Abstract
A highly sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed for the determination of tadalafil (TAD) in human plasma. TAD and its deuterated internal standard (IS), tadalafil-d3, were extracted from 200 µL plasma using Phenomenex Strata-X-C 33 µ extraction cartridges. Chromatographic analysis was carried out on Synergi™ Hydro-RP C18 (100 mm × 4.6 mm, 4 µm) column with a mobile phase consisting of methanol and 10 mM ammonium formate, pH 4.0 (90:10, v/v), delivered at a flow rate of 0.9 mL/min. Quantitation of the protonated analyte was done on a triple quadrupole mass spectrometer using multiple reaction monitoring via electrospray ionization. The precursor to product ions transitions monitored for TAD and TAD-d3 were m/z 390.3 → 268.2 and m/z 393.1 → 271.2, respectively. The calibration curve was linear over the concentration range of 0.50–500 ng/mL with correlation coefficient, r2 ≥ 0.9994. Acceptable intra-batch and inter-batch precision (≤ 3.7%) and accuracy (97.8% to 104.1%) were obtained at five concentration levels. The recovery of TAD from spiked plasma was highly precise and quantitative (98.95% to 100.61%). Further, the effect of endogenous matrix components was minimal. TAD was found to be stable under different storage conditions in human plasma and also in whole blood samples. The validated method was successfully used to determine TAD plasma concentration in a bioequivalence study with 20 mg TAD tablets in 24 healthy volunteers. Method performance was evaluated by reanalyzing 115 study samples.
Keywords: Tadalafil, Tadalafil-d3, LC-MS/MS, Sensitive, Bioequivalence study, Human plasma
1. Introduction
Oral synthetic phosphodiesterase type-5 (PDE-5) inhibitor drugs have become first-line treatment option for erectile dysfunction (ED) in males and are also used for pulmonary arterial hypertension (PAH), a debilitating chronic disease of the small pulmonary arteries [1], [2], [3]. These inhibitors bind with PDE-5 isozymes, which helps in increasing the concentrations of cyclic guanosine monophosphate (cGMP) in smooth muscle of arteries in the penis, producing smooth muscle relaxation and increasing blood flow to the corpus cavernosum for better erectile response. Currently four PDE-5 inhibitors, namely sildenafil, tadalafil (TAD), vardenafil and avanafil, are approved by the US Food and Drug Administration [4]. Sildenafil and TAD are two of the most widely used PDE-5 inhibitors worldwide. However, due to their different molecular structures they have markedly different pharmacodynamics and pharmacokinetic behavior. The key pharmacodynamic difference between sildenafil and TAD is towards selectivity for PDE isozymes. TAD is 1000 times more selective for PDE-5 than for PDE-1–4 and PDE-7–10, while sildenafil is only 41 times more selective for PDE-5 than other PDE isozymes [5]. One of the major pharmacokinetic differences is the long plasma half-life (t1/2,17.5 h) of TAD, which facilitates once-daily dosing as compared to the required three times daily dosing of sildenafil. The prolonged half-life of tadalafil is mainly due to the low volume of distribution, slow hepatic clearance, and approximately 80% bioavailability. This feature of TAD is beneficial in terms of patient convenience and adherence [2].
TAD has the longest duration of action in its class and a maximum duration of 72 h. After oral administration, the peak plasma concentration is reached in 2 h. TAD is primarily metabolized by CYP450 3A4 to its inactive catechol metabolite, which is further metabolized to its circulatory metabolite, methylcatechol glucuronide. TAD is eliminated primarily through feces, while one third of the metabolized drug is excreted in the urine. Unlike other members of its class, the absorption of TAD is unaffected by food. It is 94% plasma bound and shows linear pharmacokinetics over the dose range of 2.5–20 mg [2], [6], [7].
Several bioanalytical methods are available in the literature for the determination of TAD as a single analyte [8], [9], [10], [11], [12], [13], [14], and with other PDE-5 inhibitors [15], [16], [17], [18], [19], [20], [21], [22], [23] in different biological samples like rat plasma [9], [14], rat serum and brain tissue [17], mouse plasma [11], human and rat hair [20], human whole blood [12], [18], seminal plasma [13], human urine [15], [22] and human plasma [8], [10], [13], [16], [19], [21], [23]. Varieties of analytical techniques have been employed for its estimation including spectrofluorometry [21], gas chromatography-mass spectrometry (GC-MS) [12], [15], high performance liquid chromatography-ultraviolet (HPLC-UV) [9], [10], HPLC-fluorescence [11], liquid chromatography-tandem mass spectrometry (LC-MS/MS) [8], [13], [14], [16], [17], [19], [20], [22], [23] and ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) [18]. A comparative summary of chromatographic methods developed for TAD in different biological samples is shown in Table S1. Majority of these methods have either low sensitivity (≥ 5.0 ng/mL) [8], [9], [10], [11], [15], [16], [18], long analysis time (> 5.0 min) [9], [10], [11], [12], [15], [16], [17], [18], [20], [22], [23] or require large sample volume for processing (≥ 500 µL) [10], [12], [15], [16], [18], [22]. Thus, it is essential to develop a highly sensitive, selective and rapid bioanalytical assay especially to meet the requirement of pharmacokinetic studies. The developed method presents high sensitivity (0.50 ng/mL), and a short turnaround time (2.5 min) using 200 µL plasma samples. The method was successfully applied for a bioequivalence study in healthy Indian subjects with required accuracy and precision.
2. Experimental
2.1. Chemicals and materials
Reference standards of tadalafil (TAD, 99.59%) and tadalafil-d3 (TAD-d3, 99.99%), used as an internal standard (IS), were obtained from Vivan Life Sciences (P) Ltd. (Mumbai, India). HPLC grade methanol was procured from J T Baker, S.A.de C.V. (Estado de Mexico, Mexico), while ammonium formate and formic acid were obtained from S.D. Fine Chemicals Ltd. (Mumbai, India). Strata-X-C 33 µ reversed phase extraction cartridges (30 mg, 1 mL) were obtained from Phenomenex India (Hyderabad, India). Water used in the entire analysis was prepared using Milli-Q water purification system from Millipore (Bangalore, India). Blank human plasma in K3EDTA was obtained from Supratech Micropath (Ahmedabad, India) and was stored at –70 °C until use.
2.2. Instruments and conditions
A Shimadzu Nexera X2 UHPLC equipped with Shimadzu LCMS-8040 triple quadrupole mass spectrometer (MS) detector (Shimadzu Corporation, Kyoto, Japan) was used. Chromatographic analysis was performed on Phenomenex Synergi™ Hydro-RP C18 (100 mm × 4.6 mm, 4 µm) column using methanol and 10 mM ammonium formate, pH 4.0 adjusted with formic acid (90:10, v/v) as the mobile phase. The flow rate was kept at 0.9 mL/min. The column oven temperature and autosampler temperature was maintained at 40 °C and 5 °C, respectively. Electrospray ionization (ESI) source operating in the positive ionization mode was used for multiple reaction monitoring (MRM) LC-MS/MS analysis. The optimized MS/MS conditions for quantification of TAD and TAD-d3 are shown in Table S2. Data processing was done using Shimadzu LabSolution software.
2.3. Preparation of stock solutions, calibration standards (CSs) and quality control (QC) samples
The stock solution of TAD (500 µg/mL) was prepared by dissolving requisite amount in methanol. Working solutions were prepared by diluting the stock solution with methanol. The stock and working solutions were stored at 2–8 °C. CSs and samples were prepared by spiking blank plasma with working solutions. The concentration of CSs was in the range of 0.50–500 ng/mL. QC samples were prepared at five concentration levels (0.50, 1.50, 30.0, 200 and 400 ng/mL). All the samples prepared in plasma were kept at −70 °C until use. Stock solution of TAD-d3 (100 µg/mL) was prepared by dissolving 1.0 mg in 10.0 mL of methanol. Its working solution (1000 ng/mL) was prepared by appropriate dilution of the stock solution in methanol.
2.4. Sample extraction procedure
To an aliquot of 200 µL of spiked plasma/subject samples, 25 µL of TAD-d3 working solution was added and vortexed for 10 s. Further, 500 µL of Milli-Q water was added and vortexed again to mix. Samples were then loaded on Strata-X-C 33 µ extraction cartridges which were conditioned with 1.0 mL methanol, followed by 1.0 mL water. Washing of samples was done with 2 × 1.0 mL water, followed by drying of cartridges for 2.0 min by applying nitrogen (1.72 × 105 Pa) at 2.4 L/min flow rate. Elution of TAD and TAD-d3 was carried out using 500 µL of mobile phase solution into pre-labeled vials, and 10 µL was used for injection in the chromatographic system.
2.5. Validation procedures
The method validation was performed as per the USFDA guidelines [24] and was similar to our previous work [25], [26]. The detailed procedures and their acceptance criteria are summarized in Supplementary material.
2.6. Bioequivalence study and method reliability
The developed method was applied to measure plasma concentration of TAD for a bioequivalence study after oral administration of single dose of a test (20 mg TAD tablet from an Indian Company) and a reference (20 mg CIALIS (tadalafil) tablets from Lilly, LLC Indianapolis, IN 46,285, USA) formulation to 24 healthy Indian subjects under fasting. The study was performed as per International Conference on Harmonization, E6 Good Clinical Practice Guidelines [27]. The experimental details of the study are described in Supplementary material. The pharmacokinetic parameters of TAD were estimated by non-compartmental analysis using WinNonlin® software version 5.3 (Certara, Princeton, NJ 08540, USA). Method reliability was ascertained by reanalysis of 115 incurred samples. These samples had concentration near the Cmax and the elimination phase in the pharmacokinetic profile of the drug. According to the acceptance criterion, at least two-thirds of the original and repeat results should be within 20% of each other [28].
3. Results and discussion
3.1. LC-MS/MS method development
Though currently there are several methods to determine TAD in different biological matrices, the aim of the present work was to develop a highly sensitive and rapid method to meet the requirement of pharmacokinetic studies in healthy humans. All existing chromatographic methods have a limit of quantitation (LOQ), ≥ 2.0 ng/mL except one report in rat serum which reports LOQ of 1.0 ng/mL [17]. Further, only two methods [8], [14] have a chromatographic analysis time of less than 5.0 min. In light of this, we developed a highly sensitive, selective and rapid method to determine TAD using UHPLC-MS/MS instrumentation and solid phase extraction (SPE) employing a deuterated IS, which helped in controlling any variability during extraction and analyte ionization.
Mass spectrometer parameters were optimized by infusing 500 ng/mL solution of TAD and TAD-d3 in the positive ESI mode as reported previously [8], [13], [17]. The infusion was performed directly in the ionization source at a flow of 10 mL/min. The Q1 MS spectra of the analyte and IS showed abundant protonated molecular ions [M+H]+ at m/z 390.3 and m/z 393.1 for TAD and TAD-d3, respectively. MS/MS spectra gave highly stable and intense product ions at m/z 268.2 and m/z 271.2 corresponding to the loss of benzodioxole moiety from the precursor ions of TAD and TAD-d3, respectively (Fig. S1). Other product ion observed at m/z 240.1 can be attributed to the loss of CO molecule from m/z 268.2 and m/z 271.2, respectively. Q1 and Q3 potential and collision energy were suitably optimized to achieve the desired specificity and sensitivity of the method.
Several methods developed in human plasma have used liquid-liquid extraction (LLE) as the sample clean-up step with varying degree of success [8], [10], [16]. Recovery ranging from ~ 63% to 87% was obtained using diethyl ether-dichloromethane or diethyl ether-ethyl acetate solvent mixture in these methods. Thus, initial attempts for sample preparation of TAD were done by LLE using these solvents together with methyl tert-butyl ether-dichloromethane solvent combination. Although the recovery was quantitative (75%–82%) for higher QC samples (30.0, 200 and 400 ng/mL), it was largely inconsistent at lower (0.50 and 1.5 ng/mL) concentrations. This prompted us to employ SPE for sample clean-up, which was initiated on two different cartridges, namely Strata-X-C and Oasis HLB. Both the cartridges provided adequate recovery (> 80%), but Strata-X-C cartridge, which is mainly used for weakly basic compounds, gave about 10%–12% higher recoveries for TAD and TAD-d3 and was therefore employed in the current method.
Several C18 reversed-phase columns like Xterra MS [8], Hypersil [13], [14], Nucleodur EC [16] and Cadenza CD [19] have been used for separation of PDE-5 inhibitors. Thus, to optimize the best chromatographic conditions for adequate retention, peak shape, analytical response and analysis time, four different C18 columns having different dimensions and particle size were investigated. These included Kromasil (150 mm × 4.6 mm, 3.5 µm), Hypurity (100 mm × 4.6 mm, 5 µm), Zorbax Eclipse XDB (150 × 4.6 mm, 5 µm) and Synergi Hydro-RP (100 mm × 4.6 mm, 4 µm). Different combinations of acetonitrile/methanol and acidic buffers (ammonium formate and ammonium acetate) were tested on these columns. Although adequate response and retention was attained on all four columns, slight peak tailing was observed on all the columns except Synergi Hydro-RP. Based on this criterion, the mobile phase comprising of methanol and 10 mM ammonium formate (pH 4.0 adjusted with formic acid) (90:10, v/v) was finalized. The role of pH was also studied by changing the pH from 3.0 to 6.0. Higher pH (≥ 5) resulted in some peak asymmetry and decrease in capacity factor of TAD. Under the finalized conditions, the retention time of TAD and TAD-d3 was 1.74 and 1.73, respectively in a single run of 2.5 min (Fig. 1). Use of deuterated IS ensured acceptable method performance based on similar extraction recovery, chromatographic retention and ionization efficiency.
Fig. 1.
Representative chromatograms of (A) double blank plasma (without tadalafil and tadalafil-d3), (B) blank plasma spiked with tadalafil-d3 (100 ng/mL), (C) tadalafil (0.50 ng/mL) and tadalafil-d3 (100 ng/mL), (D) tadalafil (500 ng/mL) and tadalafil-d3 (100 ng/mL), and (E) real subject sample at Cmax after oral administration of 20 mg dose of tadalafil.
Further, the developed method is more sensitive than all existing methods in the literature. Higher sensitivity is required to have a better assessment of pharmacokinetics of TAD especially during the elimination phase. Moreover, the developed method can also be used for clinical studies with lower dose strength of TAD. The analysis time of 2.5 min is much shorter than other methods except one report [8], which is beneficial when large numbers of samples are to be analyzed in a clinical setting. Also, the sample volume used for processing (200 µL) is much less than several established methods. A detailed comparison of chromatographic methods developed for TAD in different biological matrices is shown in Table S1.
3.2. Assay validation results
The results for system suitability, ruggedness, dilution integrity and auto-sampler carryover showed satisfactory method performance as evident from the data presented in Table S3. The selectivity of the method can be seen from the chromatograms of double blank plasma, plasma spiked with TAD-d3, TAD at different concentration levels and in subject sample at Cmax in Fig. 1. There was no interference due to endogenous components at the retention time of TAD and TAD-d3. Furthermore, none of the commonly used medications by human volunteers interfered at their retention times. The calibration curves showed good linearity over the established concentration range of 0.50–500 ng/mL (r2 ≥ 0.9994). Linearity was determined using the least-square linear regression with a weighting factor of 1/x2. The mean linear equation established for TAD was y = (0.0050 ± 0.0004) x + (0.0010 ± 0.0001). The precision (% CV) and accuracy values for calibrator concentrations were in the range of 0.10%–3.23% and 96.4%–102.0%, respectively. The limit of detection (LOD) and limit of quantitation (LOQ) for TAD were 0.17 ng/mL and 0.50 ng/mL at a signal-to-noise ratio of 5 and 14, respectively. The intra-batch precision (% CV) ranged from 1.0% to 3.7% and the accuracy was within 97.9%–102.0% for TAD. Similarly for inter-batch experiments, the precision varied from 0.9% to 3.2% and the accuracy was within 97.8%–104.1% (Table S4).
The extraction recovery and matrix effect (expressed as IS-normalized matrix factors, MF) for TAD are presented in Table 1. Highly precise and quantitative recovery in the range of 98.95%–100.61% were obtained across QC levels. The mean recovery of TAD-d3 was 100.12%. The IS-normalized MFs ranged from 0.986 to 1.020. Matrix effect was also checked in lipemic and haemolysed plasma samples. This was determined by examining the precision (% CV) values of the slopes of the calibrations curves prepared from eight different plasma lots, which included six K3EDTA, one lipemic and one haemolysed plasma samples. The % CV of the slopes of calibration lines for relative matrix effect in eight different plasma lots was in the range of 1.2%–1.9%, which is within the acceptance criteria of 3%–4%.
Table 1.
Extraction recovery and matrix factor for tadalafil from human plasma.
| Quality control level (ng/mL) | Mean area response (n = 6) |
Recovery (%) |
Matrix factor |
|||||
|---|---|---|---|---|---|---|---|---|
| A (post-extraction spiking) | B (pre-extraction spiking) | C (neat samples in mobile phase) | Analyte (B/A) | IS | Analyte (A/C) | IS | IS-normalized (Analyte/IS) | |
| 400 | 5,452,803 | 5,447,895 | 5,558,413 | 99.91 | 99.84 | 0.981 | 0.976 | 1.005 |
| 200 | 2,762,572 | 2,758,151 | 2,833,407 | 99.84 | 100.05 | 0.975 | 0.981 | 0.994 |
| 30.0 | 418,560 | 421,113 | 436,910 | 100.61 | 99.63 | 0.958 | 0.972 | 0.986 |
| 1.50 | 20,735 | 20,689 | 21,895 | 99.78 | 101.52 | 0.947 | 0.928 | 1.020 |
| 0.50 | 6816 | 6744 | 7172 | 98.95 | 99.58 | 0.953 | 0.950 | 1.003 |
IS: internal standard.
Stock solutions of TAD and TAD-d3 kept for short-term stability remained stable up to 7 h, while the long-term stability of these solutions was determined up to 10 days with no significant change. The stabilities of TAD in human plasma and also in whole blood were estimated under a variety of storage and process conditions. TAD was found stable in controlled blank plasma at room temperature up to 12 h and for six freeze-thaw cycles. The analyte in extracted plasma samples was stable for 98 h under refrigerated conditions (5 °C) and for 12 h at room temperature. The concentration of spiked plasma samples of TAD stored at −20 °C and −70 °C for long-term stability showed no apparent change up to 74 days. Whole blood stability evaluated up to 2 h showed no evidence of degradation. The detailed results for stability experiments are presented in Table S5.
3.3. Application to a bioequivalence study
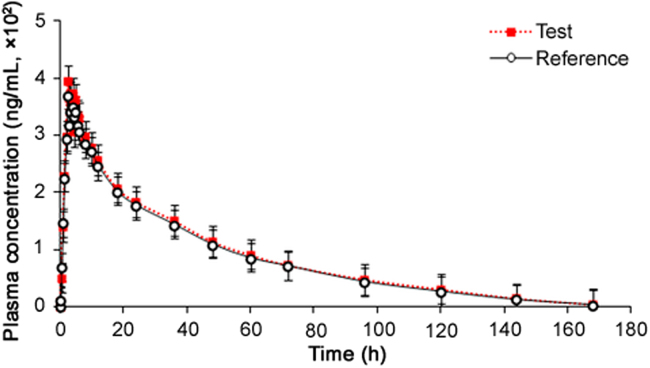
The validated method was successfully applied in a clinical study for the determination of TAD after oral administration of a single dose of 20 mg tablet in 24 healthy Indian volunteers under fasting. The mean plasma concentration–time profiles of TAD obtained for test and reference formulations are shown in Fig. 2 and pharmacokinetic parameters estimated using non-compartmental method are shown in Table 2. It was found that TAD was absorbed into the systemic circulation with its peak concentration (396.45 ng/mL) in about 2.37 h. The half life (t1/2) for elimination of TAD was 18.6 ± 2.47 h after oral administration. The method demonstrated adequate sensitivity to measure plasma concentration of TAD up to 168 h. Comparison of the results with previous reports [8], [13] showed no significant changes in any pharmacokinetic parameter. The ratios of mean log-transformed parameters, Cmax, AUC0–168 h and AUC0-inf and their 90% confidence intervals ranged from 99.2% to 110.9% for TAD, which is within the acceptance criterion of 80%–125%. These results confirm the bioequivalence of the test formulation with the reference product in terms of rate and extent of absorption. Furthermore, the assay reliability test performed with 115 incurred samples showed % change within ±15% of the initial results.
Fig. 2.
Mean plasma concentration-time profile of tadalafil after oral administration of 20 mg test and reference tablet formulations to 24 healthy Indian subjects.
Table 2.
Mean pharmacokinetic (±SD) parameters, comparison of treatment ratios and 90% CIs of natural log (Ln)-transformed parameters following oral administration of 20 mg tadalafil tablet formulation in 24 healthy Indian subjects under fasting.
| Parameter | Test | Reference | Ratio (test/ reference, %) | 90% CI (Lower – upper) | Power | Intra subject variation (% CV) |
|---|---|---|---|---|---|---|
| Cmax (ng/mL) | 396.45 ± 38.16 | 370.82 ± 44.12 | 106.9 | 101.2–110.3 | 0.9991 | 9.53 |
| AUC 0–168 h (h·ng/mL) | 14631.7 ± 1165.6 | 13933.8 ± 978.3 | 105.4 | 100.6–110.9 | 0.9995 | 10.09 |
| AUC 0-inf (h·ng/mL) | 15005.6 ± 1085.1 | 14324.6 ± 1124.2 | 104.7 | 99.2–109.4 | 0.9990 | 11.56 |
| Tmax (h) | 2.37 ± 1.23 | 2.25 ± 1.34 | – | – | – | – |
| t1/2 (h) | 18.6 ± 2.47 | 18.2 ± 2.73 | – | – | – | – |
| Kel (1/h) | 0.037 ± 0.012 | 0.038 ± 0.010 | – | – | – | – |
SD: standard deviation; CI: confidence interval; CV: coefficient of variation; Cmax: maximum plasma concentration; AUC0–168 h: area under the plasma concentration-time curve from 0 h to 168 h; AUC0-inf: area under the plasma concentration-time curve from zero hour to infinity; Tmax: time point of maximum plasma concentration; t1/2: half life of drug elimination during the terminal phase; Kel: elimination rate constant.
4. Conclusions
In summary, a sensitive, accurate and reliable LC-MS/MS method was developed and validated for the quantitation of TAD in human plasma using SPE as the sample clean-up step following current regulatory guidelines. The method is more sensitive than all the existing methods in any biological matrix and has a short turnaround time of 2.5 min. Further, the method provided highly reproducible recovery of TAD with minimal matrix interference. It was successfully applied to a bioequivalence study in healthy subjects with good accuracy and precision.
Acknowledgments
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors wish to thank the management of Synchron Research Services Pvt. Ltd, Ahmedabad for carrying out this research.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2018.01.003.
Appendix A. Supplementary material
Supplementary material
References
- 1.Gong B., Ma M., Xie W. Direct comparison of tadalafil with sildenafil for the treatment of erectile dysfunction: a systematic review and meta-analysis. Int. Urol. Nephrol. 2017;49:1731–1740. doi: 10.1007/s11255-017-1644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Washington S.L., III, Shindel A.W. A once-daily dose of tadalafil for erectile dysfunction: compliance and efficacy. Drug Des. Devel. Ther. 2010;4:159–171. doi: 10.2147/dddt.s9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falk J.A., Philip K.J., Schwarz E.R. The emergence of oral tadalafil as a once-daily treatment for pulmonary arterial hypertension. Vasc. Health Risk Manag. 2010;6:273–280. doi: 10.2147/vhrm.s6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel C.K., Bennett N. Advances in the treatment of erectile dysfunction: what's new and upcoming? F1000Res. 2016;5:1–6. doi: 10.12688/f1000research.7885.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int. J. Impot. Res. 2004;16:S11–S14. doi: 10.1038/sj.ijir.3901208. [DOI] [PubMed] [Google Scholar]
- 6.Henrie A.M., Nawarskas J.J., Anderson J.R. Clinical utility of tadalafil in the treatment of pulmonary arterial hypertension: an evidence-based review. Core Evid. 2015;10:99–109. doi: 10.2147/CE.S58457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forgue S.T., Patterson B.E., Bedding A.W. Tadalafil pharmacokinetics in healthy subjects. Br. J. Clin. Pharmacol. 2006;61:280–288. doi: 10.1111/j.1365-2125.2005.02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramakrishna N.V.S., Vishwottam K.N., Puran S. Quantitation of tadalafil in human plasma by liquid chromatography–tandem mass spectrometry with electrospray ionization. J. Chromatogr. B. 2004;809:243–249. doi: 10.1016/j.jchromb.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Cheng C.L., Chou C.H. Determination of tadalafil in small volumes of plasma by high-performance liquid chromatography with UV detection. J. Chromatogr. B. 2005;822:278–284. doi: 10.1016/j.jchromb.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Shakya A.K., Abu-awwad A.N.A., Arafat T.A. Validated liquid chromatographic–ultraviolet method for the quantitation of tadalafil in human plasma using liquid–liquid extraction. J. Chromatogr. B. 2007;852:403–408. doi: 10.1016/j.jchromb.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 11.Farthing C.A., Farthing D.E., Koka S. A simple and sensitive HPLC fluorescence method for determination of tadalafil in mouse plasma. J. Chromatogr. B. 2010;878:2891–2895. doi: 10.1016/j.jchromb.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolaou P., Papoutsis I., Athanaselis S. Development and validation of a GC/MS method for the determination of tadalafil in whole blood. J. Pharm. Biomed. Anal. 2011;56:577–581. doi: 10.1016/j.jpba.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Maa B., Shang X., Zhang Q. Rapid analysis of tadalafil in human blood plasma and seminal plasma by liquid chromatography/tandem mass spectrometry. J. Pharm. Biomed. Anal. 2013;77:149–157. doi: 10.1016/j.jpba.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.H., Oh J.H., Lee Y.J. Simple and sensitive liquid chromatography–tandem mass spectrometry methods for quantification of tadalafil in rat plasma: application to pharmacokinetic study in rats. Arch. Pharm. Res. 2013;36:457–463. doi: 10.1007/s12272-013-0046-1. [DOI] [PubMed] [Google Scholar]
- 15.Strano-Rossi S., Anzillotti L., de la Torre X. A gas chromatography/mass spectrometry method for the determination of sildenafil, vardenafil and tadalafil and their metabolites in human urine. Rapid Commun. Mass Spectrom. 2010;24:1697–1706. doi: 10.1002/rcm.4568. [DOI] [PubMed] [Google Scholar]
- 16.Rust K.Y., Wilkens H., Kaiser R. Detection and validated quantification of the phosphodiesterase type 5 inhibitors sildenafil, vardenafil, tadalafil, and 2 of their metabolites in human blood plasma by LC-MS/MS-application to forensic and therapeutic drug monitoring cases. Ther. Drug Monit. 2012;34:729–735. doi: 10.1097/FTD.0b013e31827318b8. [DOI] [PubMed] [Google Scholar]
- 17.Unceta N., Echeazarra L., Montana M. Validation of an LC–ESI-MS/MS method for the quantitation of phosphodiesterase-5 inhibitors and their main metabolites in rat serum and brain tissue samples. J. Pharm. Biomed. Anal. 2012;70:529–533. doi: 10.1016/j.jpba.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Proença P., Mustra C., Marcos M. Validated UPLC-MS/MS assay for the determination of synthetic phosphodiesterase type-5 inhibitors in postmortem blood samples. J. Forensic Leg. Med. 2013;20:655–658. doi: 10.1016/j.jflm.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama Y., Tomatsuri M., Hayashi H. Chen, Simultaneous microdetermination of bosentan, ambrisentan, sildenafil, and tadalafil in plasma using liquid chromatography/ tandem mass spectrometry for pediatric patients with pulmonary arterial hypertension. J. Pharm. Biomed. Anal. 2014;89:227–232. doi: 10.1016/j.jpba.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Lee S., Choi B., Kim J. An LC–MS/MS method for the determination of five erectile dysfunction drugs and their selected metabolites in hair. J. Chromatogr. B. 2015;978–979:1–10. doi: 10.1016/j.jchromb.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Abu El-Enin M.A., Abd Al-Ghaffar Hammouda M.E., El-Sherbiny D.T. Validated spectrofluorimetric method for determination of two phosphodiesterase inhibitors tadalafil and vardenafil in pharmaceutical preparations and spiked human plasma. Luminescence. 2016;31:173–178. doi: 10.1002/bio.2941. [DOI] [PubMed] [Google Scholar]
- 22.Campillo N., Marín J., Fenoll J. Determination of synthetic phosphodiesterase-5 inhibitors by LC-MS2 in waters and human urine submitted to dispersive liquid-liquid microextraction. Talanta. 2017;174:638–644. doi: 10.1016/j.talanta.2017.06.076. [DOI] [PubMed] [Google Scholar]
- 23.Enderle Y., Witt L., Wilkens H. Simultaneous quantification of endothelin receptor antagonists and phosphodiesterase 5 inhibitors currently used in pulmonary arterial hypertension. J. Pharm. Biomed. Anal. 2017;143:291–298. doi: 10.1016/j.jpba.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 24.Guidance for Industry, Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration Centre for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM), 2001.
- 25.A. Bhadoriya, S. Rathnam, B. Dasandi, et al., Sensitive and rapid determination of amantadine without derivatization in human plasma by LC-MS/MS for a bioequivalence study, J. Pharm. Anal. 2017. ( 10.1016/j.jpha.2017.10.003). [DOI] [PMC free article] [PubMed]
- 26.Shah P.A., Parekh J.M., Shrivastav P.S. Assessment of critical extraction and chromatographic parameters for the determination of bupropion and its three primary metabolites in human plasma by LC-MS/MS. Microchem. J. 2017;135:81–90. [Google Scholar]
- 27.Guidance for Industry: ICH E6 Good Clinical Practice, U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), Centre for Biologics Evaluation and Research (CBER), 1996.
- 28.Yadav M., Shrivastav P.S. Incurred sample reanalysis: a decisive tool in bioanalytical research. Bioanalysis. 2011;3:1007–1024. doi: 10.4155/bio.11.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material