Abstract
Treating dermatological pathologies with topical corticosteroids under occlusion is often more effective than nonocclusive therapy, especially in the treatment of psoriasis. Betamethasone valerate medicated plaster provides a controlled and localized method of dosing betamethasone valerate, a well-established corticosteroid with vasoconstrictive, anti-inflammatory, immunosuppressive, and antiproliferative properties. This self-adhesive plaster is approved for the treatment of inflammatory skin disorders that do not respond to treatment with less potent corticosteroids. As a patch, it offers all the clinical benefits of occlusive therapy such as increased penetration of topical agent into the area requiring treatment, enhanced skin hydration, and protection from local trauma or scratching. This translates into improved patient compliance, which is notoriously low in patients with dermatological conditions. This review presents the available clinical data from studies with betamethasone valerate medicated plaster in the treatment of psoriasis and other dermatoses and discusses its place in therapy for dermatological conditions.
Keywords: betamethasone valerate, corticosteroids, dermatoses, medicated plaster, pharmacokinetics, psoriasis, skin disorders, treatment
Introduction
Dermatological disorders are responsible for significant morbidity in the general population, ranging from clinically minor conditions, such as mild acne and photoaging, to malignant skin cancers. Additionally, many common skin conditions are associated with a substantial burden on patients’ quality of life (QoL).1–4 Psoriasis and atopic dermatitis (AD) are two common dermatological conditions that have been shown to significantly impair patient QoL, placing a significant burden on both healthcare systems and society.5–11
In psoriasis and AD, the primary goal of treatment is to reduce symptoms to a level that restores QoL with minimal toxicity. Topical treatment is the first line for both these dermatological disorders, while phototherapy and systemic agents are reserved for recalcitrant or moderate to severe disease.12–14 Corticosteroids are considered the cornerstone of topical treatment for patients with mild-to-moderate psoriasis and AD.12–15 Despite the extensive list of topical corticosteroids, compliance with their use remains an important issue.16–18 Therefore, the advent of newer formulations for occlusive use, which can improve patient appreciation and adherence to medication, have greatly improved the effectiveness of treatment.
This review provides an overview on the use of betamethasone valerate medicated plaster (BMVP) in the treatment of psoriasis and other dermatoses and discusses their current place in the treatment of these conditions.
Betamethasone valerate medicated plaster
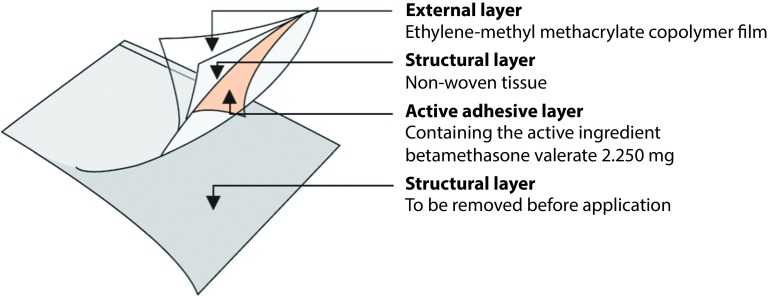
The self-adhesive medicated plaster form of betamethasone valerate (BMV) 2.250 mg is composed of a four-layer bioadhesive transparent film (Figure 1) and has been developed for occlusive use. BMVP is 75×100 mm (75 cm2) plaster and contains BMV 2.250 mg (30 μg/cm2) with a concentration of 0.1% w/w in the active adhesive layer. BMVP (Betesil®, Beteflam®, Betatape®, Cortiflam®, Cortitape®) is currently available in Europe and Canada.
Figure 1.
Schematic structure of BVMP.
The indications for BMVP include inflammatory dermatological conditions that do not respond well to lower potency topical corticosteroid therapy (Box 1).19 BMVP is identical to other BMV formulations in terms of active ingredient, strength, indications, and epidermal route of administration. However, different from other formulations, it offers the well-known clinical benefits of occlusive therapy, resulting in fast remission while being more discreet and acceptable to patients than conventional occlusive dressings. This is an important property because compliance with topical treatment of dermatological conditions is notoriously poor, and any measure that can improve it should be taken into consideration when selecting treatment.16–18 BMVP can also be applied to areas to which conventional occlusive bandage cannot be easily applied. The plaster can be cut to provide uniform distribution of the active ingredient for localized penetration into the area requiring treatment. This avoids the use of cosmetically unappealing ointments with unpleasant drawbacks such as staining of clothing. Good adherence to the skin means that it is not removed by clothing, which minimizes waste of the active ingredient. The plaster also offers a degree of protection from local trauma to the affected skin, including accidental bumps and deliberate scratching by the patient, which could otherwise impede healing. Due to the occlusive nature of the dressing, it also provides increased hydration of skin, which is of particular benefit in both psoriasis and AD. Another advantage of BMVP is that when it is removed, the horny layers of psoriatic lesions are peeled off smoothly with the plaster, which can assist in the healing of lesions. Lastly, it limits the need for pretreatment ‘cleaning’ of lesions that is often necessary in the presence of thick or recalcitrant plaques on areas that are difficult to treat.
Box 1. Inflammatory dermatological conditions unresponsive to low-potency topical corticosteroid therapy for which BMVP are indicated.19.
| Indications |
|---|
| Chronic plaque psoriasis localized in difficult-to-treat areas (e.g. knees, elbows, and anterior face of the tibia on an area not greater than 5% of BSA) |
| Eczema |
| Lichenification |
| Lichen planus |
| Granuloma annulare |
| Palmoplantar pustulosis |
| Mycosis fungoides |
Pharmacokinetic and pharmacodynamics studies on BMVP
The pharmacokinetic properties of BMVP have been evaluated in three phase I studies.20–22 In a repeat insult patch test, BMVPs were applied nine times over a period of 19 days in 28 healthy volunteers, with each application lasting 48 hours during the induction phase and 24 hours during the challenge. Blood samples were collected from 12 of these 28 subjects for pharmacokinetic analyses. No detectable betamethasone (BM) concentrations were found in the plasma of any of these 12 subjects; the limit of quantification was 0.5 mg/mL.22
Plasma concentrations of BM have also been assessed following single and repeated applications of BMVP in six healthy volunteers and six patients with psoriasis vulgaris. Six plasters daily were applied to each participant over a period of 4 days, representing the maximum daily dose in patients with psoriasis affecting a body surface area (BSA) of no more than 5%. Cmax after both single and repeated applications of BMVP was consistently <140 pg/mL, which is approximately 1000 times lower than normal plasma cortisol levels and about 750 times lower than what would be present if the same dose of BM was injected intravenously and therefore too low to cause any systemic pharmacotoxicological effects.20
Finally, the bioavailability of BMVP has been evaluated in 33 patients with psoriasis vulgaris who were randomly treated either with six BMVPs daily for 3 consecutive weeks applied for 24 hours (17 patients) or BMV 0.1% cream applied under occlusion for the first 4 days and nonoccluded for an additional 17 days (16 patients). Percutaneous absorption of BM was found to be below the limit of quantification of 0.5 mg/mL after 4 and/or after 21 days of treatment in about half of subjects receiving BMVP and in all but one subject receiving the reference BMV cream. Although systemic exposure to BM following topical patch applications seemed somewhat higher than after administration of a comparable quantity of BMV cream, overall the amount of measured active substance remained very limited.21
Pharmacodynamic studies have examined the vasoconstrictive, anti-inflammatory, and antiproliferative effects of BMVP.23–25 The anti-inflammatory and vasoconstrictive effects of topical corticosteroids are believed to occur, at least in part, secondary to the binding of the corticosteroid to lipocortin 1, resulting in inhibition of the release of pro-inflammatory compounds.
Vasoconstrictive activity has been evaluated in a phase I double-blind placebo-controlled ‘blanching test’ by comparing occlusive treatment with BMVP to a cream containing an equimolar concentration of BMV in 26 healthy volunteers. The study medications were an 11 mm diameter BMVP, BMV cream 0.1% 28.5 mg, placebo plaster, and placebo cream; both BMV medications were equivalent to 28.5 μg of the active agent. Two other BMV creams of different strengths (0.05 and 0.12%) were also tested to gather more data on the formulations available in Europe, and a seventh body area was designated as a no-treatment control. Skin colorimetry was performed before study medications were applied, when they were removed (16 hours), and at hourly intervals. The results revealed equivalent vasoconstrictive activity between BMVP and the BMV cream 0.1% and no significant difference between BMVP and 0.12% cream.23
The anti-inflammatory activity of BMVP has been compared in a phase II double-blind double-dummy study with a BMV cream 0.12% in five eczematous contact dermatitis lesions induced with nickel sulfate on the forearms of 26 healthy volunteers with known sensitivity. The anti-inflammatory effect as assessed by colorimetry was equivalent between the two BMV formulations. The lack of a significant difference was confirmed by echographic evaluation of the dermis and epidermis.24
A phase II double-blind placebo- and reference drug-controlled study in 25 patients with a psoriatic plaque measuring ≥5 cm in diameter evaluated the antipsoriatic activity of a 13 mm diameter BMVP 40 μg plaster, BMV cream 0.1% 40 μg, and clobetasol cream 0.05% 20 μg as reference drugs and placebo plaster. Four areas on each patient were treated with each of the study interventions, with a fifth area untreated as a negative control. All test medications were associated with antipsoriatic activity, as shown by reductions in the Psoriasis Area and Severity Index (PASI) score; a significantly greater reduction in the PASI score was associated with BVMP compared with the placebo patch (p<0.05).25
Efficacy studies
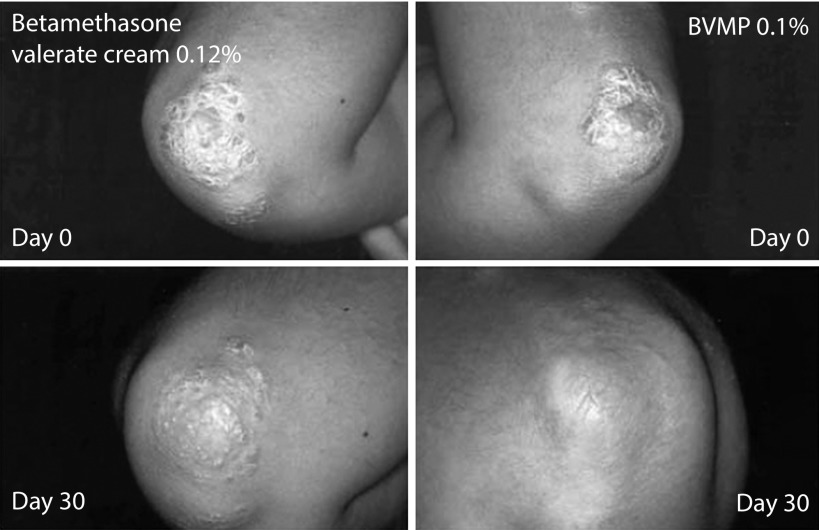
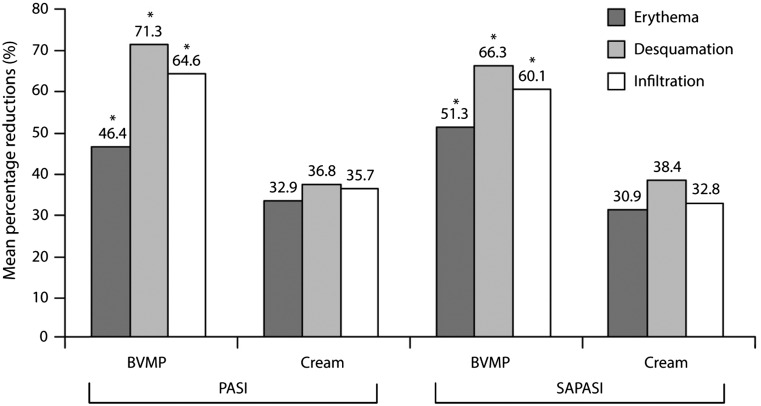
Since its introduction in the market, there have been a growing number of randomized studies evaluating the efficacy of BMVP in plaque psoriasis.26–30 A first clinical trial conducted by Pacifico et al. preliminary demonstrated the superior efficacy of BMVP compared with BMV cream in the treatment of localized chronic plaque psoriasis.29 This within-subject (right-left) randomized study involved 42 patients, mean age 46 years, with chronic psoriasis with symmetrical lesions (i.e. difference in size between corresponding lesions of ≤0.5 cm diameter), and was designed to compare the antipsoriatic efficacy of BMVP 0.1%, cut to the size of the lesion to be treated, and BMV cream 0.12% applied homogeneously over the treated lesion. Assessments included PASI and self-administered PASI (SAPASI) scores, photography, measurement of degree of skin hydration, and plasma cortisol levels (for safety assessment) performed before treatment and on day 30 (end of treatment). Treatment acceptability and compliance before, midway, during, and at the end of treatment were assessed by questionnaire. The results showed that improvements in PASI and SAPASI scores, the degree of hydration, and success rate (defined as improvements of 50 and 75% in PASI and SAPASI scores) were significantly superior with BMVP than with the cream (p<0.001) (Table 1). This superiority of BMVP over the cream was obvious in photographic analysis of the lesions (Figure 2). Furthermore, individual components of the PASI and SAPASI scores were significantly improved with BMVP compared with cream (p<0.001) (Figure 3).29 Patients preferred BMVP over the cream formulation; specifically, they mentioned that the cream was too greasy and that the plaster was easier to apply.29
Table 1.
‘Success rates’ (percentage of patients with reductions in PASI and SAPASI scores of ≥75%) and changes in baseline PASI and SAPASI scores and skin hydration following treatment of psoriatic lesions with BMVP or BMV cream 0.12%.29
| Outcome | BMVP | Betamethasone valerate cream 0.12% |
|---|---|---|
| PASI | ||
| Change in baseline score | −10.2 (−61.8%)*,** | −6.3 (−38.4%)* |
| 75% success rate | 38.1% | 11.9% |
| SAPASI | ||
| Change in baseline score | −11.5 (−59.3%)*,** | −6.6 (−34%)* |
| 75% success rate | 26.2%§ | 9.5% |
| Skin hydration | ||
| Change in baseline score | +7 (+38.8%)** | +1.98 (+11.1%) |
Statistically significant versus baseline (p<0.001);
statistically significant versus cream (p<0.001);
statistically significant versus cream (p<0.05).
BMVP, betamethasone valerate medicated plaster; PASI, psoriasis area and severity index; SAPASI, self-administered PASI.
Figure 2.
Photographs of lesions before and after 30 days of treatment with BMV cream (left) or BMVP (right).29
BMV, betamethasone valerate; BMVP, betamethasone valerate medicated plaster.
Figure 3.
Mean percentage reduction in baseline PASI and SAPASI scores following treatment of psoriatic lesions with BMVP 0.1% or BMV cream 0.12%.29.
*p<0.01 versus BMV cream.
BMV, betamethasone valerate; BMVP, betamethasone valerate medicated plaster; PASI, psoriasis area and severity index; SAPSI, self-administered PASI.
Leone et al. published findings from a prospective randomized study evaluating BMVP in combination with 308 nm monochromatic excimer light (MEL) UVB phototherapy, which unlike narrow-band UVB can target single lesions without affecting surrounding tissue. A total of 19 patients with symmetrical psoriatic lesions on elbows or knees that were unresponsive to narrow-band UVB phototherapy were administered 12 sessions of MEL UVB phototherapy three times a week bilaterally. In addition, each patient applied a BMVP each day to a lesion on a randomly selected side. The reduction in baseline PASI score after therapy was 58.7% in the lesions treated with BMVP, compared with 40.1% for lesions treated with phototherapy alone. The investigators concluded that the use of BMVP in combination with MEL UVB phototherapy may result in complete resolution of psoriatic lesions faster than phototherapy alone.26
Naldi et al. performed a prospective randomized assessor-blind parallel-group active-controlled multicenter phase III study in 231 patients with stable chronic plaque psoriasis vulgaris with two to four plaques on extensor surfaces of limbs (involving <10% of BSA) were randomized to receive BMVP 0.1% (n=116) or BMV 0.1% cream (n=115) for 3–5 weeks.27 Patients with resolution of target plaques then entered a 3-month treatment-free follow-up period.
In this study, the primary efficacy outcome was number of patients with plaque clearing (lack of active plaques after 3 weeks in target skin areas) based on Psoriasis Global Assessment (PGA) score evaluated by blinded assessors using digitized images. Other endpoints included number of patients with clearing based on patient- and investigator-evaluated PGA scores after 3 weeks and clearing after 5 weeks treatment and change from baseline in PGA score based on blinded assessment and patient and investigator evaluation. Change in target plaque size from baseline was further assessed by blinded evaluation. The authors also evaluated self-assessed itching and soreness with a 0–10 categorical scale (0 = no symptoms, 10 = very severe symptoms), treatment satisfaction, and ease of use (0 = very poor, 10 = excellent).27
The results showed that significantly more patients achieved clearing of target plaques after 3 weeks of BMVP compared with BMV cream when evaluated by blinded assessors (p<0.001) (Figure 4a). Moreover, the proportion of patients with clearing of target plaques after 3 weeks was higher in the BMVP group (p<0.001 versus BMV cream) according to evaluation by the patient self-assessment and the investigator (Figure 4a).
Figure 4.
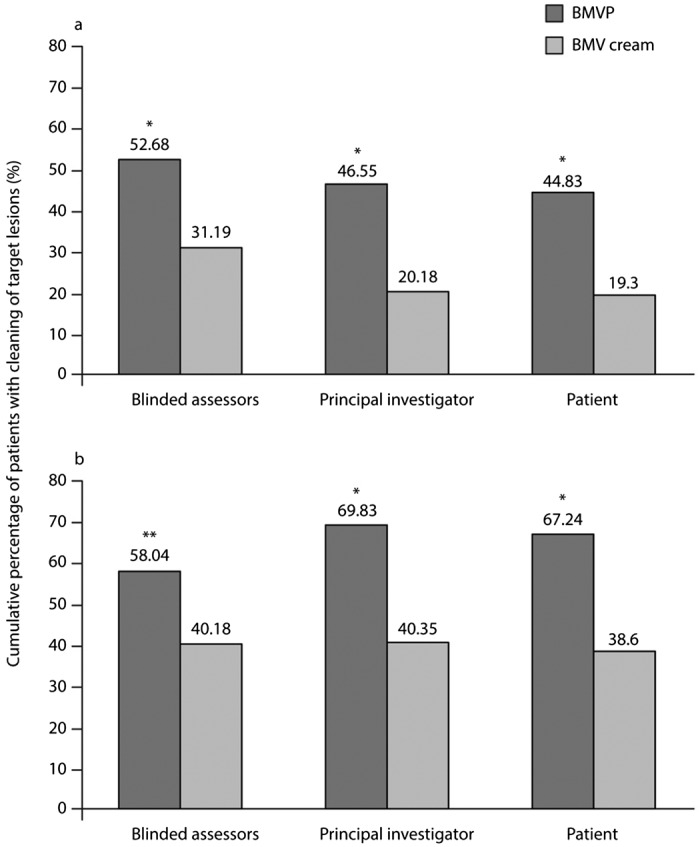
Cumulative percentage of patients with clearing of target lesions following (a) 3 and (b) 5 weeks of treatment with BMVP or BMV cream as evaluated by principal investigator, blinded assessors, and patient self-assessment.27
*p<0.001 versus BMV cream; **p=0.006 versus BMV cream.
BMV, betamethasone valerate; BMVP, betamethasone valerate medicated plaster.
Among the 61 and 89 patients in the BMVP and BMV cream groups that were not cleared at 3 weeks and who proceeded with treatment for a further 2 weeks, clearing at 5 weeks was observed in more patients treated with BMVP than in those treated with BMV cream (Figure 4b).
The total size of target plaques steadily decreased throughout the treatment period in both treatment groups; the difference reached statistical significance for the BMVP group at week 5 (p=0.017 versus BMV cream). Substantial reductions from baseline in mean PGA scores were observed in both treatments groups at weeks 3 and 5 with a significantly greater reduction in baseline PGA score with BMVP compared with BMV cream (p≤0.016).27
Similar reductions from baseline were observed in both treatment groups at weeks 3 and 5 considering severity of itching and soreness, with no significant differences between groups. Acceptability/satisfaction and ease of use were high for both treatments: satisfaction of patients was higher with BMVP while BMV cream was easier to use; compliance was similar between the two treatments.27
A multicenter prospective randomized investigator-blinded controlled noninferiority trial compared the efficacy and safety of BMVP to calcipotriol–betamethasone dipropionate (CBD) ointment during 4 weeks of treatment of patients with mild-to-moderate chronic plaque psoriasis involving less than 10% of BSA.28 The primary efficacy endpoint was the four-item psoriasis total severity score (TSS-4) at week 4, and the associated noninferiority margin was 1 point. Secondary outcome measures included PGA score and QoL assessed with the dermatology life quality index (DLQI).
A total of 324 patients were randomized to BMVP (n=165) or CBD (n=159), and considered invaluable for both safety and intention-to-treat (ITT) (all randomized patients who received at least one dose of study treatment) efficacy analyses; 32 patients in the CBD groups and 28 patients in the BMVP group did not complete the study and were therefore excluded from the per protocol (PP) population analysis. There was no significant difference between the two groups considering age, gender, body mass index, medical history, concomitant diseases, previous and concomitant medications, history of previous psoriasis, relevant treatments, and current psoriatic condition. Mean exposure was 26.3 days in the BMVP group and 26.6 days in the CBD group.28 A significant decrease in mean TSS-4 was observed at week 4 in both groups, with comparable mean change (p=0.001). The BMVP was noninferior to CBD considering adjusted mean scores at week 4, (1.981 in BMVP and 1.693 in the CBD group), with a 0.288 (95% CI: 0.610–0.034) difference between means.28
Secondary efficacy indexes were significantly reduced or improved from baseline at all post baseline time points for both PP and ITT analyses in both groups (p<0.05). No difference in the PP analysis was observed between groups in most variables including weeks 1–3 and follow-up TSS-4 total score; TSS-4 subscores for erythema, elevation and pruritus; PGA scores; active lesions disappearance at week 4, DLQI scores at weeks 1–3 and 3 months; rates and median time to relapse/rebound (56 versus 57 days in the BMVP and CBD groups ); TSS-3 total score at week 4.28 The DLQI score was slightly better in the CBD group at week 4, with no difference observed at follow-up.28
In a phase II single-center randomized 4-week study, fixed combination calcipotriol (Cal) plus BM aerosol foam and BMVP were applied once daily to six test sites (three for each treatment) in 35 patients with stable plaque psoriasis. Absolute change in total clinical score (TCS; sum of erythema, scaling, and infiltration) represented the primary efficacy endpoint, with changes from baseline in each individual clinical score, ultrasonographic changes (total skin and echo-poor band thickness) as safety secondary endpoints; a post hoc analysis evaluated change from baseline in TCS on difficult-to-treat areas.30 The mean change in total clinical score from baseline was greater for Cal/BD foam (−5.8) than BMVP. For each clinical sign, greater changes for Cal/BD foam were observed from day 8. Cal/BD foam led to a greater absolute total skin and echo-poor band thickness change compared with BMVP. Both treatments were well tolerated.30
Of note, in this preliminary study on efficacy and safety, Cal/BD aerosol foam was not administered according to the methods described in the Summary of Product Characteristics (Enstilar, Summary of Product Characteristics) – that is, the foam-treated skin areas were covered with a bandage (gauge) whose potential occlusive nature/effects were not investigated. In addition, no evaluation of untreated and covered areas was performed, meaning that there was no negative control, which may represent significant bias. As mentioned, these are only preliminary findings that need further investigation.
Another study evaluated the efficacy and safety of BMVP in patients with Prurigo nodularis, a distressing condition characterized by the presence of multiple nodules associated with intense pruritus. In this study, BMVP was compared with a moisturizing itch-relief cream containing feverfew in 11 patients who completed 4 weeks of therapy.31 After obtaining a non-pruritic state (mean of 7 days), skin lesions regressed gradually, and nodules flattened and softened within 4 weeks of treatment in 7 of 11 (63.6%) patients in both body halves. In addition, 63.7% (7/11) of patients treated with BMVP had either moderate or marked improvement compared with 27.3% of patients treated with the moisturizing itch-relief cream. Skin symptoms were effectively treated both by the BMVP and moisturizing itch-relief cream. However, lesions treated with BMVP showed greater reduction from baseline in itch (VAS score at week 4: 3.9; p<0.005), infiltration, and excoriation compared with lesions treated with moisturizing itch-relief cream (visual analogue scale (VAS) score at week 4: 5.6; p<0.005) (Figure 5). Both treatments had high cosmetic acceptability and tolerability. Moreover, 10 of 11 patients preferred the BMVP because the occlusive dressing significantly reduced the ability to scratch the lesions (p<0.05).31
Figure 5.
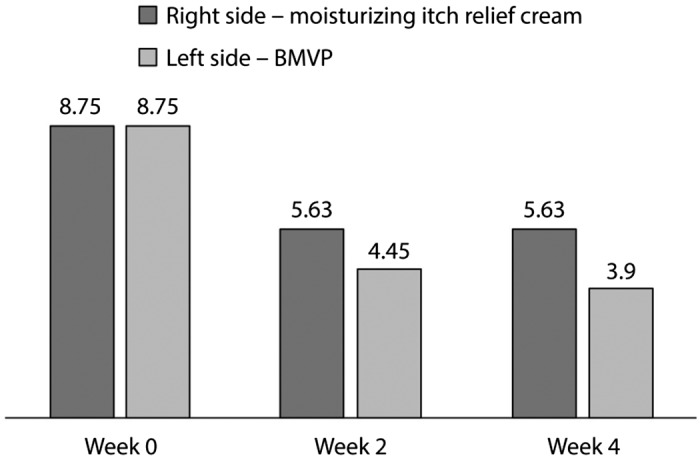
Changes in mean VAS at baseline (week 0) and after 2 and 4 weeks in the BMVP and moisturizing itch-relief cream groups.31
BMVP, betamethasone valerate medicated plaster; VAS, visual analogue scale.
The efficacy and safety of BMVP demonstrated by these randomized studies have been confirmed in large observational studies. The LIBERE study, a multicentric open-label prospective study, was performed in France by 132 investigators and included 258 patients with any inflammatory skin disease not exceeding 5% of BSA. In the patients the physician had previously decided to prescribe BMVP according to its approved indications.32 Disease severity was assessed using a PGA score with detailed evaluation of patient satisfaction assessed by physicians and patients as well as QoL (DLQI).
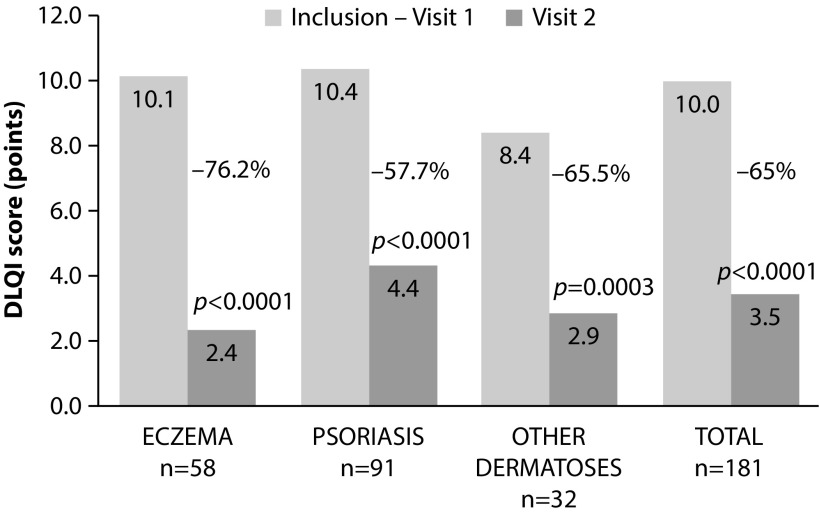
All patients were prescribed to apply up to six BMVP per day during a treatment period not exceeding thirty days. Patient assessment and data collection were performed at inclusion and at end of the study. The most common reasons for treatment were chronic plaque psoriasis (48.6%), eczema (33.9%), and other dermatoses (such as granuloma annulare, lichenifications, lichen planus, and palmoplantar pustulosis). DLQI scores decreased from 10.0±5.4 to 3.5±3.5 (p<0.0001) over the study period with absolute score reductions of 7.8±4.7 in eczema and −5.6±5.0 in other dermatoses. In patients with psoriasis, the mean absolute reduction was found to be −6.1±5.1 (Figure 6); accordingly, impairment in disease-related QoL went from strong to mild in both eczema and psoriasis and from moderate to mild in other dermatoses. Disease-related impact on QoL improved during treatment, which was rated as ‘moderate’ or ‘important’ by 71.6% of patients at the study start to ‘none’ or ‘mild’ after completion of treatment in 77.8% of patients. Ease of use and rapidity of application were rated as good or very good by 73.5 and 75.2% of patients, respectively. Independently of the type of disease, PGA was reduced from 12.5±3.1 to 4.2±3.0 during treatment (p<0.0001). Interestingly, compared with prior treatments, 90.4% of patients rated BMVP treatment as being more effective, and it satisfied both patients and physicians in the vast majority of cases (93.5 and 95.1%, respectively).32
Figure 6.
Changes in mean DLQI scores in response to BMVP treatment at baseline (visit 1) and at the end of treatment (visit 2). Reproduced under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives License (http://creativecommons.org/licenses/by-nc-nd/4.0/) from Maccari F. Improvement of inflammatory dermatoses severity and quality of life in patients treated with a betamethasone valerate plaster (LIBERE study). J Dermatolog Treat. 2016;27:59–63.
BMVP, betamethasone valerate medicated plaster; DLQI, dermatology life quality index.
Safety/tolerability data for BMVP
The safety of BMVP is largely expected to mirror the safety of its active ingredient, which is now well established since it was first approved in 1967.33–38 As such, the above-cited studies have demonstrated a high level of safety/tolerability for BMVP.
The skin atrophogenic potential of BMVP has been assessed in a randomized vehicle- and reference drug-controlled investigator-blind phase I study in 18 healthy volunteers. Skin thickness was evaluated weekly for 3 weeks using echography and clinical scores for skin atrophy and telangiectasia. Study treatments were occlusive application of BMVP, one of two marketed BMV creams 0.1%, base cream, or plaster placebo; one area was left untreated, but occluded, as a control. Skin thickness was significantly thinner for all three active controls compared with placebo and base cream (p<0.01).39
HPA-axis suppression is probably the most well known and potentially the most serious effect associated with systemic absorption of topical corticosteroids.40 A phase I study designed to evaluate the effect of BMVP on the HPA axis following systemic absorption was conducted in six healthy volunteers and six patients with psoriasis vulgaris. Subjects were treated with six BMVPs daily over 4 consecutive days, based on the dosage proposed for psoriasis with 650 cm2 of BSA involvement. Blood samples taken before, during, and after treatment were used to assess systemic exposure to the active drug and its effect on plasma cortisol levels. After a single 24-hour application of six BMVPs, there was a significant decrease in plasma cortisol levels from baseline in healthy volunteers (p=0.03), but the reduction seen in patients with psoriasis was not significant. However, during 4 days of repeated application of six BMVPs, plasma cortisol levels in healthy volunteers returned to a mean level that was not significantly different from baseline, while levels in the patients with psoriasis remained stable.20 Moreover, there were no instances when morning cortisol dropped below 70 μg/L, which is the laboratory reference level considered indicative of HPA-axis suppression (Figure 7).
Figure 7.
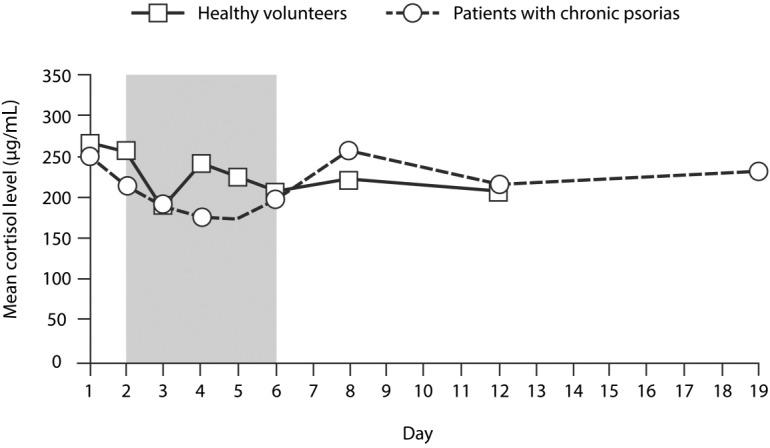
Morning plasma cortisol levels in six volunteers and six patients with psoriasis treated with BMVP (six plasters per day) for 4 consecutive days, starting on day 2 (shaded area represents the treatment period).20
BMVP, betamethasone valerate medicated plaster.
The integrity of the HPA axis was also assessed before and after 21 days of treatment using a 30-min adrenocorticotropic hormone (ACTH) test in patients with chronic psoriasis vulgaris receiving six BMVPs daily and an equivalent amount of BMV 0.1% cream as a comparator. The normal 24-hour cortisol profile and unmodified cortisol response to the rapid ACTH test ruled out any effect of treatment on the HPA axis.21
The lack of interference of the BMVPs with the HPA axis and plasma cortisol concentrations was further confirmed in the study of Pacifico et al., where morning plasma concentrations of cortisol were never below central laboratory reference values (i.e. 50 μg/L) in patients with symmetrical chronic psoriasis treated for 30 consecutive days with BMVP on one plaque and BMV 0.1% cream on the contralateral plaque. None of the participants exhibited any evidence of HPA-axis suppression.29 In the study by Saraceno et al., likewise, no cases of HPA-axis suppression were observed.31
These results suggest that though there is evidence that BMV is released from BMVP into systemic circulation and reduces cortisol levels, there is unlikely to be clinically relevant HPA-axis suppression even during intensive use of plasters. This finding is in line with current opinion that the risk of HPA-axis suppression is only an issue with topical administration of very high potency corticosteroids; additionally, the use of BMVP at the maximum recommended dosage (six plasters per day) would result in a total exposure to the active ingredient of 94.5 mg over 1 week, which is well in line with current medical practice/regulatory recommendations.26,29 In fact, the recommended oral dosage of BMV of 2.4–4.8 mg/day rarely results in HPA-axis suppression in adults. It is also worth noting that BMVP is not generally recommended for use on body areas with high absorptive capacity. These data suggest that HPA-axis suppression is unlikely to be an important concern associated with BMVP use. Safety data on localized toxicity was also reported in the study by Pacifico et al.29 No adverse events were reported during 30 days of treatment with BMVP or BMV cream 0.12% among the 42 patients, and there was no evidence of local irritation affecting any of the treated lesions. No safety or tolerability concerns were observed in later studies.27,28
In all studies carried out to date, there have been no serious adverse events or deaths reported. The nature and severity of adverse events seen in trials reflect the known and expected toxicity for topical corticosteroids. The fact that topical corticosteroid therapy is combined with phototherapy in current therapeutic practice also supports the premise that there is no risk of phototoxicity, and BMVP use is not expected to be associated with any major safety concerns.
Place of BMVP in therapy
Treating dermatological conditions under occlusion has often been shown to be more effective than nonocclusive therapy. BMVP allows a more convenient, controlled, and localized form of occlusive therapy. As such, BMVP is indicated for the treatment of inflammatory skin disorders that do not respond to treatment with less potent corticosteroids; due to its particular pharmaceutical form, BMVP is suitable for chronic plaque psoriasis localized in difficult-to-treat areas (e.g. knees, elbows, and anterior face of the tibia on an area not greater than 5% of BSA).
Although clinical study data with BMVP on conditions other than psoriasis are still relatively scarce, BMVP represents an alternative method of delivering a well-established agent,33–38 and for this reason its use might be extended to other dermatological conditions sensitive to topical BMV therapy. As for other topical corticosteroids, BMVP is not indicated for dermatological lesions caused by viruses, bacteria, or fungi.19 Lesions affected by acne (or acne rosacea), perioral dermatitis, skin ulcers, burns, frostbite, or other injuries should not be treated with BMVP. Furthermore, BMVP should not be used in patients aged <18 years due to lack of clinical data supporting such use. There are no adequate controlled studies in humans evaluating the risks associated with the use of topical corticosteroids during pregnancy or lactation. However, both systemic BMV and potent topical corticosteroid therapies have been shown to have adverse effects on fetuses in animal studies, and BMVP should only be prescribed during pregnancy when the benefit will outweigh the risks.19,41 Systemic corticosteroids are also known to be distributed in breast milk. The extent to which topical corticosteroids may also become distributed in breast milk has not been established, and caution is advised with the use of such therapy in nursing women; this precaution should be extended to BMVP.
As systemic absorption of BMV with BMVP use is negligible, there is little basis for concern regarding interactions with systemic agents even though interactions with oral BMV have been described.19 Moreover, there are no known interactions between topical BMV and other topical agents, despite their extensive use over several decades. It can, therefore, be concluded that BMVP can normally be used concurrently with other pharmacological treatment regimens without any safety or efficacy concerns related to interactions. However, medicinal products containing corticosteroids, such as BMVP, must be used with caution in patients with impaired immune system function (T-lymphocytes) or in those being treated with immunosuppressive therapy.
In conclusion, the current evidence suggests that BMVP is indicated for localized treatment of dermatological conditions (i.e. plaque psoriasis) that do not respond well to lower-potency topical corticosteroids. It offers the clinical benefits of occlusive therapy such as good hydration, which results in fast remission, and is an important and established tool in the armamentarium of clinicians managing a wide array of dermatological conditions.
Footnotes
Contributions: Drs Amici and Ly contributed in searching literature, drafting, and conceptually revising the manuscript.
Disclosure and potential conflicts of interest: Dr Amici reports personal fees from Roche, Leo Pharma, Zambon outside the submitted work. Dr Ly reports personal fees from GENEVRIER during the conduct of the study as well as personal fees from Lilly, Leo, Novartis, MEDA outside the submitted work. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors are available for download at http://www.drugsincontext.com/wp-content/uploads/2018/07/dic.212539-COI.pdf
Funding declaration: Article processing charges were funded by IBSA Institut Biochimique SA.
Correct attribution: Copyright © 2018 Amici JM, Ly S. https://doi.org/10.7573/dic.212539. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: submitted; externally peer reviewed.
Peer review comments to author: 5 July 2018
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252772009.
For all manuscript and submissions enquiries, contact the Editorial office dic.editorial@bioexcelpublishing.com
For all permissions, rights, and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Ahmed A, Leon A, Butler DC, Reichenberg J. Quality-of-life effects of common dermatological diseases. Semin Cutan Med Surg. 2013;32(2):101–109. doi: 10.12788/j.sder.0009. [DOI] [PubMed] [Google Scholar]
- 2.Finlay AY. The burden of skin disease: quality of life, economic aspects and social issues. Clin Med (Lond) 2009;9(6):592–594. doi: 10.7861/clinmedicine.9-6-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green L. The effect of skin conditions on patients’ quality of life. Nurs Stand. 2010;25(9):48–55. doi: 10.7748/ns2010.11.25.9.48.c8078. quiz 56. [DOI] [PubMed] [Google Scholar]
- 4.Karimkhani C, Dellavalle RP, Coffeng LE, et al. Global skin disease morbidity and mortality: an update from the global burden of disease study 2013. JAMA Dermatol. 2017;153(5):406–412. doi: 10.1001/jamadermatol.2016.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augustin M, Radtke MA. Quality of life in psoriasis patients. Expert Rev Pharmacoecon Outcomes Res. 2014;14(4):559–568. doi: 10.1586/14737167.2014.914437. [DOI] [PubMed] [Google Scholar]
- 6.Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 Suppl):S115–S123. [PubMed] [Google Scholar]
- 7.Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4:35. doi: 10.1186/1477-7525-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199. doi: 10.1111/j.1525-1470.2005.22303.x. [DOI] [PubMed] [Google Scholar]
- 9.Lifschitz C. The impact of atopic dermatitis on quality of life. Ann Nutr Metab. 2015;66( Suppl 1):34–40. doi: 10.1159/000370226. [DOI] [PubMed] [Google Scholar]
- 10.Raho G, Koleva DM, Garattini L, Naldi L. The burden of moderate to severe psoriasis: an overview. Pharmacoeconomics. 2012;30(11):1005–1013. doi: 10.2165/11591580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35(3):283–289. doi: 10.1016/j.det.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139(4S):S49–S57. doi: 10.1016/j.jaci.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Feldman SR. Treatment of psoriasis. [Accessed February 22, 2018]. https://www.uptodate.com/contents/treatment-of-psoriasis-in-adults. Last updated November 21, 2017.
- 14.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659. doi: 10.1016/j.jaad.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 15.van de Kerkhof PC. An update on topical therapies for mild-moderate psoriasis. Dermatol Clin. 2015;33(1):73–77. doi: 10.1016/j.det.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26( Suppl 3):61–67. doi: 10.1111/j.1468-3083.2012.04525.x. [DOI] [PubMed] [Google Scholar]
- 17.Mueller SM, Tomaschett D, Vogt DR, Itin P, Cozzio A, Surber C. Topical corticosteroid concerns from the clinicians’ perspective. J Dermatolog Treat. 2017;28(5):464–468. doi: 10.1080/09546634.2016.1255307. [DOI] [PubMed] [Google Scholar]
- 18.Snyder A, Farhangian M, Feldman SR. A review of patient adherence to topical therapies for treatment of atopic dermatitis. Cutis. 2015;96(6):397–401. [PubMed] [Google Scholar]
- 19.Italian Medicines Agency. BETESIL 2250 mg medicated plaster. Summary of product characteristics. [Accessed July, 2018]. https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_007166_035863_RCP.pdf&retry=0&sys=m0b1l3.
- 20.Ortonne JP. Data on file. IBSA Institut Biochimique SA; Lugano, Switzerland: 2003. Evaluation of the systemic effects of IBSA Tape (betamethasone valerate 0.1% tape) after repeated applications on the skin of normal healthy subjects and psoriatic patients. [Google Scholar]
- 21.Ortonne JP. Data on file. IBSA Institut Biochimique SA; Lugano, Switzerland: 2008. Evaluation of the systemic effects of IBSA BMV (betamethasone 0.1% medicated plaster) applied on the skin of psoriatic patients versus a reference 0.1% BMV cream. [Google Scholar]
- 22.Rusca A. Data on file. IBSA Institut Biochimique SA; Lugano, Switzerland: 1999. Skin sensitization potential of a 0.1% BMV tape. Repeat Insult Patch Test (RIPT) [Google Scholar]
- 23.Seidenari S. Data on file. IBSA Institut Biochimique SA; Lugano, Switzerland: 2001. Comparative study of the vasoconstrictor and anti-inflammatory activity (‘blanching test’) of Betesil® vs. betamethasone valerate cream. Double blind, double placebo study in human volunteers. [Google Scholar]
- 24.Seidenari S. Data on file. IBSA Institut Biochimique SA; Lugano, Switzerland: 2001. Studio comparativo di efficacia e tollerabilità di Betesil® e Celestoderm®, in soggetti con dermatite allergica da contatto. Studio in doppio cieco e doppio placebo. [Google Scholar]
- 25.Seidenari S. Data on file. IBSA Institut Biochimique SA; Lugano, Switzerland: 2004. Studio comparativo di Betesil®, betametasone valerato e clobetasolo propionato crema, nel test di placca psoriasica. Studio in doppio cieco. [Google Scholar]
- 26.Leone G, Pacifico A, Iacovelli P, Picardo M. Treatment of localised persistent plaque psoriasis with 308 nm monochromatic excimer light (308 nm MEL) and a new formulation of an occlusive dressing containing betamethasone valerate 0.1% [abstract] J Am Acad Dermatol. 2007;P2528:AB169. doi: 10.1016/j.jaad.2006.10.774. [DOI] [Google Scholar]
- 27.Naldi L, Yawalkar N, Kaszuba A, et al. Efficacy and safety of the Betamethasone valerate 0.1% plaster in mild-to-moderate chronic plaque psoriasis: a randomized, parallel-group, active-controlled, phase III study. Am J Clin Dermatol. 2011;12(3):191–201. doi: 10.2165/11539780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Ortonne JP, Esposito M, Chimenti S, et al. Betamethasone valerate dressing is non-inferior to calcipotriol-betamethasone dipropionate ointment in the treatment of patients with mild-to-moderate chronic plaque psoriasis: results of a randomized assessor-blinded multicentre trial. J Eur Acad Dermatol Venereol. 2014;28(9):1226–1234. doi: 10.1111/jdv.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacifico A, Daidone R, Peris K. A new formulation of an occlusive dressing containing betamethasone valerate 0.1% in the treatment of mild to moderate psoriasis. J Eur Acad Dermatol Venereol. 2006;20(2):153–157. doi: 10.1111/j.1468-3083.2006.01387.x. [DOI] [PubMed] [Google Scholar]
- 30.Queille-Roussel C, Rosen M, Clonier F, Norremark K, Lacour JP. Efficacy and safety of calcipotriol plus betamethasone dipropionate aerosol foam compared with betamethasone 17-valerate-medicated plaster for the treatment of psoriasis. Clin Drug Investig. 2017;37(4):355–361. doi: 10.1007/s40261-016-0489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saraceno R, Chiricozzi A, Nistico SP, Tiberti S, Chimenti S. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21(6):363–366. doi: 10.3109/09546630903386606. [DOI] [PubMed] [Google Scholar]
- 32.Maccari F. Improvement of inflammatory dermatoses severity and quality of life in patients treated with a betamethasone valerate plaster (LIBERE study) J Dermatolog Treat. 2016;27(1):59–63. doi: 10.3109/09546634.2015.1035692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreassi L, Giannetti A, Milani M Scale Investigators G. Efficacy of betamethasone valerate mousse in comparison with standard therapies on scalp psoriasis: an open, multicentre, randomized, controlled, cross-over study on 241 patients. Br J Dermatol. 2003;148(1):134–138. doi: 10.1046/j.1365-2133.2003.04950.x. [DOI] [PubMed] [Google Scholar]
- 34.Franz TJ, Parsell DA, Halualani RM, Hannigan JF, Kalbach JP, Harkonen WS. Betamethasone valerate foam 0.12%: a novel vehicle with enhanced delivery and efficacy. Int J Dermatol. 1999;38(8):628–632. doi: 10.1046/j.1365-4362.1999.00782.x. [DOI] [PubMed] [Google Scholar]
- 35.Mancuso G, Balducci A, Casadio C, et al. Efficacy of betamethasone valerate foam formulation in comparison with betamethasone dipropionate lotion in the treatment of mild-to-moderate alopecia areata: a multicenter, prospective, randomized, controlled, investigator-blinded trial. Int J Dermatol. 2003;42(7):572–575. doi: 10.1046/j.1365-4362.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 36.Milani M, Antonio Di Molfetta S, Gramazio R, et al. Efficacy of betamethasone valerate 0.1% thermophobic foam in seborrhoeic dermatitis of the scalp: an open-label, multicentre, prospective trial on 180 patients. Curr Med Res Opin. 2003;19(4):342–345. doi: 10.1185/030079903125001875. [DOI] [PubMed] [Google Scholar]
- 37.Samson C, Peets E, Winter-Sperry R, Wolkoff H. Betamethasone valerate – Valisone® – establishment of a new standard for topical corticosteroid potency. In: Maibach HI, Surber C, editors. Topical Corticosteroids. Basel: Karger; 1992. pp. 335–348. [Google Scholar]
- 38.Stein LF, Sherr A, Solodkina G, Gottlieb AB, Chaudhari U. Betamethasone valerate foam for treatment of nonscalp psoriasis. J Cutan Med Surg. 2001;5(4):303–307. doi: 10.1177/120347540100500404. [DOI] [PubMed] [Google Scholar]
- 39.Queille-Roussel C. Data on file. IBSA Institut Biochimique SA; Lugano, Switzerland: 2002. Evaluation of the skin atrophogenic potential of BETESIL® (betamethasone 0.1% tape) vs. betamethasone valerate 0.12% cream in normal healthy volunteers. [Google Scholar]
- 40.Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26( Suppl 3):47–51. doi: 10.1111/j.1468-3083.2012.04523.x. [DOI] [PubMed] [Google Scholar]
- 41.Sloboda DM, Newnham JP, Challis JR. Effects of repeated maternal betamethasone administration on growth and hypothalamic-pituitary-adrenal function of the ovine fetus at term. J Endocrinol. 2000;165(1):79–91. doi: 10.1677/joe.0.1650079. [DOI] [PubMed] [Google Scholar]