Abstract
Construction of C–C bonds via alkoxy radical-mediated remote C(sp3)–H functionalization is largely unexplored, as it is a formidable challenge to directly generate alkoxy radicals from alcohols due to the high bond dissociation energy (BDE) of O–H bonds. Disclosed herein is a practical and elusive metal-free alcohol-directed heteroarylation of remote unactivated C(sp3)–H bonds. Phenyliodine bis(trifluoroacetate) (PIFA) is used as the only reagent to enable the coupling of alcohols and heteroaryls. Alkoxy radicals are readily generated from free alcohols under the irradiation of visible light, which trigger the regioselective hydrogen-atom transfer (HAT). A wide range of functional groups are compatible with the mild reaction conditions. Two unactivated C–H bonds are cleaved and one new C–C bond is constructed during the reaction. This protocol provides an efficient strategy for the late-stage functionalization of alcohols and heteroaryls.
Direct remote C–H functionalization of aliphatic alcohols via alkoxy radicals is largely unexplored. Here, the authors report the C(sp3)-heteroaryl bond formation in aliphatic alcohols mediated by alkoxy radicals formed with a hypervalent iodine reagent.
Introduction
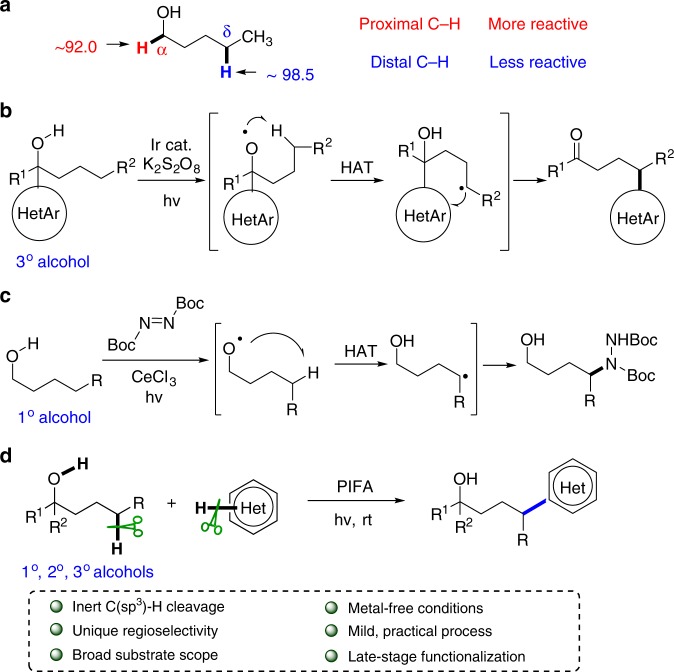
Direct functionalization of unactivated C(sp3)–H bonds represents one of the most intriguing and advanced technologies in synthetic chemistry but is still facing enormous challenges with reactivity and selectivity1–4. Radical-mediated hydrogen-atom transfer (HAT) renders an efficient entry to cleave the inert C(sp3)–H bonds that allows for subsequent substitutions5–8. Besides the classic Hofmann–Löffler reaction, recently new breakthroughs mediated by N-centered radicals have been achieved with the photoredox catalysis9–19. Alcohols are important and readily available chemicals. The radical-mediated late-stage functionalization of the C–H bonds of alcohols affords an ideal approach to the preparation of complex alcohol derivatives. According to the bond dissociation energy (BDE)20–22, the C–H bonds proximal to hydroxyl group are in higher reactivity than the distal ones (Fig. 1a)23–28. Therefore, the selective functionalization of the less reactive remote C–H bonds in alcohols is a formidable challenge.
Fig. 1.
Free alcohol-directed functionalization of remote C(sp3)–H bonds. a BDEs (kcal mol−1) of C(sp3)–H bonds in alcohols. b Intramolecular heteroarylation of tertiary alcohols. c Intermolecular amination of primary alcohols. d Intermolecular heteroarylation of alcohols
The HAT process triggered by alkoxy radicals has long been developed in order to gain the good chemo-/regio-selectivities29,30. After several decades, however, this method is still underexplored, which is mainly ascribed to the difficult homolysis of the alcoholic O–H bonds with high BDEs (ca. 105 kcal mol−1) to generate alkoxy radicals from free alcohols. On the other hand, alkoxy radicals are prone to induce the β-C–C bond fragmentation under harsh reaction conditions31–39. Consequently, alcohols are often elaborated to other surrogates, e.g., nitrite esters40–42, peroxy compounds43–46, sulfonates47–49, lead(IV) alkoxides50–52, hypohalites53–59, N-alkoxylpyridine-2-thiones60,61, and N-alkoxyphthalimides62–64, for the formation of alkoxy radicals by heat or ultraviolet irradiation. Nevertheless, these compounds are sometimes hard to handle or synthesize. In this scenario, seeking a general and mild strategy to generate alkoxy radicals from free alcohols is highly desirable.
Recently, we disclosed a tertiary-alcohol-directed heteroarylation of remote C(sp3)–H bonds by a sequence of HAT and intramolecular heteroaryl migrations (Fig. 1b)65. Alkoxy radicals were directly obtained from alcohols in the presence of iridium complex irradiated by visible light. The tertiary alcohol substrates were well designed to suit for the intramolecular mode. Soon after, Zuo et al. reported a CeCl3-catalyzed amination of remote sp3 C–H bonds of alcohols66. Only primary alcohols were applied to generate alkoxy radicals (Fig. 1c). Concerning the values and ubiquity of heteroaryl moieties in drugs and bioactive molecules, the intermolecular heteroarylation of alcohols is more significant and valuable than the intramolecular mode. Herein we report our findings in the regioselectively intermolecular heteroarylation of alcohols to furnish the Minisci-type products. This reaction demonstrates a wide scope of both alcohols and heteroaryls. Notably, all types (1°, 2°, and 3°) of alcohols are apt to afford the alkoxy radicals that trigger the regioselective heteroarylation of C(sp3)–H bonds. The metal-free conditions are mild and operationally simple, providing a practical strategy for the late-stage functionalization of alcohols and heteroaryls (Fig. 1d).
Results
Reaction conditions survey
With 4-methyl quinoline 1a and n-pentanol 2a as model substrates, we set about investigating the reaction parameters (Table 1). After a brief survey, it was found that the only use of stoichiometric amounts of hypervalent iodine(III) reagent phenyliodine bis(trifluoroacetate) (PIFA) could promote the reaction under the irradiation of blue light-emitting diodes (LEDs) (entry 1). While varying the light source to compact fluorescent light (CFL) bulb decreased the yield (entry 2), the reaction did not proceed with the use of green LEDs or in dark (entry 3). The reaction also took place at 50 °C without light irradiation albeit in a low yield (entry 4), suggesting that this is not a photocatalytic process and the light may just input energy into the reaction. Increasing the amount of PIFA slightly improved the outcome (entry 5), but using too much PIFA significantly inhibited the reaction (entry 6). Other hypervalent iodine(III) reagents were also examined. Surprisingly, the use of (diacetoxyiodo)benzene (PIDA) almost turned off the reaction (entry 8). The use of 1,2-dichloroethane (DCE) or MeCN instead of dichloromethane (DCM) delivered comparable yields (entries 9 and 10), but other solvents did not work efficiently (entries 11–14). The Suárez’s conditions (PIDA and I2)56–59, which were often applied to the intramolecular cyclization reactions via the generation of alkoxy radicals from hypohalite intermediates, were not suitable for our reaction (entry 15). This result clearly illustrated that the current reaction underwent a non-trivial pathway rather than the formation of hypohalite intermediates. The yield was further elevated to 90% by treating the reaction with high-intensive blue LEDs (entry 16). However, it was found that the reaction yield was going down along with reducing the amount of alcohols (entries 17–20).
Table 1.
Reaction parameters survey
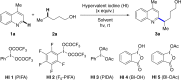
| ||||
|---|---|---|---|---|
| Entry | HI (x) | hv | Solvent | Yield (%)a |
| 1 | PIFA (2.0) | 30 W blue LEDs | DCM | 74 |
| 2 | PIFA (2.0) | 30 W CFL bulb | DCM | 57 |
| 3 | PIFA (2.0) | 30 W green LEDs, or in dark | DCM | 0 |
| 4 | PIFA (2.0) | In dark, 50 °C | DCM | 36 |
| 5 | PIFA (2.5) | 30 W blue LEDs | DCM | 76 |
| 6 | PIFA (3.0) | 30 W blue LEDs | DCM | <5 |
| 7 | F5-PIFA (2.5) | 30 W blue LEDs | DCM | 39 |
| 8 | PIDA (2.5) or BI-OH (2.5) or BI-OAc (2.5) | 30 W blue LEDs | DCM | <5 |
| 9 | PIFA (2.5) | 30 W blue LEDs | DCE | 71 |
| 10 | PIFA (2.5) | 30 W blue LEDs | MeCN | 70 |
| 11 | PIFA (2.5) | 30 W blue LEDs | CHCl3 | 38 |
| 12 | PIFA (2.5) | 30 W blue LEDs | PhCF3 | 53 |
| 13 | PIFA (2.5) | 30 W blue LEDs | DMF | <5 |
| 14 | PIFA (2.5) | 30 W blue LEDs | DMSO | 0 |
| 15 | PIDA (2.5), I2 (1.0) | 30 W blue LEDs | DCM | 0 |
| 16 | PIFA (2.3) | 100 W blue LEDs | DCM | 90 |
| 17b | PIFA (2.3) | 100 W blue LEDs | DCM | 79 |
| 18c | PIFA (2.3) | 100 W blue LEDs | DCM | 70 |
| 19d | PIFA (2.3) | 100 W blue LEDs | DCM | 43 |
| 20e | PIFA (2.3) | 100 W blue LEDs | DCM | 30 |
Reaction conditions: 1a (0.4 mmol), 2a (2.0 mmol, 5 equiv.), and HI 1–5 (as shown) in solvent (2.0 mL), rt, visible-light irradiation
aYields of isolated products
b2a (1.6 mmol, 4 equiv.)
c2a (1.2 mmol, 3 equiv.)
d2a (0.8 mmol, 2 equiv.)
e2a (0.4 mmol, 1.0 equiv.), PIFA (0.3 mmol, 0.75 equiv., added in three batches)
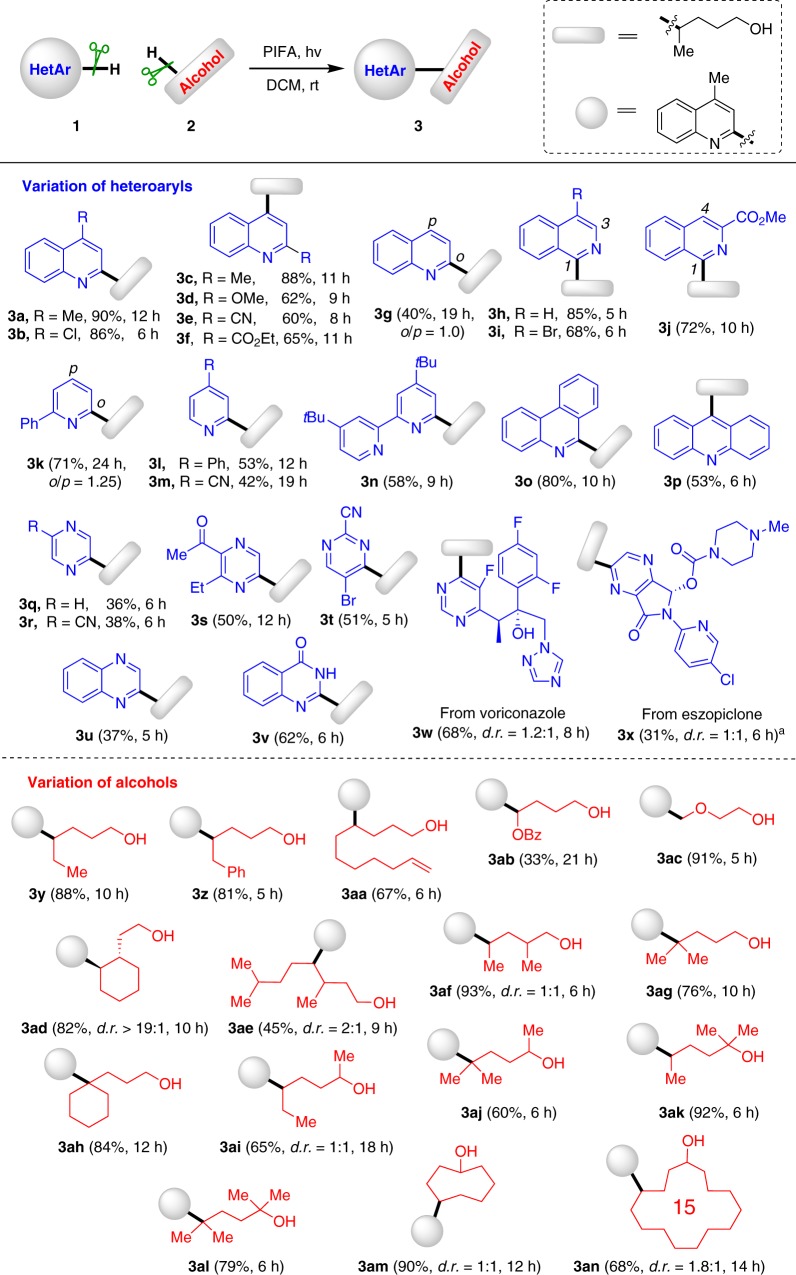
Scope of heteroaryls and alcohols
With the optimized reaction conditions in hand, we turned to examine the generality of protocol (Fig. 2). Firstly, a variety of heteroaryls were tested. The electronic properties of heteroaryls did not have much influence on the reaction, as both electron-donating (e.g., Me, OMe) and electron-withdrawing (e.g., Cl, CN, CO2Et) groups were well tolerated (3b–3f). While the reaction could take place at both the ortho- and para-positions of quinoline (3g) or pyridine (3k), the reaction of isoquinoline solely preferred the 1-position (3h–3j). The example of 3i was noteworthy, since the presence of bromide reserved a platform for the late-stage products manipulation by cross-coupling reactions. The conversion of N,N-bidentate ligand dtbpy to 3n provided a valuable tactic for the ligand modification. Other heteroaryls, such as phenanthridine (3o), acridine (3p), pyrazine (3q–3s), pyrimidine (3t), quinoxaline (3u), and 4-hydroxyquinazoline (3v) were also proved to be suitable substrates, indicating a broad scope of heteroaryls. While the pyrazine product 3r was formed along with another regio-isomer (~10%), the product 3s was delivered in a unique regioselectivity, which was determined by the heteronuclear multiple bond correlation (HMBC) analysis. Significantly, the method could be applied to complex molecules, such as voriconazole and eszopiclone, affording the corresponding products in good regioselectivities (3w and 3x). Next, a number of alcohols were investigated. The alkoxy radical-mediated 1,5-HAT exclusively occurred even in the presence of more reactive benzylic C–H bonds (3z), showing the outstanding regioselective control. It might be attributed to that the 1,5-HAT via six-membered cyclic transition state is more kinetically favorable in this reaction. A variety of Minisci-type products were readily furnished. It should be noted that the current Minisci reactions involving C(sp3)–H activation are largely dependent upon the inherent BDEs of C–H bonds, and scarcely discuss about the regioselective control67–70. The olefin moiety, which is generally susceptive to radical process, remained intact in the reaction (3aa). The C–H bonds adjacent to heteroatoms were also readily functionalized (3ab and 3ac). Remarkably, the reaction with cyclic C–H bonds proceeded stereoselectively, leading to the thermodynamically favored trans-product (3ad). In contrast, the reaction with linear C–H bonds delivered the diastereomer mixtures in a 2:1 or 1:1 ratio (3ae and 3af). In addition to primary and secondary C–H bonds, the congested tertiary C–H bonds were also readily transformed (3ag and 3ah), forming the new quaternary all-carbon centers. The applicability was further spread from primary to secondary and tertiary alcohols. Both of them were suitable precursors for the generation of alkoxy radicals to accomplish the distal C–H bond heteroarylation (3ai–3al). This method provides an efficient approach for alcohol derivatization. For instance, the heteroaryl groups could be directly introduced to the δ-position of cycloalkanols (3am and 3an). The regioselectivities were unambiguously determined by the HMBC experiments.
Fig. 2.
Scope of heteroaryls and alcohols. Reaction conditions: heteroaryl 1 (0.4 mmol), alcohol 2 (2.0 mmol), and PIFA (0.92 mmol) in DCM (2 mL), irradiated by 100 W blue LEDs, rt. Yields of isolated products are given. a1.5 equiv. TFA was added. PIFA: phenyliodine bis(trifluoroacetate)
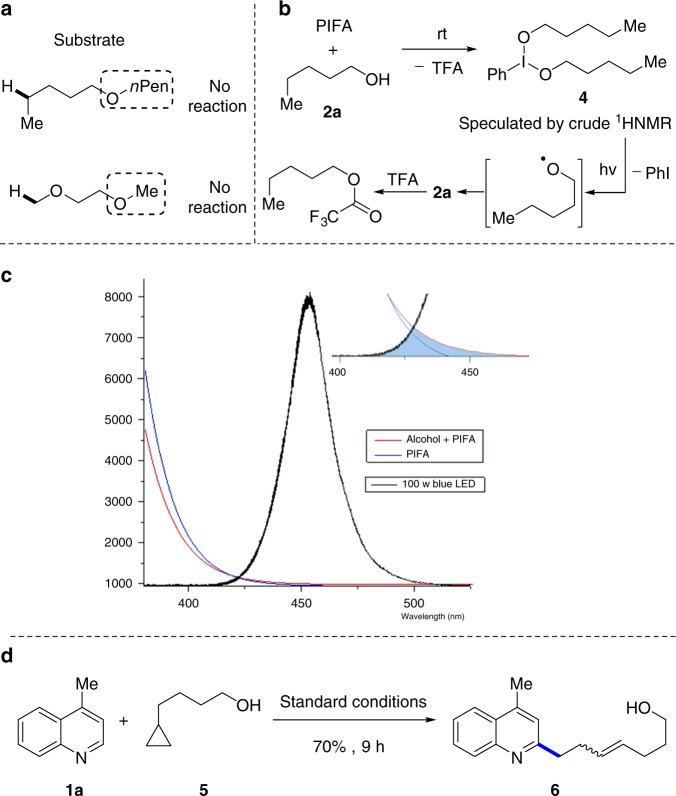
Mechanistic studies
To shed light on the mechanistic pathways, a set of experiments were carried out. Replacement of the hydroxyl group by ether entirely inhibited the reaction, verifying that the reaction was enabled by free alcohols (Fig. 3a). Mixing 2a with PIFA in d-chloroform immediately led to a metastable intermediate 4, which was speculated by crude 1H NMR. Then irradiating the mixture with blue LEDs resulted in the formation of 2a and trifluoroacetate thereof presumably via the alkoxy radical intermediate (Fig. 3b). The intermediate 4 displayed weak absorption from 420 to 450 nm, suggesting the possibility of energy transfer from blue LEDs to 4 (Fig. 3c). Finally, the radical clock experiment unambiguously provided support for the proposed radical pathway (Fig. 3d). The PIFA-promoted reaction of 1a with 5 afforded the ring-opened product 6 in 70% yield as a 4:1 mixture of E and Z isomers.
Fig. 3.
Mechanistic studies. a Reaction with ethers instead of alcohols. b NMR studies for mechanistic investigation. c Absorption spectra (PIFA and 4) and emission spectra (blue LEDs). d Radical clock experiment. PIFA: phenyliodine bis(trifluoroacetate)
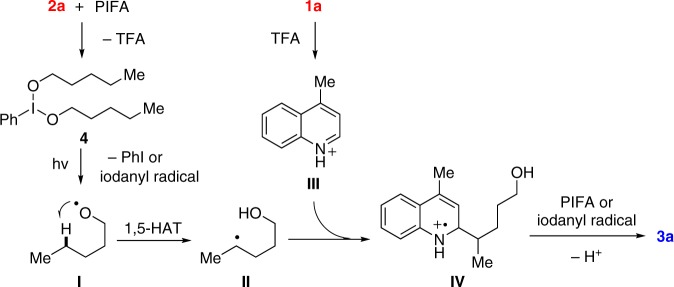
The proposed mechanism was depicted on the basis of experimental observations (Fig. 4). Initially, the mixture of 2a and PIFA results in the dialkoxyiodo benzene 4. Homolysis of 4 induced by visible-light irradiation leads to the alkoxy radical I that triggers the subsequent 1,5-HAT to generate the alkyl radical II. Meanwhile, PhI and iodanyl radical are cogenerated in the reaction. Nucleophilic addition of II to the quinoline salt III affords the radical cation IV, which is then single-electron oxidized by excess PIFA or the in situ formed iodanyl radical to afford the final product 3a.
Fig. 4.
Plausible reaction mechanism. The proposed pathways for the generation of alkoxy radical from alcohol and the subsequent Minisci-type reaction
Discussion
In summary, we have described a practical and metal-free protocol of alcohol-directed remote C(sp3)–H functionalization. The combinational use of PIFA and visible-light irradiation offers a non-trivial and mild tactic for the direct generation of alkoxy radicals from free alcohols. This strategy is expected to significantly facilitate the alkoxy radical-mediated transformations. A vast array of heteroaryls and alcohols have proven to be suitable substrates. The protocol makes a complement to the classic Minisci reactions, and may find wide use in medicinal synthesis owing to the easy operation and metal-free conditions.
Methods
General procedure for heteroarylation of remote C(sp3)–H bonds
Heteroaryl 1 (0.4 mmol) and alcohol 2 (2.0 mmol) were loaded in a reaction vial, which was subjected to evacuation/flushing with N2 three times. Then DCM (2.0 mL) followed by PIFA (0.92 mmol) was added to the mixture. The reaction was irradiated with 100 W blue LEDs and kept at room temperature (rt) under fan cooling. After the reaction completion monitored by TLC, the mixture was quenched by addition of aq. KOH until pH > 8 and then extracted with ethyl acetate (3 × 10 mL). The combined organic extracts were washed by brine, dried over Na2SO4, filtered, concentrated, and purified by flash column chromatography on silica gel (eluent: ethyl acetate/petroleum ether) to give the desired product 3.
Data availability
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
C.Z. is grateful for the financial support from Soochow University, the National Natural Science Foundation of China (21722205), the Project of Scientific and Technologic Infrastructure of Suzhou (SZS201708), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author contributions
X.W. and C.Z. conceived and designed the experiments; X.W. carried out most of the experiments; X.W., H.Z., N.T., Z.W., D.W., M.J., Y.X., M.W. and C.Z. analyzed the data; C.Z. wrote the paper; C.Z. directed the project.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/5/2018
This Article was originally published without the accompanying Peer Review File. This file is now available in the HTML version of the Article; the PDF was correct from the time of publication.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-05522-9.
References
- 1.Chen X, Engle KM, Wang DH, Yu JQ. Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newhouse T, Baran PS. If C–H bonds could talk: selective C–H bond oxidation. Angew. Chem. Int. Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi J, Yamaguchi AD, Itami K. C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- 5.Robertson J, Pillai J, Lush RK. Radical translocation reactions in synthesis. Chem. Soc. Rev. 2001;30:94–103. doi: 10.1039/b000705f. [DOI] [Google Scholar]
- 6.Chiba S, Chen H. sp3 C–H oxidation by remote H-radical shift with oxygen- and nitrogen-radicals: a recent update. Org. Biomol. Chem. 2014;12:4051–4060. doi: 10.1039/C4OB00469H. [DOI] [PubMed] [Google Scholar]
- 7.Hu XQ, Chen JR, Xiao WJ. Controllable remote C–H bond functionalization by visible-light photocatalysis. Angew. Chem. Int. Ed. 2017;56:1960–1962. doi: 10.1002/anie.201611463. [DOI] [PubMed] [Google Scholar]
- 8.Yan M, Lo JC, Edwards JT, Baran PS. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 2016;138:12692–12714. doi: 10.1021/jacs.6b08856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi GJ, Zhu Q, Miller DC, Gu CJ, Knowles RR. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature. 2016;539:268–271. doi: 10.1038/nature19811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu JCK, Rovis T. Amide-directed photoredox-catalysed C–C bond formation at unactivated sp3 C–H bonds. Nature. 2016;539:272–275. doi: 10.1038/nature19810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen DF, Chu JCK, Rovis T. Directed γ-C(sp3)–H alkylation of carboxylic acid derivatives through visible light photoredox catalysis. J. Am. Chem. Soc. 2017;139:14897–14900. doi: 10.1021/jacs.7b09306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shu W, Nevado C. Visible-light-mediated remote aliphatic C–H functionalizations through a 1,5-hydrogen transfer cascade. Angew. Chem. Int. Ed. 2017;56:1881–1884. doi: 10.1002/anie.201609885. [DOI] [PubMed] [Google Scholar]
- 13.Dauncey EM, Morcillo SP, Douglas JJ, Sheikh NS, Leonori D. Photoinduced remote functionalisations by iminyl radical promoted C–C and C–H bond cleavage cascades. Angew. Chem. Int. Ed. 2018;57:744–748. doi: 10.1002/anie.201710790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Studer A. α-Aminoxy-acid-auxiliary-enabled intermolecular radical γ-C(sp3)–H functionalization of ketones. Angew. Chem. Int. Ed. 2018;57:1692–1696. doi: 10.1002/anie.201712066. [DOI] [PubMed] [Google Scholar]
- 15.Yoon TP, Ischay MA, Du J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010;2:527–532. doi: 10.1038/nchem.687. [DOI] [PubMed] [Google Scholar]
- 16.Narayanam JMR, Stephenson CRJ. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 2011;40:102–113. doi: 10.1039/B913880N. [DOI] [PubMed] [Google Scholar]
- 17.Xuan J, Xiao WJ. Visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2012;51:6828–6838. doi: 10.1002/anie.201200223. [DOI] [PubMed] [Google Scholar]
- 18.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xuan J, Zhang ZG, Xiao WJ. Visible-light-induced decarboxylative functionalization of carboxylic acids and their derivatives. Angew. Chem. Int. Ed. 2015;54:15632–15641. doi: 10.1002/anie.201505731. [DOI] [PubMed] [Google Scholar]
- 20.Laarhoven LJJ, Mulder P, Wayner DDM. Determination of bond dissociation enthalpies in solution by photoacoustic calorimetry. Acc. Chem. Res. 1999;32:342–349. doi: 10.1021/ar9703443. [DOI] [Google Scholar]
- 21.Blanksby SJ, Ellison GB. Bond dissociation energies of organic molecules. Acc. Chem. Res. 2003;36:255–263. doi: 10.1021/ar020230d. [DOI] [PubMed] [Google Scholar]
- 22.Xue XS, Ji P, Zhou B, Cheng JP. The essential role of bond energetics in C–H activation/functionalization. Chem. Rev. 2017;117:8622–8648. doi: 10.1021/acs.chemrev.6b00664. [DOI] [PubMed] [Google Scholar]
- 23.Yi H, et al. Recent advances in radical C–H activation/radical cross-coupling. Chem. Rev. 2017;117:9016–9085. doi: 10.1021/acs.chemrev.6b00620. [DOI] [PubMed] [Google Scholar]
- 24.Guo S, Kumar PS, Yang M. Recent advances of oxidative radical cross-coupling reactions: direct α-C(sp3)–H bond functionalization of ethers and alcohols. Adv. Synth. Catal. 2017;359:2–25. doi: 10.1002/adsc.201600467. [DOI] [Google Scholar]
- 25.Zhang SY, Tu YQ, Fan CA, Zhang FM, Shi L. Iron-catalyzed C(sp3)–C(sp3) bond formation through C(sp3)–H functionalization: a cross-coupling reaction of alcohols with alkenes. Angew. Chem. Int. Ed. 2009;48:8761–8765. doi: 10.1002/anie.200903960. [DOI] [PubMed] [Google Scholar]
- 26.He T, Yu L, Zhang L, Wang L, Wang M. Direct C2-alkylation of azoles with alcohols and ethers through dehydrogenative cross-coupling under metal-free conditions. Org. Lett. 2011;13:5016–5019. doi: 10.1021/ol201779n. [DOI] [PubMed] [Google Scholar]
- 27.Zhou W, et al. Metal-free oxidative functionalization of C(sp3)–H bond adjacent to oxygen and radical addition to olefins. Org. Lett. 2015;17:1160–1163. doi: 10.1021/acs.orglett.5b00088. [DOI] [PubMed] [Google Scholar]
- 28.Jeffrey JL, Terrett JA, MacMillan DWC. O–H hydrogen bonding promotes H-atom transfer from α C–H bonds for C-alkylation of alcohols. Science. 2015;349:1532–1536. doi: 10.1126/science.aac8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartung, J. Stereoselective construction of the tetrahydrofuran nucleus by alkoxyl radical cyclizations. Eur. J. Org. Chem. 2001, 619–632 (2001).
- 30.Čeković Ž. Reactions of δ-carbon radicals generated by 1,5-hydrogen transfer to alkoxyl radicals. Tetrahedron. 2003;59:8073–8090. doi: 10.1016/S0040-4020(03)01202-X. [DOI] [Google Scholar]
- 31.Ren R, Zhu C. Radical-mediated ring-opening functionalization of cyclobutanols: a shortcut to γ-substituted ketones. Synlett. 2016;27:1139–1144. doi: 10.1055/s-0035-1561358. [DOI] [Google Scholar]
- 32.Wu X, Zhu C. Recent advances in ring-opening functionalization of cycloalkanols by C–C σ-bond cleavage. Chem. Rec. 2018;18:587–598. doi: 10.1002/tcr.201700090. [DOI] [PubMed] [Google Scholar]
- 33.Zhao H, Fan X, Yu J, Zhu C. Silver-catalyzed ring-opening strategy for the synthesis of β- and γ-fluorinated ketones. J. Am. Chem. Soc. 2015;137:3490–3493. doi: 10.1021/jacs.5b00939. [DOI] [PubMed] [Google Scholar]
- 34.Ren R, Zhao H, Huan L, Zhu C. Manganese-catalyzed oxidative azidation of cyclobutanols: regiospecific aynthesis of alkyl azides by C–C bond cleavage. Angew. Chem. Int. Ed. 2015;54:12692–12696. doi: 10.1002/anie.201506578. [DOI] [PubMed] [Google Scholar]
- 35.Ren R, Wu Z, Xu Y, Zhu C. C–C bond-forming strategy by manganese-catalyzed oxidative ring-opening cyanation and ethynylation of cyclobutanol derivatives. Angew. Chem. Int. Ed. 2016;55:2866–2869. doi: 10.1002/anie.201510973. [DOI] [PubMed] [Google Scholar]
- 36.Jia K, Zhang F, Huang H, Chen Y. Visible-light-induced alkoxyl radical generation enables selective C(sp3)–C(sp3) bond cleavage and functionalizations. J. Am. Chem. Soc. 2016;138:1514–1517. doi: 10.1021/jacs.5b13066. [DOI] [PubMed] [Google Scholar]
- 37.Yayla HG, Wang H, Tarantino KT, Orbe HS, Knowles RR. Catalytic ring-opening of cyclic alcohols enabled by PCET activation of strong O–H bonds. J. Am. Chem. Soc. 2016;138:10794–10797. doi: 10.1021/jacs.6b06517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo J, et al. Photocatalytic C–C bond cleavage and amination of cycloalkanols by cerium(III) chloride complex. Angew. Chem. Int. Ed. 2016;55:15319–15322. doi: 10.1002/anie.201609035. [DOI] [PubMed] [Google Scholar]
- 39.Jia K, Pan Y, Chen Y. Selective carbonyl-C(sp3) bond cleavage to construct ynamides, ynoates, and ynones by photoredox catalysis. Angew. Chem. Int. Ed. 2017;56:2478–2481. doi: 10.1002/anie.201611897. [DOI] [PubMed] [Google Scholar]
- 40.Barton DHR, Beaton JM, Geller LE, Pechet MM. A new photochemical reaction. J. Am. Chem. Soc. 1960;82:2640–2641. doi: 10.1021/ja01495a061. [DOI] [Google Scholar]
- 41.Barton DHR, Beaton JM. A synthesis of aldosterone acetate. J. Am. Chem. Soc. 1960;82:2641. doi: 10.1021/ja01495a062. [DOI] [Google Scholar]
- 42.Barton DHR, Beaton JM, Geller LE, Pechet MM. A new photochemical reaction. J. Am. Chem. Soc. 1961;83:4076–4083. doi: 10.1021/ja01480a030. [DOI] [Google Scholar]
- 43.Čeković Ž, Green MM. Formation of remote double bonds by ferrous sulfate–cupric acetate promoted decomposition of alkyl hydroperoxides. J. Am. Chem. Soc. 1974;96:3000–3002. doi: 10.1021/ja00816a059. [DOI] [Google Scholar]
- 44.Čeković Ž, Dimitrijević L, Djokić G, Srnić T. Remote functionalisation by ferrous ion–cupric ion induced decomposition of alkyl hydroperoxides. Tetrahedron. 1979;35:2021–2026. doi: 10.1016/S0040-4020(01)88972-9. [DOI] [Google Scholar]
- 45.Čeković Ž, Ĉvetković M. Functionallization of the δ-carbon atom by the ferrous ion induced decomposition of alkyl hydroperoxides in the presence of cupric salts. Tetrahedron Lett. 1982;23:3791–3794. doi: 10.1016/S0040-4039(00)87708-4. [DOI] [Google Scholar]
- 46.Kundu R, Ball ZT. Copper-catalyzed remote sp3 C–H chlorination of alkyl hydroperoxides. Org. Lett. 2010;12:2460–2463. doi: 10.1021/ol100472t. [DOI] [PubMed] [Google Scholar]
- 47.Beckwith, A. L. J., Hay, B. P. & Williams, G. M. Generation of alkoxyl radicals from O-alkyl benzenesulphenates. J. Chem. Soc. Chem. Commun. 0, 1202–1203 (1989).
- 48.Čeković Ž, Petrović G. Alkylation of remote non-activated δ-carbon atoms: addition of δ-carbon radicals, generated by 1,5-hydrogen transfer in alkoxy radical intermediates, to activated olefins. Tetrahedron. 1999;55:1377–1390. doi: 10.1016/S0040-4020(98)01110-7. [DOI] [Google Scholar]
- 49.Petrović, G., Saičić, R. N. & Čeković, Ž. Synthesis of acetyl scopine. Intramolecular reactions of N-carbethoxy nortropine-3α-benzenesulfenate. Synlett. 1999, 635–637 (1999).
- 50.Heusler K, Kalvoda J. Intramolecular free-radical reactions. Angew. Chem. Int. Ed. Engl. 1964;3:525–538. doi: 10.1002/anie.196405251. [DOI] [Google Scholar]
- 51.Mihailović, M. L. & Čeković, Ž. Intramolecular oxidative cyclization of alcohols with lead tetraacetate. Synthesis. 1970, 209–224 (1970).
- 52.Kalvoda, J. & Heusler, K. Die Hypojodit-reaktion (Verfahren zur intramolekularen substitution an nicht-aktivierten C-Atomen). Synthesis. 1971, 501–526 (1971).
- 53.Walling CR, Padwa A. Positive halogen compounds. VII. Intramolecular chlorinations with long chain hypochlorites. J. Am. Chem. Soc. 1963;85:1597–1601. doi: 10.1021/ja00894a013. [DOI] [Google Scholar]
- 54.Walling C, Bristol D. delta-Chloro alcohols and tetrahydrofurans from primary and secondary alkyl hypochlorites. J. Org. Chem. 1972;37:3514–3516. doi: 10.1021/jo00795a026. [DOI] [Google Scholar]
- 55.Walling CR, Clark T. Reactions of primary and secondary alkoxy radicals derived from hypochlorites. J. Am. Chem. Soc. 1974;96:4530–4534. doi: 10.1021/ja00821a028. [DOI] [Google Scholar]
- 56.Concepción JI, Francisco CG, Hernández R, Salazar JA, Suárez E. Intramolecular hydrogen abstraction. Iodosobenzene diacetate, an efficient and convenient reagent for alkoxy radical generation. Tetrahedron Lett. 1984;25:1953–1956. doi: 10.1016/S0040-4039(01)90085-1. [DOI] [Google Scholar]
- 57.Martín A, Salazar JA, Suárez E. Synthesis of chiral spiroacetals from carbohydrates. Tetrahedron Lett. 1995;36:4489–4492. doi: 10.1016/0040-4039(95)00766-6. [DOI] [Google Scholar]
- 58.Martín A, Salazar JA, Suárez E. Synthesis of chiral spiroacetals from carbohydrates. J. Org. Chem. 1996;61:3999–4006. doi: 10.1021/jo960060g. [DOI] [PubMed] [Google Scholar]
- 59.Martín A, Pérez-Martín I, Suárez E. Intramolecular hydrogen abstraction promoted by amidyl radicals. Evidence for electronic factors in the nucleophilic cyclization of ambident amides to oxocarbenium ions. Org. Lett. 2005;7:2027–2030. doi: 10.1021/ol050526u. [DOI] [PubMed] [Google Scholar]
- 60.Beckwith ALJ, Hay BP. Generation of alkoxy radicals from N-alkoxypyridinethiones. J. Am. Chem. Soc. 1988;110:4415–4416. doi: 10.1021/ja00221a051. [DOI] [Google Scholar]
- 61.Hartung J, Gallou F. Ring closure reactions of substituted 4-pentenyl-1-oxy radicals. The stereoselective synthesis of functionalized disubstituted tetrahydrofurans. J. Org. Chem. 1995;60:6706–6716. doi: 10.1021/jo00126a021. [DOI] [Google Scholar]
- 62.Kim, S., Lee, T. A. & Song, Y. Facile generation of alkoxy radicals from N-alkoxyphthalimides. Synlett. 1998, 471–472 (1998).
- 63.Zhang J, Li Y, Zhang F, Hu C, Chen Y. Generation of alkoxyl radicals by photoredox catalysis enables selective C(sp3)–H functionalization under mild reaction conditions. Angew. Chem. Int. Ed. 2016;55:1872–1875. doi: 10.1002/anie.201510014. [DOI] [PubMed] [Google Scholar]
- 64.Wang C, Harms K, Meggers E. Catalytic asymmetric C–H functionalization under photoredox conditions by radical translocation and stereocontrolled alkene addition. Angew. Chem. Int. Ed. 2016;55:13495–13498. doi: 10.1002/anie.201607305. [DOI] [PubMed] [Google Scholar]
- 65.Wu X, et al. Tertiary-alcohol-directed functionalization of remote C(sp3)–H bonds by sequential hydrogen atom and heteroaryl migrations. Angew. Chem. Int. Ed. 2018;57:1640–1644. doi: 10.1002/anie.201709025. [DOI] [PubMed] [Google Scholar]
- 66.Hu A, et al. δ-Selective functionalization of alkanols enabled by visible-light-induced ligand-to-metal charge transfer. J. Am. Chem. Soc. 2018;140:1612–1616. doi: 10.1021/jacs.7b13131. [DOI] [PubMed] [Google Scholar]
- 67.Antonchick AP, Burgmann L. Direct selective oxidative cross-coupling of simple alkanes with heteroarenes. Angew. Chem. Int. Ed. 2013;52:3267–3271. doi: 10.1002/anie.201209584. [DOI] [PubMed] [Google Scholar]
- 68.Jin J, MacMillan DWC. Direct α-arylation of ethers through the combination of photoredox-mediated C–H functionalization and the Minisci reaction. Angew. Chem. Int. Ed. 2015;54:1565–1569. doi: 10.1002/anie.201410432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quattrini MC, et al. Versatile cross-dehydrogenative coupling of heteroaromatics and hydrogen donors via decatungstate photocatalysis. Chem. Commun. 2017;53:2335–2338. doi: 10.1039/C6CC09725A. [DOI] [PubMed] [Google Scholar]
- 70.Liu W, Yang X, Zhou ZZ, Li CJ. Simple and clean photo-induced methylation of heteroarenes with MeOH. Chem. 2017;2:688–702. doi: 10.1016/j.chempr.2017.03.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author on reasonable request.