Abstract
Addictive drugs enhance dopamine release in the striatum, which can lead to compulsive drug-seeking after repeated exposure. Glial cell line-derived neurotrophic factor (GDNF) is an important regulator of midbrain dopamine neurons, and may play a mechanistic role in addiction-related behaviors. To elucidate the components of GDNF-signaling that contribute to addiction-related behaviors of place preference and its extinction, we utilized two genetically modified GDNF mouse models in an amphetamine-induced conditioned place preference (CPP) paradigm and evaluated how the behavioral findings correlate with dopamine signaling in the dorsal and ventral striatum. We utilized two knock-in mouse strains to delineate contributions of GDNF and Ret signaling using MEN2B mice (constitutively active GDNF receptor Ret), and GDNF hypermorphic mice (enhanced endogenous GDNF expression). The duration of amphetamine-induced CPP was greatly enhanced in MEN2B mice, but not in the GDNF hypermorphic mice. The enhanced duration of CPP was correlated with increased tyrosine hydroxylase (TH) expression and dopamine content in the ventral striatum. Together, our results suggest that downstream components of GDNF signaling, in this case Ret, may mediate persistent drug-seeking behavior through increased TH expression and dopamine levels in the mesolimbic dopamine neurons.
Keywords: amphetamine, conditioned place preference, dopamine, GDNF, MEN2B, striatum
1. Introduction
Striatal dopamine plays a key role in behavioral reinforcement and reward signaling. Stimulants can increase dopamine release in the striatum, and consequently dissociate these learning mechanisms from their normal drive by environmental cues (reviewed by Sulzer, 2011). After a critical number of exposures, behaviors aiming for acquisition of these reinforcing drugs eventually become highly compulsive and manifest as drug addiction (Camí and Farré, 2003; Volkow et al., 2016). Environmental stimuli previously associated with drug use (cues) play a major role to induce a conditioned response (craving and relapse), even in the absence of the drug and after long periods of abstinence (Camí and Farré, 2003; Stolerman, 1992). Hence, the dopaminergic associative learning mechanisms are the key drivers in the development and maintenance of addiction-related behavior.
Ectopically applied Glial cell line-derived neurotrophic factor (GDNF) provides trophic support for midbrain dopaminergic neuron functions, both in vitro (Burke et al., 1998; Lin et al., 1993) and in vivo (Beck et al., 1995; Hoffer et al., 1994). Perhaps a lesser known aspect of GDNF signaling is the role it may play in the behavioral effects of addictive drugs (Carnicella and Ron, 2009; Messer et al., 2000), which also carries a significant dopaminergic component from the mesolimbic pathway. Nevertheless, the role of GDNF in addiction has remained unclear, as there is evidence to support that GDNF signaling promotes or inhibits addiction processes (reviewed by Ghitza et al., 2010; Koskela et al., 2017). This ambiguity is at least in part due to shortcomings in the approaches used to evaluate the role of GDNF signaling in addiction. For example, the use of ectopic GDNF or GDNF-neutralizing antibodies to evaluate the role of GDNF in addiction may not represent the physiological context of GDNF signaling in an addiction process (Ghitza et al., 2010; Koskela et al., 2017). Furthermore, the use of heterozygous mice lacking one functional GDNF allele could trigger compensatory mechanisms that mask or interfere with the phenotype (Ghitza et al., 2010; Routtenberg and Gerlai, 1996).
To further delineate the contribution of GDNF-signaling in the addiction process, we utilized two knock-in mouse models, both of which increase two components of endogenous GDNF signaling. In multiple endocrine neoplasia type 2B (MEN2B) mice, GDNF’s main signaling receptor Ret (Rearranged during transfection) is constitutively active as a result of a single point mutation (Mijatovic et al., 2007; Smith-Hicks et al., 2000). In GDNF hypermorphic mice (Gdnfwt/hyper), endogenous GDNF expression from one Gdnf allele is enhanced due to increased mRNA stability, which doubles endogenous striatal levels of GDNF (Kumar et al., 2015). In the present study, we investigated the behavioral responses of these animal models in amphetamine-induced conditioned place preference (CPP) paradigm, where the rewarding properties of amphetamine are associated with environmental cues via repeated exposures. This conditioning phase causes animals to shift their preference towards the amphetamine-paired environment, when given a free choice (Cunningham et al., 2006). In addition, we investigated dopamine levels and metabolism in the nigrostriatal and mesocorticolimbic brain areas of these mice to determine whether indices of dopamine biosynthesis (tyrosine hydroxylase expression and phosphorylation) mediate GDNF signaling-related mechanisms associated with the behavioral phenotypes. We found that in MEN2B mice the duration of amphetamine-induced place conditioning was greatly enhanced and endured long after it was extinguished in the wild-type, whereas the Gdnfwt/hyper mice did not differ from their wild-type littermates in CPP paradigm. The enhanced CPP duration in MEN2B mice was correlated with increased levels of dopamine and tyrosine hydroxylase (TH) specifically in the ventral compartment of the striatum.
2. Material and methods
2.1. Animals
The generation and genotyping of MEN2B and GDNF hypermorphic knock-in mice has been previously described (Smith-Hicks et al., 2000; Kumar et al., 2015). The mice were bred in the animal facility at the University of Helsinki. MEN2B mice maintained in 129Ola/C57BL6 mixed background (Mijatovic et al., 2011) were first crossed to ICR strain and then several times to wild type mice from the GDNF hypermorphic colony in order to bring them to the same triple-mixed genetic background as the GDNF hypermorphic strain (129Ola/ICR/C57BL6). Male mice and littermate controls were used at 8–14 weeks of age. The mice were housed under temperature-controlled conditions at 20–22°C in a 12h/12h light/dark cycle. Each cage contained 2–6 animals, and the mice had ad libitum access to standard chow and water. Animal experiments were conducted according the 3R principles of the EU directive 2010/63/EU governing the care and use of experimental animals, and following local laws and regulations [Finnish Act on the Protection of Animals Used for Scientific or Educational Purposes (497/2013, Government Decree on the Protection of Animals Used for Scientific or Educational Purposes (564/2013)]. The protocols were authorized by the national Animal Experiment Board of Finland.
2.2. Amphetamine-induced place conditioning
For the behavioral experiments both MEN2B and GDNF hypermorphic strains were backcrossed once to C57BL6 strain. As we only had six homozygous MEN2B (M2B/M2B) mice for the behavioral experiments, they were all conditioned to the same side (Grid+). As the behavioral data from these M2B/M2B mice was completely homogenous with Wt/M2B data, we combined the groups into a common M2B group. However, as this disrupts the counterbalancing within the M2B group, we have also presented the CPP data without the M2B/M2B mice in the Figure S1A–K. Cages were changed once per week after the behavioral session of that day was completed. One week before the experiments all animals were systematically handled every day for five days to minimize experimenter-related stress during the experiment. Over the days the mice gradually received more active and intensive handling, where they were standing on a hand, lifted out of their cage and briefly immobilized to a similar grip where they would later receive injections.
CPP experiments were conducted in rectangular chamber with place preference inserts (activity monitor; MED Associates, St Albans, GA, USA). The chamber was divided into two compartments (21 x 42 x 41 cm3) that had different tactile cues and were separated by a black wall with a guillotine door. One compartment had a metal grid floor (Grid), while the other had perforated grey plastic floor with 7.5 mm holes 16 mm apart (Hole). Movement and location of the animals were recorded with infrared photobeam interruptions. Before the experiments all chambers were washed with water and soap, but during the experiment only visible excrements were removed after each session. Before the first experimental session male mice that were not part of the experiment were placed to the chambers for 30 minutes to attach mouse smell into the apparatus. White noise was used during the experiments to cover background noises.
We used an unbiased and counterbalanced experimental design (Cunningham et al., 2006), where half of the animals in each genotype were randomly assigned to receive amphetamine in one compartment and vehicle (0.9 % saline) in the other. Hence, half of the animals in each genotype were assigned to the Grid+ conditioning subgroup (Grid + amphetamine, Hole + saline) and half of them to the Grid– conditioning subgroup (Hole + amphetamine, Grid + saline). Amphetamine induced CPP is defined as a significant difference between the Grid+ and Grid– subgroups in an analysis of time spent on the grid floor (Cunningham et al., 2006, 2003). As the subgroups are matched for exposure to amphetamine, saline and each of the floor stimuli, the differences between the subgroups can be attributed to differences in learning about the paired relationship between the floor and the drug effects (Cunningham et al., 2006, 2003). Time spent on the grid floor is expressed as mean seconds per minute during the 15 minutes test session as reported by (Cunningham et al., 2006). The CPP trial was divided into 5 phases: (1) habituation, (2) conditioning, (3) preference test, (4) extinction and (5) reinstatement. From habituation until preference test the procedure was conducted five days per week with a 2-day weekend break.
The habituation phase took place 24 hours before the first conditioning and did not involve any injections. The animals were placed to the CPP chamber for 15 minutes with the guillotine door open, so they could freely explore both compartments. Time spent in each compartment and locomotor activity were measured.
In the conditioning phase, mice received a total of four injections of amphetamine (2 mg/kg, i.p., in 0.9 % saline; Division of Pharmaceutical Chemistry and Technology, Faculty of Pharmacy, University of Helsinki, Finland) and four injections of saline on alternating days. The groups were counterbalanced for the order in which they received each treatment (amphetamine or saline first). There was a 24 h delay between successive conditioning trials that took place at the same time of the day for each animal. The animals were placed into the CPP chambers immediately after receiving the injections for 30 minutes recording with the guillotine door closed.
The preference test was carried out 24 h after the final conditioning session to measure whether the animals had developed place preference to the drug-paired compartment. The preference test was identical to the habituation session. The extinction phase started 72 h after the preference test and took place three times per week with one or two days between the sessions. The extinction session was otherwise similar to habituation and preference test, except that the animals stayed in the chamber for 30 minutes, which speeds up the reversal learning (Cunningham et al., 1998). However, they were only recorded during the first 15 minutes.
Finally, reinstatement took place 24 h after the final extinction session. The reinstatement trial was similar to the extinction, but the animals received a small challenge dose of amphetamine (0.5 mg/kg, i.p.) prior being placed to the CPP box for 30 minutes recording.
2.3. Brain dissection
For brain monoamine measurements and western blot analysis samples were obtained from the relevant dissected brain regions of drug-naïve animals using a mouse brain matrix as previously described (Kopra et al., 2017). In addition, prefrontal cortex (PFC) was collected from in front of the striatal slice after removal of the olfactory bulbs.
2.4. Estimation of monoamines and their metabolites
Dopamine and its metabolites were analyzed from the dissected brain samples as described previously (Valros et al., 2015) using HPLC with electrochemical detection. The values of monoamines and their metabolites are presented as nanograms per gram of wet tissue weight.
2.5. Western blot analysis
The remaining tissue pellet from HPLC sample preparation was re-dissolved and homogenized by ultrasound in 5 mM Tris buffer (pH 8.3) containing 1 mM EDTA and 1 % SDS previously described (Salvatore et al., 2012). Next, the samples were boiled at 97 °C for 5 min and protein concentration was measured using BCA assay (Thermo Scientific, Rockford, IL, USA). Western blot samples containing 20 μg (for TH) or 5–10 μg (for pSer31 TH) of total protein were prepared in 4x Laemmli buffer (Bio-Rad, Hercules, CA, USA). In case the sample turned yellow after Laemmli buffer addition, 1 M Tris (pH 8.2) was added in 5 μl increments until blue color was restored. Samples were loaded onto Stain-Free 4–20 % gels (Bio-Rad). Calibrated standards for TH or pSer31 TH protein (Salvatore and Pruett, 2012) were prepared in mouse liver homogenate and loaded onto gels along with the samples to create standard curves. Gel run was performed at 200 V for 30 min and transferred onto Trans-Blot Turbo 0.2 μm nitrocellulose membranes (Bio-Rad). The protein loads were normalized using Ponceau S staining as previously described (Salvatore et al., 2012). Then, the membranes were blocked 1 hour in 5 % skim milk in TBS containing 0.5 % Tween20 (T-TBS), followed by primary antibody (TH; rabbit-anti-TH, 1:1000; AB 152, Millipore, Temecula, CA, USA, or pSer31 TH 1:300 (Salvatore et al., 2009b); 21st Century Biochemicals, Boston, MA, USA) incubation at 4 °C overnight. After 2 hour secondary antibody incubation (Goat anti-rabbit-HRP Conjugated; 1:2000, 170-6515, Bio-Rad), signal was detected using Li-Cor C-Digit blot scanner (Lincoln, NE, USA) and band optical densities were analyzed with Image Studio software (Li-Cor). TH or pSer31 TH protein amounts in the tissue samples were calculated based on the standard curves.
2.6. Statistical analyses
Data from more than two groups (MEN2B, three genotypes) were analyzed using a one-way ANOVA, which was followed by the Student-Newman-Keuls post hoc analysis in the case of a significant ANOVA result. Statistical analysis for pairwise comparisons was performed using Student’s t-test with two-tailed distribution and the equal variance option. In the CPP experiments a significant difference between Grid+ and Grid– subgroups provides evidence of place conditioning (Cunningham et al., 2006), so statistical comparisons were made between the means of these subgroups using Student’s t-test with two-tailed distribution and the equal variance option. A three-way ANOVA for repeated measures [genotype x treatment x time (days 1–4) as factors] was used to analyze locomotion data. All summary data are reported as the mean ± the standard error of the mean (SEM).
3. Results
3.1. Expression of place preference in MEN2B mice
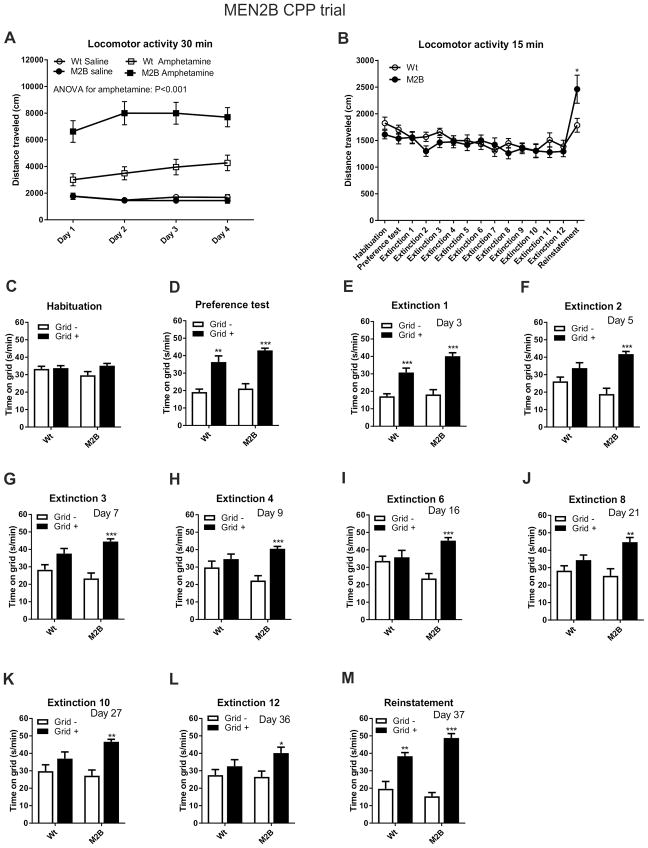
During the conditioning sessions, MEN2B mice displayed greater amphetamine-induced locomotor activation compared to their wild-type littermates, but not after saline injections (Figure 1A). This difference was also seen in the reinstatement trial (Figure 1B). The activity of MEN2B mice was similar to their controls during habituation phase and in the preference and extinction tests (Figure 1B). The mice did not display strong preference to either side of the CPP chamber (54 % of time spent on grid) during the habituation phase indicating that the system was unbiased (Figure 1C).
Fig. 1. Enhanced amphetamine-induced locomotor activity and longer expression of place preference in MEN2B mice.
(A) Locomotor activity was measured during the 30 minutes conditioning sessions after saline and amphetamine (2 mg/kg) injections that took place on alternating days. In MEN2B mice locomotor activity was unaltered after saline injections, but increased after amphetamine (repeated-measures ANOVA, p<0.001). (B) Locomotor activity was measured during the 15 minutes habituation, preference test, extinction and reinstatement sessions. In MEN2B mice locomotor activity was increased only in the reinstatement session, where animals received small priming amphetamine injections (0.5 mg/kg) (Student’s t-test, p=0.043). The magnitude of amphetamine CPP before (C) and after (D) eight conditioning trials, in multiple extinction sessions (E–L), and in reinstatement (M) is shown for mice that had amphetamine paired with the holes (Grid-; white bars) or grid (Grid+; black bars) floor. There was a clear and similar preference shift to the compartment where amphetamine was administered in both groups (B). However, while in Wt mice this preference was gone already in the second extinction session (F), in M2B mice the preference magnitude only diminished over time and was still detectable in the extinction session 12 (L). In the reinstatement session, where 0.5 mg/kg of amphetamine was administered, both groups showed again strong preference towards the amphetamine-paired compartment (M). N = 13 and 19 mice for Wt and M2B, respectively. In the CPP experiments statistical comparisons were made between the Grid+ and Grid– subgroups within each genotype using Student’s t-test. The number of days after the preference test is marked in the upper right corner of each panel. Wt, wild-type; M2B, MEN2B; *p < 0.05; **p < 0.01 and ***p < 0.001.
The four conditioning sessions with amphetamine resulted in place preference both in MEN2B and their wildtype littermates indicated by a significant difference in the time spent on the grid floor between the two conditioning subgroups [amphetamine on grid (Grid+) or amphetamine on plastic floor (Grid-)] (Figure 1D).
We next evaluated the longevity of CPP expression. The wild-type controls did not display CPP beginning with the second extinction trial (Figure 1E–F). However, in the MEN2B mice, CPP was still present at the 12th extinction test (Figure 1E–L). The total duration of CPP in the MEN2B mice lasted out to at least 36 days, being five times longer duration than in the wild-types (Figure 1L).
The priming dose of amphetamine (0.5 mg/kg) induced reinstatement of place preference in both groups, as indicated by a significant difference in the time spent on the grid floor between the two conditioning subgroups (Figure 1M).
In conclusion, MEN2B mice displayed an enhanced locomotor response to amphetamine and persistent expression of amphetamine-induced place preference. The MEN2B mutation did not affect the magnitude of place preference displayed in the preference test or the amphetamine-induced reinstatement of drug seeking.
3.2. Expression of place preference in GDNF hypermorphic mice
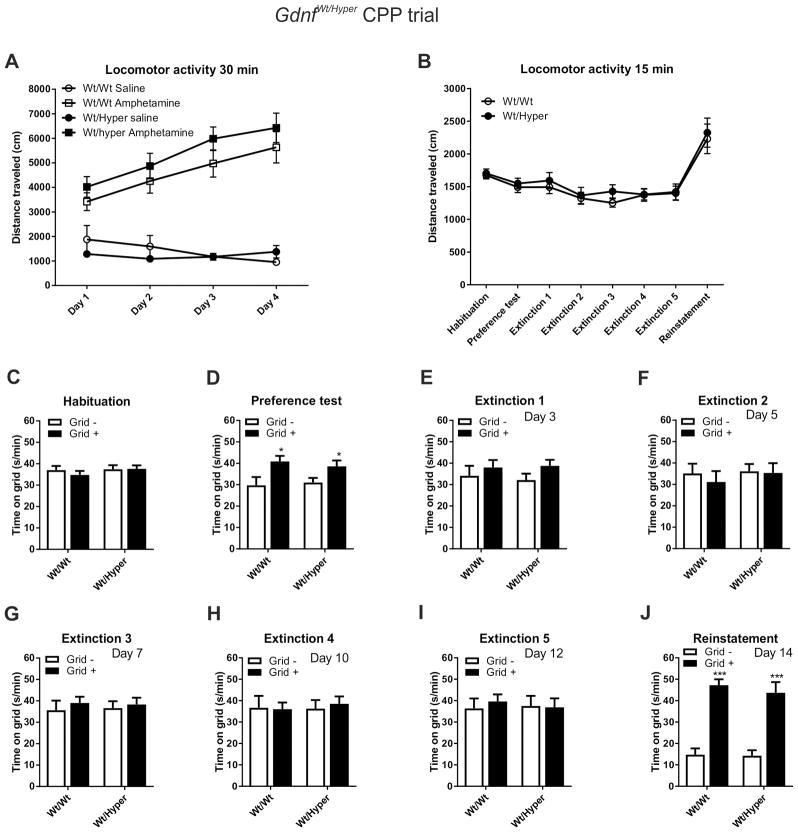
During the conditioning phase, neither saline- or amphetamine-induced locomotor activity was significantly increased in Gdnfwt/hyper mice compared to the controls (Figure 2A). However, both genotypes displayed similar and clear locomotor sensitization to the repeated amphetamine injections (Figure 2A). There were no differences in locomotor activity between the genotypes either during the habituation, preference, extinction or reinstatement tests (Figure 2B). The mice displayed a mild preference to the grid side (61 % of time spent on grid) during the habituation phase indicating that the system was very close to being unbiased (Figure 2C).
Fig. 2. Unaltered amphetamine-induced locomotion and CPP in GDNF hypermorphic mice.
(A) Locomotor activity was measured during the 30 minutes conditioning sessions after saline and amphetamine (2 mg/kg) injections that took place on alternating days. In Gdnfwt/hyper mice locomotor activity was not significantly changed after saline or amphetamine (repeated-measures ANOVA, p>0.05). (B) Locomotor activity was measured during the 15 minutes habituation, preference test, extinction and reinstatement sessions. There were no significant differences between the genotypes. The magnitude of amphetamine CPP before (C) and after (D) eight conditioning trials, in multiple extinction sessions (E–I), and in reinstatement (J) is shown for mice that had amphetamine paired with the holes (Grid-; white bars) or grid (Grid+; black bars) floor. There was a clear and similar preference shift to the compartment where amphetamine was administered in both groups (D). This preference was gone already in the first extinction session in both groups (E). There were no further changes until the 5th extinction session F–I). In the reinstatement session, where 0.5 mg/kg of amphetamine was administered, both groups similarly showed again strong preference towards the amphetamine-paired compartment (J). N = 20 and 18 mice for Wt and Gdnfwt/hyper, respectively. In the CPP experiments statistical comparisons were made between the Grid+ and Grid– subgroups within each genotype using Student’s t-test. The number of days after the preference test is marked in the upper right corner of each panel. Wt, wild-type; Hyper, GDNF hypermorphic allele; *p < 0.05 and ***p < 0.001.
The four pairings with amphetamine during the conditioning resulted in a similar CPP in both genotypes (Figure 2D). However, both genotypes no longer displayed CPP in the first extinction trial (Figure 2E) and this behavioral phenotype continued for the duration of the extinction phase of the experiment (Figure 2F–I).
The priming dose of amphetamine (0.5 mg/kg) induced reinstatement of CPP, where both genotypes showed a significant difference in the time spent on the grid floor (Figure 2J).
Taken together, amphetamine-induced locomotor activity and CPP, and its extinction, in Gdnfwt/hyper mice were not different from their wild-type littermates.
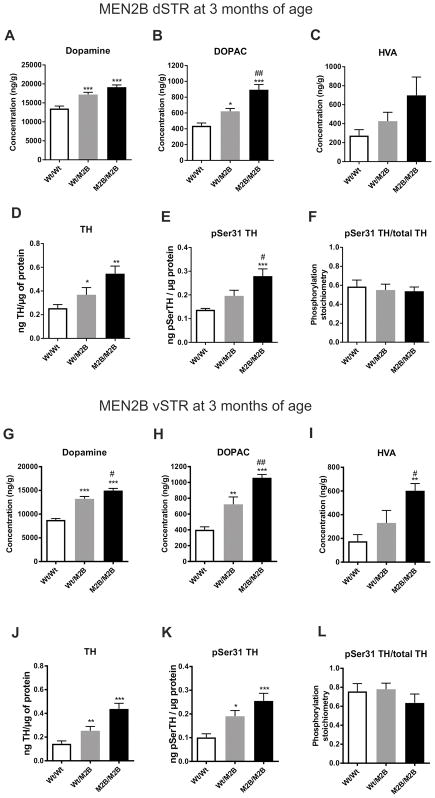
3.3. Increased dopamine and tyrosine hydroxylase expression in both striatal compartments of MEN2B mice
To elucidate possible mechanisms underlying the differences in CPP duration between the MEN2B and Gdnfwt/hyper mice, we measured the levels of dopamine and its metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the dorsal striatum (dSTR), ventral striatum (vSTR), prefrontal cortex (PFC), substantia nigra (SN) and ventral tegmental area (VTA) of drug-naïve animals. The levels of TH protein and serine 31 phosphorylated form of TH (pSer31 TH) were determined in the same dSTR and vSTR samples assessed for dopamine and metabolites (Salvatore et al., 2012). In MEN2B mice there was increased tissue dopamine (~25 % for Wt/M2B, ~40 % for M2B/M2B) and DOPAC (~40 % for Wt/M2B, ~100 % for M2B/M2B) in dSTR (Figure 3A–B), whereas HVA levels were unchanged (Figure 3C). There were parallel increases in TH and pSer31 TH levels, but no difference in the pSer31 TH levels/total TH ratio in dSTR (Figure 3D–F, see presentative bands in Figure S4), indicating that ser31 phosphorylation was unaffected. In vSTR, dopamine was also increased, and to a greater extent than in dSTR, being ~50 % for Wt/M2B and ~70 % for M2B/M2B. DOPAC was also increased (~80 % for Wt/M2B, ~160 % for M2B/M2B) (Figure 3G–H), as well as HVA levels (~240 % for M2B/M2B) (Figure 3I). Similar to the dSTR, there were parallel increases in TH and pSer31 TH levels, but no difference in the pSer31 TH levels/total TH ratio in vSTR (Figure 3J–K). Generally, in both striatal compartments the elevations in dopamine and TH tended to be higher in homozygous (M2B/M2B) than in heterozygous (M2B/Wt) animals, even though this difference did not always reach statistical significance. In MEN2B PFC dopamine and HVA levels were unchanged, but DOPAC levels were slightly increased in homozygous animals (Figure S2A–C). In SN dopamine and DOPAC were elevated, but HVA remained unchanged (Figure S2D–F). Finally, in the VTA dopamine levels were increased, while DOPAC and HVA were unchanged (Figure S2G–I).
Fig. 3. Increased levels of dopamine, its metabolites, TH and pSer31 TH in MEN2B dSTR and vSTR.
There was an increase in the dSTR dopamine (ANOVA p<0.0001) (A) and DOPAC (ANOVA p<0.0001) (B) levels, while HVA levels were not significantly elevated (ANOVA p=0.0717) (C) in MEN2B mice. There was a similar increase in the dSTR TH (ANOVA p=0.0036) (D) and pSer31 TH (ANOVA p=0.0010) (E) levels, while their ratio remained unaltered (ANOVA p=0.825) (F). In vSTR, dopamine (ANOVA p<0.0001) (G), DOPAC (ANOVA p<0.0001) (H) and also HVA (ANOVA p=0.0021) (I) levels were increased. Also TH (ANOVA p<0.0001) (J) and pSer31 TH (ANOVA p=0.0011) (K) levels were increased, while their ratio remained unchanged (ANOVA p=0.429) (L). N = 8, 9 and 7 mice for Wt/Wt and Wt/M2B and M2B/M2B, respectively. Wt, wild-type; M2B, MEN2B; dSTR, dorsal striatum; vSTR, ventral striatum; DOPAC, dihydroxyphenylacetic acid; HVA homovanillic acid; TH, tyrosine hydroxylase; *p < 0.05; **p < 0.01 and ***p < 0.001 versus Wt/Wt; #p < 0.05 and ##p < 0.01 versus Wt/M2B.
In conclusion, there were very robust and gene dose-dependent increases in the levels of dopamine as well as its primary metabolite DOPAC and expression of TH in both dorsal and ventral striatal compartments of MEN2B mice. In the other dopaminergic brain areas, there were also some similar, but less robust, increases in dopamine and metabolite levels. Generally, the relative elevations in dopamine, DOPAC and HVA in MEN2B tended to be up to two times greater in the mesolimbic (vSTR and VTA) than in the nigrostriatal (dSTR and SN) tract.
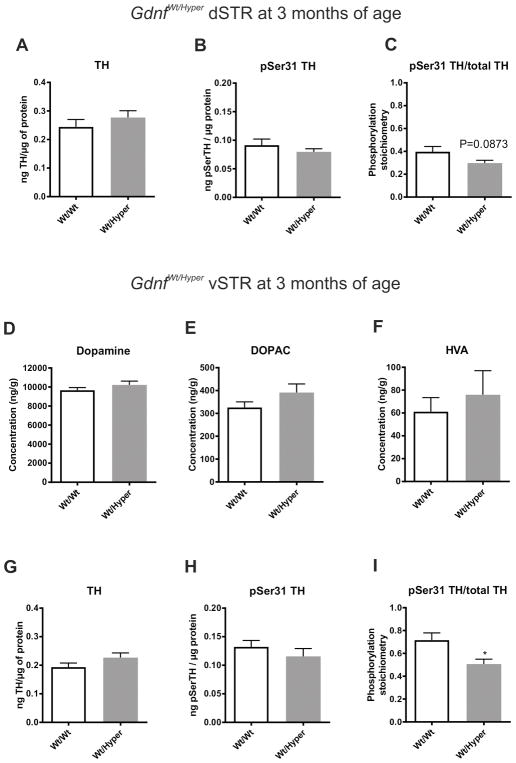
3.4. In GDNF hypermorphic mice dopamine levels are unchanged in ventral striatum
Gdnfwt/hyper mice also have elevated levels of dopamine (~25 %) and DOPAC (~45 %) in dSTR (Kumar et al., 2015). However, we did not observe any differences in TH or pSer31 TH levels or in the pSer31 TH/total TH ratio in the same samples (Figure 4A–C). In contrast, in the vSTR of the same animals, dopamine, DOPAC and HVA levels were unchanged (Figure 4D–F). Moreover, in vSTR there were no differences in TH or pSer31 TH levels, although there was a small, but significant reduction in the pSer31 TH/total TH ratio (Figure 4G–I). This is in contrast to TH expression and dopamine content seen in MEN2B mice, where both were increased in the vSTR. There were no significant differences in dopamine, DOPAC or HVA levels in PFC (Figure S3A–C) or SN (Figure S3D–F) in Gdnfwt/hyper mice, while only dopamine levels were slightly elevated in the VTA (Figure S3G–I).
Fig. 4. Levels of dopamine, its metabolites, TH and pSer31 TH in dSTR and vSTR of GDNF hypermorphic mice.
There were no differences in the dSTR TH (t-test p=0.369) (A) and pSer31 TH (t-test p=0.381) (B) levels and also their ratio remained unaltered (t-test p=0.0873) (C). There was no difference in the vSTR levels of dopamine (t-test p=0.249) (D), DOPAC (t-test p=0.150) (E) and HVA (t-test p=0.534) (F) in Gdnfwt/hyper mice. Similarly, in vSTR, TH (t-test p= p=0.141) (G) and pSer31 TH (t-test p=0.349) (H) levels were unchanged, while their ratio was reduced in Wt/Hyper mice (t-test p=0.0175) (I). N = 11 and 8 mice for Wt/Wt and Wt/Hyper, respectively. Wt, wild-type; Hyper, GDNF hypermorphic allele; dSTR, dorsal striatum; vSTR, ventral striatum; TH, tyrosine hydroxylase, DOPAC, dihydroxyphenylacetic acid; HVA homovanillic acid; *p < 0.05 versus Wt/Wt.
Taken together, the dopaminergic phenotype in Gdnfwt/hyper mice appears to be primarily limited to the dSTR. Furthermore, the lack of difference in TH expression and dopamine in the vSTR of the Gdnfwt/hyper mice, in contrast to these differences observed in the MEN2B mice, may be the mechanistic basis to explain why Gdnfwt/hyper mice do not differ from their littermate controls in the duration of amphetamine-induced CPP.
4. Discussion
Altered dopamine neurotransmission within the mesocorticolimbic system mediates the components of drug reward (Pierce and Kumaresan, 2006) and relapse to drug-seeking (Shalev et al., 2002). Mesocorticolimbic dopamine system consists of dopaminergic cell bodies in the VTA that project to several brain areas including PFC, vSTR and amygdala (Fallon and Moore, 1978; Ungerstedt, 1971). Compulsive drug-seeking and long-term vulnerability to relapse result from drug-induced neuroadaptations in the mesocorticolimbic dopamine system and the glutamatergic circuitry that is modulated by the VTA dopaminergic projections. Our present results suggest that alterations in the function of the GDNF signaling receptor Ret may mediate persistent drug-seeking behavior in association with increased TH expression in the mesolimbic dopaminergic neurons. MEN2B mice, with constitutively active Ret, developed highly persistent amphetamine-induced place preference, notably with increased TH expression and dopamine in vSTR. In contrast, CPP expression in the Gdnfwt/hyper mice with unchanged vSTR dopamine and TH levels did not differ from their wild-type littermates in the same trial.
Amphetamine is a widely abused drug that increases extracellular dopamine levels in the striatum (Ungerstedt and Pycock, 1974). Amphetamine is taken up to the nerve terminals by the dopamine transporter (DAT), where it releases dopamine from the synaptic vesicles leading to its DAT-mediated outflow to the extracellular space (Reviewed by Sulzer, 2011). Administration of amphetamine causes euphoria and alertness as well as increased arousal, concentration and locomotor activity (Camí and Farré, 2003). As amphetamine releases existing dopamine from the vesicles, it is likely that the higher striatal dopamine levels in MEN2B mice caused them to respond stronger to amphetamine. This was evidenced by increased locomotor activity after amphetamine injections and by much longer expression of amphetamine-induced CPP compared to their wild-type littermates. The amphetamine-related increase in extracellular dopamine levels in vSTR of MEN2B mice may result in more persistent neuroadaptations in neurocircuits involved in associative learning and behavioral reinforcement. Indeed, we have previously shown that MEN2B mice have increased dopamine uptake and release in the dSTR (Mijatovic et al., 2008). Striatal dopamine regulates long-term changes in synaptic strength via long-term potentiation (LTP) and depression (LTD) (Gerfen and Surmeier, 2011). Most likely these are the key neuronal mechanisms in MEN2B vSTR that underlie the persistent behavioral alterations. Finally, since 30 injection days of amphetamine with up to 20 mg/kg per day does not affect striatal tissue dopamine levels (Robinson and Camp, 1987), we felt safe to assume that the low amphetamine dose of 2 mg/kg for 4 days in this study does not alter striatal tissue dopamine levels. Future studies are needed to share further light into how MEN2B mutation manifests its effects.
To our surprise, and unlike in the regular open field test (Kumar et al., 2015), Gdnfwt/hyper mice did not run significantly more than their wild-type littermates after receiving amphetamine in the CPP trial. This might be due to interference from the CPP chamber and floor cues, the higher amphetamine dose used (2 mg/kg versus 1 mg/kg) or the shorter follow-up time (30 min versus 60 min). Nevertheless, enhanced dopamine content in the dSTR did not alter CPP in these mice, which suggests that the vSTR dopamine plays a key role in this test. Overall, our results suggest that a moderate increase in endogenous GDNF levels does not predispose to an increased risk to develop addiction.
Both mouse lines used in this study are constitutive knock-in mutants, which means that Gdnf and Ret genes are mutated in their native loci thereby avoiding artefacts associated with transgenic overexpression via random integration or viral expression. However, knock-in mice carry their mutant alleles throughout development and maturation (Kumar et al., 2015; Smith-Hicks et al., 2000). The important question in the future is to dissect whether the observed phenotype in MEN2B is caused by the effects on the development or on neurotransmission and neuroplasticity in the adult animals or a combination of the two. Another important question is why dopamine and TH levels (and the resulting amphetamine CPP) were changed in MEN2B mice, but unaltered in Gdnfwt/hyper mice. Our hypothesis is that this is at least in part because in the adult Gdnfwt/hyper mice, Gdnf expression is significantly increased only in the dorsal, not in the ventral striatum (Kumar et al., 2015). Perhaps more importantly, due to activating mutation Ret is constitutively active in MEN2B mice and unresponsive to physiological regulation of GDNF levels (Smith-Hicks et al., 2000), while in Gdnfwt/hyper mice the wild-type Ret context allows on-off signaling events in response to ligand levels. At this stage, we cannot discriminate between these two options. However, we believe that the lack of significant GDNF increase in adult vSTR of Gdnfwt/hyper mice is the most likely explanation.
Previous studies have reported that heterozygous GDNF knockout (Gdnfwt/KO) mice are more sensitive to cocaine- and methamphetamine-induced CPP (Messer et al., 2000; Niwa et al., 2007) and they also self-administer more methamphetamine (Yan et al., 2007). This contrasts with our present study, where increased GDNF signaling did not protect the animals from amphetamine-induced CPP. In fact, CPP was unchanged in Gdnfwt/hyper mice with increased levels of endogenous GDNF, and more persistent in MEN2B mice with constitutively active Ret. As suggested above, Gdnfwt/KO phenotype may be influenced by compensatory changes induced by GDNF reduction. Indeed, increased extracellular dopamine levels and DAT activity in the striatum of Gdnfwt/KO mice have been reported (Airavaara et al., 2004; Littrell et al., 2012). We conclude that about two-fold increase in endogenous GDNF expression (Kumar et al., 2015) does not affect amphetamine addiction-related behavior.
We have previously shown that TH levels and activity are robustly increased in dSTR of MEN2B mice (Mijatovic et al., 2008, 2007). Now we show that the striatal levels of TH are elevated to a similar extent and in a gene dose-dependent manner in both dorsal and ventral striatum in MEN2B mice. At the same time, the ratio of p31Ser TH to total TH remained unchanged, which suggests that GDNF/Ret signaling does not specifically increase TH phosphorylation at serine 31. This is in contrast with earlier work, where ectopic GDNF infusion to the rat striatum reduced total TH levels, but increased pSer31, 40 and 19 TH levels at a GDNF dose of 100 μg and specifically increased striatal levels of pSer31 TH at a lower dose of 30 μg (Salvatore et al., 2009a, 2004). In addition, we have previously shown that increased endogenous GDNF and ectopic GDNF applications have fundamentally different effects on brain dopamine system function (Kumar et al., 2015). Therefore, the above findings are likely specific to ectopic, high-dose GDNF application and support the use of the genetic models in this study to examine the role of GDNF signaling in the addiction process closer to the physiological range of GDNF expression.
5. Conclusions
Overall, our results suggest that the effects of Ret signaling on stimulant addiction-related behaviors are directly related to its regulatory role in mesolimbic dopamine neuron function at the level of dopamine biosynthesis. Specifically, amphetamine CPP extinction in MEN2B mice is severely stalled, which correlates with increased TH expression and dopamine levels in vSTR. Further research is required to resolve whether this also applies to other types of addictive drugs and which cellular mechanisms are involved. It would also be interesting to study whether humans carrying the corresponding MEN2B mutation (Pappa and Alevizaki, 2016) also display alterations in addiction-related behaviors. This would further support the clinical relevance of GDNF signaling in human addiction and the potential vulnerability to drug craving and relapse.
Supplementary Material
Highlights.
Constitutive Ret signaling causes long-lasting amphetamine-induced place preference
Moderate GDNF increase does not alter amphetamine-induced place conditioning
Long-lasting place preference correlates with increased mesolimbic dopamine
GDNF signalling via Ret mediates drug-seeking through increased mesolimbic dopamine
Acknowledgments
Funding
JK was supported by the Finnish Cultural Foundation, Emil Aaltonen Foundation and Otto A. Malm foundation. MS and JK were supported by the FinPharma Doctoral Program Pharmacy section. TTM is supported by grants from the Academy of Finland (267788 and 2737991), the University of Helsinki grants, the Jane and Aatos Erkko Foundation and the Sigrid Juselius Foundation. MFS supported by National Institutes of Health grant AG040261. JOA is supported from the Academy of Finland (grants 136591, 140983, and 263700) and Sigrid Juselius Foundation. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We thank Kati Rautio, Liisa Lappalainen and Susanna Wiss for technical assistance. Lauri Saastamoinen is acknowledged for the valuable ground work in setting up the behavioral methodologies.
Abbreviations
- CPP
conditioned place preference
- DOPAC
3,4-dihydroxyphenylacetic acid
- dSTR
Dorsal striatum
- GDNF
Glial cell line-derived neurotrophic factor
- HVA
Homovanillic acid
- MEN2B
Multiple endocrine neoplasia type 2B
- PFC
prefrontal cortex
- Ret
Rearranged during transfection
- SN
Substantia nigra
- TH
Tyrosine hydroxylase
- vSTR
Ventral striatum
- VTA
Ventral tegmental area
Footnotes
Author contribution
All authors designed the study. JK, MVA and MS performed the experiments and analyzed the data. TM, MFS and JOA provided materials. JK prepared the figures and wrote the manuscript. All the authors reviewed and contributed to the manuscript. GDNF hypermorphic mouse line was developed in the laboratory of Mart Saarma by JOA.
The authors do not have any conflicts of interest to declare.
References
- Airavaara M, Planken A, Gäddnäs H, Piepponen TP, Saarma M, Ahtee L. Increased extracellular dopamine concentrations and FosB/ΔFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur J Neurosci. 2004;20:2336–2344. doi: 10.1111/j.1460-9568.2004.03700.x. [DOI] [PubMed] [Google Scholar]
- Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–41. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- Burke RE, Antonelli M, Sulzer D. Glial cell line-derived neurotrophic growth factor inhibits apoptotic death of postnatal substantia nigra dopamine neurons in primary culture. J Neurochem. 1998;71:517–525. doi: 10.1046/j.1471-4159.1998.71020517.x. [DOI] [PubMed] [Google Scholar]
- Camí J, Farré M. Drug addiction. N Engl J Med. 2003;349:975–86. doi: 10.1056/NEJMra023160. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ron D. GDNF - A potential target to treat addiction. Pharmacol Ther. 2009;122:9–18. doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–70. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Henderson CM, Bormann NM. Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology (Berl) 1998;139:62–70. doi: 10.1007/s002130050690. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–579. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: A review. Neurosci Biobehav Rev. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, Lin LF, Gerhardt GA. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci Lett. 1994;182:107–11. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Kopra J, Panhelainen A, af Bjerkén S, Porokuokka L, Varendi K, Olfat S, Montonen H, Piepponen TP, Saarma M, Andressoo JO. Dampened amphetamine-stimulated behavior and altered dopamine transporter function in the absence of brain GDNF. J Neurosci. 2017;37:1581–1590. doi: 10.1523/JNEUROSCI.1673-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela M, Bäck S, Võikar V, Richie CT, Domanskyi A, Harvey BK, Airavaara M. Update of neurotrophic factors in neurobiology of addiction and future directions. Neurobiol Dis. 2017;97:189–200. doi: 10.1016/j.nbd.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kopra J, Varendi K, Porokuokka LL, Panhelainen A, Kuure S, Marshall P, Karalija N, Härma MA, Vilenius C, Lilleväli K, Tekko T, Mijatovic J, Pulkkinen N, Jakobson MM, Jakobson MM, Ola R, Palm E, Lindahl M, Strömberg I, Võikar V, Piepponen TP, Saarma M, Andressoo JO. GDNF Overexpression from the Native Locus Reveals its Role in the Nigrostriatal Dopaminergic System Function. PLoS Genet. 2015;11:e1005710. doi: 10.1371/journal.pgen.1005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Doherty D, Lile J, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science (80- ) 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Littrell OM, Pomerleau F, Huettl P, Surgener S, McGinty JF, Middaugh LD, Granholm ACC, Gerhardt Ga, Boger Ha. Enhanced dopamine transporter activity in middle-aged Gdnf heterozygous mice. Neurobiol Aging. 2012;33:427e1–14. doi: 10.1016/j.neurobiolaging.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer CJ, Eisch AJ, Carlezon WA, Whisler K, Shen L, Wolf DH, Westphal H, Collins F, Russell DS, Nestler EJ, Nestler EJ. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–57. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic J, Airavaara M, Planken A, Auvinen P, Raasmaja A, Piepponen TP, Costantini F, Ahtee L, Saarma M. Constitutive Ret activity in knock-in multiple endocrine neoplasia type B mice induces profound elevation of brain dopamine concentration via enhanced synthesis and increases the number of TH-positive cells in the substantia nigra. J Neurosci. 2007;27:4799–4809. doi: 10.1523/JNEUROSCI.5647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic J, Patrikainen O, Yavich L, Airavaara M, Ahtee L, Saarma M, Petteri Piepponen T. Characterization of the striatal dopaminergic neurotransmission in MEN2B mice with elevated cerebral tissue dopamine. J Neurochem. 2008;105:1716–1725. doi: 10.1111/j.1471-4159.2008.05265.x. [DOI] [PubMed] [Google Scholar]
- Mijatovic J, Piltonen M, Alberton P, Männistö PT, Saarma M, Piepponen TP. Constitutive Ret signaling is protective for dopaminergic cell bodies but not for axonal terminals. Neurobiol Aging. 2011;32:1486–1494. doi: 10.1016/j.neurobiolaging.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, Shen L, Noda Y, Furukawa S, Nabeshima T. An inducer for glial cell line-derived neurotrophic factor and tumor necrosis factor-alpha protects against methamphetamine-induced rewarding effects and sensitization. Biol Psychiatry. 2007;61:890–901. doi: 10.1016/j.biopsych.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Pappa T, Alevizaki M. Management of hereditary medullary thyroid carcinoma. Endocrine. 2016;53:7–17. doi: 10.1007/s12020-016-0873-1. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Gerlai R. Reverse piedpiperase: Is the knockout mouse leading neuroscientists to a watery end? Trends Neurosci. 1996;19:471–2. doi: 10.1016/S0166-2236(96)20051-7. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Gerhardt GA, Dayton RD, Klein RL, Stanford JA. Bilateral effects of unilateral GDNF administration on dopamine- and GABA-regulating proteins in the rat nigrostriatal system. Exp Neurol. 2009a;219:197–207. doi: 10.1016/j.expneurol.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS. Dichotomy of tyrosine hydroxylase and dopamine regulation between somatodendritic and terminal field areas of nigrostriatal and mesoaccumbens pathways. PLoS One. 2012;7:e29867. doi: 10.1371/journal.pone.0029867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS, Dempsey C, Fields V. Comprehensive Profiling of Dopamine Regulation in Substantia Nigra and Ventral Tegmental Area. J Vis Exp. 2012:1–7. doi: 10.3791/4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS, Spann SL, Dempsey C. Aging reveals a role for nigral tyrosine hydroxylase ser31 phosphorylation in locomotor activity generation. PLoS One. 2009b:4. doi: 10.1371/journal.pone.0008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Zhang JLL, Large DM, Wilson PE, Gash CR, Thomas TC, Haycock JW, Bing G, Stanford Ja, Gash DM, Gerhardt Ga. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J Neurochem. 2004;90:245–254. doi: 10.1111/j.1471-4159.2004.02496.x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Smith-Hicks CL, Sizer KC, Powers JF, Tischler AS, Costantini F. C-cell hyperplasia, pheochromocytoma and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B. EMBO J. 2000;19:612–22. doi: 10.1093/emboj/19.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman I. Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol Sci. 1992;13:170–6. doi: 10.1016/0165-6147(92)90059-f. [DOI] [PubMed] [Google Scholar]
- Sulzer D. How Addictive Drugs Disrupt Presynaptic Dopamine Neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic Mapping of the Monoamine Pathways in the Rat Brain. Acta Physiol Scand. 1971;82:1–48. doi: 10.1111/j.1365-201X.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Pycock C. Functional correlates of dopamine neurotransmission. Bull Schweiz Akad Med Wiss. 1974;30:44–55. [PubMed] [Google Scholar]
- Valros A, Palander P, Heinonen M, Munsterhjelm C, Brunberg E, Keeling L, Piepponen P. Evidence for a link between tail biting and central monoamine metabolism in pigs (Sus scrofa domestica) Physiol Behav. 2015;143:151–7. doi: 10.1016/j.physbeh.2015.02.049. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374:363–71. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial cell line-derived neurotrophic factor mutant mice. FASEB J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.