Abstract
The pharmaceutical industry is facing unprecedented challenges as the cost of developing new drugs has reached unsustainable levels, fueled in large parts by a high attrition rate in clinical development. Strategies to bridge studies between preclinical testing and clinical trials are needed to reduce the knowledge gap and allow earlier decisions to be made on the continuation or discontinuation of further development of drugs. The discovery and development of human induced pluripotent stem cells (hiPSCs) have opened up new avenues that support the concept of screening for cell-based safety and toxicity at the level of a population. This approach, termed “Clinical Trials in a Dish” (CTiD), allows testing medical therapies for safety or efficacy on cells collected from a representative sample of human patients, before moving into actual clinical trials. It can be applied to the development of drugs for specific populations, and it allows predicting not only the magnitude of effects but also the incidence of patients in a population who will benefit or be harmed by these drugs. This, in turn, can lead to the selection of safer drugs to move into clinical development, resulting in a reduction in attrition. The current article offers a perspective of this new model for “humanized” preclinical drug development.
Keywords: stem cells, cell-based assays, cardiac diseases, toxicology
要約
製薬業界は、主に臨床開発における高い開発中止率の影響で、新薬の開発コストが持続不可能なレベルに達しており、これまでにない問題に直面している。知識のギャップを縮小し、薬物のさらなる開発の継続または中止に関する早期の決定を可能にするためには、前臨床試験と臨床試験の間の研究を橋渡しする戦略が必要です。ヒト人工多能性幹細胞(hiPSCs)の発見と開発は、集団レベルでの細胞ベースの安全性と毒性のスクリーニングという概念を支える新たな道を切り開いた。「シャーレの中で臨床試験」(CTiD)と呼ばれるこのアプローチは、実際の臨床試験に移る前に、ヒト患者の代表的サンプルから採取した細胞に対する薬物療法の安全性または有効性の試験を可能にする。これは、特定の集団のための薬物の開発に適用することができ、効果の大きさだけでなく、集団内でこれらの薬物によって恩恵または被害を受ける患者の発生率も予測することができる。結果として、より安全な薬物を選択して臨床開発に進むことができるようになり、開発中止率の減少につながる可能性がある。本論文では、この「ヒト化」前臨床薬剤開発の新しいモデルの展望が示されている。
초록
제약 업계는 임상 개발에서의 높은 실패율을 중심으로 신약 개발 비용이 지속가능하지 않은 수준에 이르게 됨에 따라 전례 없는 도전 과제에 직면하고 있다. 전임상 시험과 임상 시험을 연계하는 전략이 지식 격차를 줄이고 신약 개발 지속 여부에 대한 조기 결정을 위해 필요하다. 인간유도만능줄기세포(hiPSCs)의 발견 및 개발로 인해 모집단 차원에서 세포 기반 안전 및 독성 검사의 개념을 뒷받침하는 새로운 방법이 생겨났다. 이와 같은 접근법은 “배양 접시 임상시험”(CTiD)이라 불리며 실제 임상 시험으로 넘어가기 전에 환자의 대표 표본에서 수집한 세포를 통해 치료법의 안전이나 효능에 대한 시험을 가능케 한다. 특정 집단을 목표로 하는 신약 개발에 적용할 수 있으며, 효과의 정도뿐만 아니라 신약으로 인해 효과를 보거나 피해를 입을 집단 내 환자의 발병률을 예측할 수 있다. 이는 임상 개발로 진행되는 안전한 신약 선택으로 이어지며 실패율을 감소시킨다. 본 논문은 “인간화된” 전임상 신약 개발에 대한 신규 모델에 대한 전망을 다룬다.
摘要
制药行业正面临着前所未有的挑战,因为研发新药的成本已经达到难以维系的水平,这很大程度上是由临床研发中的高耗损率造成的。需要在临床前试验与临床试验之间实行桥接研究战略,以缩小知识鸿沟,并使得更早地决定继续还是停止进一步研发药物成为可能。人类诱导多能干细胞(hiPSCs)的发现和开发开辟了新的途径,其支持了基于细胞对药物在人群中的安全性和毒性进行筛查的概念。这种被称为“培养皿中的临床试验”(CTiD)的方法能够在进入实际的临床试验之前,对从代表性人类患者样品中收集的细胞的疗法的安全性或功效性进行检查。它可以被用于开发针对特定人群的药物,同时它既能预测疗效的程度,又能预测在某一人群中受益于这些药物或受这些药物伤害的患者比率。这反过来又能使我们选出更安全的药物进行临床研发,从而减少耗损。本文提供了对这种新型的“人性化”临床前药物研发的展望。
Introduction
According to a 2014 study by the Tufts Center for the Study of Drug Development, the estimated average out-of-pocket cost to develop a new pharmaceutical drug now exceeds $1.4 billion, or $2.6 billion when the time value of money is taken into account.1 This represents an increase of approximately 8.5% higher than the general price of inflation, when compared to a 2003 study from the same group.2 Moreover, only 20% of that $1.4 billion is spent during the discovery and preclinical development phases (target selection, lead identification and optimization, pharmacological profiling, safety and toxicity testing), whereas 80% is spent in clinical development, applications for Investigative New Drug (IND) status, and US Food and Drug Administration (FDA) approvals.3 Whereas half of the compounds entering discovery survive preclinical testing, eight out of every nine of the remaining compounds then fail in clinical trials.4 In fact, applying this analysis to the Tufts study reveals that of the $2.6 billion spent to achieve a regulatory approval of a single new drug, only about $200 million is spent on the compound that is actually approved. The other $2.4 billion—fully 92% of the total investment—is spent on candidate drugs that failed along the way. And although the high cost of failure may be measured in dollar terms, what it really represents is thousands of person-years of scientific researchers’ time that could be redeployed against compounds that still have the potential to change the health of the world. Imagine the benefits that could come from identifying, much earlier in the development process, even just a fraction of the compounds that will ultimately fail in development.
The challenge, therefore, is to reduce attrition rates by identifying, early in the development process, drugs that will fail in clinical trials. Strategies to bridge studies between preclinical testing and clinical trials are needed to reduce the knowledge gap prior to first human exposures, and to allow earlier decisions to be made on the continuation or discontinuation of further development of drugs. Certainly, one can see how the basic concept of clinical trials has permeated little, if any, of the preclinical environment. At its most basic level, clinical trials enable a pharmaceutical sponsor to develop human population–level estimates of potential drug safety and efficacy. Today’s preclinical testing relies on a comprehensive set of safety guidelines that informs the selection of what safety tests to conduct.5 Thus, preclinical testing is primarily designed to take account of regulatory requirements. Follow-up studies may be triggered if there is a need to characterize specific adverse effects found in initial studies. Although follow-up may appear more scientifically driven than the core program, the design is nevertheless based on what is perceived by the pharmaceutical sponsor to be the data required by the regulators.6
Recent pharmaceutical industry surveys indicate that preclinical and clinical safety remains a major cause of drug attrition, and that cardiovascular toxicity represents the most frequent drug reaction, accounting for approximately 40% of all drugs withdrawn due to safety concerns.7,8 During the past few years, cardiomyocytes (CMs) derived from human induced pluripotent stem cells (hiPSCs) have shown promise for cardiac safety and toxicology screening during early drug development.9–17 hiPSC-CMs are especially attractive because they represent an unlimited source of cells that appear to recapitulate the genetic, physiologic, and pharmacological properties of human CMs and heart tissue from the donor.18–21 The discovery and development of hiPSCs have opened new possibilities of testing that support the concept of large-scale, human cell–based safety and toxicity screens, with the potential to reduce attrition of drugs in clinical development. Indeed, the noninvasive nature and unlimited supply of this patient-derived approach now allow performing surrogate clinical trials in vitro, with consequences that extend well beyond the ability to reduce the attrition of drugs. This new approach, termed “Clinical Trials in a Dish”22 (CTiD), has potential applications throughout multiple areas of drug discovery and development—from early stages of lead optimization to regulatory safety assessment.
Defining Clinical Trials in a Dish
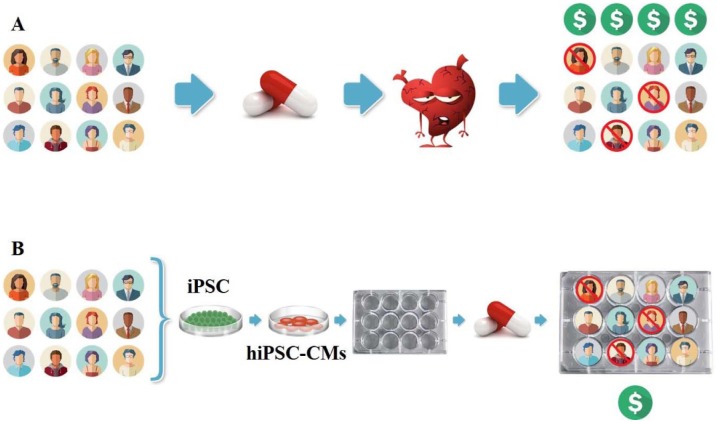
A key feature that distinguishes actual clinical trials from all other nonclinical or preclinical testing in the drug development process is the application of a drug of interest to a statistically representative cross sample of a target human population (in vivo), to understand the magnitude and distribution of effects (whether beneficial or adverse) that a population at large will experience from taking that drug. Similarly, the concept of CTiD also involves testing medical therapies for safety or efficacy, but these tests are performed in the laboratory, on cells collected from a sample of human patients, before moving into actual clinical trials. This allows defining with accuracy, in an in vitro setting, the safety of a drug when used at the level of a population ( Fig. 1 ).
Figure 1.
Similarities between Clinical Trials and Clinical Trials in a Dish (CTiD).
Human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes can be used to assess susceptibility to drug-induced cardiac toxicity. (A) In a Phase I study, healthy volunteers are recruited to assess the safety and toxicity of a drug. Cardiovascular toxicity represents the most frequent serious adverse drug reaction. (B) In a CTiD study, hiPSC-derived cardiomyocytes from the same healthy volunteers are studied in vitro. CTiD studies are efficient and can be executed at a fraction of the cost of clinical trials.
CTiD will most often use hiPSCs, or hiPSC-derived functional cells, for several reasons. First, and for example in the case of hiPSC-derived CMs, they have already been demonstrated to recapitulate their respective donor’s individual reaction to drugs. Indeed, there is now experimental evidence showing that the cardiac effects of certain drugs in healthy or diseased patients are recapitulated in hiPSC-derived heart cells from these same patients. For example, Shinozawa et al.20 evaluated the correlation between susceptibility to moxifloxacin (MOX)-induced prolongation of the QT interval on the cardiac electrocardiogram (the time interval between depolarization and repolarization of the ventricles in the heart) in 10 healthy volunteers, and that to MOX-induced prolonged repolarization in hiPSC-CMs generated from blood samples of the same individuals, in which interindividual susceptibility to QT prolongation was observed. QT prolongation is one of the most common reasons for withdrawal of drugs from the market,23,24 and MOX is a fluoroquinolone antibiotic that is commonly used as a positive control in dedicated thorough QT (TQT) studies, with the primary objective to quantify the effect of a new drug on the QT interval and assess its potential arrhythmic liability.25 The results showed that in these individuals, the QT interval was significantly positively correlated with prolongation of field potential duration (FPD; a measure analogous to the QT interval on the cardiac electrocardiogram) measured in hiPSC-CMs at clinically relevant concentrations. Genomic analysis showed no interindividual significant differences in known target binding sites for MOX. They concluded that hiPSC-CMs from healthy subjects did recapitulate the susceptibility to MOX-induced QT prolongation and provided proof of concept for in vitro CTiD.
In addition, Burridge et al.18 showed that hiPSC-CMs could recapitulate patient-specific clinical susceptibility to doxorubicin (DOX), an anthracycline chemotherapy agent, of populations at high risk of drug-induced cardiotoxicity. In that study, 12 female patients were recruited—eight with breast cancer who had been treated with DOX, comprising four patients who did not experience clinical cardiotoxicity and four patients who did experience cardiotoxicity, and four age- and gender-matched control volunteers who had never been treated with any chemotherapeutic agent. hiPSC-CMs derived from the individuals with breast cancer who experienced DOX-induced cardiotoxicity (DIC) were consistently more sensitive to DOX toxicity than hiPSC-CMs from patients who did not experience DIC. Importantly, it was noted that the differences observed between the DOX-treated hiPSC-CMs were not observed using other derived cell types from these patients, such as fibroblasts or hiPSCs.
In a different study, Stillitano et al.19 showed a strong correlation between prolongation of the corrected QT interval on the cardiac electrocardiogram from patients treated with an antiarrhythmic agent, sotalol, and effects of clinically relevant concentrations of sotalol on FPD recorded from hiPSC-CMs derived from the same patients.
Taken together, these results confirm that hiPSC-CMs are not simply models of a generic person; they are models of a specific person. That property qualifies a cohort of hiPSC donors to represent, albeit in a preclinical setting, a similar cross section as the patients recruited in a clinical trial. Certainly, healthy volunteers recruited in a first-in-human, or Phase I, study are of unknown genetic background. Therefore, analogous to the patient enrolled in a clinical trial, hiPSCs can serve as surrogates for patients in Phase I clinical trials and provide an important link between Phase I and Phase II trials. CTiD studies are efficient, allow the study of a range of clinical doses, and can be executed at a fraction of the cost of clinical trials, and outside of the rigid and heavily regulated environment of clinical testing.
Second, because hiPSCs can replicate infinitely, a single tissue sampling from a given donor can lead to the generation of a virtually infinite supply of identical and inexpensive test material representing that donor. Consequently, creating a library of samples from a representative cross section of donors now allows one to conduct an unlimited number of CTiD studies on the identical cohort of donors—thereby enabling economical, cross-test, cross-compound comparisons on the same patients, and allowing the establishment of reference lines and historical monitoring, approaches that have never been possible before.
Potential Benefits of CTiD Using iPSC-Derived Cardiomyocytes
Individual variability in drug efficacy and safety is a major challenge in drug development, but it is currently not possible, prior to clinical trials, to test how large this variability might actually be. Consequently, during the preclinical stages of safety testing, safety pharmacologists rely on a numerical minimum margin of safety that is defined by the difference between the usual effective dose of the drug of interest and the dose that causes severe side effects.8,26 However, because in the case of hiPSC-CMs, this margin is established using results obtained from a single cell line representing a single human, sponsors risk committing two types of errors: (1) false negative: assuming certain drugs are essentially safe, when in fact they are not; or (2) false positive: assuming certain drugs are essentially unsafe, when in fact they are safe.27 For example, the bulk of the population tested might be more vulnerable than the one person (more specifically, the one cell line) tested, leading to adverse effects in a population even in the presence of an (erroneously) acceptable safety margin. In contrast, the one line tested might be among the most vulnerable members of the population, in which case the defined safety margin is excessive and may lead to the attrition of a drug even if its safety profile in a population is acceptable. With CTiD, there is no need for margins to be arbitrary. Better estimates of actual safety margins can be experimentally generated from the distribution of effects associated with a population, and not a single individual representing a population.
Commonly Used Methods to Assess Toxicity
Drug-induced cardiotoxicity can affect all components and functions of the cardiovascular system, and it can be functional (altering the electrophysiology and/or mechanical functions) and/or structural (e.g., morphological damage, loss of viability, and changes in cell signaling) in nature. Current in vitro strategies to assess functional cardiotoxicity include screening of key voltage-gated ion channels using automated patch clamping of hiPSC-CMs.28,29 Even though hiPSC-CMs are physiologically relevant, these cells are relatively fragile, have complex culture requirements, and are expensive to produce, which has restricted their use on these types of systems.29 Multielectrode arrays (MEAs), however, provide noninvasive methods for studying electrophysiology.18–20,30 Cells are plated on a dish containing electrodes on the bottom, which are used to stimulate and measure extracellular field potential waveforms induced by a large number of CMs. As mentioned previously, these waveforms correlate relatively well with cardiac action potential and QT interval durations. The ability to perform prolonged recordings, and the existence of multiwells, make this technology ideal to study acute and chronic effects of drugs on cardiac electrical activity.
Access to hiPSC-CMs has also enabled the development of screens to monitor changes in cardiac contractility using impedance measurements,31 or intracellular Ca2+ transients to predict the risk that drugs may affect cardiac contractility.14,32,33 Given that most hiPSC-CMs are spontaneously active, synchronous spontaneous rhythmic beating has also been used for evaluating beat rate as well as changes in the morphology of Ca2+ transient and/or impedance signals, including peak trace amplitude, width, raise, decay, and so on. Some of these endpoints were found to be particularly good predictors of cardiac toxicity.12,17
MEA systems and Ca2+ imaging has also proven useful in noninvasive, multipoint measurements of spontaneous electrophysiological activity of cultured hiPSC-derived neurons.34,35 With electrically quiescent and nonexcitable hiPSC-derived hepatocytes, high content assays assessing multiparameter readouts of general and mechanism-specific hepatotoxicity have been developed. The endpoints measured in these assays include cell viability, nuclear shape, mitochondrial membrane potential, phospholipid accumulation, cytoskeleton integrity, and apoptosis.36
Probability of Detecting Effects Based on Cohort Size
The requirement to test pharmaceutical compounds for safety against a genetically diversified sample of humans comes from the fact that every human reacts differently to any given dose of any drug. CTiD studies are not intended to compete with clinical trials. Rather, they are intended to shed light in advance of such trials on the likely distribution of human adverse reactions that would result from clinical trials. And the number of donors required to achieve helpful degrees of statistical discrimination in CTiD studies is surprisingly small. Investigators are always concerned about the impact of a drug on vulnerable members of a population. These vulnerable patients cannot be individually identified a priori, because the same patient who has an “average” reaction to one drug in a class may be the one who has the worst reaction to another drug in the same class. Therefore, the drug must be tested against a random sample of patients. Although there is always the pressure to keep sample sizes down to minimize cost, one must not reduce the sample below that necessary for the desired discrimination. The power to detect events is directly related to the number of cell lines in the cohort (analogous to the number of patients enrolled in clinical trials). If detecting events with an incidence of 1 in 10 patients is the target, then a single hiPSC-CM line provides only a 10% chance of observing the existence of that adverse event. Testing four cell lines increases the probability to 34% ( Table 1 ). Using a cohort of 16 lines, however, yields an 81% chance that an event will be identified, and 24 lines increases the likelihood to 92%. And these probabilities are independent of the population targeted, whether it is different ethnicities, geriatric patients, children, and so on.
Table 1.
Probability of Detecting Effects Based on Cohort Size.
| Cohort Size (Individual Cell lines) (N) | 1 in 10 (Decile) Population Incidence (%) | 1 in 4 (Quartile) Population Incidence (%) |
|---|---|---|
| 24 | 92 | 99.9 |
| 16 | 81.5 | 99.0 |
| 4 | 34.4 | 68.3 |
The table provides exact probabilities for common population incidence frequencies and cohort sizes. Monte Carlo simulations were performed assuming a normal distribution of drug toxicity or sensitivity.
CTiD studies, although large enough to detect common adverse events, will not reliably detect an increased incidence of rare adverse events or events with significant latency. In fact, for most drugs, only about 500–3000 participants are studied for relatively short durations before a drug is marketed, and rare adverse events with low incidence are not detected either. Therefore, CTiD studies should be targeted to circumstances that can provide information that is pertinent to early clinical decisions but, under today’s process, would be available only after clinical trials have been completed. The benefits of such information are profound, as detailed below.
Recommendation for the Use of CTiD
Eliminating even 10% of “future failure” drugs (a realistic goal based on cardiotoxicity testing alone) could allow the redeployment of over $200 million of resources to more promising drugs. Relatedly, sponsors can compare the toxicity profiles (at a population level, rather than a single cell line level) of multiple drugs slated for clinical studies, whose therapeutic benefits appear similar but whose adverse effect profiles may vary. This allows ranking and prioritization with respect to safety risk and improves decisions as to which compounds to move into further development. For example, for high-risk and high-benefit drugs, data from CTiD studies could help better define the probability of finding patients experiencing adverse effects at, or near, the intended therapeutic dose, enabling an explicit consideration of the benefit–safety tradeoffs.
Currently, the strategy to test for cardiac safety and toxicity consists of high- and low-to-intermediate-throughput in vitro screening, as well as a battery of in vivo and ex vivo assays together with in vivo toxicity studies.37 The purpose of conducting these studies is to identify dose-limiting toxicities and to understand the safety liabilities. Once identified, safety and toxicology issues can be managed by answering a set of questions that will enable deciding how best to proceed, such as: what the safety margin is, whether the effects observed are reversible, what potential mechanisms are involved, and what the relevance of these findings to humans is. With the recent demonstration that hiPSC-CMs can recapitulate clinical individual susceptibilities to drug effects in healthy and diseased subjects, CTiD studies allow answering these critical questions in a way that was not possible before and are key in determining whether any toxicity observed has the potential to limit, or end, the development of drug prior to going into clinical trials. As such, CTiD studies are most impactful when performed using drug-like compounds that have a promising profile in preclinical models, and that are being considered for formal development. Managing toxicity will depend on the nature of the finding, however; CTiD studies are particularly well suited for prioritizing drug-like compounds that have shown equivocal chemically mediated in vitro toxicity or ambiguous results in in vivo models, or that are structurally related to a chemical series that has been shown to cause cardiac toxicity and/or that is confined to a narrow chemical space. CTiD studies of potential problematic compounds should also be performed prior to any in vivo studies to avoid harm to animals.
Repurposing Shelved Compounds
CTiD also can be deployed with the opposite goal in mind (i.e., to revive candidate drugs that were erroneously shelved in the past due to flawed concerns about safety and toxicity). The repurposing opportunity arises when a sponsor previously curtailed development during the preclinical phase, out of an overabundance of caution—that is, when it assumed, based on tests performed on CMs derived from a single donor, that an adverse reaction observed with this one donor applied to the majority of individuals within a targeted population. Because CTiD studies can better estimate the real-life distribution of responses, it offers the possibility of reigniting interest in a once-promising compound or series whose development has been stopped.
Reducing Adverse Drug–Drug Interactions
CTiD can immediately contribute to reducing the current, significant problem of adverse drug–drug interactions (DDIs). Given that an estimated 2 million serious adverse drug reactions (ADRs) occur annually in U.S. hospitals, resulting in more than 100,000 deaths (making ADRs the fourth leading cause of fatality in the United States—without adding in ADRs that occur in ambulatory settings),38 and that DDIs are estimated to represent up to 20% of all ADRs, DDIs per se may be responsible for more than 20,000 deaths per year in the United States.39 Moreover, this situation is likely to worsen in the coming years as an aging population faces more concurrent maladies, and the field of medicine migrates increasingly toward an approach of using a “cocktail” of multiple drugs to treat certain ailments (as in the case of HIV infection or certain cancers), because the rate of ADRs increases exponentially after a patient is on four or more medications.40
Clearly, the need to discover a priori whether a combination of drugs may result in a DDI effect is pressing, but current test platforms for detecting the potential for such interactions are problematic. Animal testing is expensive, resource-intensive, and subject to interspecies differences in response, and it ignores the problem of individual-to-individual variation in drug response among humans.41,42 Tissues from healthy humans can be expensive (and, from any one individual, scarce), thus limiting the number of compounds or exposures for which impacts on that one individual can be compared.
These limitations lead to a relatively narrow approach to the study of DDIs. First, the focus is overwhelmingly on pharmacokinetics (PK), even though pharmacodynamic (PD) interactions are also both common and potentially deadly. Indeed, even barely observable undesired effects can potentiate each other in a dangerous manner; for example, a combination of angiotensin-converting enzyme (ACE) inhibitors with potassium-sparing diuretics can increase potassium retention, leading to life-threatening hyperkalemia.43 Second, the number and complexity of drugs tested in combination with the candidate drug are kept low and simple. The number of drugs is minimized, the number of dose combinations is kept small, and three- and four-compound drug–drug interaction tests are virtually unheard of, despite the fact that 39% of those 65 or older already take five or more drugs concurrently.44 Third, and not surprisingly, there appears to be no attempt to develop population-level distributions of drug–drug interaction effects.
With CTiD, sponsors can economically conduct large numbers of experimentally controlled, cross-comparable assays. They can develop population-level distributions of interaction effects and test the drugs in combination with the 25 most commonly co-prescribed medications. Three- and four-drug combinations become as easy to test as simple two-drug combinations.
For example, as previously mentioned, cardiovascular toxicity remains one of the leading causes of early and late attrition in drug development, as well as a major reason for the withdrawal of marketed drugs.24–26,45,46 Although proarrhythmia represents one of the most frequent problems, the most important form of proarrhythmia is acquired long QT syndrome (LQTS), resulting in a potentially fatal form of ventricular tachycardia called torsade de pointes. QT prolongation is currently recognized as a significant concern for regulatory agencies.24,47 In the United States, about one in five patients uses at least one QT-prolonging drug (≈23% of patients); and, in the United Kingdom and Italy, up to 3% of patients are prescribed at least one noncardiac drug with proarrhythmic propensity.48,49 The problem is further complicated by patients undergoing common polytherapy, with a relatively high risk that a patient will receive at least two drugs mutually modifying their proarrhythmic potential.50–54 Under these circumstances, one can easily see the value of using hiPSC-CMs to screen for potential cardiac effects of DDIs.
The obvious choice to study DDIs would be the use of hiPSC-derived hepatocytes (hiPSC-HPs). The pharmaceutical industry, however, currently relies primarily on animal tests to define the safety of new drugs, and these tests often fail to predict human hepatic toxicity because of physiological differences between animals and humans. To date, not a single model that accurately reproduces all facets of human liver disease has been established. As discussed in greater detail in the Limitations section, the current state-of-the-art hepatic differentiation protocols produce cells that are fetal in their phenotype and function,55,56 and full adoption of hiPSC-HPs by the industry may become a reality only when more robust and consistent differentiation protocols are established, and more validation studies comparing these cells to primary hepatocytes become available. For example, in a recent article, Kajiwara et al.57 compared 28 different hiPSC lines derived from either peripheral blood cells or adult dermal fibroblasts from the same individuals and found that the major variations in hepatic differentiation from the same individual were largely attributable to donor differences, rather than to the types of the original cells. These results emphasize the importance of donor differences when comparing differentiation propensity of hiPSC clones and confirm that hiPSC-derived hepatocytes are also models of a specific person, reinforcing the idea that, once optimized, they will also be used in determining drug-induced hepatic toxicity.
In the absence of predictive hiPSC-derived hepatocyte models, DDI studies will need to focus primarily on PD interactions. Also, the ability to predict drug interactions will depend heavily on the choice of in vitro model selected as well as the endpoints measured, because some models may not be amenable to detect drugs with potentially unknown mechanistic interactions.
Additional Applications of CTiD
Protecting Ethnic Populations
The relatively low cost and high speed of CTiD will rapidly enable in vitro drug safety testing on a much wider variety of ethnically distinct populations than is economically feasible today. Currently, despite the known impact of ethnicity on drug response,58,59 many countries in the world cannot demand that pharmaceutical sponsors conduct full-scale clinical trials on their indigenous, ethnically distinct populations. The cost of clinical trials (driven by the high cost of acquiring and field-testing patients, each of whom can be used for testing only one regimen) is simply too large to bear, given many countries’ relatively small market size and/or the low price point that patients can afford to pay. As a result, many countries are faced with a no-win situation in which they must either forbid a sponsor from marketing a drug in the country, denying a potentially valuable medicine to its population, or allow a sponsor to market a drug in the country without testing, with the understanding that it may cause ADRs of indefinite severity in an unknown portion of its population.
In contrast, the cost and difficulty of obtaining the raw (cellular) material for CTiD are quite low, because it involves only the simple collection of tissue samples from a relatively small group of people representing a cross section of the target population. Furthermore, the one time fixed cost of sample acquisition can then be leveraged to test unlimited numbers of drugs, lowering the cost per test even further. Finally, there are no geographical limitations regarding where CTiD can be performed, unlike the case of clinical trials.
Although a set of CTiD studies obviously will not protect a given population to the same degree that a full-scale clinical trial could, they can certainly improve drug safety relative to the current approach of no indigenous-population testing at all. This is especially true given that 70% of all toxicity-related drug failures during clinical trials are due to mainly three types of toxicity (cardiovascular, hepatic, and CNS)60 and that CTiD are already available for cardiotoxicity and are within sight for hepatotoxicity and neurotoxicity within the next few years.61,62 Thus, sponsors can afford to conduct investigations of narrower and/or more targeted populations against an ever-broadening array of potential toxicities, and regulators in smaller or poorer countries can demand that they do so.
Protecting Children and Geriatric Patients
Protection of new populations is not limited to questions of ethnicity. For example, today’s clinical trial protocols often exclude representatives of the very populations that will ultimately consume the majority of the drug being developed.
Consider cancer treatments. Thirty-five percent of all new drugs under development today are oncology drugs.63 More than half of all cancers have an onset age of 65 or older.64 Yet, geriatric patients are vastly underrepresented in clinical trials.65,66 This is not because the pharmaceutical industry is indifferent to age, but because there are a variety of barriers to testing among geriatric patients that cannot and will not be overcome easily or soon—including comorbidities, economic constraints, physical immobility, and communication issues.
At the other end of the age spectrum, it is possible that CTiD based on hiPSCs from neonatal donors can help break the logjam in the development of pediatric drugs. It is well documented that hundreds of drugs are already in use with adults that may potentially be useful for treating children but are not approved for use in children. In fact, it is estimated that between 16% and 62% of all medicines prescribed in general pediatric wards of hospitals, and 80% of all medicines prescribed in neonatal intensive care units, are prescribed “off label.”67 The main reason for this gap is, of course, the ethical problems associated with conducting clinical trials on children.68
Although no CTiD will be a perfect substitute for a full-scale clinical trial, there must be some middle ground in which a series of PK and PD CTiD studies on cells from representative samples of neonates and children who show equal or less adverse reactions to certain drugs than their adult counterparts can pave the way toward approval of at least a portion of the most valuable medicines for pediatric use.
Limitations
A common feature of all of the applications discussed thus far is that the hiPSC-derived cells from a representative sample of donors are used to collectively estimate the magnitude and distribution of the reactions of a target population. Although these applications demand that each individual’s hiPSC-derived cells recapitulate that individual’s in vivo responses to a certain degree, they do not demand perfection. That is fortunate, because the state of stem cell science today is unquestionably imperfect, and clearly there are important issues that need to be addressed before the full potential of these cells may be realized.
Without a doubt, hiPSC-derived CMs hold tremendous promise for drug development and various therapeutic applications. These cells express expected sarcomeric proteins and ion channels and exhibit cardiac-type calcium transients and electrophysiological properties. The model is not, however, deprived of limitations. Protocols for induction of pluripotency and cardiac differentiation are still evolving. This lack of consistency in cell origin, transcription combinations, time in culture, number of passages, selection methods, and uniformity in subtype (e.g., homogeneous nodal vs. atrial vs. ventricular myocytes) adds to the heterogeneity, leading to the generation of inconsistent results that delay progress in the field.69,70 Another major limitation of currently available hiPSC-CMs is their immature phenotype.71,72 Indeed, hiPSC-CMs display many properties that are not shared with adult CMs, such as spontaneous and persistent automaticity, depolarized diastolic membrane potential, metabolic dependence on glycolysis, and lack of inotropic response to adrenergic stimulation.73-76 Several methods to enhance maturation have been proposed, including long-term culture,77,78 mechanical loading,79 electrical stimulation,80 culture substrate,81 and chemical treatments.82 None of these approaches, however, have proven sufficient to induce complete maturation equivalent to adult CMs. Nonetheless, because of their ability to recapitulate patient-specific susceptibility to drug-induced cardiotoxicity, hiPSC-CMs represent a suitable platform for CTiD.
Stem cell–derived hepatocytes have been reported to vary in metabolizing enzymes, hepatic morphology, and polarization, suggesting that they are still not comparable to primary hepatocytes.83,84 For example, CYP induction pathways have been shown to be limited in iPSC-derived hepatocytes compared to primary human hepatocytes, suggesting that nuclear receptor pathways may not be fully functional.85 Furthermore, 2D cultured human hepatocytes have been shown to undergo de-differentiation with a loss of epithelial morphology and metabolic activities after only a few days or hours in culture.86 In this field as well, differentiation protocols vary widely among different laboratories, resulting in heterogeneous cell populations expressing adult markers (e.g., high levels of albumin expression) while maintaining an immature phenotype, raising concerns about the predictive value of these cells.86–88 Therefore, although significant progress has been made in hiPSC-derived hepatocytes, there is a pressing need for more robust, consistent, and comparative studies with primary hepatocytes before full adoption of hiPSC-derived hepatocytes can be considered by the industry.
hiPSC-derived neurons (hPSNs) represent a unique model system to study the fundamental properties of the CNS and the underlying mechanisms involved in various disease phenotypes. Their value lies in their ability to recapitulate functional properties such as passive and active membrane characteristics, synaptic activity, and plasticity.89 hPSNs, however, display functional phenotypes that resemble fetal and postnatal rodent neurons. Transcriptome studies also show that the more mature hPSNs reported to date display an expression profile similar to that of midgestational human fetal brain tissue.90 Moreover, it has been reported that during differentiation, only a small percentage of neurons display synaptic markers, and most cultures still contain a large population of progenitor cells not found in most brain regions of adults.91 Nonetheless, despite these drawbacks, renewed efforts in directed differentiation, cell sorting, derivation of pure populations of transmitters, and functional phenotype-specific neurons appear poised to overcome critical hurdles and allow this technology to support its development toward potential clinical applications.92,93
Finally, although CTiD has the potential to address significant gaps in the development of pediatric and geriatric drugs, this approach must be carefully evaluated because of issues described here concerning the differentiation states of hiPSC-derived cell types. More specifically, the question remains as to whether iPSC-CMs display an immature phenotype or accurately represent a patient’s age. Indeed, some studies have reported that cellular reprogramming may “reset the aging clock” by allowing somatic cells to acquire a state that is normally associated with embryonic stem cells (ESCs).94 For example, hiPSCs can be obtained from aged individuals and yet still show key properties of ESCs, including self-renewal, elongated telomers, and round-shaped mitochondria with underdeveloped cristae.95 Even iPSCs derived from senescent and centenarian cells have been reported to exhibit a more youthful signature, displaying elongated telomers and gene expression profiles comparable to those of ESCs.96 Moreover, metabolic signatures, mitochondrial networks, handling of reactive oxygen species (ROS), telomerase expression, and other factors appear reset to a state characteristic of pluripotency.95,97,98 These data are controversial, however, because differential reports have also been published regarding the extent to which reprogramming rejuvenates aged, somatic cells and whether iPSCs exhibit aging signatures.96,99–101 In a recent article, Kang et al.102 show that iPSCs not only do not erase the signs of aging but may even reveal aging-related defects in the mitochondrial DNA that were not detectable in the whole parental tissue. One cannot exclude that conflicting reports regarding the extent to which adult somatic cells are rejuvenated may be explained, at least in part, by the distinct protocols and materials used in the different studies. Regardless, it is clear that some iPSCs and their differentiated progeny, such as CMs, may exhibit age-related defects. Whether these defects are cell line specific remains to be determined with certainty, and additional research into various aspects of maturity and aging is required.103–106
But as science improves, we should anticipate a time within just a few years when the recapitulation of clinical individual susceptibility to drug effects will be scientifically accepted for drawing conclusions about drug responses at not only the population level but also the individual level. After all, an entire industry, regenerative medicine, depends on stem cells to repair or replace a specific human’s own tissues and organs—which requires a degree of precise recapitulation far beyond anything required by the ideas proposed here.
Conclusion
The arguments provided in this article and recent publications107,108 support the view that including the study of diversity in vitro through the use of the CTiD approach has the potential to significantly affect and improve the decision-making process throughout the drug discovery and development process if approached correctly. The current “state of the art,” however, will evolve only if the industry, regulators, and clinicians are prepared to make significant changes to the current decision processes accordingly. Preclinical organizations of pharmaceutical sponsors will need to learn to take “population views,” rather than “a representative human” view, of test results. Assay results will consist of distributions of results, rather than a single, averaged data point. Organizations will have to learn how to use multiple indicators and endpoints in decision processes. CTiD data may eventually be included in IND or first-in-human study packages, and regulatory agencies will need to learn to combine clinical findings with these novel in vitro findings. Regulators may be asked to incorporate CTiD data into their approval processes, thus requiring both learning how to evaluate such data and developing quality standards for such tests. And, finally, clinicians will need to learn how to incorporate new constructs (such as data from CTiD testing) into choices of care.
Throughout the past few years, the field of hiPSC has evolved and continues to improve. It has produced advances in culturing methods and innovation in platforms allowing the development of assays that were inaccessible only a decade ago. In vitro population-based studies in the form of CTiD are now a commercial reality and are poised to revolutionize our thinking about practical, immediate, and near-term applications in the field of drug discovery and development. CTiD is a more complicated and demanding science than present in vitro testing approaches. The additional effort required, however, leads to tremendous insights that cannot be obtained so early and economically by any other approach, and in some cases not at all. These insights can make drug development more efficient and drugs safer. And, in so doing, it can dramatically change the level of contribution to society of some of the world’s most valuable research scientists working on the discovery and development of new drugs. It is evident that the time has come to consider new, more comprehensive ways of preclinical testing to reduce clinical attrition of compounds. The hope is that the CTiD approach will prevent inappropriate compound attrition, provide a more complete assessment of safety risk, reduce animal and clinical work, and potentially rescue drugs labeled with warnings based on inappropriate testing. This will enable new drugs to address unmet medical needs and reach patients more quickly. This constitutes a clear and present revolution in drug development.
Acknowledgments
The authors would like to thank Dr. Eli Fine, Dr. Jerome Chal, and Denise Sullivan for providing valued suggestions.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. DiMasi J. A., Grabowski H. G., Hansen R. W. Innovation in the Pharmaceutical Industry: New Estimates of R&D Costs. J. Health Econ. 2016, 47, 20–33. [DOI] [PubMed] [Google Scholar]
- 2. DiMasi J. A., Hansen R. W., Grabowski H. G. The Price of Innovation: New Estimates of Drug Development Costs. J. Health Econ. 2003, 22, 151–185. [DOI] [PubMed] [Google Scholar]
- 3. Palgon G. What Is the Cost of Drug Development? [Online]. https://www.liaison.com/blog/2017/05/02/cost-drug-development/ (accessed Jan 12, 2018).
- 4. Paul S. M., Mytelka D. S., Dunwiddie C. T., et al. How to Improve R&D Productivity: The Pharmaceutical Industry’s Grand Challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [DOI] [PubMed] [Google Scholar]
- 5. The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). Safety Guidelines [Online]. http://www.ich.org/products/guidelines/safety/article/safety-guidelines.html (accessed Jan 29, 2018)
- 6. Pugsley M. K., Authier S., Curtis M. J. Principles of Safety Pharmacology. Br. J. Pharmacol. 2008, 154, 1382–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laverty H., Benson C., Cartwright E., et al. How Can We Improve Our Understanding of Cardiovascular Safety Liabilities to Develop Safer Medicines? Br. J. Pharmacol. 2011, 163, 675–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferri N., Siegl P., Corsini A., et al. Drug Attrition during Pre-Clinical and Clinical Development: Understanding and Managing Drug-Induced Cardiotoxicity. Pharmacol. Ther. 2013, 138, 470–484. [DOI] [PubMed] [Google Scholar]
- 9. Mordwinkin N. M., Lee A. S., Wu J. C. Patient-Specific Stem Cells and Cardiovascular Drug Discovery. JAMA. 2013, 310, 2039–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo L., Abrams R. M., Babiarz J. E., et al. Estimating the Risk of Drug-Induced Pro-Arrhythmia Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Toxicol. Sci. 2011, 123, 281–289. [DOI] [PubMed] [Google Scholar]
- 11. Braam S. R., Tertoolen L., van de Stolpe A., et al. Prediction of Drug-Induced Cardiotoxicity Using Human Embryonic Stem Cell-Derived Cardiomyocytes. Stem Cell Res. 2010, 4, 107–116. [DOI] [PubMed] [Google Scholar]
- 12. Abi-Gerges N., Pointon A., Oldman K. L., et al. Assessment of Extracellular Field Potential and Ca2+ Transient Signals for Early QT/Pro-Arrhythmia Detection Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Pharmacol. Toxicol. Methods. 2017, 83, 1–15. [DOI] [PubMed] [Google Scholar]
- 13. Dempsey G. T., Chaudhary K. W., Atwater N., et al. Cardiotoxicity Screening with Simultaneous Optogenetic Pacing, Voltage Imaging and Calcium Imaging. J. Pharmacol. Toxicol. Methods. 2016, 81, 240–250. [DOI] [PubMed] [Google Scholar]
- 14. Pointon A., Harmer A. R., Dale I. L., et al. Assessment of Cardiomyocyte Contraction in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Toxicol. Sci. 2015, 144, 227–237. [DOI] [PubMed] [Google Scholar]
- 15. Sinnecker D., Laugwitz K. L., Moretti A. Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Development and Toxicity Testing. Pharmacol. Ther. 2014, 143, 246–252. [DOI] [PubMed] [Google Scholar]
- 16. Sirenko O., Crittenden C., Callamaras N., et al. Multiparameter In Vitro Assessment of Compound Effects on Cardiomyocyte Physiology Using IPSC Cells. J. Biomol. Screen. 2013, 18, 39–53. [DOI] [PubMed] [Google Scholar]
- 17. Sirenko O., Cromwell E. F., Crittenden C., et al. Assessment of Beating Parameters in Human Induced Pluripotent Stem Cells Enables Quantitative In Vitro Screening for Cardiotoxicity. Toxicol. Appl. Pharmacol. 2013, 273, 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burridge P. W., Li Y. F., Matsa E., et al. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Recapitulate the Predilection of Breast Cancer Patients to Doxorubicin-Induced Cardiotoxicity. Nat. Med. 2016, 22, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stillitano F., Hansen J., Kong C. W., et al. Modeling Susceptibility to Drug-Induced Long QT with a Panel of Subject-Specific Induced Pluripotent Stem Cells. eLife. 2017, 6, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shinozawa T., Nakamura K., Shoji M., et al. Recapitulation of Clinical Individual Susceptibility to Drug-Induced QT Prolongation in Healthy Subjects Using iPSC-Derived Cardiomyocytes. Stem Cell Reports. 2017, 8, 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsa E., Burridge P. W., Yu K. H., et al. Transcriptome Profiling of Patient-Specific Human iPSC-Cardiomyocytes Predicts Individual Drug Safety and Efficacy Responses In Vitro. Cell Stem Cell. 2017, 19, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ince S., Wu J. C. Envisioning Personalized Medicine and Clinical Trials in a Dish. Circulation. Res. 2015, 117, 599–602. [DOI] [PubMed] [Google Scholar]
- 23. Shah R. R. Drug-Induced Prolongation of the QT Interval: Why the Regulatory Concern? Fund. Clin. Pharmacol. 2002, 16, 119–124. [DOI] [PubMed] [Google Scholar]
- 24. Fermini B., Fossa A. A. The Impact of Drug-Induced QT Interval Prolongation on Drug Discovery and Development. Nat. Rev. Drug Discov. 2003, 2, 439–447. [DOI] [PubMed] [Google Scholar]
- 25. Darpo B. The Thorough QT/QTc Study 4 Years after the Implementation of the ICH E14 Guidance. Br. J. Pharmacol. 2010, 159, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Redfern W. S., Carlsson L., Davis A. S., et al. Relationships between Preclinical Cardiac Electrophysiology, Clinical QT Interval Prolongation and Torsade de Pointes for a Broad Range of Drugs: Evidence for a Provisional Safety Margin in Drug Development. Cardiovasc. Res. 2003, 58, 32–45. [DOI] [PubMed] [Google Scholar]
- 27. Gintant G. An Evaluation of hERG Current Assay Performance: Translating Preclinical Safety Studies to Clinical QT Prolongation. Pharmacol. Ther. 2011, 129, 109–119. [DOI] [PubMed] [Google Scholar]
- 28. Honda M., Kiyokawa J., Tabo M., et al. Electrophysiological Characterization of Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. J. Pharmacol. Sci. 2011, 117, 149–159. [DOI] [PubMed] [Google Scholar]
- 29. Rajamohan D., Matsa E., Kalra S., et al. Current Status of Drug Screening and Disease Modelling in Human Pluripotent Stem Cells. Bioessays. 2013, 35, 281–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clements M., Thomas N. High-Throughput Multi-Parameter Profiling of Electrophysiological Drug Effects in Human Embryonic Stem Cell Derived Cardiomyocytes Using Multi-Electrode Arrays. Toxicol. Sci. 2014, 140, 445–461. [DOI] [PubMed] [Google Scholar]
- 31. Scott C. W., Zhang X., Abi-Gerges N., et al. An Impedance-Based Cellular Assay Using Human iPSC-Derived Cardiomyocytes to Quantify Modulators of Cardiac Contractility. Toxicol. Sci. 2014, 142, 331–338. [DOI] [PubMed] [Google Scholar]
- 32. Itzhaki I., Rapoport S., Huber I., et al. Calcium Handling in Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. PLoS ONE. 2011, 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watanabe H., Honda Y., Deguchi J., et al. Usefulness of Cardiotoxicity Assessment Using Calcium Transient in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Toxicol. Sci. 2017, 42, 519–527. [DOI] [PubMed] [Google Scholar]
- 34. Odawara A., Katoh H., Matsuda N., et al. Physiological Maturation and Drug Responses of Human Induced Pluripotent Stem Cell-Derived Cortical Neuronal Networks in Long-Term Culture. Sci. Rep. 2016, 6, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Imamura K., Sahara N., Kanaan N. M., et al. Calcium Dysregulation Contributes to Neurodegeneration in FTLD Patient iPSC-Derived Neurons. Sci. Rep. 2016, 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sirenko O., Hesley J., Rusyn I., et al. High-Content Assays for Hepatotoxicity Using Induced Pluripotent Stem Cell–Derived Cells. Assay Drug Dev. Technol. 2014, 12, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kramer J. A., Sagartz J. E., Morris D. L. The Application of Discovery Toxicology and Pathology towards the Design of Safer Pharmaceutical Lead Candidates. Nature Reviews Drug Discovery. 2007, 6, 636–649. [DOI] [PubMed] [Google Scholar]
- 38. U.S. Food and Drug Administration. Preventable Adverse Drug Reactions: A Focus on Drug Interactions [Online]. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm110632.htm (accessed Jan 12, 2018)
- 39. Reimche L., Forster A. J., van Walraven C. J. Incidence and Contributors to Potential Drug-Drug Interactionsin Hospitalized Patients. J. Clin. Pharmacol. 2011, 51, 1043–1050. [DOI] [PubMed] [Google Scholar]
- 40. Jacubeit T., Drisch D., Weber E. Risk Factors as Reflected by an Intense Drug Monitoring System. Agents Actions. 1990, 29, 117–125. [DOI] [PubMed] [Google Scholar]
- 41. Dubois V. F. S., de Witte W. E. A., Visser S. A. G., et al. Assessment of Interspecies Differences in Drug-Induced QTc Interval Prolongation in Cynomolgus Monkeys, Dogs and Humans. Pharm. Res. 2016, 33, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Broichhausen C., Riquelme P., Ahrens N., et al. In Question: The Scientific Value of Preclinical Safety Pharmacology and Toxicology Studies with Cell-Based Therapies. Mol. Ther. 2014, 1, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cascorbi I. Drug Interactions: Principles, Examples and Clinical Consequences. Dtsch. Arztebl. Int. 2012, 109, 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qato D. M., Wilder J., Schumm L. P., et al. Changes in Prescription and Over-the-Counter Medication and Dietary Supplement Use among Older Adults in the United States, 2005 vs 2011. JAMA Intern. Med. 2016, 176, 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piccini J. P., Whellan D. J., Berridge B. R., et al. Current Challenges in the Evaluation of Cardiac Safety during Drug Development: Translational Medicine Meets the Critical Path Initiative. Amer. Heart J. 2009, 158, 317–326. [DOI] [PubMed] [Google Scholar]
- 46. Ferri N., Siegl P., Corsini A., et al. Drug Attrition during Pre-clinical and Clinical Development: Understanding and Managing Drug-Induced Cardiotoxicity. Pharmacol. Ther. 2013, 138, 470–484. [DOI] [PubMed] [Google Scholar]
- 47. Sager P. T., Gintant G., Turner J. R., et al. Rechanneling the Cardiac Proarrhythmia Safety Paradigm: A Meeting Report from the Cardiac Safety Research Consortium. Am. Heart J. 2014, 167, 292–300. [DOI] [PubMed] [Google Scholar]
- 48. Curtis L. H., Østbye T., Sendersky V., et al. Prescription of QT-Prolonging Drugs in a Cohort of about 5 Million Outpatients. Am. J. Med. 2003, 114, 135–141. [DOI] [PubMed] [Google Scholar]
- 49. De Ponti F., Poluzzi E., Vaccheri A., et al. Non-antiarrhythmic Drugs Prolonging the QT Interval: Considerable Use in Seven Countries. Br. J. Clin. Pharmacol. 2002, 54, 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boyce M. J., Baisley K. J., Warrington S. J. Pharmacokinetic Interaction between Domperidone and Ketoconazole Leads to QT Prolongation in Healthy Volunteers: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study. Br. J. Clin. Pharmacol. 2012, 73, 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Katoh T., Saitoh H., Ohno N., et al. Drug Interaction between Mosapride and Erythromycin without Electrocardiographic Changes. Jpn. Heart J. 2003, 44, 225–234. [DOI] [PubMed] [Google Scholar]
- 52. Van Der Sijs H., Kowlesar R., Klootwijk A. P. J., et al. Clinically Relevant QTc Prolongation Due to Overridden Drug-Drug Interaction Alerts: A Retrospective Cohort Study. Br. J. Clin. Pharmacol. 2009, 67, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zeuli J. D., Wilson J. W., Estes L. L. Effect of Combined Fluoroquinolone and Azole Use on QT Prolongation in Hematology Patients. Antimicrob. Agents Chemother. 2013, 57, 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mosholder A. D., Mathew J., Alexander J. J., et al. Cardiovascular Risks with Azithromycin and Other Antibacterial Drugs. N. Engl. J. Med. 2013, 368, 1665–1668. [DOI] [PubMed] [Google Scholar]
- 55. Baxter M., Withey S., Harrison S., et al. Phenotypic and Functional Analyses Show Stem Cell-Derived Hepatocyte-Like Cells Better Mimic Fetal Rather than Adult Hepatocytes. J. Hepatol. 2015, 62, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goldring C., Antoine D. J., Bonner F., et al. Stem Cell-Derived Models to Improve Mechanistic Understanding and Prediction of Human Drug-Induced Liver Injury. Hepatology. 2016, 65, 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kajiwara M., Aoi T., Okita K., et al. Donor-Dependent Variations in Hepatic Differentiation from Human-Induced Pluripotent Stem Cells. Proc. Natl. Acad. Sci. 2012, 109, 12538–12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burroughs V. J., Maxey R. W., Levy R. A. Racial and Ethnic Differences in Response to Medicines: Towards Individualized Pharmaceutical Treatment. J. Natl. Med. Assoc. 2002, 94, 1–26. [PMC free article] [PubMed] [Google Scholar]
- 59. Johnson J. A. Ethnic Differences in Cardiovascular Drug Response: Potential Contribution to Pharmacogenetics. Circulation. 2008, 118, 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cook D., Brown D., Alexander R., et al. Lesson Learned from the Fate of Astra Zeneca’s Drug Pipeline: A Five-Dimensional Framework. Nat. Rev. Drug Discov. 2014, 13, 419–431. [DOI] [PubMed] [Google Scholar]
- 61. Sirenko O., Cromwell E. F. Determination of Hepatotoxicity in iPSC-Derived Hepatocytes by Multiplexed High Content Assays. Methods Mol. Biol. 2018, 1683, 339–354. [DOI] [PubMed] [Google Scholar]
- 62. Tukker A. M., de Groot M. G. D. M., Wijnolts F. M. J., et al. Is the Time Right for In Vitro Neurotoxicity Testing Using Human iPSC-Derived Neurons? ALTEX. 2016, 33, 261–271. [DOI] [PubMed] [Google Scholar]
- 63. Long G. The Biopharmaceutical Pipeline: Innovative Therapies in Clinical Development. Boston: Analysis Group Inc., produced for Pharmaceuticals and Manufacturers of America, 2017. [Google Scholar]
- 64. National Cancer Institute. Age [Online]. https://www.cancer.gov/about-cancer/causes-prevention/risk/age (accessed Jan 12, 2018).
- 65. Zafar S. F., Heilbrun L. K., Vishnu P., et al. Participation and Survival of Geriatric Patients in Phase I Clinical Trials: The Karmanos Cancer Institute (KCI) Experience. J. Geriatr. Oncol. 2011, 2, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Herrera A. P., Snipes S. A., King D. W., et al. Disparate Inclusion of Older Adults in Clinical Trials: Priorities and Opportunities for Policy and Practice Change. Am. J. Public Health. 2010, 100, S105–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shah S. S., Hall M., Goodman D. M., et al. Off-Label Drug Use in Hospitalized Children. Arch. Pediatr. Adolesc. Med. 2007, 161, 282–290. [DOI] [PubMed] [Google Scholar]
- 68. Laughon M. M., Benjamin D. K., Jr., Capparelli E. V., et al. Innovative Clinical Trial Design for Pediatric Therapeutics. Expert Rev. Clin. Pharmacol. 2011, 4, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hartman M. E., Dai D. F., Laflamme M. A. Human Pluripotent Stem Cells: Prospects and Challenges as a Source of Cardiomyocytes for In Vitro Modeling and Cell-Based Cardiac Repair. Adv. Drug Deliv. Rev. 2016, 15, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Youssef A. A., Ross E. G., Bolli R., et al. The Promise and Challenge of Induced Pluripotent Stem Cells for Cardiovascular Applications. JACC: Basic to Translational Science. 2016, 1, 510–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Robertson C., Tran D. D., George S. C. Concise Review: Maturation Phases of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cells. 2013, 31, 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang X., Pabon L., Murry C. E. Engineering Adolescence: Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Circulation. Res. 2014, 114, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhu R., Blazeski A., Poon E., et al. Physical Developmental Cues for the Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cell Res. Ther. 2014, 5, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kane C., Terracciano C. M. Induced Pluripotent Stem Cell-Derived Cardiac Myocytes to Understand and Test Calcium Handling: Pie in the Sky? J. Mol. Cell. Cardiol. 2015, 89, 376–378. [DOI] [PubMed] [Google Scholar]
- 75. Karakikes I., Ameen M., Termglinchan V., et al. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Insights into Molecular, Cellular, and Functional Phenotypes. Circulation. Res. 2015, 117, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Koivumäki J. T., Naumenko N., Tuomainen T., et al. Structural Immaturity of Human iPSC-Derived Cardiomyocytes: In Silico Investigation of Effects on Function and Disease Modeling. Front. Physiol. 2018, 9, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Denning C., Borgdorff V., Crutchley J., et al. Cardiomyocytes from Human Pluripotent Stem Cells: From Laboratory Curiosity to Industrial Biomedical Platform. Biochim. Biophys. Acta Mol. Cell. Res. 2016, 1863, 1728–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lundy S. D., Zhu W. Z., Regnier M., et al. Structural and Functional Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cells Dev. 2013, 22, 1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tulloch N. L., Muskheli V., Razumova M. V., et al. Growth of Engineered Human Myocardium with Mechanical Loading and Vascular Coculture. Circulation. Res. 2011, 109, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nunes S. S., Miklas J. W., Liu J., et al. Biowire: A Platform for Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Nat. Methods. 2013, 10, 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Feaster T. K., Cadar A. G., Wang L., et al. Matrigel Mattress: A Method for the Generation of Single Contracting Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circulation. Res. 2015, 117, 995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang X., Rodriguez M., Pabon L., et al. Tri-iodo-l-thyronine Promotes the Maturation of Human Cardiomyocytes-Derived from Induced Pluripotent Stem Cells. J. Mol. Cell. Cardiol. 2014, 72, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lucendo-Villarin B., Rashidi H., Cameron K., et al. Pluripotent Stem Cell Derived Hepatocytes: Using Materials to Define Cellular Differentiation and Tissue Engineering. J. Mater. Chem. B. 2016, 4, 3433–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Palakkan A. A., Nanda J., Ross J. A. Pluripotent Stem Cells to Hepatocytes: The Journey So Far. Biomed. Reports. 2017, 6, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hannoun Z., Steichen C., Dianat N., et al. The Potential of Induced Pluripotent Stem Cell Derived Hepatocytes. J. Hepatol. 2016, 65, 182–199. [DOI] [PubMed] [Google Scholar]
- 86. Guguen-Guillouzo C., Guillouzo A. General Review on in Vitro Hepatocyte Models and Their Applications. Methods in Mol. Biol. 2010, 640, 1–40. [DOI] [PubMed] [Google Scholar]
- 87. Yu Y., Liu H., Ikeda Y., et al. Hepatocyte-Like Cells Differentiated from Human Induced Pluripotent Stem Cells: Relevance to Cellular Therapies. Stem Cell Res. 2012, 9, 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Palakkan A. A., Drummond R., Anderson R. A., et al. Polarization and Functional Characterization of Hepatocytes Derived from Human Embryonic and Mesenchymal Stem Cells. Biomed. Reports. 2015, 3, 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Weick J. P. Functional Properties of Human Stem Cell-Derived Neurons in Health and Disease. Stem Cells Int. 2016, 4190438, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stein J. L., de la, Torre-Ubieta L., Tian Y., et al. A Quantitative Framework to Evaluate Modeling of Cortical Development by Neural Stem Cells. Neuron. 2014, 83, 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shcheglovitov A., Shcheglovitova O., Yazawa M., et al. SHANK3 and IGF1 Restore Synaptic Deficits in Neurons from 22q13 Deletion Syndrome Patients. Nature. 2013, 503, 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chinchalongporn V., Koppensteiner P., Prè D., et al. Connectivity and Circuitry in a Dish versus in a Brain. Alzheimer’s Res. Ther. 2015, 7, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bradford A. B. Importance of Being Nernst: Synaptic Activity and Functional Relevance in Stem Cell-Derived Neurons. World J. Stem Cells. 2015, 7, 899–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Takahashi K., Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006, 126, 663–676. [DOI] [PubMed] [Google Scholar]
- 95. Prigione A., Hossini A. M., Lichtner B., et al. Mitochondrial-Associated Cell Death Mechanisms Are Reset to an Embryonic-Like State in Aged Donor-Derived iPS Cells Harboring Chromosomal Aberrations. PLoS ONE. 2011, 6, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lapasset L., Milhavet O., Prieur A., et al. Rejuvenating Senescent and Centenarian Human Cells by Reprogramming through the Pluripotent State. Genes Dev. 2011, 25, 2248–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Suhr S. T., Chang E. A., Rodriguez R. M., et al. Telomer Dynamics in Human Cells Reprogrammed to Pluripotency. PLoS ONE. 2009, 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Prigione A., Fauler B., Lurz R., et al. The Senescence-Related Mitochondrial/oxidative Stress Pathway Is Repressed in Human Induced Pluripotent Stem Cells. Stem Cells. 2010, 28, 721–733. [DOI] [PubMed] [Google Scholar]
- 99. Vaziri H., Chapman K. B., Guigova A., et al. Spontaneous Reversal of the Developmental Aging of Normal Human Cells Following Transcriptional Reprogramming. Regen. Med. 2010, 5, 345–363. [DOI] [PubMed] [Google Scholar]
- 100. Varum S., Rodrigues A. S., Moura M. B., et al. Energy Metabolism in Human Pluripotent Stem Cells and Their Differentiated Counterparts. PLoS ONE. 2011, 6, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lo Sardo V., Ferguson W., Erikson G. A., et al. Influence of Donor Age on Induced Pluripotent Stem Cells. Nat. Biotechnol. 2016, 35, 1, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kang E., Wang X., Tippner-Hedges R., et al. Age-Related Accumulation of Somatic Mitochondrial DNA Mutations in Adult-Derived Human iPSCs. Cell Stem Cell. 2016, 18, 625–636. [DOI] [PubMed] [Google Scholar]
- 103. Soria-Valles C., López-Otín C. iPSCs: On the Road to Reprogramming Aging. Trends in Molecular Medicine. 2016, 22, 713–724. [DOI] [PubMed] [Google Scholar]
- 104. Rohani L., Johnson A. A., Arnold A., et al. The Aging Signature: A Hallmark of Induced Pluripotent Stem Cells? Aging Cell. 2014, 13, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mahmoudi S., Brunet A. Aging and Reprogramming: A Two-Way Street. Curr. Opin. Cell Biol. 2012, 24, 744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Struder L., Vera E., Cornacchia D. Programming and Reprogramming Cellular Age in the Era of Induced Pluripotency. Cell Stem Cell. 2015, 16, 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Warren C. R., Cowan C. A. Humanity in a Dish: Population Genetics with iPSCs. Trends Cell Biol. 2018, 28, 46–57. [DOI] [PubMed] [Google Scholar]
- 108. Inoue H., Nagata N., Kurokawa H., et al. iPS Cells: A Game Changer for Future Medicine. EMBO J. 2014, 33, 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]