PFA fixation and further antibody staining.
EdU was visualized using the Click-it reaction kit (Life Technologies Corporation) following the manufacturer’s instructions71.
Early endocytosis compartment was visualized using the BacMam technology following manufacturer’s instructions (Life Technologies Corporation). Immunofluorescent images were acquired on a Nikon Ti-E microscope with Nikon perfect focus system equipped with a Hamamatsu Flash4.0 camera equipped with a 40×/0.75 Plan Fluor objective and/or Nikon Ti Yokogawa CSU-22 spinning disk confocal equipped with a Plan Apo VC 60×/1.4 oil objective. Images were analyzed with NIS-Elements 4.2 (Build 982) and ImageJ.
FACS
NG2-CreERT2,TdTom, Lgl1wt/wt and, NG2-CreERT2,TdTom, Lgl1fl/fl mice were analyzed by FACS 30 days or 120 days after tamoxifen injection at P30. Lateral ventricle walls and/or CC cells were isolated from Ascl1-GFP and NG2-DsRed double-transgenic mice at P60. For DNA content analysis, dissociated cells were incubated with the vital DNA marker Hoechst 33342 (Sigma)72. The antibodies to identify distinct cell populations were anti-O4–biotin (MACS Miltenyi Biotec; 1:10) conjugated to streptavidin–FITC (MACS Miltenyi Biotec; 1:10); CD140a phycoerythrin–cyanine7 [PC7]-conjugated (mouse clone APA5; 1:100 eBiosciences); CD24 phycoerythrin–cyanine7 [PC7]-conjugated (Rat IgG2b; 1:100 Life Technologies); CD140b [APC]-conjugated (life technologies clone APB5, 1:50), and Glast-APC (MACS miltenyi, 1:30). Cells were gated following the fluorescence minus one (FMO) control. For microarray analyses, CC tissue was isolated from NG2-CreERT2, TdTom, Lgl1fl/fl (n = 4) and NG2-CreERT2, TdTom, Lgl1wt/wt (n = 5) triple-transgenic, P30 mice injected twice daily with tamoxifen for 4 consecutive days and OPC were obtained by sorting for Tom-positive, PDGFRα-positive O4-negative cells.
To perform absolute cell counts, single cell suspensions were transferred to tubes containing a calibrated number of fluorescent beads (TruCount tubes, BD Biosciences)73. Prior to FACS sorting propidium iodide (PI) or Hoechst 33258 was added to a final concentration of 2 µg/mL to label the dead cells. Cells were analyzed and sorted on a FACS ARIA II (BD Biosciences). The data were analyzed with FlowJo data analysis software (Tree Star, Ashland, OR, USA).
Immunoblotting
Glioblastoma xenografts proteins were extracted using cell lysis buffer (Cell Signaling) supplemented with proteinase and phosSTOP phosphatase inhibitor cocktail (Roche). Proteins were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes, which were then probed with primary antibodies, followed by horseradish peroxidase-conjugated secondary antibody, and visualized by ECL (GE Healthcare). Antibodies specific for Hugl1 and tubulin as loading control were from Sigma.
RNA extraction and reverse transcription
For all experiments, RNAs were extracted with QIAGEN RNeasy microkit and reverse transcribed into cDNAs using high capacity RNA to cDNA kit (Applied Biosystems).
Taqman low-density arrays
For the Taqman low-density array experiment, 200–500 cells/population were sorted from the CC of young adult Ascl1-GFP and NG2-DsRed double-transgenic mice. TaqMan® Array Micro Fluidic Card were designed for neural stem cell, oligodendrocyte progenitor cell and differentiated oligodendrocyte markers, as well as well selected ACD regulators. A PreAmp pool was designed for these specific primers and a preamplification reaction was set up to 14 cycles according to manufacturer’s instructions (Custom TaqMan® PreAmp Pools Protocol, Applied Biosystems).
Whole-genome microarrays
For the microarray experiments 200–500 Lgl1 knockout and wildtype OPC cells (PDGFRα and red fluorescent protein positive O4 negative), were extracted with the RNA plus micro kit (QIAGEN) and reverse transcription into cDNAs was performed using the High Capacity Reverse Transcription Kit (Thermofisher scientific). Sample quality was controlled using the Agilent Bioanalyzer and samples were loaded on GeneChipR Mouse Gene 2.0 ST Arrays (Affymetrix). Microarray data were analyzed using Partek® Genomic Suite 6.6 with RMA normalization and differential gene expression detected with ANOVA. Ingenuity pathway analyses (QIAGEN bioinformatics) were used to identify targets and pathway differentially expressed between the Lgl1 WT and Lgl1 cKO samples.
Real-time quantitative reverse transcription PCR
Receptor-mediated endocytosis regulator genes were validated by qRT-PCR on OPC cultured from GFAPCre, dTomfl/fl, Lgl wt (n = 6) vs. Lgl1fl/fl (n = 6) mice using the following primers: Ap2a1 (5′–3′: GGAACCTCCTGGTGGATGTC, 3′–5′: AGGACCTGGGCTGAGGAAG); Epb41l2 (5′–3′: GCCCCAGTCAGGGAATTTCA, 3′–5′: TTACCGCTTACACCGGACAC); Hip1r (5′–3′: ATCTGGCCAGGAGCAGATTG, 3′–5′: AAGACTCGCACCTGTGTCTC) Eea1 (5′–3′: TCTTCAGAGGGTTTCATATGTCCC, 3′–5′: TGAGGCCTGTAGGTCTTGGA) Reps1 (5′–3′: GCAGATACTCCACCAACCAGT, 3′–5′: GTACTGTCGTCTGGTCCTGG); Syne1 (5′–3′: GACATGCAGGGAGTCACACA, 3′–5′: CTTTTTGCTGCTGGAGGCTG); Pld2 (5′–3′: ATCCTGAAGGCTCACGAACAG, 3′–5′: GAAGAAAGGGTGAAGGAGGCA); p19Arf (Forward: 5′–3′: TGAGGCTAGAGAGGATCTTGAGAAG, Reverse: 5′–3′: GTGAACGTTGCCCATCATCATC); LLgl1 (exon 2–3 Forward: 5′–3′: CCACAAGACTGTGGAGCATGG, Reverse: 5′–3′: AAA-TGC-ATC-TGG-GTG-ACG-GT).
Transferrin uptake
The CC of GFAP-Cre, dTomlox/lox, Lgl1 wt/wt(n = 4) vs. Lgl1 ox/lox (n = 4) was extracted from young adult mice (P60). After enzymatic digestion with papain (10 min, 15 U/mL)72, cells were washed with cold wash solution (PBS containing 20 mM glucose and 1% BSA). Then Transferrin Alexa Fluor 488 conjugate (Molecular probes, T13342)74 was added at 25 µg/mL and incubated at 37 °C for 15 min. Non-internalized ligand was removed from the cell surface using three 5 min wash with ice-cold freshly prepared stripping buffer (PBS/0.15% BSA, pH 3.5 adjusted with HCL) and washed three times with cold wash solution. Data analysis to quantify changes in Transferrin fluorescence in dTom+ cells was performed using a FACS Aria II (BD Biosciences) and analyzed with FlowJo data analysis software (Tree Star, Ashland, OR, USA).
Live staining of cells
Plated Lgl1 cKO and WT OPC were incubated on ice at 4 °C for 2 h with α-NG2-EC (Rabbit, courtesy of Dr. Stallcup) in Neurobasal medium (3% BSA). After two washes with neurobasal medium, cells were incubated for 1 h with an Alexa488 goat α-Rabbit. For lysosome tracking, cultured OPC were incubated with CellLight Reagent BacMam 2.0 (Molecular probe) 24 h prior to live imaging targeting the early endosome Rab5a (C10586) and lysosome-specific protein Lamp1 (C10507) at a concentration of 50 PPC.
Fluorescence time lapse microscopy
Time lapse movies were taken under a conventional widefield epifluorescence system (Nikon) equipped with a 37 °C incubation chamber and CO2 control for live cell imaging. Live-labeled cells were incubated in differentiation conditions and the system programed to take fluorescent pictures at the following frequencies: 1 frame/30 s to follow early NG2 endocytosis and 1 frame/1 h to follow its trafficking towards lysosomes.
TIRF microscopy
Live labeled cells (NG2-EC) were plated in differentiation conditions and placed on a coupled incubator for live cell imaging (37 °C, 5% CO2) under the TIRF microscope. Fluorescent pictures were taken at different frequencies: 1 frame/30 min or 1 frame/30 s. TIRF was used to provide a mean to selectively excite fluorophore very near the solid surface (<100 nm) without exciting fluorescence from regions farther from the surface. Therefore, any Alexa488 positive dot in perfect focus was considered as a docking NG2 on the membrane region that is being imaged. Different quantities of NG2 recycling over time were represented as total corrected fluorescence measured using ImageJ.
Recycling inhibition
Lgl1 cKO OPC were treated with differentiation medium containing 10 μM Monensin (TOCRIS, 5223) for 4 h. Cells were monitored under TIRF for NG2 recycling monitoring during the first hour of treatment or were switched back to normal differentiation medium for 5 days before being fixed and immunostained for O4 and NG2. Endocytosis of the proteoglycan NG2 was followed using a mouse-anti-NG2.EC antibody against the extracellular domain of NG2 (courtesy of Dr. Stallcup). OPC cultures were incubated 24 h prior to live imaging with CellLight® Reagents BacMam 2.0 (Molecular Probes) targeting the early endosome-specific protein Rab5a (ref C10586) of lysosome-specific protein Lamp1 (ref C10507) at a concentration of 50 PPC. Cells were starved 2 h prior to imaging in NBA medium, and 50 ng/mL of PDGF-AA was added right before imaging to induce endocytosis. Time-lapse movies were taken over time spans of 30–45 min with 30 s interval between the images to follow early endocytosis of NG2 antibody and 2–3 h with a 30 min interval to follow its internalization to the lysosome. After live imaging, cells were fixed 10 min in PFA4%, stained with the appropriate antibodies and imaged with a Nikon Ti Yokogawa CSU-22 spinning disk confocal equipped with a Plan Apo VC 60×/1.4 oil objective.
Endocytosis inhibitor
Monensin (TOCRIS, 5223), a selective recycling endocytosis75, was used and was not toxic at a working concentration of 10 µM.
Univariate Cox model
The prognostic significance of high Lgl1 expression (≥median) on overall survival (defined as the time between diagnosis and death due to any cause) was retrospectively tested. The follow-up period ranged from 0 to 8 years (median = 1 year). The number of events that occurred during this period and the number of censored individuals are: 86 events, with 13 censored.
Statistics and analysis
Statistical analyses were conducted as indicated in the figure legends using GraphPad Prism 5.0 and Microsoft Excel. A Mann and Whitney two-tailed paired t-test was conducted to analyze for significant differences between two treatment groups. For Kaplan–Meier survival rate analyses, the number of surviving mice were recorded daily. The data were subjected to Log-rank test in order to determine if significant differences existed in survival between the Lgl1 knockout and control groups. For statistical tests *p < 0.05,**p < 0.01,***p < 0.001. A univariate Cox models was fitted on the survival data of the 99 glioma patients (92 GBM and 7 LGG) to obtain p-values. R-function ‘survfit’ (package ‘survival’) was used to generate survival curves from the previously fitted Cox model.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
We thank Banafsheh Kadkhodaei, Ian D. Meyers, Helena Oft, Sonia Spindt and Jacky Huang for technical assistance and Valeri Vasioukhin for resources. We would like to acknowledge DeLaine Larsen and Kari Herrington at the Nikon Imaging Center at UCSF for technical support. We thank Martin Oft, Amy Sprowles, Russ Pieper, and William A. Weiss for their critical comments. S.F. is a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society. Funding was provided by the National Cancer Institute (R01CA16476-04 to C.K.P.) and the National Institute of Neurological Disorders and Stroke (R01NS080619 to C.K.P.; R01NS097551 to S.F.) of the National Institute of Health; the Loglio Research Program (to C.K.P.), the Research Allocation Program Investigator Grant UCSF (to C.K.P.), the University of California Cancer Research Coordination Committee (CA-0095729 to C.K.P.), and California Institute of Regenerative Medicine (to H.Y.C).
Author contributions
Conceptualization, M.D., M.C., and C.K.P.; Methodology, M.D., M.C., S.F., C.K.P.; Investigation, M.D., M.C., H.Y.C.; N.E.M., N.A., J.N.; Resources, W.S.; Writing-original draft, M.D., C.K.P.; Writing-review and editing, M.D., M.C., and C.K.P.; Funding acquisition, C.K.P.; Supervision, C.K.P.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mathieu Daynac, Malek Chouchane.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-05099-3.
References
- 1.Young KM, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugiarto S, et al. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell. 2011;20:328–340. doi: 10.1016/j.ccr.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boda E, et al. Early phenotypic asymmetry of sister oligodendrocyte progenitor cells after mitosis and its modulation by aging and extrinsic factors. Glia. 2015;63:271–286. doi: 10.1002/glia.22750. [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J. Neurosci. Res. 1996;43:315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Goretzki L, Burg MA, Grako KA, Stallcup WB. High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J. Biol. Chem. 1999;274:16831–16837. doi: 10.1074/jbc.274.24.16831. [DOI] [PubMed] [Google Scholar]
- 6.Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- 7.Kucharova K, Stallcup WB. The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination. Neuroscience. 2010;166:185–194. doi: 10.1016/j.neuroscience.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bin JM, Harris SN, Kennedy TE. The oligodendrocyte-specific antibody ‘CC1’ binds Quaking 7. J. Neurochem. 2016;139:181–186. doi: 10.1111/jnc.13745. [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, et al. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A. Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat. Neurosci. 2014;17:1518–1527. doi: 10.1038/nn.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assanah M, et al. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J. Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindberg N, Kastemar M, Olofsson T, Smits A, Uhrbom L. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene. 2009;28:2266–2275. doi: 10.1038/onc.2009.76. [DOI] [PubMed] [Google Scholar]
- 13.Persson AI, et al. Non-stem cell origin for oligodendroglioma. Cancer Cell. 2010;18:669–682. doi: 10.1016/j.ccr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feutlinske F, et al. Stonin1 mediates endocytosis of the proteoglycan NG2 and regulates focal adhesion dynamics and cell motility. Nat. Commun. 2015;6:8535. doi: 10.1038/ncomms9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 17.Gateff E, Mechler BM. Tumor-suppressor genes of Drosophila melanogaster. Crit. Rev. Oncog. 1989;1:221–245. [PubMed] [Google Scholar]
- 18.Peng CY, Manning L, Albertson R, Doe CQ. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. [DOI] [PubMed] [Google Scholar]
- 19.Vasioukhin V. Lethal giant puzzle of Lgl. Dev. Neurosci. 2006;28:13–24. doi: 10.1159/000090749. [DOI] [PubMed] [Google Scholar]
- 20.Sripathy S, Lee M, Vasioukhin V. Mammalian Llgl2 is necessary for proper branching morphogenesis during placental development. Mol. Cell Biol. 2011;31:2920–2933. doi: 10.1128/MCB.05431-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jossin Y, et al. Llgl1 connects cell polarity with cell-cell adhesion in embryonic neural stem cells. Dev. Cell. 2017;41:481–495 e485. doi: 10.1016/j.devcel.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beattie R, et al. Mosaic analysis with double markers reveals distinct sequential functions of Lgl1 in neural stem cells. Neuron. 2017;94:517–533 e513. doi: 10.1016/j.neuron.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Ozawa T, et al. Most human non-GCIMP glioblastoma subtypes evolve from a common proneural-like precursor glioma. Cancer Cell. 2014;26:288–300. doi: 10.1016/j.ccr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gont A, et al. PTEN loss represses glioblastoma tumor initiating cell differentiation via inactivation of Lgl1. Oncotarget. 2013;4:1266–1279. doi: 10.18632/oncotarget.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gont A, et al. Inhibition of glioblastoma malignancy by Lgl1. Oncotarget. 2014;5:11541–11551. doi: 10.18632/oncotarget.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill RA, Nishiyama A. NG2 cells (polydendrocytes): listeners to the neural network with diverse properties. Glia. 2014;62:1195–1210. doi: 10.1002/glia.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daynac M, Petritsch CK. Regulation of asymmetric cell division in mammalian neural stem and cancer precursor cells. Results Probl. Cell Differ. 2017;61:375–399. doi: 10.1007/978-3-319-53150-2_17. [DOI] [PubMed] [Google Scholar]
- 32.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds R, et al. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J. Neurocytol. 2002;31:523–536. doi: 10.1023/A:1025747832215. [DOI] [PubMed] [Google Scholar]
- 34.Dugas JC, Tai YC, Speed TP, Ngai J, Barres BA. Functional genomic analysis of oligodendrocyte differentiation. J. Neurosci. 2006;26:10967–10983. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calzolari F, et al. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat. Neurosci. 2015;18:490–492. doi: 10.1038/nn.3963. [DOI] [PubMed] [Google Scholar]
- 36.Lindberg N, et al. Oncogenic signaling is dominant to cell of origin and dictates astrocytic or oligodendroglial tumor development from oligodendrocyte precursor cells. J. Neurosci. 2014;34:14644–14651. doi: 10.1523/JNEUROSCI.2977-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 38.Huillard E, et al. Cooperative interactions of BRAFV600E kinase and CDKN2A locus deficiency in pediatric malignant astrocytoma as a basis for rational therapy. Proc. Natl Acad. Sci. USA. 2012;109:8710–8715. doi: 10.1073/pnas.1117255109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerner RG, et al. Targeting a Plk1-controlled polarity checkpoint in therapy-resistant glioblastoma-propagating cells. Cancer Res. 2015;75:5355–5366. doi: 10.1158/0008-5472.CAN-14-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhaak RG, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiyama A, Dahlin KJ, Prince JT, Johnstone SR, Stallcup WB. The primary structure of NG2, a novel membrane-spanning proteoglycan. J. Cell Biol. 1991;114:359–371. doi: 10.1083/jcb.114.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang T, et al. Lgl1 activation of rab10 promotes axonal membrane trafficking underlying neuronal polarization. Dev. Cell. 2011;21:431–444. doi: 10.1016/j.devcel.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Stein BS, Bensch KG, Sussman HH. Complete inhibition of transferrin recycling by monensin in K562 cells. J. Biol. Chem. 1984;259:14762–14772. [PubMed] [Google Scholar]
- 44.He L, Lu QR. Coordinated control of oligodendrocyte development by extrinsic and intrinsic signaling cues. Neurosci. Bull. 2013;29:129–143. doi: 10.1007/s12264-013-1318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakry D, Karram K, Trotter J. Synapses between NG2 glia and neurons. J. Anat. 2011;219:2–7. doi: 10.1111/j.1469-7580.2011.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez-Lopez S, Lerner RG, Petritsch C. Asymmetric cell division of stem and progenitor cells during homeostasis and cancer. Cell Mol. Life Sci. 2014;71:575–597. doi: 10.1007/s00018-013-1386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63:1429–1451. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ge WP, Zhou W, Luo Q, Jan LY, Jan YN. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc. Natl Acad. Sci. USA. 2009;106:328–333. doi: 10.1073/pnas.0811353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- 50.Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franklin RJ, Goldman SA. Glia disease and repair-remyelination. Cold Spring Harb. Perspect. Biol. 2015;7:a020594. doi: 10.1101/cshperspect.a020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tep C, et al. Brain-derived neurotrophic factor (BDNF) induces polarized signaling of small GTPase (Rac1) protein at the onset of Schwann cell myelination through partitioning-defective 3 (Par3) protein. J. Biol. Chem. 2012;287:1600–1608. doi: 10.1074/jbc.M111.312736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galvao RP, et al. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc. Natl Acad. Sci. USA. 2014;111:E4214–E4223. doi: 10.1073/pnas.1414389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shivas JM, Morrison HA, Bilder D, Skop AR. Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 2010;20:445–452. doi: 10.1016/j.tcb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holland EC, Hively WP, Gallo V, Varmus HE. Modeling mutations in the G1 arrest pathway in human gliomas: overexpression of CDK4 but not loss of INK4a-ARF induces hyperploidy in cultured mouse astrocytes. Genes Dev. 1998;12:3644–3649. doi: 10.1101/gad.12.23.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozawa T, et al. PDGFRA gene rearrangements are frequent genetic events in PDGFRA-amplified glioblastomas. Genes Dev. 2010;24:2205–2218. doi: 10.1101/gad.1972310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stallcup W. B. NG2 proteoglycan enhances brain tumor progression by promoting beta-1 integrin activation in both cis and trans orientations. Cancers9, 31 (2017). [DOI] [PMC free article] [PubMed]
- 58.de Vreede G, et al. The Scribble module regulates retromer-dependent endocytic trafficking during epithelial polarization. Development. 2014;141:2796–2802. doi: 10.1242/dev.105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandrasekar I, et al. Nonmuscle myosin II is a critical regulator of clathrin-mediated endocytosis. Traffic. 2014;15:418–432. doi: 10.1111/tra.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, et al. Myosin II is a negative regulator of oligodendrocyte morphological differentiation. J. Neurosci. Res. 2012;90:1547–1556. doi: 10.1002/jnr.23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dahan I, Yearim A, Touboul Y, Ravid S. The tumor suppressor Lgl1 regulates NMII-A cellular distribution and focal adhesion morphology to optimize cell migration. Mol. Biol. Cell. 2012;23:591–601. doi: 10.1091/mbc.e11-01-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanley AC, et al. The Rho GTPase Rac1 is required for recycling endosome-mediated secretion of TNF in macrophages. Immunol. Cell Biol. 2014;92:275–286. doi: 10.1038/icb.2013.90. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka Y, et al. The phospholipid flippase ATP9A is required for the recycling pathway from the endosomes to the plasma membrane. Mol. Biol. Cell. 2016;27:3883–3893. doi: 10.1091/mbc.e16-08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parsons LM, Portela M, Grzeschik NA, Richardson HE. Lgl regulates Notch signaling via endocytosis, independently of the apical aPKC-Par6-Baz polarity complex. Curr. Biol. 2014;24:2073–2084. doi: 10.1016/j.cub.2014.07.075. [DOI] [PubMed] [Google Scholar]
- 65.Portela M, Parsons LM, Grzeschik NA, Richardson HE. Regulation of Notch signaling and endocytosis by the Lgl neoplastic tumor suppressor. Cell Cycle. 2015;14:1496–1506. doi: 10.1080/15384101.2015.1026515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhuo L, et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- 67.Gong S, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 68.Arnett HA, et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- 69.Zawadzka M, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rafalski VA, et al. Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain. Nat. Cell Biol. 2013;15:614–624. doi: 10.1038/ncb2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grossauer S, et al. Concurrent MEK targeted therapy prevents MAPK pathway reactivation during BRAFV600E targeted inhibition in a novel syngeneic murine glioma model. Oncotarget. 2016;7:75839–75853. doi: 10.18632/oncotarget.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daynac M, et al. Quiescent neural stem cells exit dormancy upon alteration of GABAAR signaling following radiation damage. Stem Cell Res. 2013;11:516–528. doi: 10.1016/j.scr.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 73.Daynac M, et al. TGFbeta lengthens the G1 phase of stem cells in aged mouse brain. Stem Cells. 2014;32:3257–3265. doi: 10.1002/stem.1815. [DOI] [PubMed] [Google Scholar]
- 74.Tacheva-Grigorova SK, Santos AJ, Boucrot E, Kirchhausen T. Clathrin-mediated endocytosis persists during unperturbed mitosis. Cell Rep. 2013;4:659–668. doi: 10.1016/j.celrep.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaiser J, Stockert RJ, Wolkoff AW. Effect of monensin on receptor recycling during continuous endocytosis of asialoorosomucoid. Exp. Cell Res. 1988;174:472–480. doi: 10.1016/0014-4827(88)90316-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.