Abstract
High blood pressure in patients with diabetes mellitus results in a significant increase in the risk of cardiovascular events and mortality. The current evidence regarding the impact of intervention on blood pressure levels (in accordance with a specific threshold) is not particularly robust. Blood pressure control is more difficult to achieve in patients with diabetes than in non-diabetic patients, and requires using combination therapy in most patients. Different management guidelines recommend initiating pharmacological therapy with values >140/90 mm/Hg; however, an optimal cut point for this population has not been established. Based on the available evidence, it appears that blood pressure targets will probably have to be lower than <140/90mmHg, and that values approaching 130/80mmHg should be recommended. Initial treatment of hypertension in diabetes should include drug classes demonstrated to reduce cardiovascular events; i.e., angiotensin converting-enzyme inhibitors, angiotensin receptor blockers, diuretics, or dihydropyridine calcium channel blockers. The start of therapy must be individualized in accordance with the patient's baseline characteristics, and factors such as associated comorbidities, race, and age, inter alia.
Keywords: Diabetes, Arterial hypertension, Cardiovascular outcomes, Anti-hypertensive drugs
1. Introduction
Cardiovascular disease (CVD) continues to be the most frequent cause of morbidity and mortality in adults. Among the major cardiovascular risk (CVR) factors, arterial hypertension (AH) is the most prevalent; however, diabetes mellitus (DM) is considered “equivalent” to CVR and, by definition, is one of the most frequent causes of CVD. Among the general population, the coexistence of both conditions (AH and DM) represents a priori a higher CVR than either of them individually. Moreover, considering that both pathologies share common metabolic aspects (insulin resistance, dyslipidemia obesity, endothelial dysfunction, atherosclerosis, among other factors), it is believed that the AH population when diagnosed with DM represents a high risk of mortality from all causes, when compared against normotensive non-diabetic individuals. This suggests that to a large extent, the excessive risk may be attributed to the coexistence with AH [1]. Keeping blood pressure (BP) levels under control plays a pivotal role in patients with DM; although a significant number of studies have evaluated different BP goals with regards to cardiovascular (CV) outcomes, some questions still remain unanswered. Lowering BP using anti-hypertensive drugs (anti-AH) is the most effective and powerful intervention to reduce CV morbidity and mortality in individuals with type 2 DM (T2DM). The evidence stems from randomized clinical trials (RCTs), observational studies, and meta-analysis in different clinical settings (elderly patients with isolated systolic hypertension, essential hypertension, high CVR profile, coronary artery disease, and previous stroke, inter alia). The different international guidelines consistently recommend to start or to intensify treatment with anti-AH when BP values are >140/90 mm/Hg. However, the ideal cutoff point for the population with DM is not yet clear [2]. BP levels of <140/90, <140/85, <140/80, <130/85, <130/80 mm/Hg, have been recommended over time; however, these recommendations have not been supported with solid evidence. The purpose of this review is to make a critical literature analysis regarding the optimal BP thresholds to be targeted for the population with T2DM, and the CV outcomes associated with that goal, including a general anti-AH approach in this population.
2. Type 2 diabetes mellitus, definitions and overall burden
DM is a term used to describe a dysfunction in the metabolism of carbohydrates, lipids, and proteins of heterogeneous etiology, characterized by chronic hyperglycemia. The specific complications associated with DM include the microvascular (retinopathy, nephropathy, and neuropathy) and the macrovascular complications (CVD, cerebrovascular and peripheral arterial). Additionally, chronic hyperglycemia also refers to the so called intermediate (or transition) states between the normal level of glycaemia and the presence of DM, and includes impaired fasting glucose –IFG- (defined as fasting glycaemia of ≥100 mg/dL and <126 mg/dL) and impaired glucose tolerance –IGT- (defined as glycemic values 2 hours postprandial of 75 gm of glucose ≥140 mg/dL and <200 mg/dL). Both conditions have also been called “pre-diabetes”. IFG is the result of an early stage defect in the beta cell function and hepatic insulin resistance; while IGT is the result of peripheral insulin resistance and progressive beta cell dysfunction. Both IFG and IGT represent and define a high risk for the future development of DM and CVD [1,2,3]. The relationship between DM and CVD is complex and multifactorial, since DM may coexist with other CVR factors such as AH, dyslipidemia, cigarette smoking, obesity and a sedentary lifestyle, together with other associated conditions (increased oxidative stress, low grade inflammation, insulin resistance, endothelial dysfunction, autonomic neuropathy, and hypercoagulability). All together, they directly contribute to the development of CVD (which is the most frequent cause of death in people with DM) [4]. It has been established that DM increases the risk (2-4 fold) of CVD when compared against people without DM. Close to 70% of the patients with T2DM aged ≥65 die from CVD, whilst those with no history of CVD have a CVR “equivalent” to that of individuals with a past history of myocardial infarction (MI). Although the mortality rates attributable to CVD have declined among the population with or without DM, the global burden of CVD among patients with DM remains unacceptably high. Likewise, when comparing individuals with DM versus non-diabetics, males and females –though to a higher level in females-DM individuals have a shorter life expectancy (6 to 8 years less). At the time of diagnosing DM, most patients have at least one CVR associated or non-associated factor related to the presence of overt atherosclerotic disease. The close relationship between DM and CVD suggests that both conditions share genetic, epigenetic, and environmental factors [5,6]. In 2015, the estimated world prevalence of DM in adults (20-79 years) was 8.8%, and it is expected to rise to 10.4% by 2040. This means that the number of people with DM will grow from 415 to 642 million. Furthermore, the prevalence of IGT was 6.7%, and it is estimated that it will be 7.8% by 2040 (so the number of people with IGT will grow from 318 to 481 million). In 2012, the number of deaths attributed to DM was 3.7 million and 43% of those deaths occurred before 70 years of age and were more frequent in the low- and middle-income countries than in high-income countries. In 2015 the number of deaths associated with DM was 5.0 million [7,8].
The highest mortality rates due to DM in the world (standardized by age and per 100.000 inhabitants) can be found in the Republic of Mauritius (173.63), Republic of Fiji (146.53), Cooperative Republic of Guyana (128.59), Republic of Trinidad and Tobago (128.4), and Kingdom of Morocco (110.57). Finally, the worldwide cost of DM was established at USD 1.31 trillion, which is equivalent to 1.8% of the global gross domestic product in 2015 [9,10].
3. Arterial Hypertension, definition and global burden
The elevation of BP values is a major factor for CVR, and is one of the five primary causes of death around the world. Usually, AH has a silent pattern of clinical presentation, so a considerable number of people are undiagnosed and untreated, which considerably increases the risk and/or premature death and of other micro and macro vascular complications. According to the estimates in 2010, 31.1% of the adult population ≥20 years (1.39 billion people) had AH worldwide. AH was defined as a systolic blood pressure (SBP) ≥140 mmHg and diastolic blood pressure (DBP) ≥90 mmHg. It was also found that the prevalence of AH in low and middle-income countries was of 31.5% and 28.5%, respectively, and it was estimated that between 9 to 10 million deaths were due to AH (a figure estimated to be equivalent to 7% of the global burden of the disease) [11]. The differences existing between the number of patients with AH, access to specific treatment, and achieving the control goals, are more accentuated in the middle and low income countries. Hence, the countries that experienced the highest AH-associated mortality rates in 2014 per every 100.000 inhabitants and age adjusted, were Estonia (73.73), Guyana (56.26), Botswana (49.18), Bahamas (41.53), Philippines (38.20), Egypt (37.45), and New Guinea (8.66) [12,13]. These data are extremely worrying, in particular if we consider that of the total number of individuals with AH, only 57% are aware of their condition, 40.6% takes some kind of anti-AH medication (and only 13.2% reaches an adequate BP level control). Recently, a study compiled 814 individual trials conducted in 154 countries (from 1990 to 2015), and found that the number of people with SBP ≥140 mmHg increased from 442 million (in 1990) to 874 million (in 2015). This SBP level was responsible for 14% of the total number of deaths and of 143 million disability-associated life-years. The AH rate increased from 17,307 per 100,000 in 1990 to 20,526 per 100,000 in 2015. Although most of the SBP-associated burden was attributable to individuals with values ≥140 mmHg, almost 30% was attributable to people with SBP levels between 115-140 mmHg. Most of the deaths associated with AH were related to CVD [14]. Another recent study involved 1479 trials (from 1975 to 2015), that evaluated BP figures in 19.1 million adults. The global prevalence of AH (standardized by age) was 24.1% in males and 20.1% in females (year 2015). The number of adults with AH increased from 594 million in 1975 to 1.13 billion in 2015, with the highest increase in the low and middle-income countries [15].
AH is frequent in individuals with T2DM. The high co-existence of these two conditions in one same patient should not be considered a mere coincidence, since it is regarded as a strong determinant factor for endothelial dysfunction, atherosclerotic disease and vascular damage; from the pathophysiological point of view, both share various characteristics (for instance, those related to obesity and insulin resistance). It has been established that the prevalence of AH is 1.5-2.0 times higher in diabetics than in the non-diabetic population, indicating that a significant number of individuals with T2DM have AH at the time of diagnosis and its frequency depends on associated factors, including: level of obesity, severity of atherosclerosis, insulin resistance, advanced age (which also probably includes the presence of essential AH) and glomerular filtration rate [16,17].
Most previous studies evaluating the frequency of AH among diabetics exhibit significant issues with the design and selection of the comparator groups, and they fail to control for the presence of certain confounders (age, gender, obesity, family history, type of DM, renal dysfunction, inter alia), in addition to poorly defined SBP and DBP levels, and how BP was measured. At present, and based on observational trials, the reported prevalence of AH (defined as a value of ≥140/90 mm/Hg, or the use of anti-hypertensive medication) in individuals with T2DM is of >50%. This frequency may be influenced by the fact that when both conditions have been present for a long time (isolated) in the same individual, increase the probability of co-existence [18,19].
4. Coexistence of diabetes mellitus and arterial hypertension
In view of the above, and since both AH and DM (present in isolation) are conditions that increase the risk of CVD, it is logical to say that their coexistence could further escalate such risk. Few studies have evaluated the association between the coexistence of AH and DM and the presence of CVD. However, the risk of CVD in this group of patients is at least 5-fold higher (as compared to individuals with no AH and no DM), and is at least 3-fold higher in men and 5-7 fold higher in women. Moreover, the presence of AH is responsible for a 7.2-fold increase in the risk of mortality in individuals with DM [20,21]. Furthermore, the global differences in the distribution of frequencies observed for AH and DM, seem to be associated with certain characteristics such as overall economic development, and hence with lifestyle changes, environmental factors, alcohol use, cigarette smoking, obesity, poor physical activity, nutritional, demographic and epidemiological transition, inter alia [22].
5. Pathophysiology
The pathophysiology of AH and DM is closely interrelated, complex, and poorly understood; both conditions involve multiple organs and systems. Traditionally, it has been considered that AH in diabetic patients is volume dependent, since hyperglycemia increases osmolality in the extracellular fluid and hence, as water crosses from the intracellular to the extracellular space in order to maintain an osmotic balance, the extracellular space expands at the expense of intracellular dehydration, giving rise to a volume overload (unless of course the hyperglycemia is severe enough to cause osmotic diuresis with less likelihood of volume overload) [23].
In addition to the fact that AH is volume dependent in DM, the endothelial cells play a key role in maintaining vascular homeostasis and its dysfunction is associated with many types of CV and metabolic diseases. Consequently, endothelial dysfunction is considered to be the most important factor in the development of AH in patients with DM; it is characterized by an unbalance between vasodilatation and vasoconstriction, together with an increase in oxidative stress, vascular inflammation, alteration of the fibrinolytic and prothrombotic pathways, abnormal proliferation of smooth muscle cells and disrupted repair mechanisms [24,25].
Together with the endothelial involvement, the renin-angiotensin-aldosterone system (RAAS) acts as a key modulator of vascular function, and its hyperactivity is involved in endothelial dysfunction. RAAS comprises several components: renin, a protein and enzyme generated from cells in the kidney which it can cleave and activate another circulating protein; angiotensin and angiotensin II (ATII) in particular, is produced via certain coordinated enzyme reactions including the angiotensin converting enzyme (ACE). This peptide mainly acts through the AT1 receptor, promoting vasoconstriction, proliferation and oxidative stress. Moreover, ATII may induce (via cytokine suppression) cell remodeling and vascular injury, cell growth, vasoconstriction, oxidative stress and inflammation, subsequently activating several pro-inflammatory transcription factors such as the nuclear κB factor that regulates some adhesion molecules and the secretion of cytokines. As a whole, these molecules induce and maintain inflammation inside the vascular wall, increasing and promoting the deposit of the extracellular matrix, and the hyperplasia/hypertrophy of the smooth vascular muscle. In the proximal convoluted tubule of the kidney, ATII acts by increasing sodium reabsorption; the high levels of sodium in the body act to increase the osmolality of the blood, leading to a shift of fluid into the blood volume and extracellular space; this increases the BP of the patient. The effect of ATII on vaso-constriction takes place in systemic arterioles; here, ATII binds to the G protein-coupled receptors, leading to a secondary messenger cascade that results in potent arteriolar vasoconstriction. This acts to increase total peripheral resistance, causing an increase in BP. Finally, aldosterone is the main mineralocorticoid hormone, involved in regulating sodium and potassium homeostasis and body fluid balance by influencing the re-absorption of sodium and the excretion of potassium in the kidney. Aldosterone is traditionally thought to play a key role in regulating salt re-absorption and potassium secretion in response to two apparently opposite conditions: hypovolemia and hyper-kalemia [26,27].
In general, diabetic subjects with AH have low to normal renin circulating levels; these levels are still lower with diabetic nephropathy. However, the levels classified as “normal” in this type of patients are in fact inappropriately high for the volume expansion observed in DM, though it is clear that the absolute excess in the production of renin is an unlikely cause of AH in DM [28].
Stiffness of the arterial tree may develop both in DM per se, or jointly with AH, since in both conditions there is endothelial dysfunction and inflammation that raise the number of adhesion molecules and inflammatory cytokines, mainly ICAM-1 and TNF-α. This arterial stiffness has been replicated in several studies, showing that AH contributes to arterial stiffness and endothelial dysfunction in patients with T2DM [29].
Additionally, in T2DM, insulin resistance and hyperglycemia cause inflammation and oxidative stress; the dyslipidemia that is often present in diabetic patients with AH is also associated with arterial stiffness and vascular dysfunction. In the presence of insulin resistance, the disruption in the metabolism of glucose and lipids leads to an overproduction of aldehydes, that react non-enzymatically with free amino and sulfhydryl groups of protein amino acids to form stable conjugates called advanced glycation end-products (AGEs). AGEs act directly, as well as via receptors to alter the function of many intra and extracellular proteins. In the vessel wall, AGE formation may contribute to endothelial dysfunction and vascular stiffening. The formation of AGEs is increased in the blood and tissues of diabetic subjects as a result of hyperglycemia and is actively involved in diabetes-associated vascular stiffening and microvascular damage. Moreover, hyperinsulinemia causes sodium retention and increased activity of the sympathetic nervous system (SNS), with a subsequent increase in the level of circulating catecholamines, leading to the development of AH, which in turn activates the RAAS (also promoting inflammation) and turning this process into a vicious circle [30,31].
On the other hand, advances in genomics have enabled the description of the human genome, with cardiometabolic characteristics. The multiple findings in single nucleotide polymorphisms (SNPs) have given us a broader view of the pathogenesis of AH and T2DM. Both AH and T2DM are characterized by their complex nature, with gene-gene, gene-environment interactions and epigenetic factors. Different genes have been involved in the development of AH, including CACANA1H, IPO7, PMS1, SLC24A4, YWHAZ, GPR98, ARRDC3, C21orf, SLC25A42, genes HLA-B, inter alia. Among those involved with T2DM the following have been identified: SLC44A3, F3, RBM43, RND3, GALNTL4, CPA6, LOC729013, and 7-like 2 transcription factor (TCF7L2), with is strongly associated with the development of T2DM in several ethnic groups [32,33,34].
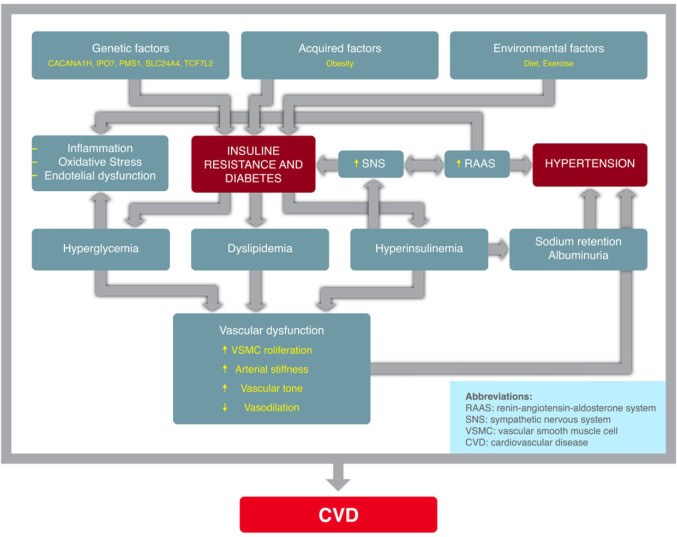
The pathophysiological association of AH in individuals with DM is as follows: insulin resistance (and the resulting hyperinsulinemia) induces the activation of the SNS and RAAS, promoting sodium and water retention (critical factors in the genesis of AH). Additionally, DM leads to an increase in vascular reactivity and the proliferation of smooth muscle vascular cells, which play a significant role in the development of AH. Hyperglycemia and the increase in total body exchangeable sodium may give rise to excess extracellular fluid and plasma volume expansion; furthermore, diabetic subjects have an increased vascular sensitivity to vasoactive hormones (in particular if the dietary sodium intake is elevated), which together with the presence of hyperinsulinemia, contributes to maintain high BP levels, because insulin promotes sodium retention and enhances the SNS activity. This is mediated by genetic and epigenetic factors, and their interaction. The pathogenesis of AH (associated with T2DM) involves an interaction of hereditary and acquired mechanisms. Both conditions share a number of pathophysiological mechanisms. Hence, it is likely that the pathogenic relationship between AH and DM is bidirectional. (Figure 1).
Figure 1.
Summary of the physiopathological mechanisms in the developement of arterial hypertension in diabetes mellitus.
6. Background of blood pressure control targets in type 2 diabetes mellitus
the various international management guidelines have focused on establishing thresholds to BP levels in T2DM individuals. These guidelines discuss one or several management options based on the available evidence, with some differences among the various options. Over time, the BP targets established have ranged from <130/85, <130/80, <140/90, <140/80, and <140/85 mm/Hg (Table 1). However, it has been frequently accepted that the target BP in T2DM individuals should be <130/80 mm/Hg.
Table 1.
Blood Pressure goals in people with Type 2 Diabetes Mellitus, from different international treatment guidelines.
| Guidelines | Year of publication | SBP (mmHg) | DBP (mmHg) |
|---|---|---|---|
| JNC VI | 1997 | ≤130 | ≤85 |
| WHO/ISH | 1999 | ≤130 | ≤85 |
| BHS | 1999 | <140 | <80 |
| CHS | 1999 | <130 | <80 |
| NKF | 2000 | <130 | <80 |
| ADA | 2001–2012 | <130 | <80 |
| ESH/ESC | 2003 | <130 | <80 |
| JNC VII | 2003 | <130 | <80 |
| WHO/ISH | 2003 | ≤130 | ≤80 |
| BHS | 2004 | <130 | <80 |
| ESH/ESC | 2007 | <130 | <80 |
| NICE | 2008 | <130 | <80 |
| KDIGO | 2012 | ≤140 | ≤90 |
| IDF | 2012 | ≤130 | ≤80 |
| IGH | 2013 | ≤140 | ≤80 |
| ADA | 2013 | <140 | <80 |
| ESH/ESC | 2013 | <140 | <85 |
| JSH | 2014 | ≤130 | ≤80 |
| ASH/ISH | 2014 | <140 | <90 |
| JNC VIII | 2014 | <140 | <90 |
| HCGC | 2017 | <130 | <80 |
| AHA/ACC/ASH | 2015 | ≤140 | ≤90 |
| NICE | 2015 | ≤140 | ≤80 |
| ACC/AHA | 2017 | ≤130 | ≤80 |
| TSOC/THS | 2017 | <130 | <80 |
| AACE/ACE | 2018 | <130 | <80 |
| ADA | 2015-2018 | <140 | <90 |
Abbreviations: AACE: American Association of Clinical Endocrinologists; ACC: American College of Cardiology; ACE: American College of Endocrinology; ADA: American Diabetes Association; AHA: American Heart Association; ASH: American Society of Hypertension; BHS: British Hypertension Society; CHS: Canadian Hypertension Society; DBP: Dyastolic Blood Pressure; ESC: European Society of Cardiology; ESH: European Society of Hypertension; HCGC: The Hypertension Canada Guidelines Committee; IDF: International Diabetes Federation; IGH: Indian guidelines on hypertension; ISH: International Society of Hypertension; JNC: Joint National Committee; JSH: Japanese Society of Hypertension; KDIGO: Kidney Disease Improving Global Outcomes; NKF: National Kidney Foundation; NICE: National Institute for Health and Care Excellence; SBP: Systolic Blood Pressure; THS: Taiwan hypertension society; TSOC: Taiwan Society of Cardiology.
It should be emphasized that the initial trials evaluating the BP control targets in DM were not large scale RCTs, or had enough power to support that goal (those were analyses based on observational or retrospective trials, sub-group analyses, or simply extrapolated the results from people with AH but no DM). In these trials, the BP levels achieved were >135/85 mm/Hg, which indicated that the recommendation to lower the BP to <130/80 mm/Hg was probably not the best. Furthermore, some trials had shown that strict BP control apparently failed to reduce CV outcomes [35,36,37]. Notwithstanding the available evidence, a target BP of <130/80 mm/Hg was still recommended in individuals with T2DM (which generated doubts and a few questions; for instance whether the initiation of pharmacological therapy for a established SBP and DBP threshold would change the results or the clinical outcomes), or rather that the pharmacological therapy for achieving a specific SBP and DBP target changes the CV outcomes or the mortality; or, whether there were any differences amongst the various anti-AH drugs in terms of CV results and mortality. There was however evidence based on observational trials, that BP levels >115/75 mmHg were associated with an increased frequency of microvascular and macrovascular events and mortality, which probably indicates that controlling BP levels in T2DM was
important for the prevention of fatal and non-fatal clinical outcomes. Furthermore, it was also considered that the existing relationship between BP and CV outcomes was consistent, continuous, and independent of other CVR factors. For instance, observational trials in adults with no history of vascular disease (VD) showed that the risk of mortality from vascular events (CV and cerebrovascular) experienced a lineal and progressive increase according to the BP levels. This was considered to be true among the general population, but not so among the elderly. It was also found that the aggressive lowering of BP levels did not necessarily translate into a substantial improvements in vascular injury. Moreover, it has been documented that when trying to reach more demanding BP goals, higher doses of anti-AH drugs had to be used, or a combination of several drugs, hence increasing the risk of adverse events, including more CV events (this effect was called the “J-curve phenomenon”). Additionally, it has been established that the relationship between BP and CV outcomes also showed a “U”-shaped curve distribution, indicating that the rate of vascular events was high, with either very low or very high BP levels and that BP values of <110-120/60-70 mm/Hg could be predictive of increased risk of vascular events [38,39,40].
7. Studies evaluating anti-AH therapy in type 2 diabetes mellitus
The uncertainty of not knowing the ideal threshold for BP in patients with DM, led to the design of studies to answer this question, as described hereunder.
By the mid-90´s The Systolic Hypertension in the Elderly Program (SHEP) evaluated the effect of therapy in adults ≥60 years old (n=4,736; 583 individuals with T2DM), which focused on establishing a goal of SBP ≥160 mm/Hg (in subjects with baseline values of SBP >180 mm/Hg and DBP <90 mm/Hg), or a 20 mm/Hg reduction in those with a baseline SBP between 160-179 mm/Hg. The active treatment group received a low dose of chlorthalidone with a step-up to atenolol or reserpine if needed. The placebo group received placebo and any active anti-AH drugs prescribed by the patient’s private physician for persistently high BP. In this trial, the rate of major CV events dropped by 34% in subjects receiving active treatment, as compared to the placebo group. Absolute risk reduction with active treatment compared with placebo was twice as great for diabetic vs non-diabetic patients (101/1,000 vs 51/1,000 randomized participants at the 5-year follow-up), reflecting the higher risk for diabetic patients [41].
In a post hoc analysis of The Systolic Hypertension in Europe (Syst-Eur) Trial, 4695 individuals ≥60 years old (492 patients had T2DM), with SBP between 160-219 mm/Hg and DBP <95 mm/Hg were assigned to receive active treatment or placebo, with the purpose of reducing the SBP levels by at least 20 mm/Hg (and to less than 150 mm/Hg). Active treatment consisted of nitrendipine, with the possible addition or substitution of enalapril or hydrochlorothiazide. At the end of follow-up (2 years), the active treatment group (with DM) experienced a drop in total mortality of 55%, CVD associated mortality by 76%, all CV events combined by 69%, and fatal and non-fatal stroke was reduced by 73%. Additionally, all the combined CV events dropped by 63% [42].
The first two trials that chronologically shed evidence about the strict BP control in T2DM were the UK Prospective Diabetes Study Group 38 (UKPDS 38) and the Hypertension Optimal Treatment randomized trial (HOT Study).
The UKPDS 38 compared the strict BP control (<150/85 mmHg) with the use of an ACE-inhibitor (captopril) or a beta blocker (atenolol) as the principal therapy, versus a less stringent control (<180/105 mm/Hg), in 1148 individuals with AH and T2DM. At the end of follow-up (8.4 years), the average BP dropped significantly in the strict control group (144/82 mm/Hg) versus the less stringent control group (154/87 mm/Hg). Moreover, the strict control group lowered the risk of DM-associated endpoints by 24%, the DM-associated deaths by 32%, the risk of stroke by 44%, and the risk of microvascular endpoints by 37%, with no significant reduction in the risk of all-cause mortality. At the end of follow-up, the strict control group required ≥3 anti-AH drugs to reach the established BP goal [43].
The HOT Study evaluated 18,790 adult individuals (aged 50-80 years) with AH (and DBP between 100-115 mmHg), that were randomly assigned to three DBP goals (≤90 mmHg, ≤85 mmHg or ≤80 mmHg). Felodipine was given as baseline therapy with the addition of other agents, according to a five-step regimen. At the end of the study, the DBP dropped by 20.3 mm/Hg, 22.3 mm/Hg, and 24.3 mm/Hg in the groups with a target of ≤90 mmHg, ≤85 mmHg or ≤80 mmHg, respectively; the lowest incidence of CV events occurred at an average DBP value of 82.6 mm/Hg. In the sub-analysis of individuals with T2DM, there was a significant reduction of CV events of 51% in the group that reached the DBP goal of ≤80 mmHg (as compared against the group that reached a goal of ≤90 mmHg). So, from the results of this trial, the recommendation was adopted to achieve a BP goal of <130/80 mm/Hg in individuals with T2DM [44].
At around the same time, The Appropriate Blood Pressure Control in Diabetes (ABCD) Trial compared the effects of moderate BP control (target DBP: 80-89 mm/Hg) against those with intensive control (target DBP: 75 mm/Hg) on the incidence and progression of DM complications. The study also compared nisoldipine with enalapril as a first-line anti-AH agent in terms of the prevention and progression of complications of DM. The study was conducted in 480 individuals with T2DM, during an average follow-up of 5.3 years. The intensive treatment arm was associated with a slow-down in the progression of microvascular disease (diabetic nephropathy and retinopathy) and with a reduction in the incidence of stroke. However, the ABCD trial did not have enough power to determine whether the intensive reduction in BP was indeed more beneficial than conventional therapy on the reduction of CV outcomes in individuals with T2DM [45].
The Heart Outcomes Prevention Evaluation (HOPE Study) assessed the effects of an ACE-inhibitor (ramipril) and Vitamin E in individuals with high CVR (n=9,541 participants, mean age 65 years); 3,577 individuals with T2DM received ramipril or placebo. After an average follow-up of 4.5 years, the participants that received the ACE-inhibitor presented a significant 25% reduction in the primary composite endpoint (MI/stroke/CV death) and a significant reduction in the risk of other individual components (37% for CV death, 22% for MI, and 33% for stroke). However, the benefit of ACE-inhibitor therapy was independent from its effect on BP [46,47].
Moreover, the Effects of losartan on renal and cardiovascular outcomes in patients with T2DM and nephropathy (RENAAL) Study evaluated 1,513 patients (aged between 31-70 years, with diabetic nephropathy) which were assigned to receive an AT1-receptor blocker (ARB) (losartan) or placebo, in addition to the traditional anti-AH drug therapy. The primary outcome was the composite of a doubling of the base-line serum creatinine concentration, end-stage renal disease, or death. Secondary end points included a composite of morbidity and mortality from CV causes, proteinuria, and the rate of progression of renal disease. By the end of the follow-up period (3.4 years), the primary endpoint was significantly reduced in the group receiving ARB (but no effect was found on the rate of mortality). The CV morbidity and mortality combined was similar between the two groups, although the rate of first hospitalization for heart failure was significantly lower with ARB [48].
The Irbesartan Diabetic Nephropathy Trial (IDNT) evaluated whether the use of an ARB (irbesartan) or a calcium channel blocker (CCB) –amlodipine- or placebo protected against the progression of diabetic nephropathy or influenced total mortality (or the rate of CV events), beyond the effect attributable to a reduction in BP, in 1,715 hypertensive individuals with T2DM (aged 30-70 years) and hypertensive nephropathy, with a BP target of ≤135/85 mm/Hg in all groups. By the end of follow-up (2.6 years in average), ARB treatment was associated with a significant 20% reduction in the compounded primary endpoint, as compared to placebo, and a 23% reduction with regards to the group that received the CCB. No differences were identified between the group that received the CCB and that on placebo, and no difference between the treatment groups in terms of CV outcomes [49].
The Perindopril Protection Against Recurrent Stroke Study (PROGRESS) assessed the benefit of using an ACE-inhibitor (perindopril) in combination with a diuretic (indapamide) or placebo in 6105 participants with prior stroke or transient ischemic attack. By the end of follow-up (average 4 years), the lowest risk of stroke relapse was found among individuals that reached an average BP level of 115/75 mm/Hg over the follow-up period. It was also shown that per each 10 mmHg reduction in the level of SBP, there was a significant 28% reduction in the risk of stroke relapse. Additionally, participants with T2DM reduced the risk of stroke relapse by 38% [50].
The Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) evaluated 42,418 high CVR subjects with AH (≥55 years). This trial was designed with a view to determine whether anti-AH therapy with drugs such as CCB (amlodipine), ACE-inhibitor (lisinopril) or α-blocker (doxazosin) would lower the incidence of coronary heart disease or other CVD events compared with treatment with a thiazide-type diuretic (chlorthalidone). The ALLHAT trial was not designed to prospectively evaluate the effect of therapy in T2DM individuals; however, 15,297 individuals from the total population had T2DM (mean age 66.6 years), and represented a pre-designed cohort for a sub-group analysis (average follow-up 4.9 years). At the end of the trial, no significant differences were found in the primary outcome (fatal and non-fatal coronary heart disease), or in the risk of other cause mortality. There was however an increase in the rate of cardiac failure with CCB (compared to diuretics), and among those that received an ACE-inhibitor, there was a higher rate of stroke and heart failure (compared against the diuretic) [51,52].
In the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, 11,140 individuals with T2DM were randomized to treatment with a fixed combination of ACE-inhibitor (perindopril) and diuretic (indapamide) or matching placebo (control), in addition to current therapy. At the end of the follow-up period (4.3 years), the active treatment group had a 9% lower risk of a macro or microvascular event, CVD mortality risk dropped by 18%, and all-cause mortality declined by 14%. The average BP in the active treatment group was 135/75 mm/Hg (as compared against the control group which was 140/77 mm/Hg) [53].
The STENO-2 Trial included 160 individuals with T2DM and persistent microalbuminuria allocated to receive intensive therapy (based on goals, through a drug combination and lifestyle changes), or conventional multifactorial therapy. The intensive therapy group had control goals for HbA1c, total cholesterol levels and triglycerides, and a BP level of <130/80. Patients were treated with RAAS blockers because of the presence of microalbuminuria, regardless of BP, and received low-dose aspirin as primary prevention. The average follow-up period was 7.8 years, but participants were followed in an observational mode for an average of 5.5 more years. At the end of the study, the intensive therapy group experienced a significant 57% decrease in the CV cause of death, and the CV events risk dropped by 59% [54].
A post hoc analysis of The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) evaluated individuals with VD or high risk T2DM (n=25,577, with average age of 66 years) with the purpose of determining whether an ARB (telmisartan) was not inferior to the ACE-inhibitor (ramipril) and whether a combination of the two drugs was superior to ramipril alone as a treatment to prevent vascular events in highrisk patients who had CVD or DM but did not have heart failure. At the end of follow-up (56 months) there were no differences among the evaluated groups with regards to the primary and secondary outcomes. In those individuals in whom the SBP dropped to <140 mm/Hg, there was a decrease in all CV outcomes, but in both groups the benefit was attenuated (and ceased to exist for coronary events) when the SBP level was below 130 mm/Hg (except for the occurrence of stroke). In the group that received combined therapy, there was a significant increase in the risk of hypotension, syncope and renal failure, as compared against monotherapy (ACE-inhibitor) [55].
Another trial, the stop atherosclerosis in native diabetics study (SANDS), compared the progression of subclinical atherosclerotic disease in individuals under specific treatment, with a view to achieve LDL cholesterol levels of ≤70 mg/dL and BP of ≤4115/75 mmHg (aggressive management group) versus standard therapy (LDL ≤100 mg/dL, and BP ≤130/85 mmHg). The study was conducted in 499 Native American men and women with T2DM without prior history of CV disease. The primary endpoint was a composite of progression of atherosclerosis as measured by common carotid artery intimal medial thickness and clinical events. Secondary endpoints included other carotid and cardiac ultrasonography measures. In accordance with the study protocol, the anti-AH medications that could be used in AH individuals were: ACE-inhibitors, ARBs, hydrochlorothiazide, CCBs, beta-blockers, α-blockers and other vasodilators. At the end of follow-up (3 years) the average SBP in the aggressive treatment group was 117 mm/Hg, whilst in the standard therapy group, was 129 mm/Hg; the average LDLc levels in the aggressive treatment group was 72 mg/dL, and in the standard treatment group was 104 mg/dL. No differences were found in the clinical events between the two treatment groups, but the individuals in the aggressive therapy group were associated with a slower progression of atherosclerosis and a higher decrease in the left ventricular mass [56].
The Avoiding Cardiovascular Events through Combination Therapy in Patients Living with Systolic Hypertension (ACCOMPLISH) trial evaluated whether treatment with an ACE-inhibitor (benazepril) combined with a CCB (amlodipine) caused improved CV than benazepril plus hydrochlorothiazide therapy. 11,506 participants with AH and high CVR (n=6,946 had DM) were assigned to one or the other treatment group. The primary end point was measured as the time to the first event (which was defined as the composite of a CV event and death from CV causes). Secondary end points were a composite of CV events, defined as the primary end point excluding fatal events, and a composite of death from CV causes, nonfatal stroke, and nonfatal MI. At the end of follow-up (36 months in average), the group receiving the ACE-inhibitor and CCB showed a significant reduction in the primary endpoint of 19.6%, and of 21% for the secondary endpoint [57].
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial (ACCORD BP) evaluated whether intensive therapy versus standard therapy with anti-AH (ACE-inhibitors, ARBs, CCBs, beta-blockers, diuretics) in 4,733 participants with T2DM and high CVR, setting a goal of <120 mm/Hg (maintaining a general SBP goal of <140 mm/Hg), lowered major CV outcomes. The compounded primary endpoint was non-fatal MI, non-fatal stroke or CV mortality. After an average follow-up of 4.7 years, the average SBP was 119.3 mm/Hg in the intensive therapy group and of 133.5 mm/Hg in the standard therapy. The compounded primary endpoint did not differ between the two treatment groups, neither did the annual all-cause mortality rate. The annual stroke rate dropped by 41% in the intensive therapy group, and serious adverse events were experienced by 3.3% of patients in the intensive therapy group and in 1.3% of the standard therapy group (p<0.001). This trial was re-analyzed considering the subgroup of participants with DM (n=4,733), randomized to achieve a significant decline in the SBP levels (119 mm/Hg) versus the standard reduction in SBP levels (136 mm/Hg). The sub-group of participants with a significant SBP reduction (and after a standard decrease in HbA1c levels), experienced a significant reduction in the risk of stroke (39%), of CV outcomes (33%), and MI (37%). Additionally, the significant HbA1c reduction also had protective CV effects, though this was not the case when the effect of both interventions was achieved (intensive SBP and HbA1c lowering) [58,59].
In The International Verapamil SR Trandolapril Study (INVEST), in a sub-group analysis of 6,400 participants (average age 66 years) with AH, T2DM and established coronary heart disease, the participants were distributed into three categories based on their SBP levels: strict control group (<130 mm/Hg), standard control group (130 to <140 mm/Hg), and an uncontrolled group (≥140 mm/Hg). Patients received first-line treatment of either a CCB or beta-blocker followed by ACE-inhibitor, a diuretic, or both. At the end of the follow-up period (2.7 years), the primary and secondary endpoints (including non-fatal MI, non-fatal stroke, and all-cause mortality), were significantly different in the three groups. For instance, the risk of all-cause mortality was significantly increased in the strict control group, although after adjusting for basal difference, the risk was not statistically superior. However, after an extended follow-up analysis (all-cause mortality) it was found that the risk was significantly higher (15%) in the strict control group versus the standard control group [60].
In the Systolic blood pressure and risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish national diabetes register, a total of 12,677 individuals with T2DM (age range 30-75 years) treated with anti-AH were analyzed, evaluating the effect of the SBP levels on the risk of fatal/non-fatal coronary heart disease, stroke and CVD (excluding subjects with a history of cardiac failure). This trial found that the risk of coronary heart disease and stroke increases progressively with an elevated baseline SBP value (with or without a history of CVD), and the J-curve phenomenon was not present with low SBP levels. The risk for coronary heart disease and stroke increased (between 8-20%, after adjusting for clinical characteristics and traditional risk factors, respectively) for each 10 mm/Hg increase in the SBP level. An SBP value less than 140 mm/Hg was associated with a 37% increase in the risk of coronary heart disease, 86% for stroke and 44% for CVD (when comparing against an average SBP value between 110-129 mm/Hg, with this latter value as a reference range ). Finally, SBP values of 130-139 mm/Hg were not associated with an increased risk of such outcomes [61].
The Secondary Prevention of Small Subcortical Strokes (SPS3) trial compared two SBP goals, high levels (130-149 mmHg) versus low levels (<130 mm Hg) individuals with recent lacunar infarction; the study population was made up of 3,020 patients with a mean age of 63 years (1,519 in the high SBP group and 1,501 in the group with low SBP levels). The participants were followed for 3.7 years in average. The use of anti-AH (ACE-inhibitors, ARBs, CCBs, beta-blockers, thiazides, and others) was determined in accordance with the opinion of the treating physician. At the end of the study, a non-significant reduction in the frequency of stroke, MI and vascular death was found in the group of low SBP levels. However, further analysis compared the association between the levels of SBP and DBP achieved versus the frequency of stroke, major vascular events, and all-cause mortality (981 of the 2,747 participants had DM), and found that after an average follow-up of 3.7 years, a J-curve phenomenon was identified between the BP levels achieved and the outcomes evaluated. The lowest risk was found with average SBP levels of 124 mm/Hg and DBP of 67 mm/Hg. For instance, a SBP level >124 mm/Hg, 1 standard deviation above (11.1 mm/Hg), was associated with a significant increase in the risk of mortality, whilst a value below that level indicated a statistically significant inverse relation. Likewise, a DBP level >67 mm/Hg, 1 standard deviation above (8.2 mm/Hg) was associated with a significant increase in the risk of stroke, while below that level, the association was significantly reversed. The lowest risk for all events was with a nadir of SBP between 120-128 mm/Hg, and DBP of 65-70 mm/Hg [62,63].
The cardiovascular outcomes at different on-treatment blood pressures in the hypertensive patients of the Valsartan Antihypertensive Long-term use Evaluation (VALUE) trial, compared the long term effects of a treatment based on the use of an ARB (valsartan) or a CCB (amlodipine) on cardiac morbidity and mortality in hypertensive individuals (n=15,244) of any ethnic origin, ≥50 years old, and with a high CRV profile. There was of group of T2DM individuals participating in the trial and the primary end point of the study was time to first cardiac event (a composite of fatal or non-fatal myocardial infarction, sudden cardiac death, death from revascularization procedures, heart failure requiring hospitalization, and emergency procedures to prevent myocardial infarction).
The secondary endpoints were all major CV events, fatal and nonfatal stroke, myocardial infarction, hospitalized heart failure, and CV or all-cause mortality. At the end of follow-up (4-6 years) the relationship between BP and CV events was adjusted by age, gender, baseline risk factors, smoking, DM, inter alia. The findings indicated that a SBP ≥140 mm/Hg and DBP≥90mmHg (compared against an SBP of 130-139mm/Hg, and DBP of 80-89mmHg) was associated with an increased incidence of the primary endpoint; furthermore, a DBP of <70 mm/Hg (compared with ≥70 mm/Hg) was not associated with an increased incidence (no J-curve phenomenon) [64].
7.1. Meta-analysis evaluating the effect of blood pressure management in individuals with type 2 diabetes mellitus
One of the meta-analysis designed to evaluate the effect of BP management in T2DM individuals, evaluated the effects of lowering the BP on the risk of MI and stroke. This meta-analysis involved RCTs designed with a parallel group, comparing anti-AH agents (ACE-inhibitors, ARBs, CCBs, diuretics and beta-blockers alone or in combination). Eligible studies compared agents against placebo or another active treatment, and the trial duration needed to be at least one year. 31 RCTs were included (n=73,913 participants). In general, the participants allocated to the experimental therapy showed a significant 9% reduction in the risk of stroke, 11% for MI; the allocation to a stricter BP control (versus a more lenient control), significantly reduced the risk of stroke by 31%, but not so the risk of MI. The meta-regression analysis found that the risk of stroke was significantly reduced by 13% per each 5 mm/Hg decline in the SBP levels, and by 11.5% per each 2 mm/Hg drop in the DBP levels. However, the risk of MI showed no significant association with the scope of BP decrease, or when comparing a stricter BP control versus a more lenient control [65].
Another meta-analysis evaluated the efficacy and safety of intensive BP therapy versus the standard therapy in patients with T2DM. RCTs and quasi-randomized trials were eligible for inclusion (n=7,312 patients). The trials had to involve adults with T2DM and compare anti-AH therapy interventions designed to achieve intensive versus standard BP targets. Intensive targets were defined as an upper limit of 130 mmHg for SBP and 80 mmHg for DBP. Standard targets were defined as 140 to 160 mmHg (for SBP) and 85 to 100 mmHg (for DBP). Mean follow-up duration ranged from 1.9 to 5.3 years. Intensive and standard BP target groups did not differ significantly in terms of risk of death or MI. Risk of stroke was significantly lower in the intensive target group (35%). Rates of serious adverse events were significantly higher in the intensive target group (3.3 versus 1.7%) [66].
Another meta-analysis evaluated the BP goals and macrovascular or microvascular events in individuals with T2DM and among those with IFG or IGT; only RCTs using anti-AH therapy were involved (n=37,736). The participants should have achieved SBP levels of ≤135 mm/Hg in the intensive control group, and of ≤140 mm/Hg in the standard control group. The comparative groups were intensive versus standard BP lowering; benazepril/amlodipine versus benazepril/hydrochlorothiazide; candesartan versus placebo; doxazosine versus chlorthalidone; enalapril versus nifedipine; fosinopril/amlodipine versus amlodipine; perindopril versus placebo; perindopril-indapamide versus placebo; ramipril versus placebo; and valsartan versus placebo. The results indicated that the intensive BP control group (SBP ≤135 mm/Hg) achieved a significant 10% reduction in all-cause mortality, as compared against the BP standard control group. No differences were found between the two study groups in terms of outcomes such as CV mortality MI, heart failure, angina pectoris, and revascularization. However, the intensive control group showed a significant 17% reduction in the risk of stroke. Finally, the intensive control group showed a significant reduction in microalbuminuria and overt nephropathy, but there were no other differences in other renal or microvascular outcomes. It was further concluded that SBP goals between 130-135 mm/Hg are acceptable for this population and that SBP goals of <120 mm/Hg could be considered for high-risk of stroke populations. However, the recommendation was that setting SBP goals of <130 mm/Hg should only be done upon a risk-benefit analysis of such intervention [67].
One further meta-analysis of RCTs determined the associations between treatment with anti-AH and VD in T2DM and evaluated the risk of all-cause mortality, CV events, coronary, heart disease events, stroke, heart failure, retinopathy, new onset or worsening of albuminuria, and renal failure (100,354 participants). In order to establish whether the associations between anti-AH therapies and outcomes varied in accordance with the SBP level, the trials were stratified into categories (≥140 mm/Hg and <140 mm/Hg); moreover, to determine whether the associations between anti-AH therapies and outcomes varied according to the BP levels achieved, the studies were stratified into SBP categories of ≥130 mm/Hg and <130 mm/Hg. The anti-AH agents evaluated were ACE-inhibitors, ARBs, beta-blockers, diuretics, and CCBs against another class. The results indicated that per every 10 mm/Hg of SBP lowering, there was a significant 13% reduction in the risk of mortality an 11% reduction in CV events, 12% in coronary heart disease, 27% in stroke, 17% in albuminuria and 13% in retinopathy. When the studies were stratified based on the mean SBP baseline level (≥140 mm/Hg or <140 mm/Hg), the risk of outcomes other than stroke retinopathy and renal failure was lower in the studies where the participants had a higher baseline SPB. The associations among the various anti-AH therapies used and the outcomes were not significantly different and independent from the drug class with the exception for stroke and heart failure; diuretics showed a significantly lower risk of heart failure, as well as the ARBs, whilst CCBs were associated with higher risk of heart failure and lower risk of stroke, and beta-blockers were associated with higher risk of stroke [68].
More recently, a meta-analysis of RCTs (n=73,738 participants) evaluated the effect of anti-AH therapy on CV mortality and morbidity in individuals with T2DM at different BP levels, comparing any type of anti-AH drug versus placebo, or two anti-AH drugs versus a single drug, or different BP goals. The findings showed that if the SBP baseline levels were <150 mm/Hg, the anti-AH therapy significantly lowered the risk of all-cause mortality (11%), the risk of CV mortality (25%), MI (26%), stroke (23%), and end-stage renal disease (18%). If the baseline SBP levels ranged between 140-150 mm/Hg, additional therapy significantly reduced the risk of all-cause mortality (13%), MI (16%), and heart failure (20%). However, if the SBP baseline levels were <140 mm/Hg, additional treatment increased the risk of CV mortality and all-cause mortality in a non-significant manner. Meta-regression analyses showed a 15 percentage point worse treatment effect on CV mortality for each 10 mm Hg lower baseline SBP, crossing the zero line from benefit towards harm at 141 mm/Hg [69].
Another recent meta-analysis (n= 260,210) compared the effects of lowering the SBP and DBP levels at different values (SBP ≥140, or 130 to <140, or <130 mm/Hg; and the DBP levels ≥80 or <80 mm/Hg) with different classes of anti-AH drugs (ACE-inhibitors, ARBs, beta-blockers, CCBs, diuretics), in individuals with or without T2DM, with regards to CV and renal outcomes. In total, the data from 61,772 individuals with T2DM and 191,353 individuals without T2DM were analyzed. The findings indicated that in patients achieving an SBP level below 140 mm/Hg, the relative and absolute risk of most CV outcomes was significantly lower in people with DM than in non-DM individuals (among the DM population, the risk of stroke was significantly reduced by 27%, the risk for coronary artery disease dropped by 29%, heart failure by 25%, and all-cause mortality by 18%). However, no significant reduction was found in the risk for CV mortality, whilst in those that achieved a SBP level of <130 mm/Hg, these differences were no longer present or were reversed; in other words, there was a higher reduction in the non-DM outcomes. Furthermore, there was a significant reduction in the risk of end-stage renal disease (21%), only in subjects with DM (when the SBP level achieved was at least 140 mm/Hg, but not when the SBP level achieved was <140 mm/Hg). Overall, all of the anti-AH evaluated lowered the CVR when compared against placebo (with and without DM); however, the ACE-inhibitors were the only class that showed higher efficacy in DM individuals than in non-diabetics [70].
The results of these trials conclude that the current knowledge about the importance of controlling BP levels in individuals with T2DM stems from clinical trials originally designed mainly to evaluate the impact of glycaemia control in individuals with T2DM, or based on the analysis of CV outcomes in sub-groups of subjects with T2DM that were part of studies on AH therapy (Table 2). Such results have been characterized by a high heterogeneity and lack of uniformity. However, the available evidence indicates that individuals with T2DM who achieve BP goals have fewer vascular events, and the development of microvascular complications (in particular, diabetic nephropathy) is limited.
Table 2.
Studies evaluating anti-AH therapy in Type 2 Diabetes Mellitus - a summary.
| Study (ref.) | Inclusion criteria | Intervention | Control | Effect of the intervention on risk of CV events |
|---|---|---|---|---|
| SHEP [41] | High SBP, DM2 | Chlorthalidone + atenolol | Placebo | Reduction |
| Syst-Eur [42] | High SBP, DM2 | Nitrendipine ± enalapril ± HCTZ | Placebo | Reduction |
| UKPDS 38 [43] | DM2, AH | Captopril | Atenolol | Reduction |
| HOT [44] | DM2, AH | Felodipine ± ACE-inhibitors, beta-blockers, diuretics | Standard therapy | Reduction |
| ABCD [45] | DM2, AH | Nisoldipine | Enalapril | Increase |
| HOPE [46,47] | CVD + 1 additional CVR factor, DM2 | Ramipril | Placebo | Reduction |
| RENAAL [48] | DM2, nephropathy, AH | Losartan | Placebo | No difference |
| IDNT [49] | AH, DM2, nephropathy | Irbesartan | Amlodipine o placebo | No difference |
| PROGRESS [50] | DM2, AH, stroke | Perindopril ± indapamide | Placebo | Reduction |
| ALLHAT [51,52] | DM2, AH | Amlodipine | Chlorthalidone | No difference |
| ADVANCE [53] | DM2, AH | Perindopril + indapamide | Placebo | Reduction |
| Steno-2 [54] | DM2, mAlb | Intensive treatment | Conventional treatment | Reduction |
| ONTARGET [55] | VD, or DM2 with high CVR | Telmisartan ± ramipril | Placebo, o Ramipril | No difference |
| SANDS [56] | DM2, AH | Aggressive treatment | Standard therapy | Reduction |
| ACCOMPLISH [57] | AH, DM2 and high CVR | Amlodipine + benazepril | HCTZ + benazepril | Reduction |
| ACCORD [58,59] | DM2 and high CVR | Intensive treatment | Standard therapy | Reduction |
| INVEST [60] | DM2, AH, CVD | Verapamil | Atenolol | No difference |
| Swedish National Diabetes Register [61] | DM2 + use of anti-AH | NA | NA | Increase |
| SPS3 [62,63] | Lacunar infarction, DM2 | Intensive treatment | Standard therapy | Reduction |
| VALUE [64] | AH, high CVR, DM2 | Valsartan | Amlodipine | Increase |
| Reboldi G, et al. [65] | Anti-AH in DM2 (MA) | Monotherapy or combined treatment | Placebo, active treatment | Reduction |
| McBrien K, et al. [66] | Anti-AH in DM2 (MA) | Intensive treatment | Standard therapy | Reduction |
| Bangalore S, et al. [67] | Anti-AH in DM2, prediabetes (MA) | Intensive treatment | Standard therapy | Reduction |
| Emdin CA, et al. [68] | Anti-AH in DM2 (MA) | ACE-inhibitors, BRAs, beta-blockers, diuretics, CCB | Placebo, active treatment | Reduction |
| Brunström M, et al. [69] | Anti-AH in DM2 (MA) | 1 or 2 anti-AH drugs | Placebo, monotherapy with anti-AH | Reduction |
| Thomopoulos C, et al. [70] | Anti-AH in DM2 (MA) | Anti-AH (monotherapy or combined treatment) | Placebo, no treatment, or anti-AH drugs | Reduction |
ACE-inhibitors: angiotensin converting enzyme inhibitors; AH: arterial hypertension; Anti-AH: anti-hypertensives drugs; ARBs: angiotensin II type 1-receptor blockers; CCB: calcium channel blocker; CV: cardiovascular; CVD: cardiovascular disease; CVR: cardiovascular risk; HCTZ: hydrochlorothiazide; mAlb: microalbuminuria; MA: meta-analysis; NA: not applicable; SBP: systolic blood pressure; T2DM: Type 2 diabetes mellitus; VD: vascular Disease
8. Which should be the target blood pressure recommended for individuals with type 2 diabetes mellitus?
Adequate management of BP levels in individuals with T2DM is a cost-effective measure, since the benefits of such intervention on CV outcomes outweigh those achieved through glycaemia control per se. However, the recommended goals for SBP and DBP have been controversial and targets of SBP <130 mm/Hg have been widely accepted by international guidelines for diabetic individuals with AH. Nevertheless, notwithstanding the fact that RCTs have reported that the lowering by several mm/Hg in intensive therapy groups (versus the control groups) is associated with a reduction in the risk of CV events, the SBP levels achieved in such trials have always been >130 mm/Hg. These results initially led to setting SBP goals within a more conservative range (between 130-139 mm/Hg), which was also confirmed by several meta-analysis that found that SBP levels between 130-139 mm/Hg achieved greater benefits in this population; it was further documented that SBP values of <130 mm/Hg were associated with a higher risk of adverse events (or that the beneficial effects of BP lowering were attenuated, with regards to SBP numbers between 130-139 mm/Hg). Until then, the benefit of lowering the SBP to <130 mm/Hg in individuals with T2DM and AH seemed to be exclusive to lowering the risk of ischemic or hemorrhagic stroke (when compared against SBP levels of >130 and <140 mm/Hg) [71]. Nevertheless, in a further analysis of the results of the ACCORD trial, the population with T2DM allocated to intensive therapy and that reached SBP levels of 119 mm/Hg (versus the standard group, which reached levels of 136 mm/Hg), following the initial allocation to an intensive versus a standard decrease in the HbA1c levels, the results indicated that those individuals that achieved an intensive reduction in the SBP levels (and a standard decrease in the HbA1c levels) experienced a significant 39% reduction in the risk of stroke, 33% reduction in combined CV outcomes and 37% reduction in the risk of MI. This same trial showed that those individuals that could reach SBP goals of <120 mm/Hg experienced a significant increase in the risk of CV events as compared to those that remained within the 120-139 mm/Hg range [72]. The results of the ACCORD trial weakened the conventional believe that “the lower the better”.
Even with the results of previous studies, currently it is not possible to ascertain whether an SBP level of <130 mm/Hg in a population with T2DM and AH is better than an SBP level of <140 mm/Hg. What seems to be conclusive however, is that SBP levels <120 mm/Hg do not seem to benefit this population in terms of CV outcomes. However, the established DBP goals under the various management guidelines have been <80, <85, and <90 mm/Hg; for instance, in the HOT trial, a 51% reduction in the incidence of CV events was documented among individuals that reached DP levels of ≤80 mmHg versus those that achieved levels between ≤85 and ≤90 mmHg. While in the UKPDS 38 study, patients with T2DM and AH, who achieved DBP levels of 83 mm/Hg with treatment, had 21% lower risk of MI, 44% lower risk of stroke, and 37% less risk of microvascular endpoints, versus those patients that achieved DBP levels of 87 mm/Hg. Both the HOT and UKPDS 38 trials determined the established recommendation by several guidelines to reach DBP targets of ≤80 mmHg or ≤85 mm/Hg.
Whilst a DBP level of <80 mm/Hg reduces the risk of vascular events in patients with T2DM, the overall reduction in CVR is achieved with DBP levels between 80-89 mm/Hg. Additionally, it must be kept in mind that achieving SBP levels in the range of 130-139 mm/Hg, almost always leads to DBP levels of <90 or <85 mm/Hg, and even <80 mm/Hg [73,74]. Achieving these DBP targets (between 70-79 mm/Hg) has not been associated with undesirable effects in individuals achieving SBP control levels, though there are doubts about the “J-curve phenomenon” which may develop with a DBP level <80 mm/Hg [75].
9. Managing blood pressure levels in type 2 diabetes mellitus and the J-curve phenomenon
From the physiological perspective, the constant perfusion of vital organs (heart, brain, kidneys) becomes compromised when the BP levels progressively decrease; therefore, anti-AH therapy and achieving very strict goals in the population with AH may give rise to a turning point in which very low BP levels may affect the perfusion and function of multiple organs. This has been proven in multiple studies in which levels of SBP and DBP below certain number have been associated with a higher incidence of CV events (J-curve phenomenon) [76,77,78]. Considering that coronary flow is a function of the perfusion pressure and of the coronary arteries resistance, the perfusion pressure is the difference between the aortic diastolic pressure and the tele-diastolic left ventricular pressure. Therefore, a low BP level (particularly of the DBP) may compromise coronary perfusion, and since coronary perfusion occurs in diastole, diastolic hypotension may lead to coronary hypoperfusion, mainly in people with compromised coronary flow (Figure 2). This may explain why in experimental trials in which DBP levels of <90 mm/Hg are achieved using sodium nitroprusside a marked reduction in coronary blood flow develops in hypertensive individuals with left ventricular hypertrophy (LVH), while in those without LVH, no disruptions were found in coronary perfusion when reaching DBP levels close to 70 mm/Hg [79]. It has also been found that patients with established coronary heart disease that achieve DBP levels of <80 mm/Hg, experience a significant increase in the incidence of CV events. So, hypertensive individuals with cardiovascular comorbidity (LVH, coronary artery disease) are more prone to develop a J-curve phenomenon [80]. This can be shown for instance when analyzing the BP levels in the Treating to New Targets (TNT) Trial, which showed that in individuals with coronary artery disease, there was a persistent J-curve phenomenon (or a non-lineal relationship) between the BP levels and CV events. This indicated that SBP values <110-120 mm/hg and DBP <60-70 mm/Hg, involved a higher risk of CV events (except for stroke, where there was a potential reduction in the risk of those events by achieving such SBP levels) [81]. Likewise, other studies have found an association between a nadir of DBP of <70 mm/Hg, <80, or 85-89 mm/Hg and the occurrence of vascular events in T2DM. The HOT trial did not find any differences in CV outcomes between treatment groups when allocated to a DBP goal of ≤90, ≤85, or ≤80 mmHg; moreover, among individuals with previous cardiac ischemic disease, those who achieved DBP levels of 80 mm/Hg, had a high rate of MI as compared to those with 85 mm/Hg. Such increased risk of MI with a reduction in DBP was not present in patients with no ischemic cardiac disease. In the INVEST trial, individuals with established coronary artery disease and AH had fewer CV events when the DBP level was <90 mm/Hg, whilst the risk increased when the value was <70 mm/Hg. Moreover, in the ONTARGET study, the incidence of CV events dropped when the BP levels decreased from 145/82 mm/Hg to 133/76 mm/Hg, but increased again among the individuals that reached lower BP targets (125/72 and 116/68 mm Hg).
Figure 2.
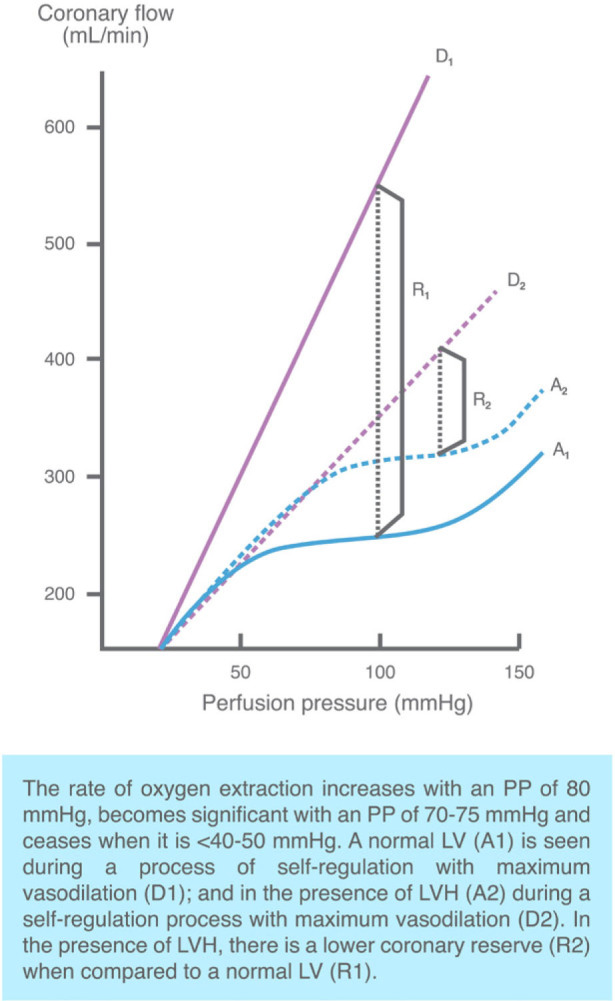
“J-curve phenomenon” and self-regulation of coronary flow in patients with AH and LVH
Several pathophysiological mechanisms have been suggested to explain the presence of the J-curve phenomenon. One of them is that is an epiphenomenon in individuals with underlying debilitating chronic conditions (for instance, cancer) in whom a low BP level contributes to increased mortality; another mechanism is that a low BP level is an epiphenomenon of cardiac dysfunction (for instance, a very low ejection fraction). It has also been said that the J-curve phenomenon represents an epiphenomenon of increased arterial stiffness, since a low DBP level may be a marker for high pulse pressure and hence the increase in mortality. And finally, it is also true that low DBP levels compromise coronary perfusion, increasing the risk of CV events [82,83].
All of the above indicates that the J-curve phenomenon should be considered when establishing BP targets in hypertensive individuals (with or without DM). In patients with T2DM, the nadir may be even lower than in non-T2DM. So, if T2DM is considered an “equivalent” of CVR, hypothetically this population should have at least the same BP nadir to cause coronary hypoperfusion as the population without T2DM, but with LVH, or established coronary artery disease. Finally, probably there is not just one single nadir for individuals with T2DM, but rather several of them, depending on the baseline characteristics of the patients (functional class, ejection fraction, diastolic dysfunction, aortic insufficiency, overweight, smoking, associated comorbidities and the presence of other CVR factors, use of multiple anti-AH drugs, inter alia).
10. Pharmacological therapy of the arterial hypertension in individuals with type 2 diabetes mellitus
The various management guidelines providing recommendations for the pharmacological management of AH in patients with T2DM agree on several matters [84,85,86,87,88,89] that are listed hereunder:
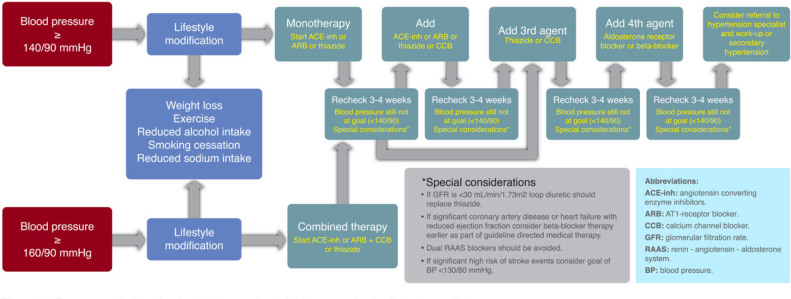
The initial therapy shall include anti-AH drugs proven to reduce CV outcomes. The BP threshold considered at the beginning of treatment (monotherapy) is ≥140/90 mm/Hg (Figure 3).
All major anti-AH drug classes (i.e., ACE-inhibitors, ARBs, dihydropyridine CCBs, and thiazide-type diuretics) should be recommended as first line agents for this population.
In patients with albuminuria (≥30 mg/gm creatinine), the first line anti-AH drugs are ACE-inhibitors and ARBs.
ACE-inhibitors and ARBs can slow progression of nephropathy and retinopathy, and hence these are the first line anti-AH drugs for such individuals.
In the absence of albuminuria, risk of progressive kidney disease is low, and ACE-inhibitors and ARBs have not been found to afford superior cardioprotection when compared with other anti-AH agents. Therefore, in patients without albuminuria, initial monotherapy can consist of an ACE-inhibitor, ARBs, thiazide-type diuretic, or CCBs.
In patients with established CV disease or with heart failure, beta-blockers may be recommended, keeping in mind that this type of drug has not been shown to lower mortality in the absence of this condition. Moreover, a loop diuretic is likely to be required in individuals with renal disease or heart failure who have a propensity to fluid retention.
BP control is more difficult to achieve in patients with DM than in those without DM, so the use of combination therapy is a requirement in most patients.
There is very limited evidence on the efficacy, safety and CV outcomes with drugs such as α-blockers (although they may help in patients with associated prostate disease), and with drugs affecting the metabolism of aldosterone (spironolactone or eplerenone); however, these may be considered in individuals that are AH treatment-resistant (monitoring the renal function and the serum potassium levels, particularly when combining with ACE-inhibitors, ARBs or other types of diuretics).
Combined therapy shall be considered based on BP levels of ≥150/100 mm/Hg, with a stronger indication for BP levels of ≥160/100 mm/Hg.
The choice of any of the major anti-AH drug classes is the foundation for pharmacological therapy for AH in T2DM; however, this decision must be made on an individualized, case-bycase basis, considering factors such as: ethnicity, potential adverse events, glycaemia control, costs, drug interaction, CVD, heart failure, renal function, inter alia. These factors may impact other aspects such as compliance, efficacy, outcomes and safety.
Figure 3.
Recommendations for the treatment of arterial hypertension in diabetes mellitus
11. Final thoughts
The results from the above trials have led to the consolidation of several recommendations regarding the BP level that may be achieved in individuals with T2DM. It is however difficult to establish an optimum level for this particular population. The current evidence shows that an SBP target between 130-139 mm/Hg protects against CV and renal complications and it has been proven that achieving an SBP level as close to 130 mm/Hg leads to an additional and significant benefit versus higher values. A target of <130 mm/Hg may be beneficial for individuals with a high risk of cerebrovascular events –for instance patients with prior stroke history- and in patients with nephropathy and significant proteinuria. Finally, SBP levels of <120 mm/Hg should not be considered for this population, since there is no evidence that those levels significantly reduce the frequency of CV outcomes or mortality, but on the contrary, may be associated with the J-curve phenomenon. A DBP between 80-89 mm/Hg may be established as the initial goal in these patients; however, there may be additional benefits when lowering these numbers to a range between 70-79 mm/Hg (provided that the rate of adverse events is not increased). It must be kept in mind that BP goals may vary in the population with T2DM (for example as a result of factors such as age, target organ, newly diagnosed T2DM, ethnicity, frailty, presence of adverse effects, comorbidities, etc.), and consequently the BP goal must be individualized in these patients. Of course, a very demanding goal practically makes it mandatory to use multiple anti-AH agents to achieve the desired BP level, and hence increases the risk of adverse events and poor compliance. Few studies have been made with the purpose of establishing BP goals in individuals with T2DM >80 years old. A goal of <150/90 mm/Hg may be reasonable, although the risk-benefit balance must be weighted when setting more demanding goals in these individuals.
12. Conclusions
In individuals with T2DM and AH, an SBP goal between 130-139 mm/Hg and DBP between 80-89 mm/Hg has proven to be safe and lowers the risk of CV outcomes. SBP levels of <130 mm/Hg should be reserved for patients at high risk of cerebrovascular events. DBP levels between 70-79 mm/Hg are ideal, as long as the risk of adverse events or the J-curve phenomenon are not increased (particularly in individuals with established coronary heart disease or with LVH). These goals must be individualized in accordance with the baseline characteristics of the patients and their comorbidities. Finally, SBP levels of <120 mm/Hg or DBP <70 mm/Hg have failed to show fewer CV outcomes and may even increase the occurrence of such events.
Footnotes
Disclosures: H V-U has served on advisory boards and/or on speaker bureaus for AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Novo and Sanofi. MF C-A has no conflicts of interest to declare.
Review criteria: We searched the MEDLINE and Clinical-Trials.gov databases using the search terms “diabetes”, “arterial hypertension”, “cardiovascular outcomes” and “anti-hypertensive drugs”, alone and in combination. We focused on articles published in the past 25 years, in English but did not exclude commonly referenced and highly regarded older publications.
Contributors: H V-U and MF C-A designed the study, planned the analyses, did the data mining and wrote the manuscript. All authors reviewed and approved the final manuscript.
Conflict of interest: The authors report no conflicts of interest in this work.
References
- [1].International Diabetes Federation. 3rd. IDF Diabetes Atlas; http://www.diabetesatlas.org/resources/previous-editions.html (accessed Nov 18, 2017) [Google Scholar]
- [2].Carey RM, Whelton PK.. ACC/AHA Hypertension Guideline Writing Committee. Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Synopsis of the 2017 American College of Cardiology/American Heart Association Hypertension Guideline. Ann Intern Med. 2017;2018;168(5):351–358. doi: 10.7326/M17-3203. [DOI] [PubMed] [Google Scholar]
- [3].Nolan C, Damm P, Prentki Ml.. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378(9786):169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- [4].Taylor R.. Type 2 diabetes: etiology and reversibility. Diabetes Care. 2013;36(4):1047–1055. doi: 10.2337/dc12-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Keating S, Plutzky J, El-Osta A.. Epigenetic changes in diabetic and cardiovascular risk. Circ Res. 2016;118:1706–1722. doi: 10.1161/CIRCRESAHA.116.306819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R. et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].World Health Organization. Global report on diabetes. Geneva, Switzerland: 2016. ISBN 978 92 4 156525 7 (NLM classification: WK 810) [Google Scholar]
- [8].Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH. et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- [9].World health ranking. http://www.worldlifeexpectancy.com/cause-of-death/diabetes-mellitus/by-country/ (accessed Feb 02, 2018) [Google Scholar]
- [10].Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, Vollmer S.. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017;5(6):423–430. doi: 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- [11].Huffman MD, Lloyd-Jones DM.. Global Burden of Raised Blood Pressure Coming Into Focus. JAMA. 2017;317(2):142–143. doi: 10.1001/jama.2016.19685. [DOI] [PubMed] [Google Scholar]
- [12].World health ranking. http://www.worldlifeexpectancy.com/cause-of-death/hypertension/by-country/ (accessed Jan 11, 2018) [Google Scholar]
- [13].World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013-2020. 2013 http://apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf?ua=1 (accessed Dec 21, 2017) [Google Scholar]
- [14].Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L. et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317(2):165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- [15].NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet. 2017;389(10064):37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu G, Jousilahti P, Tuomilehto J.. Joint effects of history of hypertension at baseline and type 2 diabetes at baseline and during follow-up on the risk of coronary heart disease. Eur Heart J. 2007;28:3059–3066. doi: 10.1093/eurheartj/ehm501. [DOI] [PubMed] [Google Scholar]
- [17].Menke A, Casagrande S, Geiss L, Cowie CC.. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- [18].Chen G, McAlister FA, Walker RL, Hemmelgarn BR, Campbell NR.. Cardiovascular outcomes in Framingham participants with diabetes: the importance of blood pressure. Hypertension. 2011;57:891–897. doi: 10.1161/HYPERTENSIONAHA.110.162446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Franklin SS, Thijs L, Li Y, Hansen TW, Boggia J, Liu Y. et al. International Database on Ambulatory blood pressure in Relation to Cardiovascular Outcomes Investigators. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61:964–971. doi: 10.1161/HYPERTENSIONAHA.111.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tatsumi Y, Ohkubo T.. Hypertension with diabetes mellitus: significance from an epidemiological perspective for Japanese. Hypertens Res. 2017;40(9):795–806. doi: 10.1038/hr.2017.67. [DOI] [PubMed] [Google Scholar]
- [21].Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR.. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75) Diabetologia. 2006;49:1761–1769. doi: 10.1007/s00125-006-0297-1. [DOI] [PubMed] [Google Scholar]
- [22].Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K. et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Whaley-Connell A, Sowers JR.. Insulin Resistance in Kidney Disease: Is There a Distinct Role Separate from That of Diabetes or Obesity? Cardiorenal Med. 2017;8(1):41–49. doi: 10.1159/000479801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F.. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- [25].Haas AV, McDonnell ME.. Pathogenesis of Cardiovascular Disease in Diabetes. Endocrinol Metab Clin North Am. 2018;47(1):51–63. doi: 10.1016/j.ecl.2017.10.010. [DOI] [PubMed] [Google Scholar]
- [26].Neves MF, Cunha AR, Cunha MR, Gismondi RA, Oigman W.. The Role of Renin-Angiotensin-Aldosterone System and Its New Components in Arterial Stiffness and Vascular Aging. High Blood Press Cardiovasc Prev. 2018 doi: 10.1007/s40292-018-0252-5. [DOI] [PubMed] [Google Scholar]
- [27].Bian J, Zhang S, Yi M, Yue M, Liu H.. The mechanisms behind decreased internalization of angiotensin II type 1 receptor. Vascul Pharmacol. 2018;pii doi: 10.1016/j.vph.2018.01.008. S1537-1891(17)30304-X. [DOI] [PubMed] [Google Scholar]
- [28].Rosano GMC, Seferovic P, Farmakis D, Filippatos G.. Renin inhibition in heart failure and diabetes: the real story. Eur J Heart Fail. 2018;20(1):149–151. doi: 10.1002/ejhf.1072. [DOI] [PubMed] [Google Scholar]
- [29].Hussain M, Awan FR.. Hypertension regulating angiotensin peptides in the pathobiology of cardiovascular disease. Clin Exp Hypertens. 2017:1–9. doi: 10.1080/10641963.2017.1377218. [DOI] [PubMed] [Google Scholar]
- [30].Safar ME.. Arterial stiffness as a risk factor for clinical hypertension. Nat Rev Cardiol. 2018;15(2):97–105. doi: 10.1038/nrcardio.2017.155. [DOI] [PubMed] [Google Scholar]
- [31].Libianto R, Batu D, MacIsaac RJ, Cooper ME, Ekinci EI.. Pathophysiological Links Between Diabetes and Blood Pressure. Can J Cardiol. 2018;34(5):585–594. doi: 10.1016/j.cjca.2018.01.010. [DOI] [PubMed] [Google Scholar]
- [32].Ahn SY, Gupta C.. Genetic Programming of Hypertension. Front Pediatr. 2018;5:285. doi: 10.3389/fped.2017.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP.. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front Endocrinol (Lausanne) 2018;9:2. doi: 10.3389/fendo.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Adebamowo SN, Tekola-Ayele F, Adeyemo AA, Rotimi CN.. Genomics of Cardiometabolic Disorders in Sub-Saharan Africa. Public Health Genomics. 2017;20(1):9–26. doi: 10.1159/000468535. [DOI] [PubMed] [Google Scholar]
- [35].Grossman Y, Shlomai G, Grossman E.. Treating hypertension in type 2 diabetes. Expert Opin Pharmacother. 2014;15(15):2131–2140. doi: 10.1517/14656566.2014.947267. [DOI] [PubMed] [Google Scholar]
- [36].Pavlou DI, Paschou SA, Anagnostis P, Spartalis M, Spartalis E, Vryonidou A. et al. Hypertension in patients with type 2 diabetes mellitus: Targets and management. Maturitas. 2018;112:71–77. doi: 10.1016/j.maturitas.2018.03.013. [DOI] [PubMed] [Google Scholar]
- [37].Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA. et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359(12):1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].World Health Organization-International Society of Hypertension Blood Pressure Lowering Treatment Trialists’ Collaboration. Protocol for prospective collaborative overviews of major randomized trials of blood-pressure lowering treatments. J Hypertens. 1998;16:127–137. [PubMed] [Google Scholar]
- [39].Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, LaRosa JC.. J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J. 2010;31(23):2897–2908. doi: 10.1093/eurheartj/ehq328. [DOI] [PubMed] [Google Scholar]
- [40].Dudenbostel T, Oparil S.. J Curve in Hypertension. Curr Cardiovasc Risk Rep. 2012;6(4):281–290. doi: 10.1007/s12170-012-0246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Curb JD, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H. et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996;276(23):1886–1892. [PubMed] [Google Scholar]
- [42].Tuomilehto J, Rastenyte D, Birkenhäger WH, Thijs L, Antikainen R, Bulpitt CJ. et al. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. Systolic Hypertension in Europe Trial Investigators. N Engl J Med. 1999;340(9):677–684. doi: 10.1056/NEJM199903043400902. [DOI] [PubMed] [Google Scholar]
- [43].UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- [44].Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S. et al. Effects of intensive blood pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351(9118):1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- [45].Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW.. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338:645–652. doi: 10.1056/NEJM199803053381003. [DOI] [PubMed] [Google Scholar]
- [46].Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P.. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- [47].Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- [48].Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH. et al. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- [49].Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB. et al. Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- [50].PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood pressure lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- [51].ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive And Lipid-Lowering Treatment To Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- [52].Barzilay JI, Davis BR, Pressel SL, Cutler JA, Einhorn PT, Black HR. et al. ALLHAT Collaborative Research Group. Long-term effects of incident diabetes mellitus on cardiovascular outcomes in people treated for hypertension: the ALLHAT Diabetes Extension Study. Circ Cardiovasc Qual Outcomes. 2012;5(2):153–162. doi: 10.1161/CIRCOUTCOMES.111.962522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L. et al. ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- [54].Gaede P, Lund-Andersen H, Parving HH, Pedersen O.. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- [55].Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H. et al. ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- [56].Howard BV, Roman MJ, Devereux RB, Fleg JL, Galloway JM, Henderson JA. et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA. 2008;299(14):1678–1689. doi: 10.1001/jama.299.14.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V. et al. ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- [58].Cushman WC, Evans GW, Byington RP, Goff DC Jr., Grimm RH Jr., Cutler JA. et al. ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Margolis KL, O’Connor PJ, Morgan TM, Buse JB, Cohen RM, Cushman WC. et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37(6):1721–1728. doi: 10.2337/dc13-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, Pepine CJ.. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304(1):61–68. doi: 10.1001/jama.2010.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cederholm J, Gudbjörnsdottir S, Eliasson B, Zethelius B, Eeg-Olofsson K, Nilsson PM.. Systolic blood pressure and risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish national diabetes register. J Hypertens. 2010;28(10):2026–2035. doi: 10.1097/HJH.0b013e32833c8b75. [DOI] [PubMed] [Google Scholar]
- [62].Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA. et al. SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Odden MC, McClure LA, Sawaya BP, White CL, Peralta CA, Field TS. et al. Achieved Blood Pressure and Outcomes in the Secondary Prevention of Small Subcortical Strokes Trial. Hypertension. 2016;67(1):63–69. doi: 10.1161/HYPERTENSIONAHA.115.06480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mancia G, Kjeldsen SE, Zappe DH, Holzhauer B, Hua TA, Zanchetti A. et al. Cardiovascular outcomes at different on-treatment blood pressures in the hypertensive patients of the VALUE trial. Eur Heart J. 2016;37(12):955–964. doi: 10.1093/eurheartj/ehv633. [DOI] [PubMed] [Google Scholar]
- [65].Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P.. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29(7):1253–1269. doi: 10.1097/HJH.0b013e3283469976. [DOI] [PubMed] [Google Scholar]
- [66].McBrien K, Rabi DM, Campbell N, Barnieh L, Clement F, Hemmelgarn BR. et al. Intensive and Standard Blood Pressure Targets in Patients With Type 2 Diabetes Mellitus: Systematic Review and Meta-analysis. Arch Intern Med. 2012;172(17):1296–1303. doi: 10.1001/archinternmed.2012.3147. [DOI] [PubMed] [Google Scholar]
- [67].Bangalore S, Kumar S, Lobach I, Messerli FH.. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799–2810. doi: 10.1161/CIRCULATIONAHA.110.016337. [DOI] [PubMed] [Google Scholar]
- [68].Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A.. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313(6):603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- [69].Brunström M, Carlberg B.. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717. doi: 10.1136/bmj.i717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Thomopoulos C, Parati G, Zanchetti A.. Effects of blood-pressure-lowering treatment on outcome incidence in hypertension: 10 - Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta-analyses of randomized trials. J Hypertens. 2017;35(5):922–944. doi: 10.1097/HJH.0000000000001276. [DOI] [PubMed] [Google Scholar]
- [71].Shaikh A.. A Practical Approach to Hypertension Management in Diabetes. Diabetes Ther. 2017;8(5):981–989. doi: 10.1007/s13300-017-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cloutier L, Lamarre-Cliché M.. Hypertension in Adults with Diabetes: a Review of Blood Pressure Measurement Methods, Targets and Therapy. Canadian Journal of Diabetes. 2018 doi: 10.1016/j.jcjd.2018.01.012. [DOI] [PubMed] [Google Scholar]
- [73].de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P. et al. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273–1284. doi: 10.2337/dci17-0026. [DOI] [PubMed] [Google Scholar]
- [74].Dojki FK, Bakris GL.. Blood Pressure Control and Cardiovascular/Renal Outcomes. Endocrinol Metab Clin North Am. 2018;47(1):175–184. doi: 10.1016/j.ecl.2017.10.008. [DOI] [PubMed] [Google Scholar]
- [75].Zanchetti A.. Blood pressure targets of antihypertensive treatment: up and down the J-shaped curve. Eur Heart J. 2010;31:2837–2840. doi: 10.1093/eurheartj/ehq281. [DOI] [PubMed] [Google Scholar]
- [76].Lucas SJ, Tzeng YC, Galvin SD, Thomas KN, Ogoh S, Ainslie PN.. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension. 2010;55:698–705. doi: 10.1161/HYPERTENSIONAHA.109.146290. [DOI] [PubMed] [Google Scholar]
- [77].Dorresteijn JA, van der Graaf Y, Spiering W, Grobbee DE, Bots ML, Visseren FL.. Secondary Manifestations of Arterial Disease Study Group. Relation between blood pressure and vascular events and mortality in patients with manifest vascular disease: J-curve revisited. Hypertension. 2012;59:14–21. doi: 10.1161/HYPERTENSIONAHA.111.179143. [DOI] [PubMed] [Google Scholar]
- [78].Alderman MH, Ooi WL, Madhavan S, Cohen H.. Treatment-induced blood pressure reduction and the risk of myocardial infarction. JAMA. 1989;262:920–924. [PubMed] [Google Scholar]
- [79].Polese A, De Cesare N, Montorsi P, Fabbiocchi F, Guazzi M, Loaldi A, Guazzi MD.. Upward shift of the lower range of coronary flow autoregulation in hypertensive patients with hypertrophy of the left ventricle. Circulation. 1991;83:845–853. doi: 10.1161/01.cir.83.3.845. [DOI] [PubMed] [Google Scholar]
- [80].Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A. et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery Disease be dangerous? Ann Intern Med. 2006;144:884–893. doi: 10.7326/0003-4819-144-12-200606200-00005. [DOI] [PubMed] [Google Scholar]
- [81].Rizvi AA.. Addressing hypertension in the patient with type 2 diabetes mellitus: pathogenesis, goals, and therapeutic approach. Eur Med J Diabetes. 2017;5(1):84–92. [PMC free article] [PubMed] [Google Scholar]
- [82].Chrysant SG.. Current status of aggressive blood pressure control. World J Cardiol. 2011;3(3):65–71. doi: 10.4330/wjc.v3.i3.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rahman F, McEvoy JW.. The J-shaped Curve for Blood Pressure and Cardiovascular Disease Risk: Historical Context and Recent Updates. Curr Atheroscler Rep. 2017;19(8):34. doi: 10.1007/s11883-017-0670-1. [DOI] [PubMed] [Google Scholar]
- [84].Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA. et al. Consensus Statement By the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2018 Executive Summary. Endocr Pract. 2018;24(1):91–120. doi: 10.4158/CS-2017-0153. [DOI] [PubMed] [Google Scholar]
- [85].James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- [86].American Diabetes Association’s Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(1):S1–159. [Google Scholar]
- [87].Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;pii:HYP.0000000000000065. doi: 10.1161/HYP.0000000000000065. [DOI] [Google Scholar]
- [88].Leung AA, Daskalopoulou SS, Dasgupta K, McBrien K, Butalia S, Zarnke KB. et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557–576. doi: 10.1016/j.cjca.2017.03.005. [DOI] [PubMed] [Google Scholar]
- [89].de Boer IH, Bakris G, Cannon CP.. Individualizing Blood Pressure Targets for People With Diabetes and Hypertension: Comparing the ADA and the ACC/AHA Recommendations. JAMA. 2018 doi: 10.1001/jama.2018.0642. [DOI] [PubMed] [Google Scholar]