Abstract
Cancer immunotherapy is aimed at stimulating tumor-specific cytotoxic T lymphocytes and their subsequent trafficking so that they may reach, and persist in, the tumor microenvironment, recognizing and eliminating malignant target cells. Thus, characterization of the phenotype and effector functions of CD8+ T lymphocytes infiltrating human solid tumors is essential for better understanding and manipulating the local antitumor immune response, and for defining their contribution to the success of current cancer immunotherapy approaches. Accumulating evidence indicates that a substantial subpopulation of CD3+CD8+ tumor-infiltrating lymphocytes are tissue resident memory T (TRM) cells, and is emerging as an activated tumor-specific T-cell subset. These TRM cells accumulate in various human cancer tissues, including non-small-cell lung carcinoma (NSCLC), ovarian and breast cancers, and are defined by expression of CD103 [αE(CD103)β7] and/or CD49a [α1(CD49a)β1] integrins, along with C-type lectin CD69, which most likely contribute to their residency characteristic. CD103 binds to the epithelial cell marker E-cadherin, thereby promoting retention of TRM cells in epithelial tumor islets and maturation of cytotoxic immune synapse with specific cancer cells, resulting in T-cell receptor (TCR)-dependent target cell killing. Moreover, CD103 integrin triggers bidirectional signaling events that cooperate with TCR signals to enable T-cell migration and optimal cytokine production. Remarkably, TRM cells infiltrating human NSCLC tumors also express inhibitory receptors such as programmed cell death-1, the neutralization of which, with blocking antibodies, enhances CD103-dependent TCR-mediated cytotoxicity toward autologous cancer cells. Thus, accumulation of TRM cells at the tumor site explains the more favorable clinical outcome, and might be associated with the success of immune checkpoint blockade in a fraction of cancer patients.
Keywords: CD8 tissue resident memory T (TRM) cells, CD103 integrin, cytotoxic T lymphocytes, onco-immunology, cancer immunotherapy
Introduction
CD8+ T lymphocytes play an essential role in defense against cancers through recognition by T-cell receptors (TCR) of specific antigenic peptides presented on the surface of malignant cells by major histocompatibility complex class I (MHC-I) molecules, and elimination of the tumor target, mainly by releasing the content of cytolytic granules containing perforin and granzymes. To destroy their target, cytotoxic T lymphocytes (CTL) must first migrate to the tumor site, infiltrate the tumor tissue, and interact with the cancer cell, to finally trigger effector functions leading to transformed cell eradication. Integrins and their ligands (1) play a crucial role in promoting antitumor T-cell activities by regulating T-cell migration and retention within the tumor, adhesion to antigen-presenting cells and co-stimulation resulting in CTL activation and functions (2). Cytokines and chemokines are also involved in coordinating circulation, homing, retention, and activation of T lymphocytes. Although some of them are known to contribute to tumor cell proliferation and dissemination by inhibiting tumor-specific T-cell responses, others promote infiltration and activation of T lymphocytes in a hostile tumor ecosystem, resulting in tumor cell destruction (3). In this regard, TGF-β, abundant in the tumor microenvironment, was reported to be an immunosuppressive factor used by malignant cells to escape from the immune response (4). This cytokine inhibits expression of lymphocyte-function-associated antigen-1 (LFA-1, also known as αLβ2 or CD11a) integrin and LFA-1-mediated T-cell functions (5). Paradoxically, this cytokine induces CD103 (also known as αEβ7 or HML-1) integrin expression on activated intraepithelial CD8+ T lymphocytes, and enhanced CD103-dependent T-cell adhesion and signaling (6, 7).
LFA-1 and CD103 are the predominant integrins expressed by intraepithelial T lymphocytes (IEL) and CD8+ tumor-infiltrating lymphocytes (TIL). While the contribution of LFA-1 and its ligand ICAM-1 (CD54) to TCR-mediated CTL activities is well documented (8), much less is known about the role of CD103 and its ligand, the epithelial cell marker E-cadherin, to T-cell-mediated cytolytic activity. CD103 has been associated with cytotoxicity of CD8+ T cells in several human pathologies, including graft-versus-host disease (GVHD) (9), allogeneic transplant rejection (10–12), autoimmune diseases (13, 14), and cancer (6, 15). This integrin, together with the activation marker CD69 and the integrin CD49a [also known as α1β1 or very late antigen-1 (VLA-1)], defines a recently identified subtype of CD8+ T lymphocytes called “tissue-resident memory T (TRM) cells,” possibly endowed with potent cytotoxic activities. Moreover, there is an emerging consensus that TRM cells frequently accumulate in multiple human tumors, especially of epithelial origin, and play an essential role in tumor-specific T-cell responses and, likely, in control of malignant diseases. TRM cells are also surrogate markers of the efficacy of cancer vaccines (16, 17), and a low number of this T-cell subset among TIL may correlate with failure of immune checkpoint blockade therapy in most cancer patients. In this review, we focus on CD8+ TRM cells accumulating in human solid tumors, mainly non-small-cell lung carcinoma (NSCLC), and current insight implicating CD103 integrin in regulating TRM functions and CTL-mediated antitumor immune responses, with potential prognosis and immunotherapeutic applications.
Phenotypic and Molecular Features of TRM Cells in Tumors
It is now generally agreed that a population of TRM cells accumulates in tumors of epithelial origin, such as ovarian, pancreatic, colorectal, and lung tumors (15, 18–20), as well as those of non-epithelial origin, including malignant glioma and melanoma (21, 22). These TRM cells express a broad range of integrins and chemokine receptors, probably involved in their migration to the tumor site, and may interfere with their egress from the tumor tissue. Transcriptional studies pointed to expression of CXCR3 and CXCR6 by TRM cells infiltrating human lungs (23). Intratumoral TRM cells express high levels of CCR5 and CCR6 chemokine receptors that may confer T-cell homing to the inflammatory tumor microenvironment (15). Moreover, CCR5 is recruited at the immune synapse formed between T cells and tumor target cells upon interaction of CD103 with E-cadherin, promoting retention of TRM cells at the tumor site by inhibiting their sensitivity to a CCL5 chemotactic gradient (7). By contrast, TRM cells do not express CX3CR1, a chemokine receptor that mediates transmigration through the endothelium, supporting the hypothesis that this T-cell population has reached its final destination and does not need to exit from the lung tissue (23). Lung tumor TRM lack expression of lymph node homing receptors CCR7 and CD62L, as well as the receptor for sphingosine 1-phosphate, S1PR1 (15), which mediates the egress of T cells from lymphoid organs (24). Indeed, downregulation of SIPR1 appears to be a prerequisite for retention of CD8+CD103+ TRM cells in peripheral tissues (25, 26).
With regard to adhesion/costimulatory molecules, the expression profile of intratumoral TRM cells seems to be compatible with their capacity to reside in tumor tissue and their inability to recirculate in the bloodstream. In melanoma, CD8+ TRM cells were found to co-express CD69, CD103, and VLA-1 (CD49a or α1β1 integrin), with the latter reported to cause long-term retention of activated T cells in peripheral tissues (27). Human lung tumor CD8+ TRM cells are characterized by downregulation of CD28 and upregulation of CD69 and CD103 and CD49a integrins, which are most likely induced by TGF-β in the tumor microenvironment (15, 28). TGF-β plays a pivotal role in formation and maintenance of TRM, at least in part via induction of CD103. Indeed, TGF-β is directly involved in CD103 expression in tumor-specific T cells upon engagement of TCR with specific tumor peptide–MHC-I complexes (7), through binding of Smad2/3 and NFAT-1 transcription factors to promoter and enhancer elements of the ITGAE gene, which encodes the CD103 (αE) subunit (29). This cytokine is also involved in dampening expression of the LFA-1 integrin on TIL, thus participating in T-cell residency within the tumor (15, 30). In LCMV chronic infection, but not acute infection, TGF-β signaling inhibits migration of CD8+ effector T lymphocytes from the spleen to the gut by dampening expression of integrin α4β7 during the formation phase of TRM cells (31). Consequently, CD8+ Tgfbr2−/− T cells migrate normally to the intestine, but their retention in the gut epithelium is impaired. In contrast, TGF-β signaling does not impact α4β7 integrin expression and T-cell migration to the gut after acute bacterial infection (32). Moreover, E-cadherin, which is downregulated by TGF-β in cancer cells during epithelial-to-mesenchymal transition [for a review see Ref. (33)], appeared to promote accumulation of a subset of CD8+ memory T cells in murine submandibular glands by a mechanism independent of CD103 (34). This cytokine has been identified as a potential therapeutic target in cancer because of its role in supporting tumor progression and in inducing immunosuppression. In this regard, it has been shown that targeting the TGF-β pathway inhibits tumor growth by promoting antitumor immunity associated with increased CD8+ T-cell numbers (35). However, the consequence of such cancer immunotherapy approaches on TRM cells, the maintenance of which is dependent of TGF-β, has not been addressed.
T-cell inhibitory receptors are important for maintaining self-tolerance and regulating the immune response in peripheral tissues (36). Among these immune checkpoints, cytotoxic T-lymphocyte-associated antigen (CTLA)-4 and Tim-3 appeared to be associated with tumor antigen-specific CD8+ T-cell dysfunction in melanoma patients (37). CD103+ TRM cells have been shown to express a wide range of inhibitory receptors, such as CTLA-4, Tim-3, and programmed cell death-1 (PD-1), associated with their capacity to maintain peripheral tolerance (25, 38). Data from our group and other groups revealed that intratumoral CD8+CD103+ TRM cells frequently express PD-1, Tim-3, and Lag-3, which are likely involved in their exhausted state and their dysfunctioning at the tumor site (15, 28, 39, 40). Notably, TGF-β is also involved in PD-1 induction on CD8+ T cells, contributing to T-cell anergy and a sustained tolerance (41). Neutralization of TGF-β results in downregulation of PD-1 expression in T cells causing graft rejection. Mechanistically, PD-1 is regulated by the NFATc1 transcription factor (42), and is enhanced by a TGF-β/SMAD3-dependent signaling pathway (43). Expression of PD-1 on TIL is described as a biomarker of CD8+ tumor-reactive T cells in cancer patients (44). Thus, the PD-1+ status of tumor TRM cells suggests that they are enriched with antigen-specific CD8+ T cells that may be used as targets in cancer immunotherapy.
Alongside upregulation of genes encoding PD-1, CTLA-4 and Tim-3, CD8+ TIL display increased expression levels of genes encoding transcription factors EGR1 and Nr4a2 (25, 38), as well BATF and NAB1, suggesting a role in TRM establishment in the tumor (28). CD8+CD103+ TIL also express an increased level of T-bet (45) and the Runx3 transcription factor, which programs their residency in tumors (46). Indeed, Runx3 deficiency impaired TIL accumulation without affecting migration to the tumor, associated with an increase in tumor growth. By contrast, KLF2 transcription factor was diminished in TRM cells from human TILhi tumors (28), while Notch activity appeared to be required for maintenance of CD103+ TRM cells in mouse tumors (23). Therefore, additional studies are needed to better characterize the transcriptional features of CD8+CD103+ TRM cells in human tumors, and transcriptional factors that govern their residency in malignant tissues. Overall, the TRM cell subset is characterized by a Runx3+, Notch+, Hobit+, Blimp1+, BATF+, EOMESneg, and Tbetlow transcription factor profile (23, 46–49) and is defined by the surface expression of CD103, CD49a, and CD69 [for reviews see Ref. (50–52)]. It also expresses the inhibitory receptors PD-1, CTLA-4, and Tim-3 (15, 38, 53), and is promoted by particular route of immunization targeting tissue dendritic cells (17, 54, 55) and specific environmental factors mainly TGF-β, IL-33, and IL-15 (56–59).
Functional Activities of Intratumoral TRM Cells
Thus far, little is known about CD8+CD103+ TRM functions in tumor tissues. Immune checkpoint expression by CD103+ TIL suggested that CD8+ TRM cells in tumors are enriched with tumor antigen-specific CTL. These T cells were found to express transcripts encoding products linked to cytotoxic functions of CD8+ T lymphocytes, including IFNG, GZMA, GZMB, SEMA7A, KLRB1, CCL3, STAT1, RAB27A, IL21R, and FKBP1A (28). Expression of granzyme A, granzyme B, and perforin by CD8+CD103+ TIL was also observed at the protein level, together with the CD107a (LAMP-1) degranulation marker and the Ki-67 proliferation marker (15, 28, 45, 60).
Functional studies showed that CD8+CD103+ TIL are able to secrete inflammatory cytokines, including interferon (IFN)γ and TNFα (28, 46). Moreover, interaction of CD103 with E-cadherin on tumor target cells optimizes cytokine release, since siRNA targeting E-cadherin partially inhibited IFNγ production (61). Cytotoxicity experiments indicated that freshly isolated CD103+ TIL were able to kill autologous tumor cells following neutralization of the PD-1–PD-L1 interaction with anti-PD-1 or anti-PD-L1 blocking antibodies (15). This cytotoxic activity is most likely mediated by CD103+ T cells, since anti-CD103 neutralizing monoclonal antibodies (mAb) compromise this function. Consistently, cytotoxicity of CD103+ T-cell clones toward autologous E-cadherin+ tumor cells is inhibited anti-CD103 blocking mAb (6). Another noteworthy aspect of our contribution to the field is the demonstration that CD103 is an important molecule required for polarization of cytotoxic granules at the immune synapse formed between CTL clones and autologous tumor cells, and that siRNA targeting E-cadherin inhibited TCR-mediated target cell killing (6). Moreover, CD103 contributes to recruitment of CD103+ TRM cells within epithelial tumor islets, and intratumoral early T-cell signaling (30).
A role for the VLA-1 integrin in the differentiation and functions of TRM cells was reported in a mouse tumor model (27). VLA-1+ T cells, co-expressing or not CD103, secreted high levels of IFNγ upon re-stimulation, and this cytokine production was impaired by anti-VLA-1 or anti-CD103 mAb. Moreover, blockade of VLA-1 or CD103 severely compromised control of tumor growth in vivo. Similar studies revealed that CD8+CD103+ TRM cells accumulate and protect mice against melanoma in a CD103-dependent manner, and these TRM cells play a pivotal role in perpetuating antitumor immunity (22). Conversely, it has been reported that anti-latency-associated peptide (LAP) antibodies targeting the LAP/TGF-β complex induce a decrease in CD8+CD103+ T cells in mouse spleen and lymph nodes, and that this peculiar T-cell subset displays a tolerogenic feature (62). Murine CD8+CD103+ regulatory T cells have also been described in autoimmune diseases where they are induced by TGF-β and display suppressive activities independently of granzyme B (63). Moreover, CD8+CD103+ T cells are crucial for prevention of chronic GVHD lupus in mice by suppressing T helper and B cell responses through a non-cytotoxic mechanism involving TGF-β and IL-10 signals (64). However, further studies are needed to permit the distinction between human CD8+CD103+ CTL and CD8+CD103+ T regulatory cells, even though granzyme B expression appears as a good marker, and determine the exact contribution of both subsets in autoimmune [for a review see Ref. (65)] and cancer diseases.
Bidirectional Signaling of CD103 Dictates its Activation and Functions
Integrins are heterodimeric transmembrane receptors that mediate cell-extracellular matrix adhesion and cell–cell interactions (2). Among a family of 24 members (1), the CD103 integrin, formed by αE (CD103) and β7 subunits, is exclusively expressed by leukocytes, in particular IEL (66), psoriatic skin epidermal CD8+ T cells (67), cervico-vaginal antigen-specific CTL (68), and CD8+ T lymphocytes infiltrating various human tumors (6, 18–20, 60, 69). The restricted distribution of the CD103 integrin is attributed to expression of the αE subunit, since the β7 subunit is widely expressed in T cells (70).
On naive T lymphocytes, integrins have weak affinity for their ligands. However, stimulation of T lymphocytes through TCR or chemokine receptors initiates an “inside-out” signal that induces integrin activation by triggering integrin-extended conformation and clustering, thereby enhancing their affinity for their ligands. Firm adhesion of integrins to their ligands triggers an “outside-in” signal that has costimulatory functions in TCR signaling, thereby contributing to T-cell activation, migration, and cytotoxicity (71–73). Until recently, the signaling pathways of CD103 integrin and the molecules involved in its bidirectional activation were not clearly elucidated. Like the other integrins, CD103 activation is regulated by TCR engagement. In this context, it has been shown that cross-linking of TCR on IEL or cell treatment with phorbol myristate acetate increased the avidity of CD103 for E-cadherin and provided a mechanism for lymphocyte adherence and activation (74). Furthermore, the CCR9 ligand, CCL25, induced CD103-mediated adhesion of CD8+ IEL to E-cadherin, suggesting a role for this chemokine receptor/chemokine pair in promoting functions of CD103 via inside-out signaling (75). Similarly, the CCL7 chemokine has been shown to favor adhesion and retention of CD103-expressing T cells during renal allograft rejection, by promoting the adhesive properties of CD103 (76).
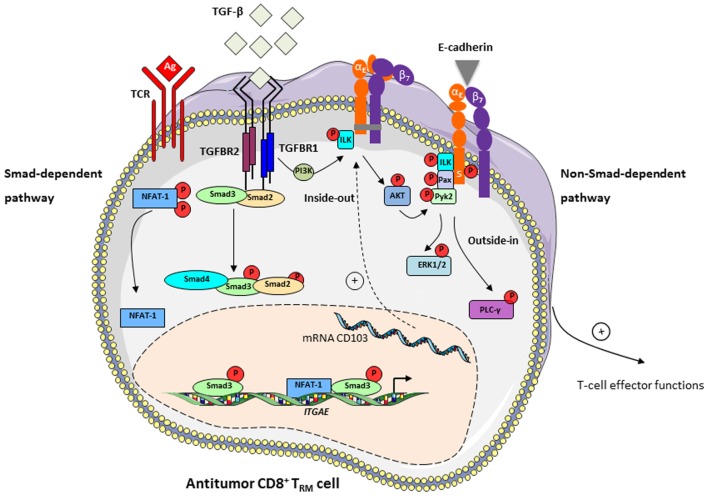
TGF-β is responsible for inducing CD103 integrin in CD8+ T lymphocytes (6, 77) by regulating expression of both ITGAE (29, 78) and ITGB7 (79) genes encoding αE and β7 chains, respectively. In addition, in contrast to all other integrins, TGF-β regulates CD103 activation and signaling within epithelial tissues (Figure 1). Indeed, we previously demonstrated that the interaction of TGF-β with its receptors TGFBR on the surface of CD8+CD103+ T cells induces recruitment and phosphorylation of integrin-linked kinase (ILK) by TGFBR1 (activin receptor-like kinase-5) (30). We further showed that phosphorylated-ILK interacted with the CD103 subunit intracellular domain, resulting in phosphorylation of protein kinase B (PKB)/AKT, thereby initiating integrin inside-out signaling leading to activation of CD103 and strengthening of CD103-E-cadherin adhesion.
Figure 1.
TGF-β induces CD103 expression in tumor-specific T cells and participates in integrin bidirectional signaling. Left: TGF-β controls CD103 expression in tumor antigen (Ag)-specific T cells upon interaction of T-cell receptor (TCR) with specific tumor peptide–major histocompatibility complex class I complexes, via a Smad-dependent pathway. TGF-β binds to TGFBR at the surface of CD8+ T lymphocytes and leads to recruitment and phosphorylation of Smad2 and Smad3 and their subsequent nuclear translocation. Transcription factors NFAT-1, translocated into the nucleus upon TCR engagement, and Smad2/3 bind to promoter and enhancer elements of the ITGAE gene, which encodes the CD103 (αE) subunit, and activates CD103 expression (29). Right: TGF-β participates in CD103 intracellular signaling via a non-Smad-dependent pathway. Interaction of TGF-β with TGFBR on CD8+CD103+ TRM cells induces recruitment and phosphorylation of integrin-linked kinase (ILK). Phosphorylated (P)-ILK interacts with the CD103 subunit intracellular domain, resulting in phosphorylation of protein kinase B/AKT and initiating integrin inside-out signaling leading to activation of CD103 (30). CD103-E-cadherin tight adhesion initiates an outside-in signal by promoting phosphorylation of paxillin (Pax) and Pyk2, and subsequent binding of phosphorylated-paxillin to the αE subunit tail where a phosphorylatable Ser (S) in the ES1163IRKAQL motif plays an important role (80). Adhesive interaction of E-cadherin with CD103 also triggers activation of PI3K/extracellular signal-regulated kinase (ERK) and phospholipase C/PKC pathways (60), providing intracellular signals that promote CD8+ TRM effector functions, including actin cytoskeleton reorganization, T-cell spreading and migration, cytokine release and polarized exocytosis of cytotoxic granules leading to target cell destruction.
The mechanism regulating the CD103 outside-in signaling pathway is not fully understood. Studies from our group have shown that CD103-E-cadherin tight adhesion initiates an outside-in signal by promoting phosphorylation of the focal-adhesion-associated adaptor protein paxillin and proline-rich tyrosine kinase-2 (Pyk2), and subsequent binding of phosphorylated-paxillin to the CD103 subunit tail (80). In addition, the adhesive interaction of E-cadherin with CD103 on TIL triggers phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and phospholipase C γ1 proteins, providing intracellular signals that promote CTL effector functions (60). These studies emphasize a unique costimulatory role of the CD103 integrin in activation of tumor-specific CTL, by triggering polarization of cytotoxic granules at the immune synapse and subsequent TCR-mediated cytotoxicity (60), and in proliferation of CD103+ thymocyte cells (81). Engagement of CD103 with E-cadherin also determines cell shape and motility of CD103+ lymphocytes (82), and recruitment of CD8+ TRM cells within epithelial tumor islets, in an actin-polymerization-dependent fashion (30, 80). Moreover, TGF-β enhances T-cell adhesion and movement toward tumor regions by increasing CD103 expression levels and promoting intracellular T-cell signals leading to integrin activation (30). CD103 also contributes to retention of TRM cell subpopulations by interacting with E-cadherin and mediating arrest of T lymphocytes on epithelial tissues (32, 61). Thus, CD103 appears to be a unique integrin for adjusting T-cell adhesion and migratory potential in a TGF-β-rich tumor microenvironment, as well as retention of tumor-specific CD8+ TRM cells and local antitumor effector functions (Figure 1).
Prognostic Value of TRM Cells in Human Cancers
CD8+CD103+ TRM cells have emerged as predictive markers of patient survival in several malignant diseases, including ovarian, lung, endometrial, and breast cancers (15, 20, 28, 83, 84). Indeed, in a large cohort of high-grade serous ovarian cancers (20) and a cohort of early-stage NSCLC (15), an enhanced CD103+ TIL subset correlated with improved patient survival. CD103+ TIL were also associated with a favorable prognosis in urothelial cell carcinoma of the bladder, and could represent a favorable prognostic predictor of overall and recurrence-free survival (83). In that retrospective study, CD8+ T cells were identified as the principal cellular sources of CD103, and the density of intratumoral CD103+ cells was inversely associated with tumor size. More recent studies also defined the CD103 integrin as a biomarker of good prognosis in cohorts of breast (85) and lung cancer (17, 28, 84). Notably, TRM infiltration in lung cancer correlated with better clinical outcome in both univariate and multivariate analyses, independently of CD8+ T cells (17). In addition, high numbers of intratumoral CD103+ TIL were significantly associated with prolonged disease-free survival and overall survival in patients with pulmonary squamous cell carcinoma, but not in those with pulmonary adenocarcinoma (84). The epithelial localization of CD103+ TIL has an even more significant predictive value compared to the stromal location, suggesting that intraepithelial CD8+CD103+ cells encompass a higher proportion of tumor-specific TRM cells (15, 85). This intratumoral positioning of CD103+ TIL was correlated with expression of E-cadherin on tumor cells in bladder cancer (83), but not in ovarian or breast cancer (20, 85). Moreover, this predominant location in intratumoral regions, rather than in the stroma, was associated with the capacity of CD103 to promote recruitment of TIL in epithelial tumor islets (30). Thus, TRM cells appear to be key components in antitumor immunity, and their presence at the tumor site predicts a favorable prognosis in many cancers of different histological types. Paradoxically, their dominant expression of checkpoint receptors suggests that may be functionally exhausted. However, their location in close contact with tumor cells, their ability to proliferate in situ, to produce granzyme B and other cytotoxic molecules and pro-inflammatory cytokines, support the hypothesis that TRM cells are enriched in tumor-specific CD8+ T cells that could trigger specific cytotoxic activity toward target cells in physiological conditions and following neutralization of PD-1–PD-L1 interaction, as we demonstrated ex vivo (15) and possibly also during anti-PD-1/anti-PD-L1 cancer immunotherapy. Accordantly, recent studies revealed an expansion of CD8+CD103+ TRM cells during anti-PD-1 treatment in melanoma (86).
Concluding Remarks
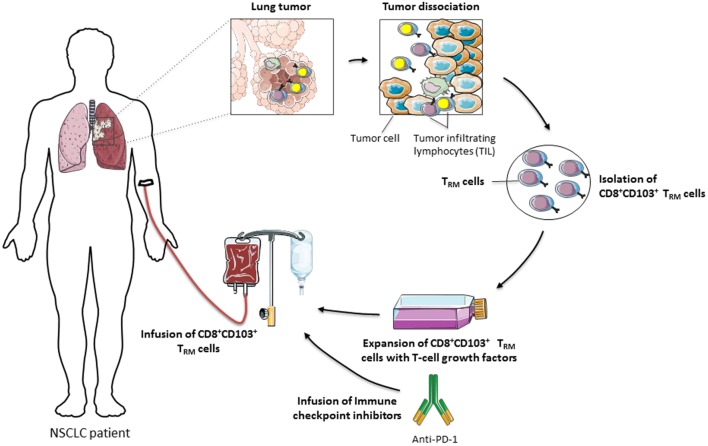
Overall, CD8+ TRM cells that accumulate in human tumor lesions appear to be important effectors in antitumor CTL responses. Their retention within the tumor ecosystem may control tumor growth and explain more favorable prognoses in certain cancer patients. Moreover, CD103 emerges as a key molecule in CD8+ TRM activation, the expression of which is probably adjusted in the tumor microenvironment by TGF-β. This integrin not only promotes T-cell adhesion to target cells through interaction with its unique known ligand E-cadherin but also provides positive signals triggering diverse T-cell effector functions, such as spreading, migration, proliferation, and cytotoxicity (Figure 1). Nevertheless, additional studies and tools are required to further decipher CD103 structure and bidirectional signaling, and to determine whether this integrin also undergoes conformational changes within the tumor ecosystem in order to control the affinity to its ligand E-cadherin and to regulate its functional properties. In this regard, identification of new partners and associated molecules controlling integrin intracellular signals and regulating the dynamics of CD103 are essential in order to optimize the antitumor reactivity of CD8+ TRM cells. They would also help to determine the true contribution of CD8+CD103+ TRM cells and the identified costimulatory molecules in the success of immune checkpoint blockade immunotherapies in a minor subpopulation of cancer patients, and to improve current T-cell-based cancer immunotherapeutic approaches such as adoptive T-cell therapies (Figure 2).
Figure 2.
Adoptive transfer of CD8+CD103+ TRM cells for cancer immunotherapy. CD8+CD103+ TRM cells, suspected to express tumor-reactive T-cell receptors, are isolated from tumor-infiltrating lymphocytes (TIL), amplified ex vivo in the presence of T-cell growth factors, including IL-2, and then reinjected into cancer patients, in combination or not with immune checkpoint inhibitors, such as anti-programmed cell death-1, to reverse T-cell exhaustion and optimize the antitumor cytotoxic T lymphocyte response.
Author Contributions
SC, MB, and FM-C coordinated the writing of the manuscript. SC, MB, MK, CN, and FM-C participated in drafting and editing the text and figures. All authors gave final approval to the version submitted.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Institut national du Cancer (INCa) PL-Bio 2016 (n° INCA_10557), Association pour la Recherche sur le Cancer (ARC) (n° PJA20161204720), Groupement des Entreprises françaises de Lutte contre le Cancer (GEFLUC) (n° R15080LL) and Ligue contre le cancer « Comité des Yvelines » (n° 9FI12414QLCZ). SC is a recipient of a fellowship from INCa.
Abbreviations
CTL, cytotoxic T lymphocytes; CTLA, cytotoxic T-lymphocyte-associated antigen; PD-1, programmed cell death-1; IFN, interferon; LFA-1, lymphocyte-function-associated antigen-1; mAb, monoclonal antibodies; NSCLC, non-small-cell lung carcinoma; MHC-I, major histocompatibility complex class I; TCR, T-cell receptor; TIL, tumor-infiltrating lymphocytes.
References
- 1.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res (2010) 339(1):269–80. 10.1007/s00441-009-0834-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol (2004) 22:157–80. 10.1146/annurev.immunol.22.012703.104649 [DOI] [PubMed] [Google Scholar]
- 3.Franciszkiewicz K, Boissonnas A, Boutet M, Combadiere C, Mami-Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res (2012) 72(24):6325–32. 10.1158/0008-5472.CAN-12-2027 [DOI] [PubMed] [Google Scholar]
- 4.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell (2005) 8(5):369–80. 10.1016/j.ccr.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 5.Sela U, Mauermann N, Hershkoviz R, Zinger H, Dayan M, Cahalon L, et al. The inhibition of autoreactive T cell functions by a peptide based on the CDR1 of an anti-DNA autoantibody is via TGF-beta-mediated suppression of LFA-1 and CD44 expression and function. J Immunol (2005) 175(11):7255–63. 10.4049/jimmunol.175.11.7255 [DOI] [PubMed] [Google Scholar]
- 6.Le Floc’h A, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, et al. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med (2007) 204(3):559–70. 10.1084/jem.20061524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franciszkiewicz K, Le Floc’h A, Jalil A, Vigant F, Robert T, Vergnon I, et al. Intratumoral induction of CD103 triggers tumor-specific CTL function and CCR5-dependent T-cell retention. Cancer Res (2009) 69(15):6249–55. 10.1158/0008-5472.CAN-08-3571 [DOI] [PubMed] [Google Scholar]
- 8.Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A (2005) 102(18):6437–42. 10.1073/pnas.0502467102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, et al. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med (2005) 201(10):1647–57. 10.1084/jem.20041044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadley GA, Charandee C, Weir MR, Wang D, Bartlett ST, Drachenberg CB. CD103+ CTL accumulate within the graft epithelium during clinical renal allograft rejection. Transplantation (2001) 72(9):1548–55. 10.1097/00007890-200111150-00013 [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Wang D, Yuan R, Parker CM, Farber DL, Hadley GA. CD103 expression is required for destruction of pancreatic islet allografts by CD8(+) T cells. J Exp Med (2002) 196(7):877–86. 10.1084/jem.20020178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan R, El-Asady R, Liu K, Wang D, Drachenberg CB, Hadley GA. Critical role for CD103+CD8+ effectors in promoting tubular injury following allogeneic renal transplantation. J Immunol (2005) 175(5):2868–79. 10.4049/jimmunol.175.5.2868 [DOI] [PubMed] [Google Scholar]
- 13.Fujihara T, Fujita H, Tsubota K, Saito K, Tsuzaka K, Abe T, et al. Preferential localization of CD8+ alpha E beta 7+ T cells around acinar epithelial cells with apoptosis in patients with Sjogren’s syndrome. J Immunol (1999) 163(4):2226–35. [PubMed] [Google Scholar]
- 14.Oshitani N, Watanabe K, Maeda K, Fujiwara Y, Higuchi K, Matsumoto T, et al. Differential expression of homing receptor CD103 on lamina propria lymphocytes and association of CD103 with epithelial adhesion molecules in inflammatory bowel disease. Int J Mol Med (2003) 12(5):715–9. [PubMed] [Google Scholar]
- 15.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpreville V, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol (2015) 194(7):3475–86. 10.4049/jimmunol.1402711 [DOI] [PubMed] [Google Scholar]
- 16.Nizard M, Roussel H, Tartour E. Resident memory T cells as surrogate markers of the efficacy of cancer vaccines. Clin Cancer Res (2016) 22(3):530–2. 10.1158/1078-0432.CCR-15-2364 [DOI] [PubMed] [Google Scholar]
- 17.Nizard M, Roussel H, Diniz MO, Karaki S, Tran T, Voron T, et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat Commun (2017) 8:15221. 10.1038/ncomms15221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French JJ, Cresswell J, Wong WK, Seymour K, Charnley RM, Kirby JA. T cell adhesion and cytolysis of pancreatic cancer cells: a role for E-cadherin in immunotherapy? Br J Cancer (2002) 87(9):1034–41. 10.1038/sj.bjc.6600597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn E, Hawkins N, Yip YL, Suter C, Ward R. CD103+ intraepithelial lymphocytes – a unique population in microsatellite unstable sporadic colorectal cancer. Eur J Cancer (2003) 39(4):469–75. 10.1016/S0959-8049(02)00633-0 [DOI] [PubMed] [Google Scholar]
- 20.Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res (2014) 20(2):434–44. 10.1158/1078-0432.CCR-13-1877 [DOI] [PubMed] [Google Scholar]
- 21.Masson F, Calzascia T, Di Berardino-Besson W, de Tribolet N, Dietrich PY, Walker PR. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol (2007) 179(2):845–53. 10.4049/jimmunol.179.2.845 [DOI] [PubMed] [Google Scholar]
- 22.Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol (2017) 2(10):eaam6346. 10.1126/sciimmunol.aam6346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, Stark R, et al. Programs for the persistence, vigilance and control of human CD8(+) lung-resident memory T cells. Nat Immunol (2016) 17(12):1467–78. 10.1038/ni.3589 [DOI] [PubMed] [Google Scholar]
- 24.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol (2012) 30:69–94. 10.1146/annurev-immunol-020711-075011 [DOI] [PubMed] [Google Scholar]
- 25.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol (2013) 14(12):1294–301. 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- 26.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol (2013) 14(12):1285–93. 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray T, Fuertes Marraco SA, Baumgaertner P, Bordry N, Cagnon L, Donda A, et al. Very late antigen-1 marks functional tumor-resident CD8 T cells and correlates with survival of melanoma patients. Front Immunol (2016) 7:573. 10.3389/fimmu.2016.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol (2017) 18(8):940–50. 10.1038/ni.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mokrani M, Klibi J, Bluteau D, Bismuth G, Mami-Chouaib F. Smad and NFAT pathways cooperate to induce CD103 expression in human CD8 T lymphocytes. J Immunol (2014) 192(5):2471–9. 10.4049/jimmunol.1302192 [DOI] [PubMed] [Google Scholar]
- 30.Boutet M, Gauthier L, Leclerc M, Gros G, de Montpreville V, Theret N, et al. TGFbeta signaling intersects with CD103 integrin signaling to promote T-lymphocyte accumulation and antitumor activity in the lung tumor microenvironment. Cancer Res (2016) 76(7):1757–69. 10.1158/0008-5472.CAN-15-1545 [DOI] [PubMed] [Google Scholar]
- 31.Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity (2013) 39(4):687–96. 10.1016/j.immuni.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrancois L. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity (2014) 40(5):747–57. 10.1016/j.immuni.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H, Shen Y, Hong J, Xia Q, Zhou F, Liu X. The contribution of TGF-beta in epithelial-mesenchymal transition (EMT): down-regulation of E-cadherin via snail. Neoplasma (2015) 62(1):1–15. 10.4149/neo_2015_002 [DOI] [PubMed] [Google Scholar]
- 34.Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci U S A (2011) 108(40):16741–6. 10.1073/pnas.1107200108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, et al. Targeting the TGFbeta pathway with galunisertib, a TGFbetaRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer (2018) 6(1):47. 10.1186/s40425-018-0356-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med (2010) 207(10):2175–86. 10.1084/jem.20100637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol (2012) 189(7):3462–71. 10.4049/jimmunol.1201305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol Res (2015) 3(8):926–35. 10.1158/2326-6066.CIR-14-0239 [DOI] [PubMed] [Google Scholar]
- 40.Boddupalli CS, Bar N, Kadaveru K, Krauthammer M, Pornputtapong N, Mai Z, et al. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight (2016) 1(21):e88955. 10.1172/jci.insight.88955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baas M, Besancon A, Goncalves T, Valette F, Yagita H, Sawitzki B, et al. TGFbeta-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. Elife (2016) 5:e08133. 10.7554/eLife.08133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol (2008) 181(7):4832–9. 10.4049/jimmunol.181.7.4832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park BV, Freeman ZT, Ghasemzadeh A, Chattergoon MA, Rutebemberwa A, Steigner J, et al. TGFbeta1-mediated SMAD3 enhances PD-1 expression on antigen-specific T cells in cancer. Cancer Discov (2016) 6(12):1366–81. 10.1158/2159-8290.CD-15-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest (2014) 124(5):2246–59. 10.1172/JCI73639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang P, Huang B, Gao Y, Yang J, Liang Z, Zhang N, et al. CD103(+)CD8(+) T lymphocytes in non-small cell lung cancer are phenotypically and functionally primed to respond to PD-1 blockade. Cell Immunol (2018) 325:48–55. 10.1016/j.cellimm.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 46.Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, Phan AT, et al. Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature (2017) 552(7684):253–7. 10.1038/nature24993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol (2005) 6(12):1236–44. 10.1038/ni1268 [DOI] [PubMed] [Google Scholar]
- 48.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity (2010) 33(2):229–40. 10.1016/j.immuni.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science (2016) 352(6284):459–63. 10.1126/science.aad2035 [DOI] [PubMed] [Google Scholar]
- 50.Amsen D, van Gisbergen K, Hombrink P, van Lier RAW. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat Immunol (2018) 19(6):538–46. 10.1038/s41590-018-0114-2 [DOI] [PubMed] [Google Scholar]
- 51.Gebhardt T, Palendira U, Tscharke DC, Bedoui S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol Rev (2018) 283(1):54–76. 10.1111/imr.12650 [DOI] [PubMed] [Google Scholar]
- 52.Topham DJ, Reilly EC. Tissue-resident memory CD8(+) T cells: from phenotype to function. Front Immunol (2018) 9:515. 10.3389/fimmu.2018.00515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mackay LK, Wakim L, van Vliet CJ, Jones CM, Mueller SN, Bannard O, et al. Maintenance of T cell function in the face of chronic antigen stimulation and repeated reactivation for a latent virus infection. J Immunol (2012) 188(5):2173–8. 10.4049/jimmunol.1102719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu CI, Becker C, Wang Y, Marches F, Helft J, Leboeuf M, et al. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-beta. Immunity (2013) 38(4):818–30. 10.1016/j.immuni.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enamorado M, Iborra S, Priego E, Cueto FJ, Quintana JA, Martinez-Cano S, et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8(+) T cells. Nat Commun (2017) 8:16073. 10.1038/ncomms16073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol (2012) 188(10):4866–75. 10.4049/jimmunol.1200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, et al. T-box transcription factors combine with the cytokines TGF-beta and IL-15 to control tissue-resident memory T cell fate. Immunity (2015) 43(6):1101–11. 10.1016/j.immuni.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 58.Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, Vezys V, et al. IL-15-independent maintenance of tissue-resident and boosted effector memory CD8 T cells. J Immunol (2016) 196(9):3920–6. 10.4049/jimmunol.1502337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strutt TM, Dhume K, Finn CM, Hwang JH, Castonguay C, Swain SL, et al. IL-15 supports the generation of protective lung-resident memory CD4 T cells. Mucosal Immunol (2018) 11(3):668–80. 10.1038/mi.2017.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Floc’h A, Jalil A, Franciszkiewicz K, Validire P, Vergnon I, Mami-Chouaib F. Minimal engagement of CD103 on cytotoxic T lymphocytes with an E-cadherin-Fc molecule triggers lytic granule polarization via a phospholipase Cgamma-dependent pathway. Cancer Res (2011) 71(2):328–38. 10.1158/0008-5472.CAN-10-2457 [DOI] [PubMed] [Google Scholar]
- 61.Franciszkiewicz K, Le Floc’h A, Boutet M, Vergnon I, Schmitt A, Mami-Chouaib F. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res (2013) 73(2):617–28. 10.1158/0008-5472.CAN-12-2569 [DOI] [PubMed] [Google Scholar]
- 62.Gabriely G, da Cunha AP, Rezende RM, Kenyon B, Madi A, Vandeventer T, et al. Targeting latency-associated peptide promotes antitumor immunity. Sci Immunol (2017) 2(11):eaaj1738. 10.1126/sciimmunol.aaj1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Lan Q, Lu L, Chen M, Xia Z, Ma J, et al. Phenotypic and functional characteristic of a newly identified CD8+ Foxp3- CD103+ regulatory T cells. J Mol Cell Biol (2014) 6(1):81–92. 10.1093/jmcb/mjt026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhong H, Liu Y, Xu Z, Liang P, Yang H, Zhang X, et al. TGF-beta-induced CD8(+)CD103(+) regulatory T cells show potent therapeutic effect on chronic graft-versus-host disease lupus by suppressing B cells. Front Immunol (2018) 9:35. 10.3389/fimmu.2018.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu H, Liao W, Li Q, Long H, Yin H, Zhao M, et al. Pathogenic role of tissue-resident memory T cells in autoimmune diseases. Autoimmun Rev (2018). 10.1016/j.autrev.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 66.Kilshaw PJ, Murant SJ. A new surface antigen on intraepithelial lymphocytes in the intestine. Eur J Immunol (1990) 20(10):2201–7. 10.1002/eji.1830201008 [DOI] [PubMed] [Google Scholar]
- 67.Pauls K, Schon M, Kubitza RC, Homey B, Wiesenborn A, Lehmann P, et al. Role of integrin alphaE(CD103)beta7 for tissue-specific epidermal localization of CD8+ T lymphocytes. J Invest Dermatol (2001) 117(3):569–75. 10.1046/j.0022-202x.2001.01481.x [DOI] [PubMed] [Google Scholar]
- 68.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest (2012) 122(12):4606–20. 10.1172/JCI63287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cresswell J, Wong WK, Henry MJ, Robertson H, Neal DE, Kirby JA. Adhesion of lymphocytes to bladder cancer cells: the role of the alpha(E)beta(7) integrin. Cancer Immunol Immunother (2002) 51(9):483–91. 10.1007/s00262-002-0305-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andrew DP, Rott LS, Kilshaw PJ, Butcher EC. Distribution of alpha 4 beta 7 and alpha E beta 7 integrins on thymocytes, intestinal epithelial lymphocytes and peripheral lymphocytes. Eur J Immunol (1996) 26(4):897–905. 10.1002/eji.1830260427 [DOI] [PubMed] [Google Scholar]
- 71.Dustin ML, de Fougerolles AR. Reprogramming T cells: the role of extracellular matrix in coordination of T cell activation and migration. Curr Opin Immunol (2001) 13(3):286–90. 10.1016/S0952-7915(00)00217-X [DOI] [PubMed] [Google Scholar]
- 72.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity (2007) 26(1):17–27. 10.1016/j.immuni.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 73.Lek HS, Morrison VL, Conneely M, Campbell PA, McGloin D, Kliche S, et al. The spontaneously adhesive leukocyte function-associated antigen-1 (LFA-1) integrin in effector T cells mediates rapid actin- and calmodulin-dependent adhesion strengthening to ligand under shear flow. J Biol Chem (2013) 288(21):14698–708. 10.1074/jbc.M112.430918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Higgins JM, Mandlebrot DA, Shaw SK, Russell GJ, Murphy EA, Chen YT, et al. Direct and regulated interaction of integrin alphaEbeta7 with E-cadherin. J Cell Biol (1998) 140(1):197–210. 10.1083/jcb.140.1.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ericsson A, Svensson M, Arya A, Agace WW. CCL25/CCR9 promotes the induction and function of CD103 on intestinal intraepithelial lymphocytes. Eur J Immunol (2004) 34(10):2720–9. 10.1002/eji.200425125 [DOI] [PubMed] [Google Scholar]
- 76.Al-Hamidi A, Pekalski M, Robertson H, Ali S, Kirby JA. Renal allograft rejection: the contribution of chemokines to the adhesion and retention of alphaE(CD103)beta7 integrin-expressing intratubular T cells. Mol Immunol (2008) 45(15):4000–7. 10.1016/j.molimm.2008.06.011 [DOI] [PubMed] [Google Scholar]
- 77.Hadley GA, Bartlett ST, Via CS, Rostapshova EA, Moainie S. The epithelial cell-specific integrin, CD103 (alpha E integrin), defines a novel subset of alloreactive CD8+ CTL. J Immunol (1997) 159(8):3748–56. [PubMed] [Google Scholar]
- 78.Robinson PW, Green SJ, Carter C, Coadwell J, Kilshaw PJ. Studies on transcriptional regulation of the mucosal T-cell integrin alphaEbeta7 (CD103). Immunology (2001) 103(2):146–54. 10.1046/j.1365-2567.2001.01232.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim SP, Leung E, Krissansen GW. The beta7 integrin gene (Itgb-7) promoter is responsive to TGF-beta1: defining control regions. Immunogenetics (1998) 48(3):184–95. 10.1007/s002510050422 [DOI] [PubMed] [Google Scholar]
- 80.Gauthier L, Corgnac S, Boutet M, Gros G, Validire P, Bismuth G, et al. Paxillin binding to the cytoplasmic domain of CD103 promotes cell adhesion and effector functions for CD8(+) resident memory T cells in tumors. Cancer Res (2017) 77(24):7072–82. 10.1158/0008-5472.CAN-17-1487 [DOI] [PubMed] [Google Scholar]
- 81.Kutlesa S, Wessels JT, Speiser A, Steiert I, Muller CA, Klein G. E-cadherin-mediated interactions of thymic epithelial cells with CD103+ thymocytes lead to enhanced thymocyte cell proliferation. J Cell Sci (2002) 115(Pt 23):4505–15. 10.1242/jcs.00142 [DOI] [PubMed] [Google Scholar]
- 82.Schlickum S, Sennefelder H, Friedrich M, Harms G, Lohse MJ, Kilshaw P, et al. Integrin alpha E(CD103)beta 7 influences cellular shape and motility in a ligand-dependent fashion. Blood (2008) 112(3):619–25. 10.1182/blood-2008-01-134833 [DOI] [PubMed] [Google Scholar]
- 83.Wang B, Wu S, Zeng H, Liu Z, Dong W, He W, et al. CD103 tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J Urol (2015) 194(2):556–62. 10.1016/j.juro.2015.02.2941 [DOI] [PubMed] [Google Scholar]
- 84.Koh J, Kim S, Kim MY, Go H, Jeon YK, Chung DH. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget (2017) 8(8):13762–9. 10.18632/oncotarget.14632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. CD103 and intratumoral immune response in breast cancer. Clin Cancer Res (2016) 22(24):6290–7. 10.1158/1078-0432.CCR-16-0732 [DOI] [PubMed] [Google Scholar]
- 86.Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, et al. CD103(+) Tumor-resident CD8(+) T cells are associated with improved survival in immunotherapy-naive melanoma patients and expand significantly during anti-PD-1 treatment. Clin Cancer Res (2018) 24(13):3036–45. 10.1158/1078-0432.CCR-17-2257 [DOI] [PubMed] [Google Scholar]