Abstract
The current study was carried out to assess in vitro and in vivo effects of Moringa oleifera seed methanolic extract on Fasciola hepatica to develop an alternative source of treatment. The in vitro ovicidal effect of M. oleifera seed extract on immature F. hepatica eggs has provided evidence of inhibitory activity on the vitality and hatchability of F. hepatica eggs. This inhibitory activity was concentration-dependent and also correlated strongly with the exposure time. In the in vivo trial, the oral administration of F. hepatica experimentally infected rabbits with doses of 150 mg/kg BW prepared extract per day for 3 consecutive days on the 63rd day post infection confirmed potent fasciolicide activity of the extract. A gradual decrease in fecal egg count (FEC) was detected from the 1st day post treatment until reaching 100% FEC reduction by the 7th day post treatment. No flukes could be found at post mortem examinations. Significant increments of serum total protein, globulin, the activities of ALT and AST, total cholesterol, triglycerides and urea were recorded during the period of infection, which were improved by treatment. Remarkable histopathological alterations were observed in the infected liver and gallbladder tissues which decreased clearly in the treated rabbits. This study proposes that the used extract has promising and potent fasciolicide activity.
Keywords: Fasciolosis, F. hepatica, Moringa oleifera, In vitro, In vivo, Anthelmintic activity, Rabbits
Introduction
Fasciolosis is a parasitic disease widespread in animals and human beings, with considerable economic and health importance (Shalaby et al. 2016; Gabrashanska et al. 2016). Fasciola gigantica (F. gigantica) is considered the endogenous species of Fasciola, found in the Nile Delta, Egypt, while Fasciola hepatica (F. hepatica) was thought to be present only in imported animals (Lotfy et al. 2002). For a number of years it has posed a major problem in ruminant livestock production but, due to climatic changes, there has been a worrying upsurge in the incidence and spread of the disease (Mitchell 2002). In Bulgaria, this helminthiasis is caused by F. hepatica. Local foci that are more commonly observed could be converted under certain conditions into localities for the enzootic course of the disease. This is most often due to poor pastures in rainy years which create favorable conditions for the increase of the population of the intermediate host Galba truncatula (Georgieva et al. 2012). Most of the studies on the pathogenesis of fasciolosis are focused on the hepatic changes, as the liver is the main target organ. Mechanic-traumatic, toxic, immunologic and carcinogenic effects of parasites F. hepatica were examined in different infected hosts during their migration inside the body or permanent localization in the liver (Gabrashanska et al. 2016). Hepatic lesions produced by migration of the F. hepatica juvenile flukes in the liver of various host species are associated with mechanical damage of organ parenchyma, resulting in hemorrhage, necrosis, fibrosis, cirrhosis and hyperplasia of the bile duct epithelium, inflammation of the liver and bile ducts accompanied by loss of condition, digestive disturbances, reduction in productivity, hypoalbuminemia, anemia, and weight loss (Mendes et al. 2012; Gabrashanska et al. 2016).
Different types of anthelmintics have been used in the treatment and control of fasciolosis, the therapeutic effect of closantel was recorded as an adulticide in treating chronic fasciolosis (Solana et al. 2016). Although the combination of oxyclozanide and oxfendazole was proven to have potential effect against Fasciola species (Khan et al. 2016), it is widely known that triclabendazole (TCBZ) is the drug of choice for treatment because of its ability to kill both adult and immature stages (Kelley et al. 2016). Unfortunately, resistance against many anthelmintics, including TCBZ, has evolved; a situation that affects disease control and necessitates trying other alternatives, especially medicinal plants (Ullah et al. 2017). Bioactive plant products have been given much attention because they are ecologically safe and culturally more acceptable than their synthetic counterparts (Singh et al. 1996). Different medicinal plant extracts have been successfully used as anthelmintics against fasciolosis (Moazeni and Khademolhoseini 2016; Ullah et al. 2017). Moringa oleifera Lam. belongs to the family Moringaceae and is known as a vibrant and excellent source of phytochemicals, which are involved strongly in medicinal applications and functional food preparations (Saini et al. 2016). The antiparasitic effect of M. oleifera extracts has been evaluated in different studies, and a potent effect was recorded against different developmental stages of Haemonchus contortus (Tayo et al. 2014). Complete elimination of Trypanosoma brucei brucei from infected mice was recorded by Bulus and Addau (2013). M. oleifera extracts showed promising results as an alternative treatment to schistosomiasis (Almanzor et al. 2014), while the gum of M. oleifera showed an anti-filarial effect (Murthy et al. 2011). These findings motivated this study to explore the in vitro ovicidal effect of M. oleifera seed methanolic extract on development of F. hepatica eggs and evaluate in vivo effects on F. hepatica experimentally infected rabbits, with special reference to the examination of some biochemical and histopathological effects in rabbit sera and tissues.
Materials and methods
Ethical approval
The experiments were conducted in compliance with the requirements and recommendations of the International Animal Ethics Committee and the ethical Committee of the National Research Centre and the current Egyptian Law and Regulations for the protection of experimental animals to minimize negative states (harm) and improve feeding and housing conditions, under certificate number (17134).
Preparation of M. oleifera seed methanolic extract
M. oleifera seeds were brought from the Egyptian Scientific Society for M. oleifera at the National Research Centre, Dokki, Giza. Seeds were milled to powder and kept in a closed bottle. The powdered seeds (500 g) were submerged in methanol (1:10) at room temperature for 2 days with discontinuous shaking and then filtered through filter paper. The concentration of methanolic extracts was made under reduced pressure using a rotary evaporator at 40 °C. The concentrated methanolic extract was kept at 4 °C until utilized. When utilized, the prepared extract was dissolved in distilled water with the addition of a few drops of Tween-80 (Harborne 1984).
Collection of F. hepatica metacercariae
Experimental infection for snails was performed using miracidia hatched from mature F. hepatica eggs. Metacercariae were collected from snail’s culture of Galba truncatula (Nanev et al. 2017). Examination of metacercariae was performed under an optical microscope: metacercariae with excretory granules were deemed viable.
In vitro study
Collection and counting of F. hepatica eggs
Three rabbits were experimentally infected orally using 50 selected viable F. hepatica encysted metacercariae per animal (Poljakova-Krusteva 1970). After detection of F. hepatica eggs in feces on the 63rd day post infection, using a sedimentation technique according to Mooney et al. (2009), the rabbits were humanely slaughtered. Immature F. hepatica eggs were obtained from the gallbladders. The eggs were handled according to the method of Hegazi et al. (2007). The number of immature eggs per 1 ml of solution was recorded and the collected eggs were kept in the refrigerator at 4 °C until used.
Ovicidal activity of the extract
The efficacy of M. oleifera seed methanolic extract was investigated against immature F. hepatica eggs at concentrations of 1, 5, 10, 25 and 50 mg/ml with different exposure times; 24, 48 and 72 h in test tubes, which each contained around 1000 eggs. At the end of the exposure period, repeated washing and sedimentation using distilled water was performed to exclude any remnants of the tested extract. A control negative untreated test tube with 1000 eggs suspended in distilled water was included. All the test tubes were incubated at 26 °C for embryonation. The eggs were examined every 3 days until the control eggs approached hatching, as evidenced by movement of miracidia within the eggs (Roweliffe and Ollerenshaw 1959). The day of hatching was that on which most of the eggs with developed active miracidia hatched after exposure to artificial light for 15 min. The number of hatching eggs out of 100 eggs found in five separate microscopic fields was counted. Hatching mean percentage was estimated according to Hegazi et al. (2007). Then, the reduction percentage of F. hepatica egg hatching was determined using the following formula:
In vivo trial
Experimental design
Eighteen local breed rabbits, 1.5–2 kg weight, free from parasites as proved by daily examination of their fecal samples for 5 successive days using a sedimentation technique (Tsotetsi et al. 2013), were utilized. The rabbits were maintained in clean metal cages, and nourished on balanced rations and water ad libitum. Three groups of rabbits were involved with six animals in each. Group I served as uninfected untreated animals (negative control), group II was experimentally infected with F. hepatica (positive control), while group III was treated with M. oleifera seed methanolic extract. The experimental infection of groups II and III was achieved via oral dose of 50 selected viable F. hepatica encysted metacercariae per animal on the 1st day of the experiment (Poljakova-Krusteva 1970). Infection was confirmed through detection of the characteristic Fasciola eggs in feces on the 63rd day post infection. Group III (the treated group) was given an oral dose of 150 mg/kg BW M. oleifera seed methanolic extract per day for 3 successive days, according to Almanzor et al. (2014).
Fecal egg count per gram
Fecal samples of each animal were collected separately for 10 consecutive days post treatment. Determination of egg count per gram of feces (EPG) was carried out using the sedimentation technique as described by Mooney et al. (2009). The efficacy of the extract was estimated by the reduction of mean EPG following the formula mentioned by Foreyt (1988):
Fluke recovery
On day 84 post infection, dissection of all infected and treated rabbits was done, and livers and gallbladders were examined carefully. Flukes were assembled and counted according to the method of Kendall et al. (1967).
Biochemical studies
Blood samples were collected weekly for each animal from the ear vein of all infected and treated rabbits. Serum samples were separated and kept at − 20 °C until further biochemical analyses. Total proteins (Henary et al. 1974) albumin (Doumas et al. 1971), glucose (Trinder 1969), activity of alanine aminotransferase (ALT) (Reitman and Frankel1957), total cholesterol (Allain et al. 1974), triglycerides (Fossati and Prencipe 1982), creatinine (Houot 1985), and urea (Patton and Crouch 1977) were assessed. Test kits supplied by bioMérieux-France, were utilized.
Histopatholgical studies
Tissue specimens from livers and gallbladders were handled rapidly after slaughter of rabbits, fixed in 10% neutral formalin and stained with hematoxylin and eosin (H & E) stain as mentioned by Bancroft and Gamble (2002).
Statistical analysis
The obtained data was analyzed for the mean and standard deviation (SD). Statistical comparison between the means of different treatments was conducted using one-way ANOVA with the SPSS program, version 10. A P value of < 0.05 was assumed for statistical significance.
Results
In vitro study
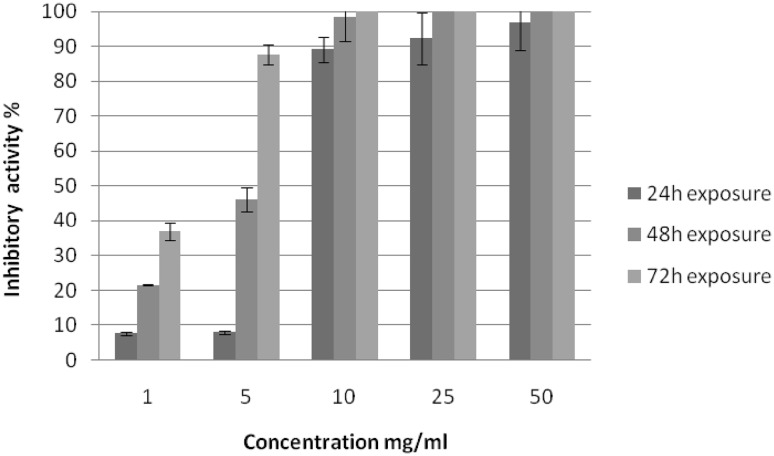
Five different concentrations of M. oleifera seed methanolic extract, ranging from 1 to 50 mg/ml, were tested against the vitality and hatchability of F. hepatica eggs at various exposure times, where no inhibitory effect of the solvent on immature F. hepatica eggs was observed (Fig. 1). The results revealed that the inhibitory activity of the extract increased with increasing extract concentration and exposure time. Following 24 h of exposure, a slight inhibitory effect of the used extract, 7.7 and 8.0%, was observed at concentrations of 1 and 5 mg/ml, respectively. This inhibitory effect increased with increasing application concentration, until reaching the highest rate (96.9%) at a concentration of 50 mg/ml. Yet, no complete failure of development of treated eggs was observed. Following 48 and 72 h of exposure, the inhibitory activity of the extract was higher than that recorded after 24 h of exposure at all tested concentrations of the extract. Complete failure of development and death of all immature eggs first appeared at concentrations of 25 and 10 mg/ml, respectively.
Fig. 1.
Inhibitory activity of Moringa oleifera seed methanolic extract on hatchability of F. hepatica eggs
At low concentrations of M. oleifera seeds methanolic extract, high rate of dead miracidia inside the eggs (developed non-hatched eggs) was observed (Fig. 2). While, at high concentrations complete failure of egg development and dead eggs containing dark embryos without any development were observed in comparison with control non-treated eggs (Fig. 2).
Fig. 2.
Shapes of normal F. hepatica eggs and F. hepatica eggs treated with Moringa oleifera seed methanolic extract. a F. hepatica egg obtained from fecal sample of experimentally infected rabbits × 100. b Normal (untreated) F. hepatica eggs collected from the gall bladder of experimentally infected rabbit × 100. c Normal hatched F. hepatica eggs × 400. d Treated F. hepatica eggs showing various deformities × 400. e Undeveloped miracidium inside egg × 200. f Egg containing dark embryo × 100
In vivo study
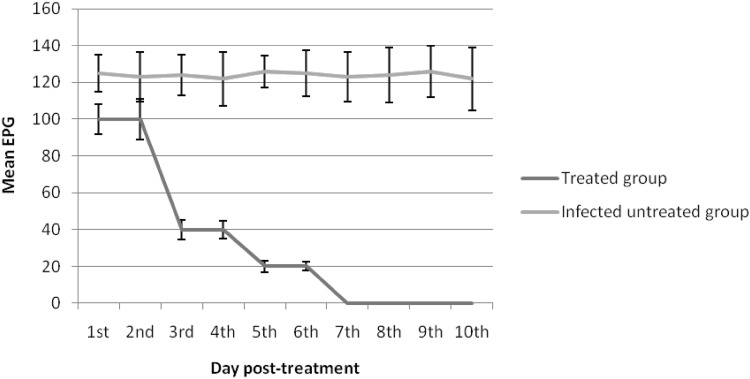
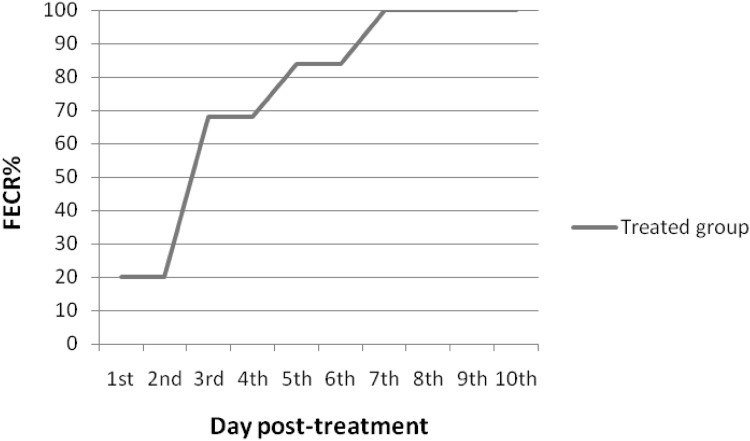
Fecal examination revealed that all the experimentally infected rabbits were passing eggs on day 63 post infection. Mean fecal egg count (FEC) at 0 day (before the treatment) was 125 ± 13.75 eggs per gram (EPG) feces. Gradual decreasing of FEC was detected from 1st day post treatment until the end of the experiment. On the 1st and 2nd days post treatment, FEC in the treated group began to decrease, showing a 20% reduction in comparison with the positive control group. Meanwhile, on day 3 after treatment, FEC in the treated group decreased significantly (P < 0.05) showing 68% reduction. Complete absence of egg shedding was recorded on the 7th day post treatment until the end of the experiment, 84th day post infection (Figs. 3 and 4).
Fig. 3.
Anthelmintic efficacy of M. oleifera seed methanolic extract against F. hepatica as evident by mean egg count per gram of feces in rabbits
Fig. 4.
Fecal egg count reduction (FECR) percentage in the treated rabbits
All the experimentally infected rabbits were sacrificed on day 84 post infection and the number of flukes recovered from each of the livers on autopsy was recorded. The average number of flukes in the control non-treated rabbits was 3.2 ± 1.5, while no flukes could be recovered from the treated rabbits.
Serum biochemical changes
The results showed significant (P < 0.05) increase in the serum total protein and globulin post infection, while this level diminished over the course of treatment; however, albumin level and A/G ratio showed a significant decrease during infection and treatment. Serum glucose level revealed non-significant changes (Table 1). A significant rise was detected in the activities of ALT, AST, total cholesterol, triglycerides and urea during the period of infection, which decreased with treatment. Slight significant reduction of creatinine level was recorded during infection and treatment (Table 2).
Table 1.
Changes in serum proteins and glucose concentrations of rabbits experimentally infected with F. hepatica before and after treatment using M. oleifera seed methanolic extract
| Parameters periods (week) | Total proteins (g/dl) | Albumin (g/dl) | Total globulins (g/dl) | A/G ratio | Glucose (mg/dl) | |
|---|---|---|---|---|---|---|
| After infection | 0 | 7.24 ± 0.38 cd | 3.97 ± 0.10ab | 3.26 ± 0.28e | 1.25 ± 0.08bc | 101.69 ± 2.57abc |
| 1 | 7.12 ± 0.11d | 3.47 ± 0.00de | 3.65 ± 0.10de | 0.95 ± 0.03def | 100.50 ± 0.80abc | |
| 2 | 7.22 ± 0.02 cd | 3.35 ± 0.03ef | 3.87 ± 0.01d | 0.87 ± 0.01def | 87.10 ± 2.25d | |
| 3 | 6.29 ± 0.11e | 3.79 ± 0.07bc | 2.50 ± 0.17f | 1.56 ± 0.14a | 72.47 ± 1.37e | |
| 4 | 7.44 ± 0.14 cd | 3.43 ± 0.02de | 4.00 ± 0.17 cd | 0.87 ± 0.04def | 107.46 ± 3.86ab | |
| 5 | 7.35 ± 0.12 cd | 3.31 ± 0.04ef | 4.04 ± 0.16 cd | 0.83 ± 0.04ef | 99.59 ± 4.34bc | |
| 6 | 8.05 ± 0.25b | 4.04 ± 0.01a | 4.00 ± 0.26 cd | 1.03 ± 0.07cde | 100.48 ± 1.03abc | |
| 7 | 7.08 ± 0.04d | 3.92 ± 0.13ab | 3.16 ± 0.17e | 1.26 ± 0.11b | 108.14 ± 1.39a | |
| 8 | 6.89 ± 0.15d | 3.59 ± 0.03 cd | 3.30 ± 0.18e | 1.10 ± 0.07abc | 105.93 ± 0.16ab | |
| 9 | 8.69 ± 0.02a | 3.18 ± 0.10f | 5.51 ± 0.08a | 0.58 ± 0.03 g | 95.59 ± 0.11c | |
| After treatment | 1* | 8.17 ± 0.12b | 3.46 ± 0.03de | 4.71 ± 0.10b | 0.73 ± 0.01 fg | 103.43 ± 4.39abc |
| 2* | 7.74 ± 0.20bc | 3.25 ± 0.11ef | 4.49 ± 0.11bc | 0.72 ± 0.02 fg | 101.46 ± 2.67abc | |
| 3* | 6.95 ± 0.10d | 3.32 ± 0.12ef | 3.63 ± 0.16de | 0.92 ± 0.07def | 100.12 ± 1.61abc | |
Means in the same column with different superscript letters (a, b, c…) are significant at P < 0.05
The asterisk (*) refers to the weeks of treatment
Table 2.
Changes in serum biochemical parameters of rabbits experimentally infected with F. hepatica before and after treatment using M. oleifera seed methanolic extract
| Parameters periods (weeks) | Alanine aminotransferase (IU/l) | Aspartate aminotransferase (IU/l) | Total cholesterol (mg/dl) | Triglycerides (mg/dl) | Urea (mg/dl) | Creatinine (mg/dl) | |
|---|---|---|---|---|---|---|---|
| After infection | 0 | 28.47 ± 0.67 fg | 28.37 ± 4.78f | 39.20 ± 0.76f | 38.21 ± 3.05ef | 48.15 ± 2.08 cd | 0.95 ± 0.04a |
| 1 | 38.32 ± 1.08 cd | 40.84 ± 0.12de | 44.53 ± 3.46de | 37.86 ± 3.16ef | 45.92 ± 0.60 cd | 1.01 ± 0.00a | |
| 2 | 42.03 ± 2.25c | 46.66 ± 1.75bc | 46.68 ± 0.26 cd | 40.36 ± 0.79de | 37.77 ± 1.00f | 0.88 ± 0.00b | |
| 3 | 39.49 ± 1.81c | 48.32 ± 0.59bc | 49.60 ± 2.02c | 44.64 ± 0.11d | 40.63 ± 0.58ef | 0.55 ± 0.02d | |
| 4 | 53.99 ± 0.99a | 58.26 ± 0.47a | 40.13 ± 1.39ef | 55.00 ± 1.36c | 65.88 ± 1.76a | 0.99 ± 0.03a | |
| 5 | 52.18 ± 0.30ab | 44.65 ± 0.36 cd | 43.52 ± 0.61def | 44.18 ± 0.22d | 43.75 ± 0.26de | 0.88 ± 0.01b | |
| 6 | 53.20 ± 0.47a | 50.12 ± 0.19b | 57.72 ± 0.13b | 57.75 ± 0.11c | 63.78 ± 0.20a | 0.85 ± 0.01b | |
| 7 | 38.61 ± 0.81 cd | 39.07 ± 0.23e | 62.67 ± 1.43a | 64.64 ± 1.47b | 55.80 ± 2.32b | 0.37 ± 0.01e | |
| 8 | 42.88 ± 3.42c | 46.90 ± 0.35bc | 65.88 ± 1.78a | 65.27 ± 0.64b | 40.82 ± 0.32ef | 0.65 ± 0.03c | |
| 9 | 48.04 ± 0.45b | 51.16 ± 0.17b | 63.19 ± 0.74a | 75.07 ± 1.33a | 48.95 ± 2.79c | 0.88 ± 0.01b | |
| After treatment | 1* | 34.57 ± 0.41de | 37.13 ± 0.68e | 27.47 ± 1.69 g | 35.27 ± 6.10ef | 40.79 ± 0.40ef | 1.00 ± 0.01a |
| 2* | 27.51 ± 0.64 g | 20.62 ± 0.42 g | 44.80 ± 0.85cde | 36.89 ± 0.49ef | 44.69 ± 0.77cde | 0.85 ± 0.02b | |
| 3* | 32.25 ± 1.09ef | 27.74 ± 0.77f | 26.64 ± 0.62 g | 33.93 ± 1.64f | 45.83 ± 0.90 cd | 0.87 ± 0.01b | |
Means in the same column with different superscript letters (a, b, c…) are significant at P < 0.05
The asterisk (*) refers to the weeks of treatment
Histopathological alterations
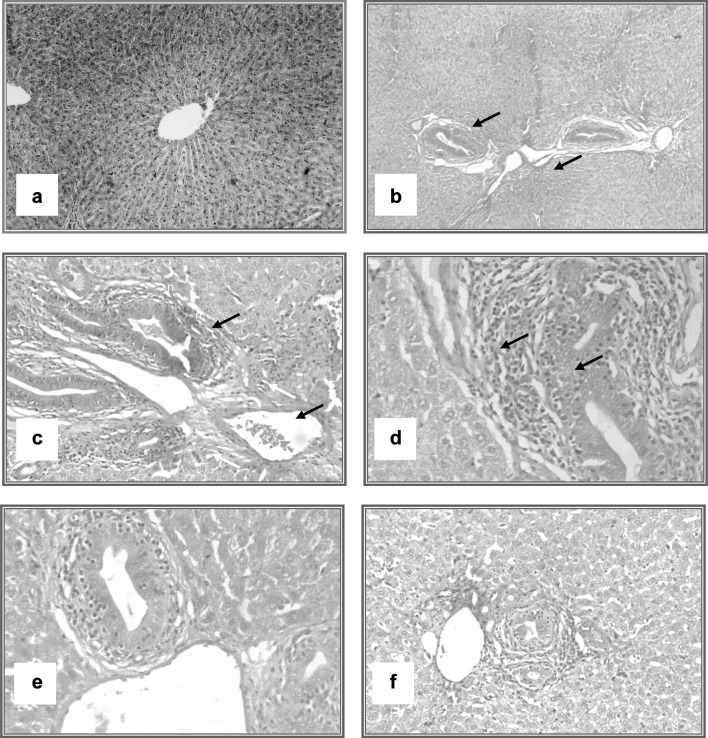
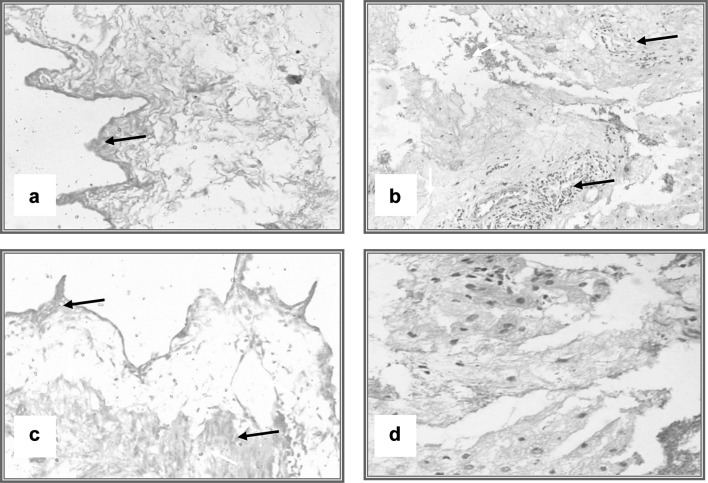
Microscopic examination of the infected rabbits’ liver tissue demonstrated presence of degenerative and necrotic changes of the hepatocytes, focal inflammatory cell infiltration and fibrous connective tissue proliferation present at the portal area, associated with hemorrhage. These alterations were improved via treatment with M. olifera seed methanolic extract in the infected treated group by lessening bile duct hyperplasia, congestion of blood vessels and leukocytic infiltration (Fig. 5). The examination of gallbladders of infected rabbits indicated epithelial hyperplasia, congestion and severe leukocytic infiltration; these changes were reduced in treated rabbits’ gallbladder tissues (Fig. 6).
Fig. 5.
Histopathological changes of liver of rabbits; pre and post treatment of experimental F. hepatica infection. a Liver of rabbit showing normal histological picture of the liver (H & E × 100). b Liver of rabbit (9 weeks post infection), revealing marked portal tract changes in the form of hyperplastic bile duct, congested vessels (H & E × 40). c and d Liver of rabbits (9 weeks post infection), demonstrating marked portal tract changes in the form of hyperplastic bile duct, congested vessels and leukocytic infiltration (arrows) (H & E × 100). e Liver of rabbit (9 weeks post infection), showing higher magnification of the previous lesions (H & E × 400). f Liver of rabbit (post treatment) displaying decrease in bile duct hyperplasia, congestion of blood vessels and leukocytic infiltration (H & E × 100)
Fig. 6.
Histopathological changes of the gall bladder of rabbits pre and post treatment of experimental F. hepatica infection. a–c The gall bladder of infected rabbits, showing epithelial hyperplasia, congestion and sever leukocytic infiltration (H & E × 100). d The gall bladder of a post-treated rabbit, demonstrating decrease in hyperplasia, congestion and leukocytic infiltration
Discussion
The current study was carried out to assess in vitro ovicidal and in vivo flukicidal effects of M. oleifera seed methanolic extract on F. hepatica to develop an alternative source of treatment. It provided evidence of inhibitory activity of the extract on the vitality and hatchability of F. hepatica eggs. This inhibitory activity was concentration-dependent and also correlated strongly with the exposure time. In other reports, the inhibitory activity of some synthetic and natural fasciolicides increased with increments of their concentrations and prolongation of egg exposure time (Hegazi et al. 2007; Moazeni and Khademolhoseini 2016). Tayo et al. (2014) proved that inhibition of H. contortus egg embryonation could induced by using of aqueous extract of M. oleifera leaf extracts by (94.5% ± 4%) and (92.8% ± 6.2) for the ethanolic extract. Also they recorded inhibition of H. contortus egg hatchability by (90.2% ± 8.4%) for the aqueous extract and by (99% ± 2%) for the ethanolic extract at concentration of 5 mg/ml. Cabardo and Portugaliza (2017) mentioned that aqueous extract of M. oleifera seeds caused inhibition of H. contortus egg hatchability by (81.72%), while during using the ethanolic extract, it was (95.89%) at concentration of 15.6 mg/ml extract. This inhibitory effect of the extract on vitality and hatchability of F. hepatica eggs might be attributed to tannins as the main secondary metabolite in M. oleifera seeds (Cabardo and Portugaliza 2017). Tannins might permeat the different layers of the egg and suppress the formation of miracidia by affecting the embryo. Vargas-Magaña et al. (2014) illustrated three reasonable mechanisms that could induce inhibition of egg hatching using plant extracts, including an effect on the permeability of the egg shell, suppression of some enzymes for egg hatching, and an impact on the hatching receptors present in egg shells. Unfortunately, insufficient data on the ovicidal effect of medicinal plants against F. hepatica eggs was available.
In vivo, the anthelmintic activity of M. oleifera seeds was confirmed by a gradual decrease of FEC from the 1st day post treatment to the end of experiment and elimination of liver flukes in the treated rabbits. These results appeared to be in line with those obtained by Maqbool et al. (2004) who screened the efficacy of aqueous extract of some indigenous medicinal plants; Fumaria parviflora, Caesalpinia crista, Nigella sativa, and Saussurea lappa on improvement from fasciolosis in buffaloes. In a related study, Nassef et al. (2014) reported that the use of soybean extract with genistein at a single oral dose of 100 mg/kg BW against the experimentally F. gigantica infected rabbits induced FEC reduction 41.6 and 95.8% on the 3rd and the 7th day post treatment, respectively. Other medicinal plants, Albizzia anthelmintica, Mimoaseae stem bark and Balanites aegyptiaca could induce reduction in fluke counts reaching to 95.5, 93.2 and 97.7%, respectively (Koko et al. 2000). Moreover, Almanzor et al. (2014) reported that the administration of M. oleifera leaf ethanolic extract at a dose of 150 mg/kg BW for Schistosoma japonicum infected mice caused a decrease of the number of recovered worms. The anthelmintic activity of the extract in the present study might be due to the content of the various parts of the Moringa tree being good sources of unique glucosinolates, flavonoids and phenolic acids (Coppin et al. 2013). So the effect of M. oleifera seed methanolic extract against F. hepatica might be assigned to the antiparasitic action of benzyl isothiocyanat, which suppressed the energy metabolism and motor systems of the parasites (Kumar et al. 1991; Chimedza et al. 2017).
Regarding the protein profile in this study, the increase in total serum proteins during infection and pretreatment might reflect a rise in the globulins, predominately the immunoglobulins (Kaneko et al. 1992). It might be due to the increase of γ-globulin in response to the antigenic stimulation of the migrating juvenile flukes (Chapman et al. 1979). Indeed, Gajewska et al. (2005) reported that migration of flukes could involve damage of liver tissue, causing alteration of the level of plasma protein concentration (albumin, globulin) and hepatic enzymes released in the blood. Increased values of AST, ALT, triglycerides, and the total cholesterol during the infection period might be due to the resulting hepatocellular necrosis and degeneration (Boone et al. 2005; Taleb et al. 2007; Değer et al. 2008). In the present study, administration of M. oleifera seed methanolic extract in the infected treated group could induce reduction of AST and ALT activities, as well as urea, cholesterol and triglycerides levels, which might be due to the hepatoprotective effect of the prepared extract and the existence of flavonoids, steroids, and phenolic acids and their esters among M. oleifera seed extract constituents (Saini et al. 2016). Also, the hypocholesterolemic effect of M. oleifera extract could be a result of its direct influence on the liver or an indirect effect on thyroid hormones which impact reactions in nearly all the pathways of lipid metabolism (Ghasi et al. 2000; Mehta et al. 2003).
On the other hand, histopathological findings showed that infected rabbit liver and gallbladder tissues showed severe histological alterations, atrophy, and dysfunction. This might be explained by Hodžić et al. (2013) who found that the pathological changes brought about by mechanical and toxic effects of F. hepatica impacted the complex vascular and biliary systems in the liver which are essential for maintaining normal function of the liver. Moreover, the microscopic examination of liver and gallbladder tissues of infected rabbits revealed severe leukocytic infiltrations that agreed with Paulo et al. (2010) who mentioned that the eosinophil granulocytes were mostly supposed to be attracted by immune complexes which would be motivated by histamine release, causing the body to generate more endogenous histamine along with other inflammatory molecules into the body. The pathological alterations of the liver and gallbladder of rabbits were improved via treatment with the prepared extract, showing decreases in hyperplasia, congestion, and leukocytic infiltration. Hence, it was suggested that the significant changes after treatment might be due to regenerative changes in the parenchyma and the normalization of liver function (Hodžić et al. 2013). Also, the different biological activities embracing antiproliferation, hepatoprotective, anti-inflammatory, antinociceptive, antiatherosclerotic, oxidative DNA damage protective, antiperoxidative, cardioprotective and the popular medicinal uses of M. oleifera might be related to the presence of functional bioactive compounds, such as; phenolic acids, flavonoids, alkaloids, phytosterols, natural sugars, vitamins, minerals, and organic acids (Saini et al. 2016).
The present study has a head start in evaluating M. oleifera seed methanolic extract against fasciolosis. It is perceived that the in vivo results of application of M. oleifera extract against F. hepatica infection are consistent with the in vitro experimental results. This study proposed that the extract used has promising and potent fasciolicide activity, with certain limitations. However, further large-scale in vivo investigations using variable multiple doses of the prepared extract are required to check the efficacy and achieve a safe and easily applicable herbal drug against fasciolosis.
Acknowledgements
This work was funded from a co-project between the Academy of Scientific Research in Egypt and the Bulgarian Academy of Science, entitled “Recent studies of pathogenetic mechanism of neoplastic fasciolosis and advanced control methods.”
Authors’ contributions
OMK designed, supervised and directed the experiment. VN, NT, and MG carried out the experimental infection to provide metacercaria. OMK, NMFH, DS, EBA, and HAS conducted the in vitro and in vivo experiments and the laboratory work of the samples. SA performed the biochemical and histopathological studies. OMK, NMF, DS, EBA, SNA and HAS analyzed and discussed the resultant data. OMK, NMFH and HAS implemented writing the manuscript. OMK and NMFH revised and reviewed the manuscript for publication. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Omnia M. Kandil, Phone: +01005414113, Email: kandil_om@yahoo.com
Noha M. F. Hassan, Email: nohamhassan555@yahoo.com
Doaa Sedky, Email: doaa_sedky@yahoo.com.
Emad B. Ata, Email: emadvet2003@yahoo.com
Somia A. Nassar, Email: somianassar@ymail.com
Hatem A. Shalaby, Email: shalaby85@gmail.com
Veselin Nanev, Email: veselinnanev@gmail.com.
Neli Tsocheva-Gaytandzhieva, Email: tsocheva_n@abv.bg.
Margarita Gabrashanska, Email: m.gabrashanska@gmail.com.
References
- Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- Almanzor DE, Clemente RJC, Fornillos MA, Gomez FRM, Ladiao BD, Calzada Tamodtamod R. In vivo trials of Moringa oleifera Lam. extracts as antischistosomal treatment on Schistosoma japonicum infected mice. Sanghiran Multidiscip J. 2014;2:49–56. [Google Scholar]
- Bancroft JD, Gamble M. Theory and practice of histological techniques. Oxford Phila: Elsevier Sci; 2002. pp. 130–175. [Google Scholar]
- Boone L, Meyer D, Cusick P, Ennulat D, Provencher Bolliger A, Everds N, Meador V, Elliott G, Honor D, Bounous D, Jordan H. Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet Clin Pathol. 2005;34:182–188. doi: 10.1111/j.1939-165X.2005.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Bulus T, Addau FT. Comparative antitrypanosomal screening of methanolic extracts of Khaya senegalensis and Moringa oleifera. Sci World J. 2013;8:1–6. [Google Scholar]
- Cabardo DE, Jr, Portugaliza HP. Anthelmintic activity of Moringa oleifera seed aqueous and ethanolic extracts against Haemonchus contortus eggs and third stage larvae. Int J Vet Sci Med. 2017;5:30–34. doi: 10.1016/j.ijvsm.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CB, Knopf PM, Hicks JD, Mitchell GF. IgG1 hypergamma globulinaemia in chronic parasitic infections in mice: magnitude of the response in mice infected with various parasites. Aust J Exp Biol Med Sci. 1979;57:369–387. doi: 10.1038/icb.1979.38. [DOI] [PubMed] [Google Scholar]
- Chimedza JA, Graf BL, Simmler C, Kim Y, Kuhn P, Pauli GF. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE. 2017;12:1–20. doi: 10.1371/journal.pone.0182658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin JP, Xu Y, Chen HD. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J Funct Foods. 2013;5:1892–1899. doi: 10.1016/j.jff.2013.09.010. [DOI] [Google Scholar]
- Değer Y, Ertekin A, Değer S, Mert H. Lipid peroxidation and antioxidant potential of sheep liver infected naturally with distomatosis. Türkiye Parazitoloji Dergisi. 2008;32:23–26. [PubMed] [Google Scholar]
- Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Foreyt WJ. Evaluation of clorsulon against immature Fascioloides magna in cattle and sheep. Am J Vet Res. 1988;49:1004–1006. [PubMed] [Google Scholar]
- Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- Gabrashanska M, Tsocheva-Gaytandzhieva N, Nanev V, Vladov I (2016) Antioxidants in the liver of Fasciola hepatica infected rabbits after mixed basic salts application. In: Proceedings of the Seventh Workshop of Experimental Models and Methods in Biomedical Research, 16–18 May, Sofia, Bulgaria, EO7, pp 92–97
- Gajewska A, Smaga-Kozłowska K, Wiśniewski M. Pathological changes of liver in infection of Fasciola hepatica. Wiad Parazytol. 2005;51:115–123. [PubMed] [Google Scholar]
- Georgieva K, Georgieva S, Mizinska Y, Stoitsova SR. Fasciola hepatica miracidia lectin binding and stimulation of in vitro miracidium-to-sporocyst transformation. Acta Parasitol. 2012;57:46–52. doi: 10.2478/s11686-012-0007-8. [DOI] [PubMed] [Google Scholar]
- Ghasi S, Nwobodo E, Ofili JO. Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam. in high-fat diet fed Wistar rats. J Ethnopharmacol. 2000;69:21–25. doi: 10.1016/S0378-8741(99)00106-3. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Phytochemical methods. 2. New York: Chapman and Hall; 1984. [Google Scholar]
- Hegazi AG, Abd El Hady FK, Shalaby HA. Inhibitory effect of Egyptian propolis on Fasciola gigantica eggs with reference to its effect on Clostridium oedematiens and correlation to chemical composition. Pak J Biol Sci. 2007;10:3295–3305. doi: 10.3923/pjbs.2007.3295.3305. [DOI] [PubMed] [Google Scholar]
- Henary RJ, Cannon DC, Winkleman JW. Clinical chemistry principles and techniques. 2. New York: Harper and Roe; 1974. [Google Scholar]
- Hodžić A, Zuko A, Avdić R, Alić A, Omeragić J, Jažić A. Influence of Fasciola hepatica on serum biochemical parameters and vascular and biliary system of sheep liver. Iran J Parasitol. 2013;8:92–98. [PMC free article] [PubMed] [Google Scholar]
- Houot O (1985) Interpretation of clinical laboratory tests. Ed. by Siest, G., Henny, J., Schiele, F. and Young, D.S. Biochemical Publications, pp 220–234
- Kaneko JJ, Harvey JW, Bruss ML. Clinical biochemistry of domestic animals. 5. California: Academic Press; 1992. [Google Scholar]
- Kelley JM, Elliott TP, Beddoe T, Anderson G, Skuce P, Spithill TW. Current threat of triclabendazole resistance in Fasciola hepatica. Trends Parasitol. 2016;32:458–469. doi: 10.1016/j.pt.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Kendall SB, Hebert NJ, Parfitt JW, Peirce MA. Resistance to reinfection with Fasciola hepatica in rabbits. Exp Parasitol. 1967;20:242–247. doi: 10.1016/0014-4894(67)90044-6. [DOI] [PubMed] [Google Scholar]
- Khan MN, Sajid MS, Rizwan HM, Qudoos A, Abbas RZ, Riaz M, Khan MK. Comparative efficacy of six anthelmintic treatments against natural infection of Fasciola species in sheep. Pak Vet J. 2016;37:65–68. [Google Scholar]
- Koko WS, Galal M, Khalid HS. Fasciolicidal efficacy of Albizia anthelmintica and Balanites aegyptiaca compared with albendazole. J Ethnopharmacol. 2000;71:247–252. doi: 10.1016/S0378-8741(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Kumar D, Mishra SK, Tandan SK, Tripathi HC. Mechanism of anthelmintic action of benzyl isothiocyanate. Fitoterapia. 1991;62:403–410. [Google Scholar]
- Lotfy WM, El-Morshedy HN, Abou El-Hoda M, El-Tawila MM, Omar EA, Farag HF. Identification of the Egyptian species of Fasciola. Vet Parasitol. 2002;103:323–332. doi: 10.1016/S0304-4017(01)00613-6. [DOI] [PubMed] [Google Scholar]
- Maqbool A, Hayat CS, Tanveer A. Comparative efficacy of various indigenous and allopathic drugs against fasciolosis in buffaloes. Veterinarski Arhiv. 2004;74:107–114. [Google Scholar]
- Mehta K, Balaraman R, Amin AH, Bafna PA, Gulati OD. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J Ethnopharmacol. 2003;86:191–195. doi: 10.1016/S0378-8741(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Mendes EA, Vasconcelos AC, Lima WDS. Histopathology of Fasciola hepatica infection in Merionesunguiculatus. Rev Patol Trop. 2012;41:55–62. doi: 10.5216/rpt.v41i1.17747. [DOI] [Google Scholar]
- Mitchell GBB. Update on fasciolosis in cattle and sheep. In Pract. 2002;24:378–385. doi: 10.1136/inpract.24.7.378. [DOI] [Google Scholar]
- Moazeni M, Khademolhoseini AA. Ovicidal effect of the methanolic extract of ginger (Zingiber officinale) on Fasciola hepatica eggs: an in vitro study. J Parasit Dis. 2016;40:662–666. doi: 10.1007/s12639-014-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney B, Good JP, Hanrahan G, Mulcahy T, de Waal T. The comparative efficacy of four anthelmintics against a natural acquired Fasciola hepatica infection in hill sheep flock in the west of Ireland. Vet Parasitol. 2009;164:201–205. doi: 10.1016/j.vetpar.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Murthy P, Saxena K, Lakshmi V, Kushwaha V, Verma S, Sharma R. Antifilarial activity of gum from Moringa oleifera Lam. on human lymphatic filaria Brugia malayi. Chron Young Sci. 2011;2:201–206. doi: 10.4103/2229-5186.93025. [DOI] [Google Scholar]
- Nanev V, Vladov I, Gabrashanska M, Kandil OM, Hassan NMF, Sedky D, Tsocheva-Gaytandzhieva NT. Rat spleen trace element contents undercompound effect on chronic fasciolosis basic zinc-copper compound. C R Acad Bulg Sci. 2017;70(8):1115–1120. [Google Scholar]
- Nassef NE, El-Kersh WM, El Sobky MM, Harba NM, El Refai Khalil SA. In vitro and in vivo assessment of the effect of soybean extract on Fasciola gigantica infection in comparison with triclabendazole. Menoufia Med J. 2014;27:93–102. doi: 10.4103/1110-2098.132768. [DOI] [Google Scholar]
- Patton CJ, Crouch SR. Calorimetric determination of urea. Anal Chem. 1977;49:464–469. doi: 10.1021/ac50011a034. [DOI] [Google Scholar]
- Paulo RC, Roberta FJC, Celina WM, Carlos d’ AMF. Histamine, histamine receptors and antihistamines: new concepts. An Bras Dermatol. 2010;85:195–210. doi: 10.1590/S0365-05962010000200010. [DOI] [PubMed] [Google Scholar]
- Poljakova-Krusteva O (1970) Pathogenic studies of hepatic changes in different hosts experimentally infected with Fasciola hepatica. Ph. D. Thesis, Bulgarian Academy of Sciences, Sofia, 6–69 (in Bulgarian)
- Reitman SMD, Frankel S. A colorimeter method for determination of serum glutamic oxaloacetic acid and glutamic pyruvic acid transferases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Roweliffe SA, Ollerenshaw OB. Observations on bionomics of the eggs of Fasciola hepatica. Ann Trop Med Parasit. 1959;54:172–181. doi: 10.1080/00034983.1960.11685973. [DOI] [PubMed] [Google Scholar]
- Saini RK, Sivanesan I, Keum YS. Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. Biotech. 2016;6:203. doi: 10.1007/s13205-016-0526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby HA, El Namaky AH, Kamel ROA. In vitro tegumental alterations on adult Fasciola gigantica caused by mefloquine. J Parasit Dis. 2016;40:145–151. doi: 10.1007/s12639-014-0466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Singh DK, Misra TN, Agarwal RA. Molluscicides of plant origin. Biol Agric Hortic. 1996;13:205–252. doi: 10.1080/01448765.1996.9754782. [DOI] [Google Scholar]
- Solana MV, Meray Sierra R, Scarcella S, Neira G, Solana HD. In vivo assessment of closantel ovicidal activity in Fasciola hepatica eggs. Exp Parasitol. 2016;160:49–53. doi: 10.1016/j.exppara.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Taleb DF, Soliman EK, el-Khalek A. Effect of fascioliasis on hematological, serum biochemical and histopathological changes in sheep. Egypt J Sheep Goat Sci. 2007;2:15–34. [Google Scholar]
- Tayo GM, Poné JW, Komtangi MC, Yondo J, Ngangout AM, Mbida M. Anthelminthic activity of Moringa oleifera leaf extracts evaluated in vitro on four developmental stages of Haemonchus contortus from goats. Am J Plant Sci. 2014;5:1702–1710. doi: 10.4236/ajps.2014.511185. [DOI] [Google Scholar]
- Trinder P. Enzymatic methods for glucose determination. Ann Clin Biochem. 1969;6:24–28. doi: 10.1177/000456326900600108. [DOI] [Google Scholar]
- Tsotetsi AM, Njiro S, Katsande TC, Moyo G, Baloyi F, Mpofu J. Prevalence of gastrointestinal helminths and anthelmintic resistance on small-scale farms in Gauteng Province, South Africa. Trop Anim Health Prod. 2013;45:751–761. doi: 10.1007/s11250-012-0285-z. [DOI] [PubMed] [Google Scholar]
- Ullah R, Rehman A, Zafeer MF, Rehman L, Khan YA, Khan MAH, Abidi SMA. Anthelmintic potential of thymoquinone and curcumin on Fasciola gigantica. PLOS ONE. 2017;12(2):e0171267. doi: 10.1371/journal.pone.0171267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Magaña JJ, Torres-Acosta JFJ, Aguilar-Caballero AJ, Sandoval-Castro CA, Hoste H, Chan-Pérez JI. Anthelmintic activity of acetone–water extracts against Haemonchus contortus eggs: interactions between tannins and other plant secondary compounds. Vet Parasitol. 2014;206:322–327. doi: 10.1016/j.vetpar.2014.10.008. [DOI] [PubMed] [Google Scholar]