Abstract
Here, accumulation of glucosinolates and expression of glucosinolates biosynthesis genes in green and red mustard hairy roots were identified and quantified by HPLC and qRT-PCR analyses. The total glucosinolates content of green mustard hairy root (10.09 µg/g dry weight) was 3.88 times higher than that of red mustard hairy root. Indolic glucosinolates (glucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin) in green mustard were found at 30.92, 6.95, and 5.29 times higher than in red mustard hairy root, respectively. Conversely, levels of glucotropaeolin (aromatic glucosinolate) was significantly higher in red mustard than in green mustard. Accumulation of glucoraphasatin, an aliphatic glucosinolate, was only observed only in red mustard hairy roots. Quantitative real-time PCR analysis showed that the expression level of genes related to aliphatic and aromatic glucosinolate biosynthesis were higher in red mustard, exception BjCYP83B. The expression of BjCYP79B2, which encodes a key enzyme involved in the indolic glucosinolate biosynthetic pathway, was higher in green mustard than in red mustard. Additionally, to further distinguish between green mustard and red mustard hairy roots, hydrophilic and lipophilic compounds were identified by gas chromatography–mass spectrometry and subjected to principal component analysis. The results indicated that core primary metabolites and glucosinolate levels were higher in the hairy roots of green mustard than in those of red mustard.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1393-x) contains supplementary material, which is available to authorized users.
Keywords: Glucosinolate, Hairy roots, Mustard, Brassica juncea, Metabolite profiling
Introduction
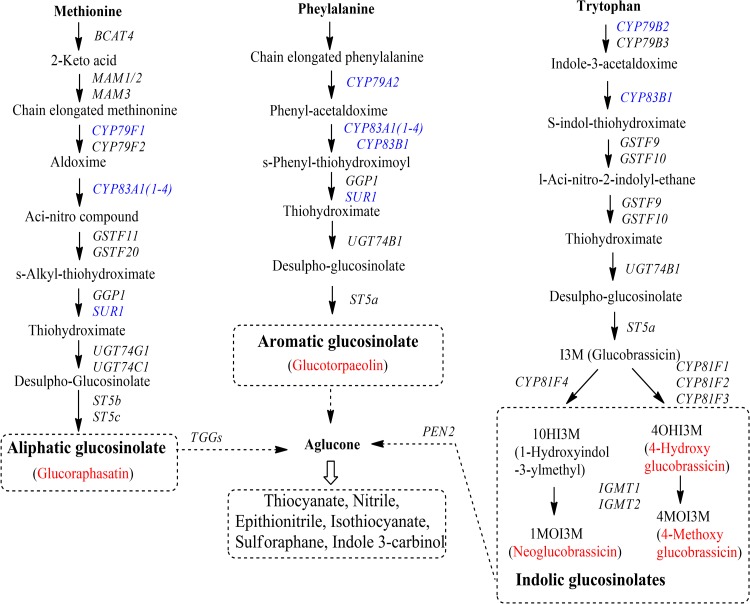
Glucosinolates (GSLs) are a large group of plant secondary metabolites that contain sulfur and nitrogen and are derived from glucose and an amino acid. These compounds likely contribute to plant defenses against pests and diseases (Halkier and Du 1997). GSLs are largely found in members of the family Brassicaceae, such as Nasturtium officinale (Il Park et al. 2011), Brassica napus (Velasco et al. 2008), cabbage (B. oleracea) (Choi et al. 2014), Chinese cabbage (B. rapa ssp) (Kim et al. 2013a), and B. juncea (Yang et al. 2014). Mustard (B. juncea) has been shown to be a rich source of pharmaceutical compounds, including ascorbic acid, GSLs, phenolics, β-carotene, and flavonoids (Ismail and Cheah 2003; Guo et al. 2005; Antonious et al. 2009; Lin and Harnly 2010; Lin et al. 2011; Kim et al. 2016). GSLs are synthesized from amino acids such as methionine, alanine, valine, phenylalanine, isoleucine, tyrosine, and tryptophan, and can be divided into aliphatic, indolic, and aromatic GSLs that are predominantly derived from methionine, tryptophan, and phenylalanine, respectively (Fig. 1). The genes involved in the GSL biosynthesis pathways have been identified in many plant species. However, only a few genes, such as cytochrome P450 83A1 (CYP83A1) (Meenu et al. 2015), dihomomethionine N-hydroxylase (CYP79F1) (Sharma et al. 2016), and transcription factor MYB28 (Augustine et al. 2013), have been reported in mustard.
Fig. 1.
Glucosinolate biosynthetic pathways in plant. Red color denotes the GSLs measured in this study by HPLC analysis and blue color indicates enzymatic activities for which gene expression was monitored via real-time PCR
Hairy root cultures obtained by genetic transformation in plants by Agrobacterium rhizogenes are characterized by infinite and rapid growth and metabolic stability (Wielanek and Urbanek 1999); these cultures have generated interest as an alternative to cell suspensions for the production of secondary metabolites for use as pharmaceuticals, food additives, and in cosmetics (Giri and Narasu 2000; Ludwig-Muller et al. 2014; Murthy et al. 2008; Srivastava and Srivastava 2007). Recently, the GSL content was measured in hairy root cultures generated using A. rhizogenes transformation of plant species such as watercress (Il Park et al. 2011), Barbarea verna (Wielanek et al. 2009), Arabis caucasica (Wielanek et al. 2009), Tropaeolum majus (Wielanek and Urbanek 1999, 2006), Arabidopsis thaliana (Kastell et al. 2013a), Sinapis alba (Kastell et al. 2013b), B. rapa (Kastell et al. 2013b), and broccoli (B. oleracea var. italica) (Kim et al. 2013b). However, GSL production by mustard hairy root has not been reported to date.
In metabolomics analysis, the use of chemometric techniques like principal components analysis (PCA) and partial least square-discriminate analysis allow for the classification of samples with diverse biological status, classification according to their origin, or quality (Kim et al. 2014). Metabolic profiling using gas chromatography–mass spectrometry (GC–MS) in conjunction with data mining tools is a technique commonly used for the comprehensive characterization of plant genotypes and in the field of functional genomics (Roessner et al. 2001). Through chemical derivatization of these hydrophilic metabolites, GC–MS allows researchers to determine the levels of primary metabolites such as organic acids, amino acids, and sugars (Li et al. 2014). Using chemical derivatization of these lipophilic metabolites, Kim et al. (2015) characterized the policosanols and phytosterols in nine leafy vegetables include mustard, oak, Chinese mallow, chicon, kale, lettuce, tatsoi, and amaranth (Kim et al. 2015). The present study describes the expression of GSL biosynthesis genes and their accumulation in green mustard and red mustard hairy roots. Furthermore, hydrophilic and lipophilic metabolic profiling in mustard hairy roots using GC–MS was applied to determine the GSL variation between these mustard varieties and analyze the relationship between their levels in each.
Materials and methods
Plant material and induction of hairy roots
Green and red mustard (Brassica juncea) seeds were purchased from Asia Seeds Ltd. (Seoul, Korea). Dehulled seeds were sterilized with 70% (v/v) ethanol for 30 s and 1% (v/v) sodium hypochlorite solution for 10 min and were then germinated on half-strength Murashige and Skoog (1/2 MS) medium (Murashige and Skoog 1962) with 2.5 mM MES and 0.8% agar at pH 5.7 before being incubated at 25 °C under light/dark (16/8 h) conditions. Agrobacterium rhizogenes strain R1000 cultured on Luria–Bertani (LB) medium was used for infection of 2-week-old B. juncea seedlings. Leaves of mustard green and red were infected with A. rhizogenes strain R1000 for 10 min before being blotted dry with sterile filter paper, transferred to agar-solidified MS medium, and incubated in the dark at 25 °C. After 48 h of co-cultivation, samples were washed and transferred to plates containing agar-solidified 1/2MS with 250 mg/l of cefotaxime before being grown in the dark for 2 or 3 weeks. Hairy roots were then isolated and cultured singly on agar-solidified 1/2MS with 250 mg/l of cefotaxime. A single fast-growing clone from green or red mustard was used for further experimentation. Once fully grown the hairy roots were transferred to 1/2MS liquid medium and grown at 25 °C on gyratory equipment (100 rpm). Hairy roots were harvested after 4 weeks and immediately stored at − 80 °C for extraction of total DNA or RNA, analysis of GSL compounds, or GC–MS analysis.
Genomic DNA isolation and PCR analysis
Total DNA was isolated from green and red mustard hairy roots using the Plant Genomic DNA Mini Kit (Geneaid Biotech Ltd., New Taipei City, Taiwan) and the quality was determined by gel electrophoresis using a 1.2% agarose gel. For amplification, primers were designed to amplify the rol A, B, C, or D coding sequence, including rol A-F 5′ CATGTTTCAGAATGGAATTA and rol A-R 5′ AGCCACGTGCGTATTAATCC, rol B-F 5′ TCACAATGGATCCCAAATTG and rol B-R 5′ TTCAAGTCGGCTTTAGGCTT, rol C-F 5′ ATGGCTGAAGACGACCTGTGT and rol C-R 5′ TTAGCCGATTGCAAACTTGCA, rol D-F 5′ ATGGCCAAACAACTTTGCGA and rol D-R 5′ TTAATGCCCGTGTTCCATCG that amplified 360, 900, 514, or 1035 bp regions, respectively. The PCR amplification program was 95 °C for 3 min followed by 30 cycles of 95 °C for 30 s; 30 s annealing at 43 °C, 59 °C, 55 °C, or 57 °C for rol A, rol B, rol C, or rol D, respectively; and 72 °C for 1 min ending with a final extension at 72 °C for 10 min. After amplification, 20 µL of amplified PCR products were examined by gel electrophoresis on a 1.2% agarose gel.
RNA isolation and cDNA synthesis
The Easy BLUE total RNA kit (iNtRON, Seongnam, Korea) was used to extract total RNA; the quality of total extracted RNA from the hairy roots of different lines was determined by 1% agarose gel electrophoresis. ReverTra Ace-kit (Toyobo Co., Ltd, Osaka, Japan) was used for cDNA synthesis and 20-fold dilutions of these cDNA samples were used as the template for quantitative real-time PCR (qRT-PCR).
qRT-PCR analysis
qRT-PCR primers (Table 1) were designed using the Primer 3 website (http://bioinfo.ut.ee/primer3-0.4.0/primer3/) (Untergasser et al. 2012) based on the sequence of genes in the GSL pathway such as BjCYP79F1 (Accession Number KT254222.1), BjCYP83A1-1 (Accession Number KF111567.1), BjCYP83A1-2 (Accession Number KF111568.1), BjCYP83A1-3 (Accession Number KF111569.1), and BjCYP83A1-4 (Accession Number KF111570.1). Primer sequences for BjCYP79A2, BjCYP79B2, BjCYP83B1, and BjSUR1 were obtained from Yang et al. (2014). For qRT-PCR, the 20 µL reaction mixture contained 2 µL primers (10 µM), 5 µL diluted cDNA (20-fold dilution), 10 µL 2 × SYBR Green, and 3 µL water. The PCR conditions were: 3 min at 95 °C; 41 cycles of 15 s at 95 °C, 15 s at 56 °C, and 15 s at 72 °C and a final extension of 10 min at 72 °C. The expression of genes was calculated by relative quantification using the BjActin gene (GenBank: HM565958.1) as the reference; each reaction was completed in triplicate. PCR products were analyzed by Bio-Rad CFX Manager 2.0 software (Bio-Rad Laboratories, Hercules, CA, USA).
Table 1.
Primers used for real-time PCR
| Primers | Forward primer sequences (5′–3′) | Reverse primer sequences (5′–3′) |
|---|---|---|
| BjActin | CCGACCGTATGAGCAAGGAAAT | TTCCTGTGGAACCTGACTCAT |
| BjCYP79B2 | ACGAGATAAACCGGAGATCG | CGGAGAGACATCTCAACGAA |
| BjCYP83B1 | GGCCATGACAGAACTCGTTA | CCTCTCCTTGTTGAGCCCTA |
| BjCYP79A2 | GAGGCTCACCCTATTCCTGA | CCGGAGCATTTAGTCACGTA |
| BjCYP79F1 | GACAGGTAGCCACATTCACGTC | GACCAGAGAAAGCTCCTTCGAG |
| BjCYP83A1-1 | GGAGATGAGGAAGATGGGAATG | TTATATCGACAGGCTCGGCTCT |
| BjCYP83A1-2 | CCGCAGTTCTCTTCTTCATCCT | ACCGTTGTGGGTTAAGGTTCTG |
| BjCYP83A1-3 | ATTCCTCTCCTTGTCCCTCGTT | CGTAGTCCGTCCCTTTGAAGTC |
| BjCYP83A1-4 | CGAACCTGTGATTCCTCTCCTT | CCTCTCTGGTCTGAACTCGTCA |
| BjSUR1 | GACCAAACGCAAACATCTTG | AGAAGATCGAACTTGCGGAT |
High-performance liquid chromatography (HPLC) analysis
Extraction of desulfo-glucosinolates (DS-GSLs) and HPLC analysis were performed as presented by Kim et al. (2013a) (Kim et al. 2013a). Samples (100 mg) were subjected to extraction with 4.5 mL MeOH 70% (v/v) at 70 °C for 5 min in a water bath and centrifuged (12,000 rpm at 4 °C) for 10 min. The extract solution was loaded onto a DEAE-Sephadex A-25 mini-column and the GSLs were treated in the column with a 75 µL arylsulfatase solution (H-1 type from Sigma, St. Louis, MO, USA). Following an overnight desulfation reaction (16–18 h) at room temperature, DS-GSL samples were eluted into a 2 mL microcentrifuge tube with 0.5 mL (threefold sample volume) of ultrapure water. An Agilent Technologies 1260 series HPLC system (Agilent Technologies, Palo Alto, CA, USA) was used for the analysis at a wavelength of 227 nm, column oven temperature of 40 °C, and flow rate of 0.4 mL/min. An Inertsil ODS-3 chromatographic column (GL Sciences, Tokyo, Japan) was used with an internal diameter of 150 × 3.0 mm and a particle size of 3 µm. A mobile phase composed of A (water) and B (CH3CN) with gradient programs was used as follows: 0% B at 0 min, 0% B at 2 min, 10% B at 7 min, 31% B at 16 min, 31% B at 19 min, 0% B at 21 min (total 27 min). We used the standard methods reported by ISO 9167-1 (1992) to identify and quantify individual GSLs. Briefly, individual GSLs were identified by comparing both the retention times and the peak areas with a sinigrin standard. Measurements were performed in triplicate.
Extraction of hydrophilic metabolites and GC–MS analysis
The hydrophilic metabolites were extracted and analyzed according to the method of Kim et al. (2013c). Freeze-dried samples (10 mg) were added to 1 mL of mixed methanol:water:chloroform solvent (2.5:1:1 by vol.) and 0.06 mL of ribitol (200 µg/mL H2O) was used an internal standard. Samples were incubated at 37 °C for 30 min with 1200 rpm shaking using a thermomixer comfort (model 5355, Eppendorf AG, Hamburg, Germany). After mixing, the samples were centrifuged at 16,000×g for 3 min at 4 °C. For each sample, 0.8 mL of the upper layer was pipetted into a new tube and 0.4 mL of water was added. After mixing and centrifuging at 16,000×g for 3 min, 0.9 mL of the upper layer was pipetted into a new tube. Finally, the liquid phase was completely dried using a centrifugal concentrator (CC-105, TOMY, Tokyo, Japan) and freeze-drier (MCFD8512, IlShinBioBase, Gyeonggi, Korea). For derivatization, 0.08 mL of methoxamine (MOX) reagent was added to the dried samples and they were incubated at 30 °C for 90 min with 1200 rpm shaking. Next, 0.08 mL of N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) was added and the samples were incubated again at 37 °C for 30 min with 1200 rpm shaking. The derivatized samples were analyzed with the GCMS-QP2010 Ultra system (Shimadzu, Japan), with a DB-5 column (30 m, 0.25 mm inner diameter, and film thickness of 1 µm). Helium was used as the carrier gas with a flow rate through the column of 1.0 mL/min. The injector temperature was 280 °C and the split ratio was set at 1:10. The temperature program was as follows: 100 °C for 4 min, followed by an increase to 320 °C at a rate of 10°C/min, and an 11 min hold at 320 °C. The interface temperature was 280 °C and ion source 200 °C. The scanned mass range and the ionization voltage were 45–600 (m/z) and 70 (eV), respectively. Each sample was performed in triplicate. The retention index for each peak was calculated by comparing the retention times against those of a C7–C30 alkane series. Mainlib and replib in the NIST (National Institute of Standards and Technology) Library, Wiley Chemical Structural Libraries (Excalibur software, Thermo Inc.), and OA TMS DB5 Library (Shimadzu, Japan) were used as standard chemicals when identifying the compounds. Chromatogram acquisition and detection of mass spectral peaks were performed using Shimadzu GC–MS solution software (ver. 4.11) that provided a GC–MS metabolite database equipped with retention indices.
Extraction of lipophilic metabolites and GC–MS analysis
Extraction of lipophilic metabolites and GC–MS analysis were carried out as previously described by Kim et al. (2015). To analyze lipophilic compounds, 50 mg of freeze-dried powder was extracted with 3 mL ethanol containing 0.1% ascorbic acid (w/v). Next, 5 µL of 5α-cholestane (10 µg/mL hexane) was added as an internal standard. After vortexing, samples incubated in a water-bath at 85 °C for 5 min. Next, 120 µL of potassium hydroxide (80%, w/v) was added and the samples were vortexed and incubated at 85 °C for 10 min. After saponification, the samples were put on ice for 5 min, 1.5 mL each of deionized water and hexane were added, and samples were vortexed and then centrifuged for 5 min at 4 °C (1200 rpm). The supernatant was transferred to a new tube, and the pellet was re-extracted by 1.5 mL of hexane. The hexane layer was dried in a centrifugal concentrator. After adding 30 µL of MSTFA and 30 µL of pyridine, the sample was incubated at 60 °C for 30 min at 1200 rpm for derivatization. The derivatized sample was analyzed with the GCMS-QP2010 Ultra system, with an Rtx-5MS column (30 m, 0.25 mm inner diameter, and film thickness of 0.25 µm). Setup was 1.0 mL/min for flow rate of helium gas, the split ratio was set at 1:10 and injector temperature at 290 °C. The temperature program was as follows: 150 °C for 2 min, followed by an increase to 320 °C at 15°C/min and then a hold at 320 °C for 10 min. The temperatures of the interface and ion source were 280 °C and 230 °C, respectively. The scanned mass range was 85–600 (m/z). Each sample was performed in triplicate.
Statistical analysis
Quantification and analysis were each performed in triplicate and analyzed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA, 2009). PCA (SIMCA-P version 13.0; Umetrics, Umeå, Sweden) was used to evaluate the relationships in terms of similarities or dissimilarities among groups of multivariate data from GC–MS data. Likewise, samples were clustered on the basis of their metabolite profiles through hierarchical clustering analysis (HCA), with Pearson correlation and average linkage.
Results and discussion
Hairy root induction with A. rhizogenes strain R1000
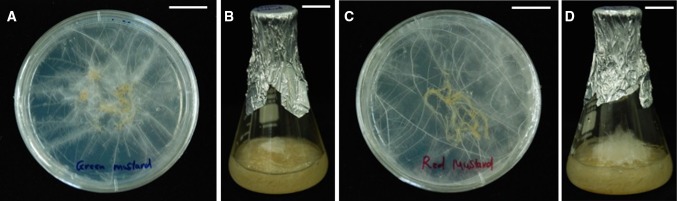
Agrobacterium rhizogenes strain R1000 induced extensive root development from wounding sites on B. juncea. Hairy roots initially emerged from the infected leaf explants 5–7 days after inoculation. Over the next 2–3 weeks the hairy roots began to grow more rapidly. While the non-transformed roots failed to grow on MS medium devoid of phytohormones, the transformed hairy roots grew pleiotropically and showed extensive branching. Approximately 4 weeks after co-cultivation with A. rhizogenes, the hairy roots were excised from the necrotic explant tissues and subcultured on fresh agar-solidified medium containing 250 mg/L cefotaxime (Fig. 2a, c). Five green mustards hairy roots and six red mustards hairy roots lines were created. After consecutive transfers to fresh medium over 1–2 months, one hairy root line grows better of each mustard cultivars were transferred to liquid culture (Fig. 2b, d) where they grew well in 100 mL flasks for 3 weeks. PCR amplification of DNA from the hairy roots of two mustard cultivars with primers specific for the Rol A, B, C, and D sequences indicated that the rol gene of the Ri plasmid in A. rhizogenes is responsible for the induction of hairy roots in plant species. Hairy roots gave the expected bands for rol A (360 bp), rol B (900 bp), rol C (514 bp), and rol D (1035 bp) genes, whereas the wild-type (control) root was negative for rol genes, thus confirming transformation (data not shown).
Fig. 2.
Green (a, b) and red (c, d) mustard hairy roots. The scale bars represent 2 cm
Expression of GSL biosynthesis genes in hairy roots of B. juncea
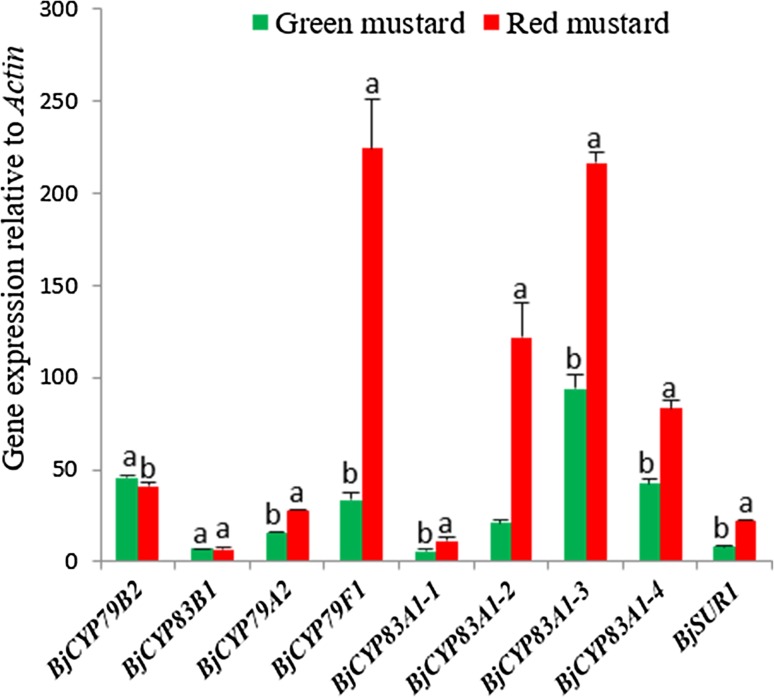
qRT-PCR was used to determine the expression of GSL biosynthetic genes in mustard hairy roots. Nine genes in GSL biosynthesis pathways (Fig. 1) were examined. The expression levels of BjCYP79B2, BjCYP83B1, BjCYP79A2, BjCYP79F1, BjCYP83A1-1, BjCYP83A1-2, BjCYP83A1-3, BjCYP83A1-4, and BjSUR1 are shown in Fig. 3. Expression of genes related to aliphatic and aromatic GSLs biosynthesis, including BjCYP79F1, BjCYP83A1-1, BjCYP83A1-2, BjCYP83A1-3, BjCYP83A1-4, BjSUR1, and BjCYP79A2, were higher in red mustard than in green mustard. In particular, the expression of BjCYP79F1 (6.72-fold), BjCYP83A1-2 (5.79-fold), and BjSUR1 (2.9-fold) were significantly higher in red mustard hairy roots than in green mustard hairy roots. Conversely, expression of BjCYP79B2, which is a key enzyme in the indolic GSL biosynthetic pathway, was higher in green mustard than in red mustard. Additionally, BjCYP83B1 showed similar expression patterns in both cultivars. These results suggest that the higher expression of BjCYP79F1, BjCYP83A1-1, BjCYP83A1-2, BjCYP83A1-3, BjCYP83A1-4, BjSUR1, and BjCYP79A2 in red mustard may lead to greater accumulation of aliphatic and aromatic GSL in red mustard than in green mustard. Conversely, the higher expression BjCYP79B2 in green mustard than in red mustard may result in higher accumulation of indole GSLs in green mustard.
Fig. 3.
Expression levels of genes in glucosinolate biosynthesis pathway in green and red mustard hairy root lines. Three replications for each sample were used in the real-time PCR analyses. Letters a and b indicate significant differences among cultivar (p < 0.05)
GSL content of hairy roots of B. juncea
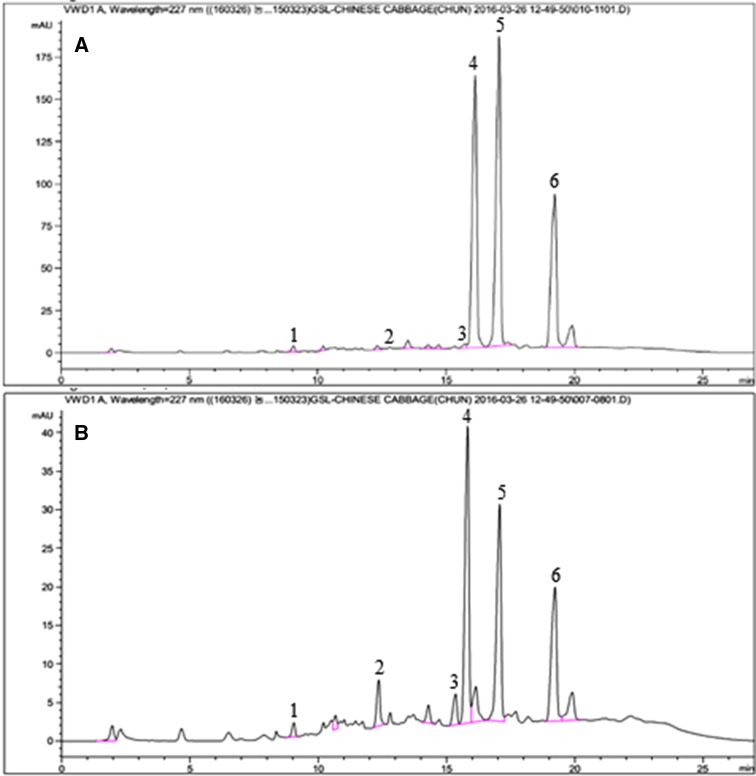
Recently, GSL compounds have been investigated and reported in the leaves of B. juncea. For example, Carlson et al. (1987) identified four GSL compounds including sinigrin, gluconapin, glucobrassicin, and gluconasturtiin, while several GSL compounds such as glucoiberin, sinigrin, gluconapin, glucoiberverin, glucobrassicanapin, glucobrassicin, and gluconasturtiin were identified (Cole 1997) from the leaves of B. juncea. Additionally, Kim et al. (2016) discovered a total of 11 individual GSL compounds, including two new compounds, in the leaves of B. juncea var. integrifolia. Prior to this study, the accumulation of GSL compounds in mustard hairy root cultures had not previously been reported. In this study, green and red mustard were transformed with A. rhizogenes strain R1000. Using HPLC analysis, six GSLs, including glucoraphasatin, 4-hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, neoglucobrassicin, and glucotropaeolin, were identified in hairy roots of mustard (Table 2; Fig. 4). The total GSL content of green mustard hairy root (10.09 µg/g dry weight) was 3.88 times higher than that of red mustard hairy root (2.60 µg/g dry weight). The main compound observed in red mustard hairy root was glucoraphasatin, which accounted for 45.4% of total GSLs; in green mustard hairy root, the main compounds were glucobrassicin and 4-methoxyglucobrassicin (accounting for 39.8% and 39.2% total GSLs, respectively). This is similar to results previously reported for kale hairy root (Lee et al. 2016). In green mustard hairy root, the concentration of glucobrassicin was highest, followed by 4-methoxyglucobrassicin, neoglucobrassicin, glucotropaeolin, and 4-hydroxyglucobrassicin; glucoraphasatin was not detected. In red mustard hairy root, however, the concentration of glucoraphasatin was the highest, followed by that of 4-methoxyglucobrassicin, glucotropaeolin, neoglucobrassicin, and glucobrassicin; 4-hydroxyglucobrassicin was not detected in red mustard hairy root. The accumulation of glucobrassicin (4.02 µg/g dry weight), 4-methoxyglucobrassicin (3.96 µg/g dry weight), and neoglucobrassicin (1.85 µg/g dry weight) in green mustard were 30.92, 6.95, and 5.29 times higher than in red mustard hairy root, respectively. The accumulation of glucobrassicin in red mustard was 0.13 µg/g dry weight, similar to the results observed in watercress hairy roots, the dry weight of which ranged from 0.1 to 0.2 µg/g (Il Park et al. 2011). The higher accumulation of aliphatic GSL (glucoraphasatin) in red mustard correlated with high expression of BjCYP79F1, BjCYP83A1-1, BjCYP83A1-2, BjCYP83A1-3, BjCYP83A1-4, and BjSUR1 genes in red mustard hairy root. We also observed a higher level of aromatic GSL (glucotropaeolin) in red mustard than in green mustard, potentially because of the higher expression of BjCYP79A2 in red mustard. The high expression of BjCYP79B2 in green mustard correlated positively with the higher levels of several indolic GSLs (4-hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin) in green mustard than in red mustard.
Table 2.
Glucosinolates content (µg/g dry weight) in hairy root of green and red mustard
| No. | Trivial name | Green mustard | Red mustard |
|---|---|---|---|
| 1 | Glucoraphasatin | ND | 1.18 ± 0.07 |
| 2 | 4-Hydroxyglucobrassicin | 0.10 ± 0.02 | ND |
| 3 | Glucobrassicin | 4.02 ± 0.23 | 0.13 ± 0.01 |
| 4 | 4-Methoxyglucobrassicin | 3.96 ± 0.25 | 0.57 ± 0.03 |
| 5 | Neoglucobrassicin | 1.85 ± 0.12 | 0.35 ± 0.01 |
| 6 | Glucotropaeolin | 0.17 ± 0.00 | 0.38 ± 0.02 |
| 7 | Total glucosinolate | 10.09 ± 0.62 | 2.60 ± 0.12 |
Values are the means from three independent experiments ± SD (n = 3). Mean values indicated by the same letter are not significantly different at least significant differences (LSD) (p = 0.05)
ND not detected
Fig. 4.
HPLC chromatograms of glucosinolates analysis from hairy root of green (a), and red (b) mustard. 1— Glucotrpaeolin; 2—4-hydroxyglucobrassicin; 3—glucoraphasatin; 4—glucobrassicin; 5—4-methoxyglucobrassicin; 6—neoglucobrassicin
Metabolic profile of B. juncea hairy roots
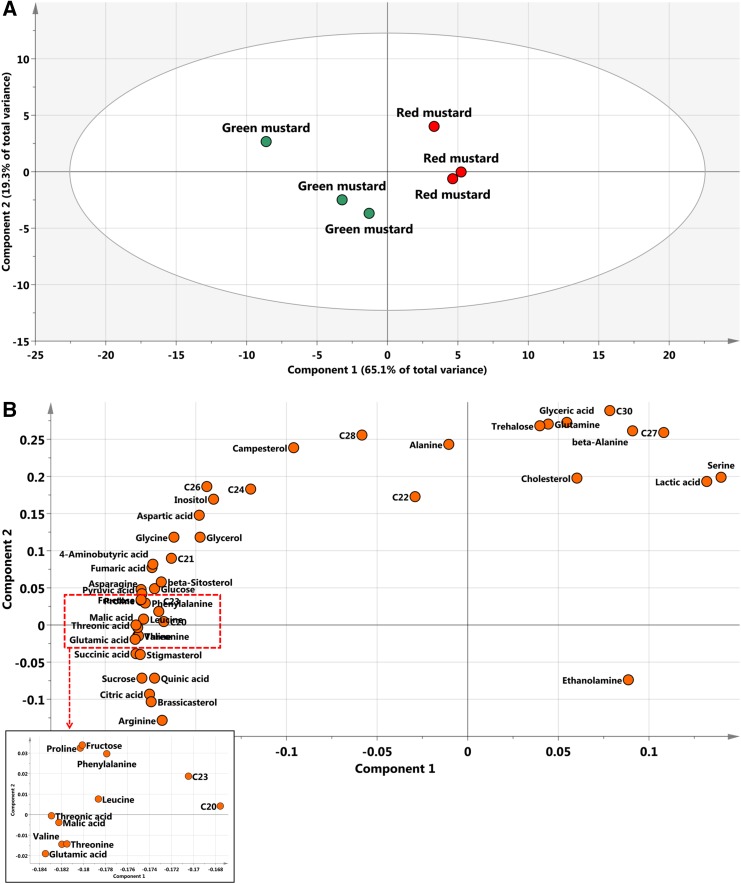
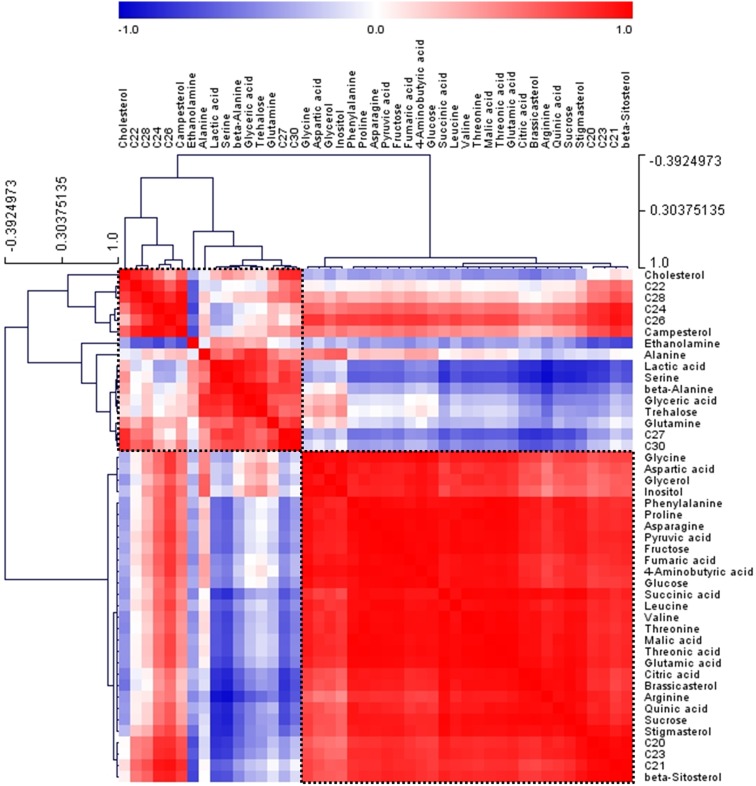
To identify and compare differences in metabolites between green and red mustard hairy roots, GC–MS was used. The LabSolutions GC–MS solution (version 4.11, Shimadzu) software was used to assist with peak location. Peak identification of hydrophilic metabolites was performed by comparing the reference compounds and using an in-house library. Quantification was performed using selected ions and quantitative calculations were based on the peak area ratios relative to that of the internal standard (IS). In two cultivars, 31 hydrophilic metabolites (nine organic acids, 15 amino acids, four sugars, two sugar alcohols, and one amine) (Online Resource 1) and 14 lipophilic (nine policosanols and five phytosterols) (Online Resources 2 and 3) were detected. GC–MS was previously used to analyze lipophilic metabolites in rice by Kim et al. (2012), while 22 lipophilic compounds were also detected in nine vegetables consumed in Korea using GC–MS (Kim et al. 2015). The corresponding retention times and their fragment patterns are given in Online Resource 2; these were similar to those previously reported (Kim et al. 2015). PCA was conducted to evaluate the relationships between the two mustard cultivars. The abscissa represents the principal component one (PC1) score, while the ordinate represents the principal component two (PC2) score. As shown in Fig. 5, the first and second PCs of the PCA score plot accounted for 89.4% of the total variance. The PCA results with metabolite profiles showed the absence of significant variances between the two mustard cultivars (Fig. 5). The PC1, accounting for 65.1% of the total variance, resolved the cultivars according to their total metabolites. The metabolic loadings of principal components (PC1 and PC2) were compared to investigate the contributors to the components. In PC1, the corresponding loading was negative for most of the metabolites. The loading indicated that the levels of most amino acids, pyruvic acid, and intermediates of the tricarboxylic acid cycle such as succinic acid, fumaric acid, and citric acid were higher in green mustard than in red mustard. The primary metabolites were tightly associated with the production of secondary metabolites. Thus, the differences in the GSL content of the two cultivars appeared to follow the same pattern as was observed with their precursors. Pearson’s correlation analyses of the accessions were performed to characterize detailed relationships between the 45 metabolites in mustard. The result matrix with HCA shown in Fig. 6 indicates that the correlation coefficients defined two major metabolite clusters (boxed within dotted lines). Intermediates of the tricarboxylic cycle such as succinic acid, fumaric acid, and citric acid were clustered.
Fig. 5.
a Scores and b loading plots of principal components 1 and 2 of the PCA results. C20, eicosanol; C21, heneicosanol; C22, docosanol; c23, tricosanol; C24, tetracosanol; C26, hexacosanol; C27, heptacosanol; C28, octacosanol; C30, triacontanol
Fig. 6.
Correlation matrix of 45 metabolites from two mustard cultivars. Each square indicates Pearson’s correlation coefficient for a pair of compounds, and the value of the correlation coefficient is represented by the intensity of blue or red, as indicated on the color scale. Hierarchical clusters are represented in a cluster tree. C20, eicosanol; C21, heneicosanol; C22, docosanol; c23, tricosanol; C24, tetracosanol; C26, hexacosanol; C27, heptacosanol; C28, octacosanol; C30, triacontanol
Conclusion
In this study, hairy root cultures of two mustard cultivars (green and red) were generated by transformation with the bacterium A. rhizogenes strain R1000. The expression levels of genes associated with the GSL biosynthetic pathway were examined in both green and red mustard hairy roots. Moreover, to fully distinguish between green mustard and red mustard hairy roots, their hydrophilic and lipophilic compounds were identified by GC–MS and PCA. The results indicate that the expression of almost all the genes related to aliphatic and aromatic GSL biosynthesis was higher in red mustard; the expression of genes involved in indolic GSL biosynthesis was higher in green mustard and was closely associated with the accumulation of indolic, aliphatic, and aromatic GSLs in mustard hairy roots. PC1 of the PCA indicates that the level of core primary metabolites and GSLs were higher in green mustard hairy roots than in red mustard. These results will facilitate further dissection of the mechanisms regulating secondary metabolite biosynthesis in mustard hairy roots. These results also show that induced hairy roots could be used as “chemical factories” to produce bioactive substances such as GSLs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning (2016M3A9A5919548). This work was supported by a grant from the Next-Generation BioGreen 21 Program (SSAC, Project # PJ013328) Rural Development Administration, Republic of Korea.
Author contributions
S.U. Park designed the experiments and analyzed the data. D.M. Cuong, J.K. Kim, S.J. Bong, S.-A Baek, J. Jeon, and J.S. Park wrote the manuscript, performed the experiments, and analyzed the data.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Contributor Information
Do Manh Cuong, Email: domanhcuong87hy@gmail.com.
Jae Kwang Kim, Email: kjkpj@inu.ac.kr.
Sun Ju Bong, Email: asop_258@naver.com.
Seung A Baek, Email: bsa1103@inu.ac.kr.
Jin Jeon, Email: jeonjin519@gmail.com.
Jong Seok Park, Email: jongseok@cnu.ac.kr.
Sang Un Park, Phone: +82-42-821-5730, Email: supark@cnu.ac.kr.
References
- Antonious GF, Bomford M, Vincelli P. Screening Brassica species for glucosinolate content. J Environ Sci Health B. 2009;44:311–316. doi: 10.1080/03601230902728476. [DOI] [PubMed] [Google Scholar]
- Augustine R, Majee M, Gershenzon J, Bisht NC. Four genes encoding MYB28, a major transcriptional regulator of the aliphatic glucosinolate pathway, are differentially expressed in the allopolyploid Brassica juncea. J Exp Bot. 2013;64:4907–4921. doi: 10.1093/jxb/ert280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson DG, Daxenbichler ME, Vanetten CH, Kwolek WF, Williams PH. Glucosinolates in crucifer vegetables: broccoli, brussels sprouts, cauliflower, collards, kale, mustard greens, and kohlrabi. J Am Soc Hortic Sci. 1987;112:173–178. [Google Scholar]
- Choi SH, Park S, Lim YP, Kim SJ, Park JT, An G. Metabolite profiles of glucosinolates in cabbage varieties (Brassica oleracea var. capitata) by season, color, and tissue position. Hortic Environ Biotech. 2014;55:237–247. doi: 10.1007/s13580-014-0009-6. [DOI] [Google Scholar]
- Cole RA. The relative importance of glucosinolates and amino acids to the development of two aphid pests Brevicoryne brassicae and Myzus persicae on wild and cultivated brassica species. Entomol Exp Appl. 1997;85:121–133. doi: 10.1046/j.1570-7458.1997.00242.x. [DOI] [Google Scholar]
- Giri A, Narasu ML. Transgenic hairy roots: recent trends and applications. Biotechnol Adv. 2000;18:1–22. doi: 10.1016/S0734-9750(99)00016-6. [DOI] [PubMed] [Google Scholar]
- Guo DP, Guo YP, Zhao JP, Liu H, Peng Y, Wang QM, Chen JS, Rao GZ. Photosynthetic rate and chlorophyll fluorescence in leaves of stem mustard (Brassica juncea var. tsatsai) after turnip mosaic virus infection. Plant Sci. 2005;168:57–63. doi: 10.1016/j.plantsci.2007.07.019. [DOI] [Google Scholar]
- Halkier BA, Du LC. The biosynthesis of glucosinolates. Trends Plant Sci. 1997;2:425–431. doi: 10.1016/S1360-1385(97)90026-1. [DOI] [PubMed] [Google Scholar]
- Il Park N, Kim JK, Park WT, Cho JW, Lim YP, Park SU. An efficient protocol for genetic transformation of watercress (Nasturtium officinale) using Agrobacterium rhizogenes. Mol Biol Rep. 2011;38:4947–4953. doi: 10.1007/s11033-010-0638-5. [DOI] [PubMed] [Google Scholar]
- Ismail A, Cheah SF. Determination of vitamin C, b-carotene and riboflavin contents in five green vegetables organically and conventionally grown. Malays J Nutr. 2003;9:31–39. [PubMed] [Google Scholar]
- Kastell A, Smetanska I, Schreiner M, Mewis I. Hairy roots, callus, and mature plants of Arabidopsis thaliana exhibit distinct glucosinolate and gene expression profiles. Plant Cell Tiss Org. 2013;115:45–54. doi: 10.1007/s11240-013-0338-7. [DOI] [Google Scholar]
- Kastell A, Smetanska I, Ulrichs C, Cai Z, Mewis I. Effects of phytohormones and jasmonic acid on glucosinolate content in hairy root cultures of Sinapis alba and Brassica rapa. Appl Biochem Biotechnol. 2013;169:624–635. doi: 10.1007/s12010-012-0017-x. [DOI] [PubMed] [Google Scholar]
- Kim JK, Ha SH, Park SY, Lee SM, Kim HJ, Lim SH, Suh SC, Kim DH, Cho HS. Determination of lipophilic compounds in genetically modified rice using gas chromatography-time-of-flight mass spectrometry. J Food Compos Anal. 2012;25:31–38. doi: 10.1016/j.jfca.2011.06.002. [DOI] [Google Scholar]
- Kim YB, Li X, Kim SJ, Kim HH, Lee J, Kim H, Park SU. MYB transcription factors regulate glucosinolate biosynthesis in different organs of Chinese cabbage (Brassica rapa ssp. pekinensis) Molecules. 2013;18:8682–8695. doi: 10.3390/molecules18078682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Park WT, Uddin MR, Kim YB, Nam SY, Jho KH, Park SU. Glucosinolate biosynthesis in hairy root cultures of broccoli (Brassica oleracea var. italica) Nat Prod Commun. 2013;8:217–220. [PubMed] [Google Scholar]
- Kim JK, Choi SR, Lee J, Park SY, Song SY, Na JH, Kim SW, Kim SJ, Nou IS, Lee YH, Park SU, Kim HR. Metabolic differentiation of diamondback moth (Plutella xylostella (L.)) resistance in cabbage (Brassica oleracea L. ssp. capitata) J Agric Food Chem. 2013;61:11222–11230. doi: 10.1021/jf403441t. [DOI] [PubMed] [Google Scholar]
- Kim YB, Chun JH, Kim HR, Kim SJ, Lim YP, Park SU. Variation of glucosinolate accumulation and gene expression of transcription factors at different stages of Chinese cabbage seedlings under light and dark conditions. Nat Prod Commun. 2014;9:533–537. [PubMed] [Google Scholar]
- Kim TJ, Lee KB, Baek SA, Choi J, Ha SH, Lim SH, Park SY, Yeo Y, Park SU, Kim JK. Determination of lipophilic metabolites for species discrimination and quality assessment of nine leafy vegetables. Appl Biol Chem. 2015;58:909–918. doi: 10.1007/s13765-015-0119-6. [DOI] [Google Scholar]
- Kim HW, Ko HC, Baek HJ, Cho SM, Jang HH, Lee YM, Kim JB. Identification and quantification of glucosinolates in Korean leaf mustard germplasm (Brassica juncea var. integrifolia) by liquid chromatography–electrospray ionization/tandem mass spectrometry. Eur Food Res Technol. 2016 doi: 10.1007/s00217-016-2648-6. [DOI] [Google Scholar]
- Lee SY, Bong. SJ, Kim JK, Park. SU. Glucosinolate biosynthesis as influenced by growth media and auxin in hairy root cultures of Kale (Brassica oleracea var. acephala) Emirates. J Food Agric. 2016;28:5. doi: 10.9755/ejfa.2016-01-064. [DOI] [Google Scholar]
- Li X, Kim JK, Park SY, Zhao S, Kim YB, Lee S, Park SU. Comparative analysis of flavonoids and polar metabolite profiling of Tanno-original and Tanno-high rutin buckwheat. J Agric Food Chem. 2014;62:2701–2708. doi: 10.1021/jf4049534. [DOI] [PubMed] [Google Scholar]
- Lin LZ, Harnly JM. Phenolic component profiles of mustard greens, Yu Choy, and 15 other Brassica vegetables. J Agric Food Chem. 2010;58:6850–6857. doi: 10.1021/jf1004786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LZ, Sun JH, Chen P, Harnly J. UHPLC-PDA-ESI/HRMS/MSn analysis of anthocyanins, flavonol glycosides, and hydroxycinnamic acid derivatives in red mustard greens (Brassica juncea coss variety) J Agric Food Chem. 2011;59:12059–12072. doi: 10.1021/jf202556p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Muller J, Jahn L, Lippert A, Puschel J, Walter A. Improvement of hairy root cultures and plants by changing biosynthetic pathways leading to pharmaceutical metabolites: strategies and applications. Biotechnol Adv. 2014;32:1168–1179. doi: 10.1016/j.biotechadv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Meenu AR, Majee M, Pradhan AK, Bisht NC. Genomic origin, expression differentiation and regulation of multiple genes encoding CYP83A1, a key enzyme for core glucosinolate biosynthesis, from the allotetraploid Brassica juncea. Planta. 2015;241:651–665. doi: 10.1007/s00425-014-2205-0. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–479. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Murthy HN, Hahn EJ, Paek KY. Adventitious roots and secondary metabolism. Chin J Biotechnol. 2008;24:711–716. doi: 10.1016/S1872-2075(08)60035-7. [DOI] [PubMed] [Google Scholar]
- Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie A. Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell. 2001;13:11–29. doi: 10.1105/tpc.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Mukhopadhyay A, Gupta V, Pental D, Pradhan AK. BjuB.CYP79F1 regulates synthesis of propyl fraction of aliphatic glucosinolates in oilseed mustard Brassica juncea: functional validation through genetic and transgenic approaches. PLoS One. 2016;11:e0150060. doi: 10.1371/journal.pone.0150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Srivastava AK. Hairy root culture for mass-production of high-value secondary metabolites. Crit Rev Biotechnol. 2007;27:29–43. doi: 10.1080/07388550601173918. [DOI] [PubMed] [Google Scholar]
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3 new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco P, Soengas P, Vilar M, Cartea ME, del Rio M. Comparison of glucosinolate profiles in leaf and seed tissues of different Brassica napus crops. J Am Soc Hortic Sci. 2008;133:551–558. [Google Scholar]
- Wielanek M, Urbanek H. Glucotropaeolin and myrosinase production in hairy root cultures of Tropaeolum majus. Plant Cell Tiss Org Cult. 1999;57:39–45. doi: 10.1023/A:1006398902248. [DOI] [Google Scholar]
- Wielanek M, Urbanek H. Enhanced glucotropaeolin production in hairy root cultures of Tropaeolum majus L. by combining elicitation and precursor feeding. Plant Cell Tiss Org Cult. 2006;86:177–186. doi: 10.1007/s11240-006-9106-2. [DOI] [Google Scholar]
- Wielanek M, Krolicka A, Bergier K, Gajewska E, Sklodowska M. Transformation of Nasturtium officinale, Barbarea verna and Arabis caucasica for hairy roots and glucosinolate-myrosinase system production. Biotechnol Lett. 2009;31:917–921. doi: 10.1007/s10529-009-9953-0. [DOI] [PubMed] [Google Scholar]
- Yang JH, Song N, Zhao X, Qi XH, Hu ZY, Zhang MF. Genome survey sequencing provides clues into glucosinolate biosynthesis and flowering pathway evolution in allotetrapolyploid Brassica juncea. BMC Genom. 2014 doi: 10.1186/1471-2164-15-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.