Abstract
Magnusiomyces capitatus is a rare cause of fungal infection in immunocompromised patients, mainly seen in hematological malignancies. M capitatus infections are extremely rare in immunocompetent patients, as it is part of normal human microbial flora. We are presenting an extremely rare case of M capitatus peritonitis in an otherwise immunocompetent patient who suffered from gastrointestinal leakage due to pancreatitis. Fungal identification was performed at reference laboratory by phenotypic characteristics and DNA sequencing of target internal transcribed spacer region of the rRNA gene and the D1-D2 domain of the large-subunit rRNA gene and susceptibility testing by Clinical and Laboratory Standards Institute guidelines (document M27-S4) broth dilution method. He was successfully treated with a combination of surgical repair and voriconazole single therapy.
Keywords: Magnusiomyces capitatus, Geotrichum capitatum, Dipodascus capitatus, Trichosporon captiatum, Saprochaete capitata, Blastoschizomyces capitatus, peritonitis
Introduction
Magnusiomyces capitatus, previously known as Geotrichum capitatum, Dipodascus capitatus, Trichosporon captiatum, Saprochaete capitata, or Blastoschizomyces capitatus,1 is a rare cause of fungal infection in immunocompromised patients, mainly seen in hematological malignancies.2-17 M capitatus is extremely rare in immunocompetent patients, as it is part of normal human microbial flora.18 Presented here is a case of peritonitis infection with M capitatus without underlying malignancies.
Case Report
A 32-year-old alcoholic male with liver steatosis presented with hemorrhagic necrotizing pancreatitis with peritonitis and retroperitoneum involvement. He was started on conservative therapy and percutaneous irrigation and drainage. Unfortunately, he rapidly deteriorated on hospital day 4 into acute abdominal compartment syndrome with acute respiratory distress. He was intubated and underwent damage control laparotomy resulting in pancreatic necrosectomy with subtotal pancreatectomy, splenectomy, repair of superior mesenteric vein, and wedge liver biopsy. Intraoperatively, peripancreatic necrosis was noted to extend proximally to diaphragm with extensive dissection throughout the retroperitoneum and at the root of the small bowel retroperitoneal area. During his second relaparotomy on hospital day 5 for removal of abdominal packing, incidental duodenal and gastric enterotomies were noted and repaired. Retroperitoneal edema was much improved. Cholecystectomy was performed for eosinophilic cholecystitis. Large Davol sump drains were placed for postoperative irrigation. Whittman patch and wound vacuum-assisted closure were placed. He required prolonged intensive care unit (ICU) admission with mechanical ventilation. Four additional operations were required to reapproximate his abdominal fascia. Skin was eventually closed on hospital day 17.
His course was also complicated by pleural effusions, pulmonary embolism, and persistent fevers and leukocytosis. Pleural effusions were therapeutically drained and were culture negative. Heparin was bridged to warfarin for his pulmonary embolism. Meropenem, linezolid, and micafungin were started empirically on hospital day 19.
Peritoneal fluid was collected on hospital day 19 and sent for culture, which grew Klebsiella oxytoca and vancomycin-resistant Enterococcus faecium (VITEK2, bioMérieux, Durham, NC). There was suspicion of incomplete drainage of intraabdominal fluid, and so a retroperitoneal drain was placed by interventional radiology on hospital day 31. Culture of this retroperitoneal fluid grew vancomycin-resistant enterococci E faecium (VITEK2, bioMérieux) and M capitatus (identification by phenotypic characterization and DNA sequencing of targets internal transcribed spacer region of the rRNA gene and the D1-D2 domain of the large-subunit rRNA gene and the D1-D2 domain of the large-subunit rRNA gene by University of Texas Health Science, San Antonio, TX; see Figures 1-3). Peritoneal fluid was collected again from hospital day 40, and it grew M capitatus, K oxytoca, and Streptococcus sanguinis (VITEK2, bioMérieux). He also developed eosinophilia (absolute eosinophil count of 800) on hospital days 42 to 46.
Figure 1.
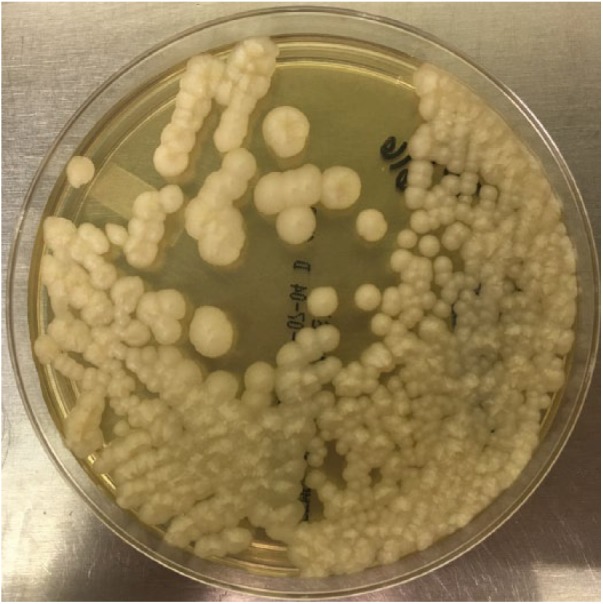
Magnusiomyces capitatus, peritoneal fluid, 5-day culture on Sabouraud Dextrose Agar, Emmons media (Thermo Scientific, Remel, Lenexa, KS).
Figure 2.
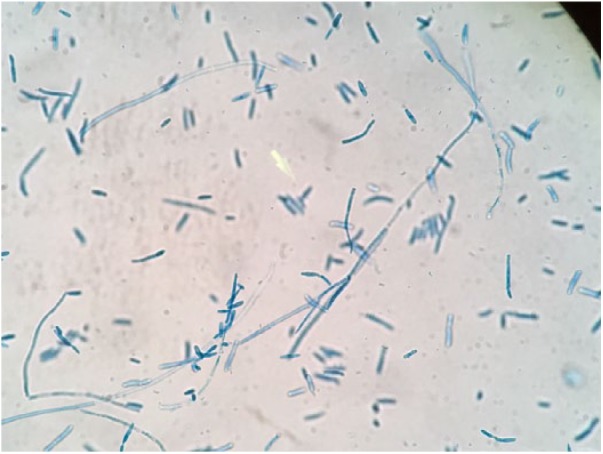
Magnusiomyces capitatus, peritoneal fluid, 2-day culture, lactophenol cotton blue stain, 40× magnification.
Figure 3.
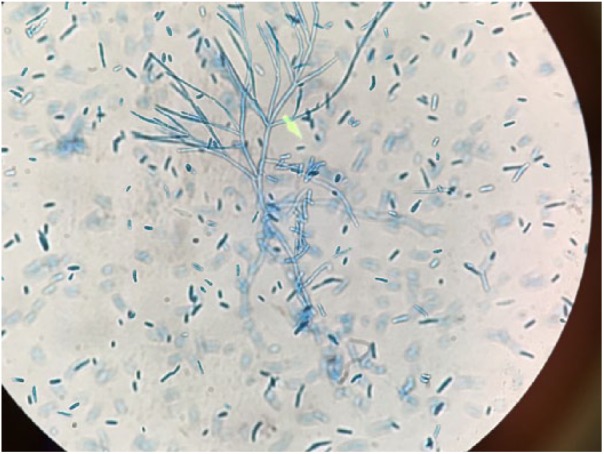
Magnusiomyces capitatus, retroperitoneal fluid, 7-day culture, lactophenol cotton blue stain, 40× magnification.
Meropenem was de-escalated to a 2-week course of ceftriaxone on hospital day 45 (changed to ciprofloxacin at discharge). Linezolid was discontinued after a 2-week course was completed. A 12-week course of voriconazole (minimum inhibitory concentration = 0.25 µg/mL by Clinical and Laboratory Standards Institute broth dilution M27-S4 method by the University of Texas Health Science, San Antonio, TX; see Table 1) was started on hospital day 45. Warfarin for his pulmonary embolism was switched to enoxaparin due to drug-drug interaction of warfarin with voriconazole. He started to improve and was eventually discharged home on hospital day 50 with follow-up in outpatient clinic, ambulating and tolerating food.
Table 1.
Magnusiomyces capitatus, Retroperitoneal Fluid, Antifungal Sensitivities by the University of Texas Health Science, San Antonio, TX, by the Clinical and Laboratory Standards Institute Broth Dilution (M27-S4) Method. No Established Break Points.
| Antifungal Agent | Minimum Inhibitory Concentration |
|---|---|
| Amphotericin B | 1 µg/mL |
| Natamycin | 8 µg/mL |
| Fluconazole | 8 µg/mL |
| Itraconazole | 1 µg/mL |
| Posaconazole | 0.5 µg/mL |
| Voriconazole | 0.25 µg/mL |
| Isavuconazole | 0.25 µg/mL |
At 12-week follow-up, the patient reported abstinence from alcohol since initial hospital admission. The patient’s wife was supportive during the entire hospital stay as well as the post hospital recovery, ensuring wound dressing changes and medication compliance. Liver function was monitored every 3 to 4 weeks as an outpatient throughout the 12-week course of voriconazole. Liver function was within normal limits. He completed a 90-day course of anticoagulation.
Discussion
To our knowledge, this is an extremely rare case of M capitatus peritonitis in an otherwise immunocompetent patient who suffered from gastrointestinal leakage due to pancreatitis, likely from the gastric and duodenal enterotomies found and repaired on hospital day 5. He was successfully treated with a combination of surgical repair and voriconazole.
Literature review suggests an intrinsic resistance to echinocandins19; however, in vitro and in vivo activity of antifungals may differ. Liposomal amphotericin B and azoles, specifically voriconazole and posaconazole, have had reported clinical success.7,16,20 In vitro studies with flucytosine, fluconazole, and itraconazole showed poor susceptibilities.21 No susceptibility break points have been determined yet.
The newest triazole, isavuconazole, demonstrated excellent in vitro activity against M capitatus.22 In the SECURE trial, a phase 3, randomized, controlled, noninferiority clinical trial against aspergillus and other filamentous fungi, isavuconazole was equally tolerable but had better pharmacokinetics and fewer drug-related adverse events compared with voriconazole.23 Due to identical minimum inhibitory concentration of our patient’s isolate to voriconazole and isavuconazole (see Table 1), voriconazole was selected as the initial triazole antifungal therapy so that isavuconazole could be reserved for rescue therapy in the event that voriconazole did not improve clinical status. Recently, ICU admissions have been linked to the development of M capitatus infection. In Italy, a non-neutropenic patient in the ICU after cardiac surgery developed M capitatus fungemia.24 In Croatia, a fatal M capitatus respiratory tract infection was diagnosed posthumously in a patient who became febrile 7 days into his ICU admission for polytrauma.25 Moreover, a recent survey of M capitatus infections in the ICU and hematology-oncology unit within a single hospital in Turkey found the strains to be genetic clones. However, microbiological investigations of the hospital environment failed to find the isolate.26 While M capitatus is considered a ubiquitous environmental organism and part of the normal human gastrointestinal flora,18 to the authors’ knowledge, there has not been any case reports or studies tracing M capitatus to a hospital fomite. More studies are needed to determine a true correlation between ICU admissions and M capitatus infections.
Vancomycin-resistant E faecium, S sanguinis, and K oxytoca likely had a gastrointestinal instead of a cutaneous source. While coinfection may have caused peritonitis in this patient, his clinical status did not improve until the addition of the appropriate antifungal covering this particular strain of M capitatus.
Acknowledgments
The authors wish to acknowledge the contributions of the following: Danna Mejia, Jocelyn Oats, and Joan Buddecke.
Footnotes
Authors’ Note: This case report was presented as a poster at the Solomon Scholars Research Program at UCLA Department of Medicine, in June 2017; American College of Physicians Southern California Chapters Regions I, II, and III at Marina del Rey, California, in September 2017; as well as American Federation for Medical Research Western Medical Research Conference in Carmel, California, in January 2018.
Declaration of Conflicting Interests: The authors(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Ethical approval to report this case was obtained from the Kern Medical Center Institutional Review Board (Study #17037).
Informed Consent: Informed consent for patient information to be published in this article was not obtained because patient or legal representative was not available in time for publication. The information in the investigator’s written request for “Waiver of Consent” coupled with the written research proposal disclosing the data use plan were reviewed by the Kern Medical Center Institutional Review Board to determine that under the conditions of study approval, there should be minimal or less risk for exposure of patient identity. The Kern Medical Center Institutional Review Board approved the request for the Waiver of Consent as part of its ethics approval of the study.
ORCID iD: Carlos D’Assumpcao  https://orcid.org/0000-0001-9967-9612
https://orcid.org/0000-0001-9967-9612
References
- 1. de Hoog GS, Smith MT. Ribosomal gene phylogeny and species delimitation in Geotrichum and its teleomorphs. Stud Mycol. 2004;50:489-515. [Google Scholar]
- 2. Martino P, Venditti M, Micozzi A, et al. Blastoschizomyces capitatus: an emerging cause of invasive fungal disease in leukemia patients. Rev Infect Dis. 1990;12:570-582. [DOI] [PubMed] [Google Scholar]
- 3. Pagano L, Morace G, Ortu-La Barbera E, Sanguinetti M, Leone G. Adjuvant therapy with rhGM-CSF for the treatment of Blastoschizomyces capitatus systemic infection in a patient with acute myeloid leukemia. Ann Hematol. 1996;73:33-34. [DOI] [PubMed] [Google Scholar]
- 4. Schiemann R, Glasmacher A, Bailly E, et al. Geotrichum capitatum septicemia in neutropenic patients: case report and review of the literature. Mycoses. 1998;41:113-116. [DOI] [PubMed] [Google Scholar]
- 5. DeMaio J, Colman L. The use of adjuvant interferon-gamma therapy for hepatosplenic Blastoschizomyces capitatus infection in a patient with leukemia. Clin Infect Dis. 2000;31:822-824. [DOI] [PubMed] [Google Scholar]
- 6. Pérez-Sanchez I, Anguita J, Martín-Rabadan P, et al. Blastoschizomyces capitatus infection in acute leukemia patients. Leuk Lymphoma. 2000;39:209-212. [DOI] [PubMed] [Google Scholar]
- 7. Gadea I, Cuenca-Estrella M, Prieto E, et al. Genotyping and antifungal susceptibility profile of Dipodascus capitatus isolates causing disseminated infection in seven hematological patients of a tertiary hospital. J Clin Microbiol. 2004;42:1832-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martino R, Salavert M, Parody R, et al. Blastoschizomyces capitatus infection in patients with leukemia: report of 26 cases. Clin Infect Dis. 2004;38:335-341. [DOI] [PubMed] [Google Scholar]
- 9. Girmenia C, Pagano L, Martino B, et al. GIMEMA infection program, invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: a retrospective multicenter study from Italy and review of the literature. J Clin Microbiol. 2005;43:1818-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonini A, Capatti C, Parmeggiani M, et al. Galactomannan detection in Geotrichum capitatum invasive infections: report of 2 new cases and review of diagnostic options. Diagn Microbiol Infect Dis. 2008;62:450-452. [DOI] [PubMed] [Google Scholar]
- 11. Özkaya-Parlakay A, Cengiz AB, Karadağ-Öncel E, et al. Geotrichum capitatum septicemia in a hematological malignancy patient with positive galactomannan antigen: case report and review of the literature. Turk J Pediatr. 2012;54:674-678. [PubMed] [Google Scholar]
- 12. Saghrouni F, Abdeljelil JB, Youssef YB, et al. Geotrichum capitatum septicemia in patients with acute myeloid leukemia. Report of their cases. Med Mycol Case Reports. 2012;1:88-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miglietta F, Vella A, Faneschi ML, et al. Geotrichum capitatum septicaemia in a haematological patient after acute myeloid leukaemia relapse: identification using MALDI-TOF mass spectrometry and review of the literature. Infez Med. 2015;23:161-167. [PubMed] [Google Scholar]
- 14. Trabelsi H, Néji S, Gargouri L, et al. Geotrichum capitatum septicemia: case report and review of the literature. Mycopathologia. 2015;179:465-469. [DOI] [PubMed] [Google Scholar]
- 15. Karapinar DY, Karadaş N, Siviş ZO, et al. Rare severe mycotic infections in children receiving empirical caspofungin treatment for febrile neutropenia. Braz J Infect Dis. 2015;19:549-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cofrancesco E, Viviani MA, Boschetti C, Tortorano AM, Balzani A, Castagnone D. Treatment of chronic disseminated Geotrichum capitatum infection with high cumulative dose of colloidal amphotericin B and itraconazole in a leukaemia patient. Mycoses. 2016;38:377-384. [DOI] [PubMed] [Google Scholar]
- 17. Tanuskova D, Horakova J, Svec P, et al. First case of invasive Magnusiomyces capitatus infection in Slovakia. Med Mycol Case Rep. 2017;16:12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouza E, Muñoz P. Invasive infections caused by Blastoschizomyces capitatus and Scedosporium spp. Clin Microbiol Infect. 2004;10(suppl 1):76-85. [DOI] [PubMed] [Google Scholar]
- 19. Arendrup MC, Boekhout T, Akova M, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20(suppl 3):76-98. [DOI] [PubMed] [Google Scholar]
- 20. Birrenbach T, Bertschy S, Aebersold F, et al. Emergence of Blastoschizomyces capitatus yeast infections, Central Europe. Emerg Infect Dis. 2012;18:98-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Girmenia C, Pizzarelli G, D’Antonio D, Cristini F, Martino P. In vitro susceptibility testing of Geotrichum capitatum: comparison of the E-Test, disk diffusion, and Sensititre colorimetric methods with the NCCLS M27-A2 broth microdilution reference method. Antimicrob Agents Chemother. 2003;47:3985-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson GR, 3rd, Wiederhold NP, Sutton DA, Fothergill A, Patterson TF. In vitro activity of isavuconazole against Trichosporon, Rhodotorula, Geotrichum, Saccharomyces and Pichia species. J Antimicrob Chemother. 2009;64:79-83. [DOI] [PubMed] [Google Scholar]
- 23. Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760-769. [DOI] [PubMed] [Google Scholar]
- 24. Cavanna C, Lallitto F, Mangione F, Tamarozzi F, Marone P, Ceriana P. Fungemia due to Saprochaete capitata in a non-neutropenic patient hospitalized in an intensive care unit after cardiac surgery. J Mycol Med. 2017;27:281-284. [DOI] [PubMed] [Google Scholar]
- 25. Radic M, Barisic IG, Kuscevic D, Novak A, Tonkic M, Rubic Z. Geotrichum capitatum respiratory tract infection in a patient with polytrauma. Infez Med. 2015;23:270-274. [PubMed] [Google Scholar]
- 26. Koç AN, Atalay MA, Timur D, Demir G, Kaynar L. Molecular epidemiology and antifungal susceptibility of Saprochaete capitata (Blastoschizomyces capitatus) isolates causing nosocomial infection in Kayseri/Turkey. Infect Dis (Lond). 2016;48:596-603. [DOI] [PubMed] [Google Scholar]


