Abstract
In the twenty-first century, the occurrence of allergic diseases has increased. Prevention and control of house dust mites (HDMs) are required as they play a major role in allergic conditions. The present work aimed to detect HDM allergy (Dermatophagoides pteronyssinus and Dermatophagoides farinae) among allergic patients attending the Allergy and Immunology Unit, Zagazig University. Ninety-six patients with a history of allergic diseases were included in this study. They were examined for allergy to D. pteronyssinus and D. farinae using different diagnostic tools: the skin prick test (SPT) and measurement of specific IgE antibodies to HDM allergen extracts. Ninety-six allergic patients were recruited in this study [60 females (62.5%) and 36 males (37.5%) aged between 5–60 years]. SPT (81.2 and 79.2%) and IgE (70.9 and 75%) gave positive results for both D. pteronyssinus and D. pteronyssinus, respectively. The common risk factors were use of cotton bedding > 10 years old, older homes > 20 years, crowded homes, family history, home dampness and homes at the ground floor. It was concluded that allergies to D. pteronyssinus and D. farinae contribute to allergic diseases in Zagazig City. Use of the SPT and IgE level is a promising diagnostic tool in the diagnosis of D. pteronyssinus and D. farinae.
Keywords: Skin prick test, Dermatophagoides farinae, Dermatophagoides pteronyssinus, Specific IgE, Allergy, Zagazig city
Introduction
Allergic diseases are considered a global health problem worldwide, with the prevalence increasing by 5% each year (Clausens et al. 2008). More than 300 million patients suffered from and 255,000 died from asthma in 2005 (Schneider et al. 2013). The principle sources of indoor allergens ,including HDMs, are animal dander, molds and cockroaches (Sears et al. 1989). Of these allergens, HDMs, especially D. pteronyssinus and D. farinae, are considered the major perennial indoor allergen sources inducing allergic sensitization worldwide (Sporik et al. 1990). House dust mites were detected as widely spread aeroallergens by multicenter studies in Europe, Asia, South America, Australia and Africa (Thomas 2010). They cause a significant number of allergic diseases such as allergic rhinitis (AR), atopic asthma (AA) and atopic dermatitis (AD) (Calderón et al. 2015). They not only affect the quality of life, but also cause significant morbidity and mortality (Asero et al. 2012). HDMs inhabit different areas in homes and workplaces that are rich in their survival necessities. They are found in carpets, bedding, clothing, soft toys and furnishings (Yu et al. 2015). Their preferable food is shed skin scales from people and animals, which can be colonized by fungi, bacteria and yeast, even though they can also benefit from various organic detritus sources present in different houses (Kort 1990). About 24 HDM allergens have been identified, which mostly represent extracts of HDM feces (Pittner et al. 2004). The definitive diagnosis of allergic diseases is made by a careful history taking, physical examination and results of laboratory tests (Moghtaderi et al. 2015). There are many ways to detect allergen-specific IgE, such as the skin prick test (SPT), enzyme-linked immunosorbent assay (ELISA), radioallergosorbent test (RAST) and elimination challenge method (Minami et al. 2013). The skin prick test remains a valuable epidemiologic tool in the diagnosis of allergic diseases. It is informative, safe and easy to perform, but has some disadvantages, such as pain, anaphylactic reactions and patient discomfort (Moghtaderi et al. 2015). Reliable and accurate assessment of specific IgE antibodies to HDM allergens is critical to identify patients with atopic allergy, identify causative agents of allergic diseases and help to provide an important step in selecting patients suitable for allergen immunotherapy using HDM extract (Yunginger et al. 2000). Therefore, this study was performed to determine the prevalence of HDM sensitization among different types of allergic patients in Zagazig City and focus on the proper diagnostic tools leading to proper diagnosis of HDM allergic diseases.
Subjects and methods
Study type
This cross-sectional study was done at the Allergy and Immunology Unit and Medical Parasitology Department, Faculty of Medicine, Zagazig University, from March to October 2017.
Subjects
Ninety-six patients with histories of allergic diseases such as atopic asthma, allergic rhinitis, atopic dermatitis and allergic conjunctivitis were recruited with these exclusion criteria: children < 5 years old, patients > 60 years old, pregnant females, patients receiving immunotherapy, associated broncho-pulmonary disorders, and patients with infectious and chronic chest diseases, e.g., severe persistent asthma. All selected patients were subjected to the following:
Full case history and complete clinical examination This included the age, sex, residence (urban or rural), type of allergic symptoms, family history of allergy and a completed questionnaire on the characteristics of the home as a building structure, degree of humidity in the home, bedding types, presence of poultry or pets at home, cleaning frequency and number of inhabitants.
Skin prick test (SPT) This test is based upon the indirect measurement of the reactivity of cutaneous mast cells in the presence of a specific IgE due to histamine release when certain allergens are injected into the skin (Bernstein et al. 2008). The skin site was cleaned with 70% alcohol before the test and left to dry. The forearm was marked and labeled with a marker pen according to to the tested allergens and the negative and positive controls. The distance between skin marks should not be < 2 cm. A drop from each of the allergen extracts of D. pteronyssinus and D. farinae allergen solution (Der p and Der f; Stallergen, France), saline solution as a negative and histamine dihydrochloride (10 mg/ml) as a positive control solution was put beside each mark. Then, a sterile prick lancet was used to prick the skin (Bernstein and Storms 1995). Results were read after 15–20 min; the reaction appeared as redness, swelling and skin itching (flare and wheal response). Wheals were measured in millimeters according to the standard guideline (Osterballe and Weeke 1979).
Specific IgE Abs detection of HDM allergens in the blood Blood samples were collected aseptically by vein puncture from all patients. Then, the serum was separated (Tu et al. 2013). The cellulose disc-based Enzyme Allergo Sorbent Test (EAST; RIDASCREEN® Spec. IgE, R-Biopharm, Germany), a special type of enzyme-linked immunosorbent assay (ELISA), was used to determine specific IgE antibodies quantitatively in human serum. All microwell plates and reagents were brought to room temperature (20–25 °C) for 10–15 min before use. Then, the reagents were mixed and the wash buffer prepared. The allergen DiscC, DiscS and DiscA filled a microwell plate. This was followed by complete removal of all buffers present in wells by suction. Then, 50 µl of each standard (in duplicate), control and patient serum was pipetted into the wells; subsequently, the plate was covered and incubated at 37 °C for 60 min. Diluted wash buffer was used to wash the wells. This step was repeated six times. In the last step, the buffer was removed completely, and the discs remained in the wells. Conjugate was added to each well and incubated at 37 °C for 60 min. Substrate solution (AllergySub S) was added to each well and incubated at 37 °C for 15 min. Finally, stopping of the reaction was done by adding 50 µl of stop reagent (Allergy Stop R) to each well. Reading the results was done at 405-nm wavelength against a reference wavelength of 620 nm.
Statistical analysis
The computerization and statistical analysis of the collected data were done using the SPSS program (Statistical Package for Social Science), version 18.0. Qualitative data were shown as frequencies and relative percentages. Calculation of the difference between qualitative variables within the groups was performed by the single chi-square test. A statistically significant difference was considered when P < 0.05.
Ethical consideration
The objectives and methodology of the study were explained to the patients or parents/guardians prior to sample collection, and a written or thumb-printed informed consent was obtained. The study was approved by the Committee of Research, Publications and Ethics of the College of Medicine, Zagazig University, Egypt.
Results
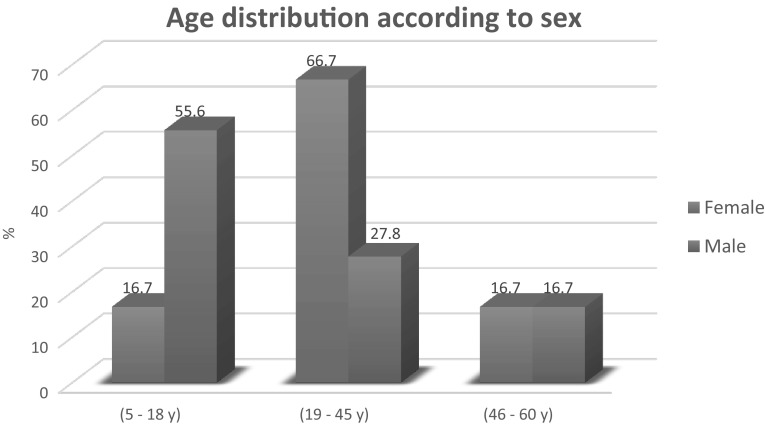
Study population Ninety-six allergic patients were recruited in this study [60 females (62.5%) and 36 males (37.5%)]. Of the 96 subjects, in the 5–18-year age group, 20 (55.6%) were males and 10 (16.7%) were females. In the 19–45-year age group, 40 (66.7%) were female and 10 (27.8%) were males. In the 46–60-year age group, ten (16.7%) were females and six (16.7%) were males, as shown in Fig. 1.
Skin prick test Regarding SPT, Table 1 showed that only 18 (18.8%) and 20 (20.8%) patients had negative SPT results for both Der p and Der f, respectively, while 78 (81.2%) and 76 (79.2%) had positive results for both Der p and Der f, respectively, with varying degrees of sensitivity. A highly statistically significant agreement between them (P < 0.001) was recorded.
Determination of specific IgE As shown in Table 2, there was significant accordance between the levels of specific IgE and both Der p and Der f (P = 0.04). Only 29.1 and 25% showed negative IgE level results for both Der p and Der f, respectively, while 70.9 and 75% had variable levels of specific IgE to Der p and Der f. The sensitivity, specificity, PPV, NPV and accuracy of specific IgE in the Der p and Der f allergens were (85.9, 94.4, 98.5, 60.7 and 87.5%) and (93.4, 95, 98.6, 79.2 and 93.8%), respectively, with highly statistically significant agreement between the two tests according to the kappa test (P < 0.001), as shown in Tables 3 and 4.
Risk factors accompanying allergic conditions The difference between the Der p and Der f allergen sensitized and non-sensitized groups detected by SPT regarding family history, poor housekeeping and bird nests was statistically significant, but non-significant between the other risk factors, as shown in Figs. 2 and 3.
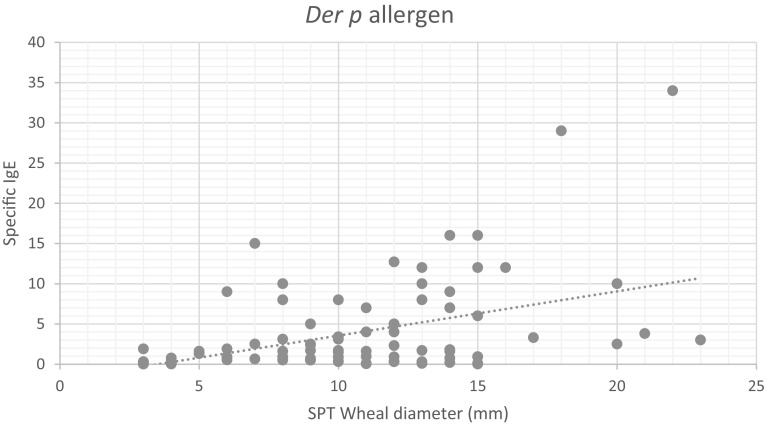
Correlation between SPT wheal diameter and specific IgE associated Der p and Der f allergen among 96 examined cases. The results showed that a positive correlation between the SPT wheal diameter and specific IgE level was significant with r values of 0.39 and 0.41 regarding Der p and Der f allergens, respectively, and P < 0.001, as shown in Figs. 4, 5.
Fig. 1.
Age and sex distribution of the 96 examined patients
Table 1.
Interpretation of the SPT results of the 96 examined cases
| Wheal size (mm) | Interpretation | SPT to Der p allergen extract | SPT to Der f allergen extract | ||
|---|---|---|---|---|---|
| No | % | No | % | ||
| 3–4 | Negative | 18 | 18.8 | 20 | 20.8 |
| 5–10 | Mildly sensitive | 30 | 31.2 | 32 | 33.3 |
| 10–15 | Moderately sensitive | 40 | 41.7 | 38 | 39.6 |
| > 15 | Markedly sensitive | 8 | 8.3 | 6 | 6.3 |
| % of Agreement (kappa) | 93.4% | ||||
| P value | < 0.001 | ||||
Table 2.
EAST classes and level of allergen-specific IgE titer to Der p and Der f allergens among the 96 examined cases
| EAST classes | Specific IgE concentration (IU/ ml) | Allergen-specific IgE content | sIgE to Der p | sIgE to Der f | ||
|---|---|---|---|---|---|---|
| No | % | No | % | |||
| 0 | 0.00–0.34 | None found | 28 | 29.1 | 24 | 25 |
| I | 0.35–0.69 | Low | 6 | 6.3 | 8 | 8.3 |
| II | 0.70–3.49 | Increased | 32 | 33.3 | 34 | 35.4 |
| III | 3.50–17.49 | Significant increased | 28 | 29.2 | 26 | 27.1 |
| IV | 17.50–49.99 | High | 2 | 2.1 | 4 | 4.2 |
| V | 50.00–99.99 | Very high | 0 | 0 | 0 | 0 |
| VI | ≥ 100.00 | Extremely high | 0 | 0 | 0 | 0 |
| % of Agreement (kappa) | 52.6% | |||||
| P value | 0.04 | |||||
Table 3.
Validity of specific IgE in the detection of Der p allergen among 96 examined cases
| Specific IgE | SPT | Total | Kappa | P value | |
|---|---|---|---|---|---|
| +ve | −ve | ||||
| +ve | 67 | 1 | 68 | 0.61 | < 0.001 |
| −ve | 11 | 17 | 28 | ||
| Total | 78 | 18 | 96 | ||
| Validity | Sensitivity: 85.9% PPV: 98.5% |
Specificity: 94.4% NPV: 60.7% |
|||
| Accuracy | 87.5% | ||||
PPV positive predictive value, NPV negative predictive value
Table 4.
Validity of specific IgE in detection of Der f allergen among 96 examined cases
| Specific IgE | SPT | Total | Kappa | P value | |
|---|---|---|---|---|---|
| +ve | −ve | ||||
| +ve | 71 | 1 | 72 | 0.71 | < 0.001 |
| −ve | 5 | 19 | 24 | ||
| Total | 76 | 20 | 96 | ||
| Validity | Sensitivity: 93.4% PPV: 98.6% |
Specificity: 95% NPV: 79.2% |
|||
| Accuracy | 93.8% | ||||
Fig. 2.
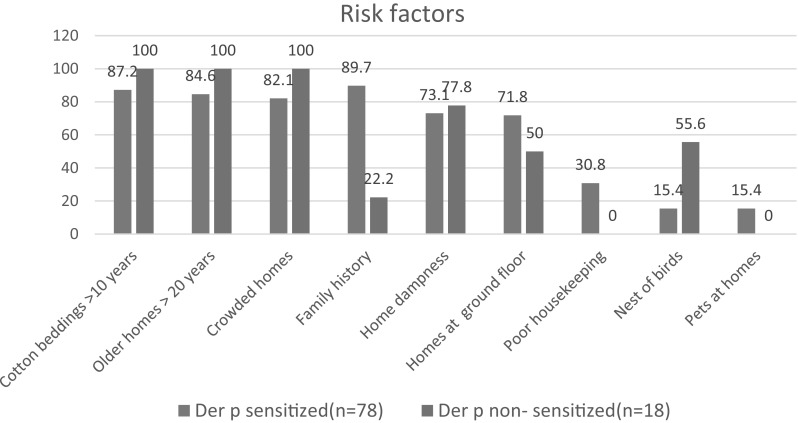
Risk factors of Der p allergen sensitization by SPT of the studied group
Fig. 3.
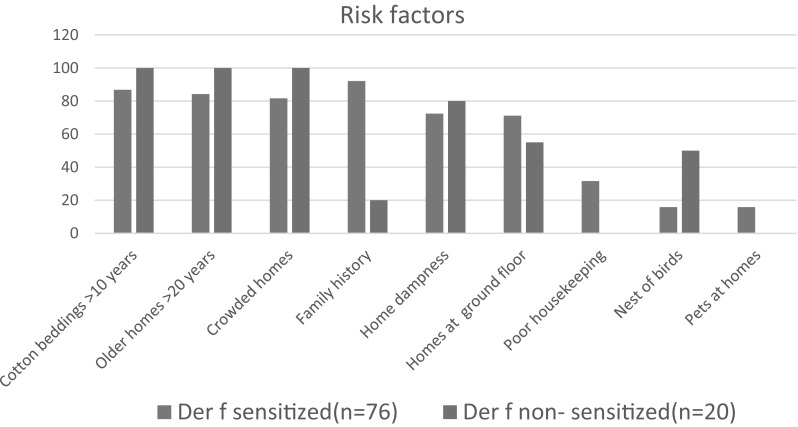
Risk factors of Der f allergen sensitization by SPT of the studied group
Fig. 4.
Correlation between SPT wheal diameter and specific IgE specific to Der p allergen
Fig. 5.
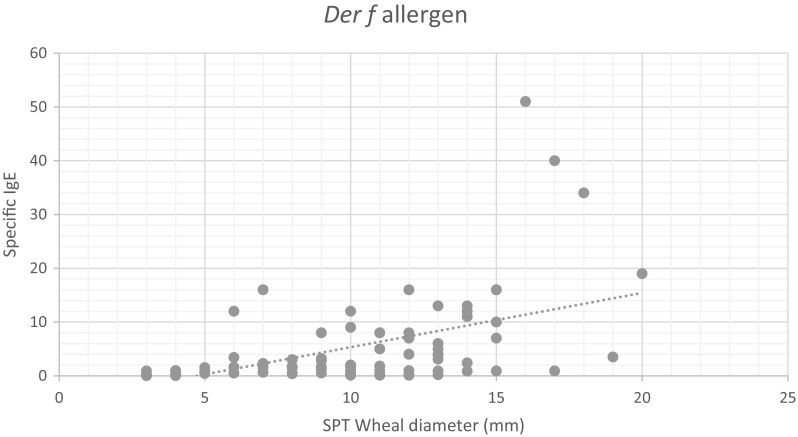
Correlation between SPT wheal diameter and specific IgE specific to Der f allergen
Discussion
The prevalence of allergic diseases such as atopic asthma, allergic rhinitis, atopic dermatitis and atopic rhinoconjunctivitis has increased in recent years, and they often cannot be completely controlled by modern medicine (Kubo et al. 2017). Children represent as much as 40% of the affected people in developed countries (Asher et al. 2006). Asthma is considered a major public health problem affecting more than 300 million people worldwide (Schneider et al. 2013). It has been suggested that the geographical situation of Egypt and its favorable climatic conditions together with other factors contribute to the abundance of HDM; consequently, HDM allergy occurs more commonly than any other allergens among Egyptian asthmatic patients (Hassan and Hagrass 2017). This work was performed to determine the prevalence of house dust mite allergy (D. pteronyssinus and D. farinae) in allergic patients attending the Allergy and Immunology Unit, Zagazig University, Zagazig City, using different diagnostic tools: SPT and estimation of specific IgE antibodies to HDM allergen extracts.
Regarding the gender distribution of allergic diseases, the present study included 60 (62.5%) females and 36 (37.5%) males, with a statistically significant difference between the number of females and males (P < 0.05): there were 20 males (55.6%) and 10 females (16.7%) in the 5–18-year age group, 40 females (66.7%) and 10 males (27.8%) in the 19–45-year age group and 10 females (16.7%) and six males (16.7%) in the 46–60-year age group. Several studies supported these results. Antonios et al. (2012) reported that there were three times more asthmatic female than male patients, indicating that that bronchial asthma was associated with females with an obvious sex bias. Also, females were reported to have a higher prevalence of asthma than males among preparatory school students in the Al Maadi and Al Maasara regions (Al Dhduh et al. 2015). Furthermore, Erel et al. (2017) reported that females were more susceptible to HDM allergy, especially D. pteronyssinus and D. farinae, than males (P < 0.05). The rate of HDM sensitization was 39% for females and about 29% for males. Sabry (2011) reported that 20–40% of asthmatic females of reproductive age suffer from worsening of their symptoms during their menstrual period, suggesting that sex hormones may have a major role in the biologic sex difference. These obvious sex differences may be attributed to many factors, such as the biologic sex differences including immunologic and pulmonary factors and environmental and sociocultural differences. Also, females complain of more severe symptoms, higher anxiety, more frequent doctor visits and more use of medications (Thomas et al. 2004). Genetic factors may also be involved, such as cyclooxygenase-2 (COX-2) gene homozygosity, which have been related to females. The COX pathways play an important role in the course of bronchial asthma (Holmquist and Vesterberg 2003). Additionally, total levels of IgE might be inheritable, especially in females, because of the existence of two alleles at the X-linked locus (Somani 2008). In contrast, Lin et al. (2015) recorded that the sensitization rates to both D. farinae and D. pteronyssinus were significantly higher in males than females (P < 0.05). This may be attributed to higher exposure to asthmatic triggers among men, such as smoking or occupational exposures with a subsequent increase in IgE levels and allergic sensitization (Krishnan et al. 2001). Also, Dowdee and Osseque (2007) found that the ratio of male to female children was 4:1; this ratio may invert after puberty and may be associated with the smaller airway diameters relative to lung volume among male children. Kim et al. (2013) stated that the total IgE levels were higher in males. However, the total IgE levels in girls increased with age from 3 to 6 years, while in boys a plateau was reached during the same age range. These findings showed that there was a disparity in the development of total IgE during the early years of life between girls and boys.
Regarding the different risk factors of allergic diseases among the examined cases, this study showed that the most common risk factor among the studied group was the use of cotton bedding > 10 years, homes > 20 years old, crowded homes, family history, home dampness and homes at the ground floor, representing 89.6, 87.5, 85.4, 77.1, 75 and 67.7%, respectively, while the least common risk factors were poor housekeeping, bird nests and pets at home, representing 25, 22.9 and 12.5%, respectively. Also, there was a statistically significant difference between the sensitized and non-sensitized groups of Der p and Der f allergens detected by SPT regarding family history, poor housekeeping and bird nests, but a non-significant difference between other risk factors. These results agreed with Antonios et al. (2012), who reported that 100% of patients had old furniture and old beds with cotton bedding and 90% lived in crowded homes; 60% of cases had a positive family history, and only 15% had pets in their homes. This family history may involve genetic factors and the surrounding environment because all family members are usually exposed to the same environmental conditions regarding humidity and temperature. The predictor of a high allergen load in beds is the age of the bed and mattress because a lot of dust tends to collect in older beds, representing an increase in the number of skin scales over time (Mihrshahi et al. 2002). An association between the age of the house and density of the mite population was observed because older homes were suggested to have a larger mite population than younger ones (Colloff 2009). This was attributed to older homes perhaps being damper and more poorly repaired than new ones (Custovic et al. 2002). Assarehzadegan et al. (2013) reported that mites can be transported outside houses to public places on the skin, hair or clothes. An active population of mites will be formed if favorable feeding conditions, temperature and humidity are available in the new environment. Sakaki and Suto (1994) reported that there was a positive correlation between the number of residents in homes and the average mite population densities. The family size, besides the number of rooms in the house, was strongly associated with the prevalence of mites in old homes in Japan. Additionally, Chen et al. (2007) reported that homes with many residents seem to have higher allergen concentrations in floor dust samples. This was attributed to the daily changes in indoor temperature and humidity levels determined by the different habits of the residents because more crowded rooms provide better chance for increasing humidity and the collection of dust via perspiration and domestic cleaning. Homes with good housekeeping practices are characterized by having significantly lower mite population densities than those with poor ones (Bigliocci et al. 1996). Frequent physical practices such as vacuuming and steam cleaning are effective and practical methods to decrease the level of allergens in low-income urban houses (Vojta et al. 2001). Also, Yu et al. (2015) reported that the main habitats of HDMs in indoor environments are mattresses, carpets, furniture, pillows and other places where humans rest. The size of the HDM population depends on the availability of food resources, degree of moisture and temperature. Al Dhduh et al. (2015) recorded that among students a prevalent family history regarding the absence or presence of asthma symptoms was 52.1 and 47.9%, respectively, with a statistically significant difference (P < 0.001). On the other hand, Mosbech et al. (1988) reported that there is no evidence that a certain kind of bedding is better than another (synthetic, cotton or wool blankets) to decrease the load of mite allergens. Regular hot washing (> 55 °C) can be done to properly remove allergens and get rid of mites.
Regarding the skin prick test (SPT), this study showed only 18 (18.8%) and 20 (20.8%) patients had negative SPT results for both Der p and Der f, respectively, while 78 (81.2%) and 76 (79.2%) had positive results for both Der p and Der f, respectively, as follows: 31.2 and 33.3% were mildly sensitive to both Der p and Der f, respectively, while 41.7% were moderately sensitive to Der p and 39.6% were moderately sensitive to Der f. Only 8.3 and 6.3% were markedly sensitive to both Der p and Der f, respectively. There was highly statistically significant agreement between them (P < 0.001). These results were in agreement with Lin et al. (2015), who recorded that the positivity rate of SPT to both D. farinae and D. pteronyssinus was 60.0% among children with allergic rhinitis (AR) living in Qingdao, China. A study in Shiraz, southwestern Iran, in 2001 reported that 44% of allergic patients with atopic dermatitis were sensitized to HDM allergens by SPT. The sensitization level to HDM allergens was 22.7% among patients with AR (Moghtaderi et al. 2015). Also, Antonios et al. (2012) recorded 95% positive reactions to both D. pteronyssinus and D. farinae allergens using SPT among asthmatic patients in Gharbia Governorate, Egypt. A study conducted to detect the rate of sensitization to multiple types of HDM using SPT allergen extracts in a group of asthmatic Egyptian children in Greater Cairo Province, Egypt, reported that 12% of the studied children showed sensitization to D. pteronyssinus, 11% to D. farinae and 7% to L. destructor, while 6 and 4% were sensitized to T. putrescentiae and A. siro, respectively. D. pteronyssinus and D. farinae were the most predominant species (Hosny et al. 2014). Caraballo et al. (2016) demonstrated that the assessment of the D. pteronyssinus sensitization by skin prick resulted in a range from 10.8% (in a cross-sectional population in Butajira, southern Ethiopia) to > 70% (in Singapore). In Saudi Arabia, Gheith et al. (2016) stated that the most common allergens differed from one area to another. For example, in Al-Khobar, SPT to D. farinae was 49.1 and to D. pteronyssinus 39.3%, while in Jeddah, it was 51.5% to D. farinae and 48.5% to D. pteronyssinus. These variable percentages of skin test results were attributed to the geographic nature and variability among ecologic conditions such as the temperature, relative humidity and environmental factors affecting the density of mite populations in homes. In Germany, Haftenberger et al. (2013) reported that the prevalence of sensitization to HDM allergens using SPT was 16% among adults and 22% in children.
The skin prick test (SPT) using a crude extract has been considered a standard diagnostic tool for the detection of allergic sensitization to a specific allergen, especially when combined with case history and clinical examination (Erel et al. 2017). It can give an idea about the immunotherapy of allergic patients (Dowdee and Osseqe 2007). It is simple, fast, safe and highly specific (Bousquet et al. 2007). The main advantage of SPT compared with an in vitro measurement of specific IgE antibodies is that the interpretation of the skin test can be done 20 min after applying the allergen to the skin. Moreover, skin testing gives an indication of the allergic sensitization, which may impact the patient's behavior (Heinzerling et al. 2013). However, there are many disadvantages to this method. There are many conditions under which SPT cannot be performed, including significant dermatographism, diffuse skin disease, an inability to stop taking drugs that may interfere with skin testing or the use of an allergen extract that may induce a systemic reaction in certain individuals (Stone et al. 2010).
Regarding immunologic evaluation of allergic patients by quantitative measurement of specific IgE antibodies in serum samples using EAST, the present study reported that only 29.1 and 25% had negative IgE level results for both Der p and Der f, respectively, while 70.9 and 75% had variable levels of specific IgE to Der p and Der f, respectively. There was a statistically significant agreement between them (P < 0.05). Fluctuating levels of specific IgE to HDM allergens among allergic patients in different regions were reported. For instance, in Egypt, Antonios et al. (2012) reported that the positive levels of specific IgE to both D. pteronyssinus and D. farinae allergens were 95% among asthmatic patients.
Additionally, Alshishtawy et al. (1991) recorded that D. pteronyssinus and D. farinae allergens were the highest level aeroallergens inducing asthma in Tanta, Egypt. In Saudi Arabia, Emad et al. (2006) reported 97 and 93.7% positive levels of specific IgE to both D. farinae and D. pteronyssinus allergens, respectively. In Australia, among atopic children and adults, the prevalence of sensitization of HDM to Der p 1 and Der p 2 was 91 and 86%, respectively (Pittner et al. 2004). According to the validity of specific IgE in the detection of Der p and Der f allergen extracts in correlation with SPT, the present work reported that the sensitivity, specificity, PPV, NPV and accuracy of specific IgE in measuring Der p allergen were 85.9, 94.4, 98.5, 60.7 and 87.5%, respectively. There was a highly statistical significant agreement between the two tests (P < 0.001) while the sensitivity, specificity, PPV, NPV and accuracy of specific IgE in measuring Der f allergen were 93.4, 95, 98.6, 79.2 and 93.8%, respectively. There was highly statistical significant agreement between the two tests (P < 0.001). These data were in accordance with Chung et al. (2010) who suggested that in vitro tests may be of lower sensitivity and/or specificity than SPT depending on the allergens and method utilized. Also, van der Zee et al. (1988) reported that there were discrepancies between the results of skin tests and IgE antibody methods showing that more than 80% of these discordances comprised positive skin test reactions in patients with undetectable allergen-specific IgE antibodies in their sera. However, Antonios et al. (2012) reported that both SPT and specific IgE tests had sensitivity and specificity of 100% and a positive and negative predictive value of 100% for both D. farinae and D. pteronyssinus. Application of a uniform, practical interpretation of different studies testing the application of both SPT and specific IgE to determine aeroallergen sensitization is difficult because of the availability of several practical methods (e.g., different allergen extracts and skin test devices), differences in study populations and variable analytical tools (Masse et al. 2011).
Regarding the correlation between SPT wheal diameter and specific IgE-associated Der f andDer p allergens, this work reported that there was positive, significant correlation between the SPT wheal diameter and specific IgE level in both Der f and Der p allergens. These data agreed with Antonios et al. (2012), who recorded that there was a correlation (100%) between the specific IgE levels and results of SPT in the detection of allergic cases of both D. farinae and D. pteronyssinus. Also, El Shami and Alaba (1989) reported that the results for the detection of a specific IgE were good and in accordance with the results of skin tests with high concordance and correlation.
These results agreed with De Vos (2014) who showed that there were fair to moderate correlations between the strength of the SPT reactions and levels of specific IgE. Also, it was reported that if only one type of immunologic testing (SPT or specific IgE) was done, a significant number of allergic cases could not be detected. Therefore, both SPT and specific IgE tests can be considered complementary tools to each other, but practically it is not always necessary to perform both SPT and specific IgE testing to avoid unnecessary costs. Therefore, the choice of the proper diagnostic method should be made on an individual base. Therefore, several studies suggest the measurement of serum allergen-specific IgE antibodies has a result similar to skin tests in the diagnosis of type I allergy, but with higher reproducibility and high value, especially under conditions that prevent SPT as it is not affected by anti-inflammatory and antihistamine therapies, which interfere with the proper interpretation of the test results. Also, measurement of serum allergen-specific IgE is considered the first choice for patients with widespread eczema dermographism and urticaria, which interferes with skin prick testing (Somani 2008). It was concluded that HDMs, especially D. pteronyssinus and D. farinae, are present at high concentrations among allergic patients attending the Allergy and Immunology Unit, Zagazig University, Zagazig City. In addition, the simultaneous use of both SPT and specific IgE tests should be considered in future studies aiming to determine sensitized population because using one test alone may not detect the exact range of allergic sensitizations and allow missed diagnoses. Also, implementation of strict control measures is necessary to reduce the mite population and allergen levels and to prevent different forms of allergic diseases, especially respiratory allergies, in this area.
Author contributions
AERT and SEE both designed the study design and research topics and initiated the research idea. SAAR initiated the research idea, shared in performing the laboratory work, wrote and reviewed the manuscript. AMFAG shared in performing the laboratory work and interpretation of the results. AHA shared in collecting the allergic cases to be examined from the Allergy and Immunology Unit. AMD helped perform laboratory work and collect references.
Compliance with ethical standards
Conflict of interests
The authors declare that there is no conflict of interests.
References
- Al-Dhduh MA, Sabri NAM, Fouda EM. Prevalence and severity of allergic diseases among Egyptian pediatric in different Egyptian areas. Int J Pharm Sci Res. 2015;2:107. [Google Scholar]
- Alshishtawy MM, Abdella AM, Gelber LE, Chapman MD. Asthma in Tanta, Egypt: serologic analysis of total and specific IgE antibody levels and their relationship to parasite infection. Int Arch Allergy Appl Immunol. 1991;196(4):348–354. doi: 10.1159/000235520. [DOI] [PubMed] [Google Scholar]
- Antonios SN, Ashour DS, Elkholy MG, Hanterra ME, Elmehy DA. House dust mites as a risk factor in chest allergic patients in Gharbia Governorate, Egypt. J Egypt Med Microbiol. 2012;21(1):51–62. doi: 10.12816/0004867. [DOI] [Google Scholar]
- Asero R, Mistrello G, Ariano R, Colombo G, Conte ME, Crivellaro M, De Carli M, Torre FD, Emiliani F, Rizzini FL, Longo R, Macchia D, Minale P, Murzilli F, Nebiolo F, Quercia O, Senna GE, Villalta D. Shrimp allergy in Italian adults: a multicenter study showing a high prevalence of sensitivity to novel high molecular weight allergens. Int Arch Allergy Immunol. 2012;157:3–10. doi: 10.1159/000324470. [DOI] [PubMed] [Google Scholar]
- Asher M, Montefort S, Bjorksten B, Lai CK, Strachan DP, Wieland SK. worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- Assarehzadegan MA, Shakurnia A, Amini A. The most common aeroallergens in a tropical region in Southwestern Iran. J World Allergy Organ. 2013;6(1):7. doi: 10.1186/1939-4551-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IL, Storms WW. Practice parameters for allergy diagnostic testing. Joint Task Force on Practice Parameters for the Diagnosis and Treatment of Asthma. The American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1995;75(2):543–625. [PubMed] [Google Scholar]
- Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, Sicherer S, Golden DB, Khan DA, Nicklas RA, Portnoy JM, Blessing-Moore J, Cox L, Lang DM, Oppenheimer J, Randolph CC, Schuller DE, Tilles SA, Wallace DV, Levetin E, Weber R. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(3):1–148. doi: 10.1016/S1081-1206(10)60305-5. [DOI] [PubMed] [Google Scholar]
- Bigliocci F, Frusteri L, Carrieri MP, Maroli M. Distribution and density of house dust mites Dermatophagoides spp. (Acarina: Pyroglyphidae) in the mattresses of two areas of Rome, Italy. Parassitologia. 1996;38:543–546. [PubMed] [Google Scholar]
- Bousquet PJ, Chinn S, Janson C, Kogevinas M, Burney P, Jarvis D. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy. 2007;62(3):301–309. doi: 10.1111/j.1398-9995.2006.01293.x. [DOI] [PubMed] [Google Scholar]
- Calderon MA, Linneberg A, Kleine-Tebbe J, De Blay F, Virchow JC, Demoly P. Respiratory allergy caused by house dust mites: What do we really know? J Allergy Clin Immunol. 2015;136(1):38–48. doi: 10.1016/j.jaci.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Caraballo L, Zakzuk J, Lee BW, Acevedo N, Soh JY, Sánchez-Borges M, Hossny E, García E, Rosario N, Ansotegui I, Puerta L, Sánchez J, Cardona V. Particularities of allergy in the tropics. World Allergy Organ J. 2016;9:20. doi: 10.1186/s40413-016-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Mielk A, Fahlbusch B, Bischof W, Herbarth O, Borte M, Wichmann HE, Heinrich J. Social factors, allergen, endotoxin and dust mass in mattresses. Indoor Air. 2007;17:384–393. doi: 10.1111/j.1600-0668.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- Chung BY, Kim HO, Park CW, Lee CH. Diagnostic usefulness of the serum specific IgE, the skin prick test and the atopy patch test compared with that of the oral food challenge test. Ann Dermatol. 2010;22(4):404–411. doi: 10.5021/ad.2010.22.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausens M, Kristjansson S, Haraldsson A, Bjorksten B. High prevalence of allergic diseases and sensitization in low allergen country. Acta Paediatr. 2008;97(9):1216–1220. doi: 10.1111/j.1651-2227.2008.00887.x. [DOI] [PubMed] [Google Scholar]
- Colloff MJ. Dust mites. Collingwood: Australia CSIRO; 2009. [Google Scholar]
- Custovic A, Simpson BM, Murray CS, Lowe L, Woodcock A. The National Asthma campaign manchester asthma and allergy study. Pediatr Allergy Immunol. 2002;13(15):32–37. doi: 10.1034/j.1399-3038.13.s.15.3.x. [DOI] [PubMed] [Google Scholar]
- De Vos G. Skin testing versus serum-specific IgE testing: Which is better for diagnosing aeroallergen sensitization and predicting clinical allergy? Curr Allergy Asthma Rep. 2014;14(5):430. doi: 10.1007/s11882-014-0430-z. [DOI] [PubMed] [Google Scholar]
- Dowdee A, Osseqe J. Assessment of childhood allergy for the primary care practitioner. J Am Acad Nurse Pract. 2007;19(2):52–63. doi: 10.1111/j.1745-7599.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- El Shami AS, Alaba O. Liquid-phase. In vitro allergen specific IgE assay with in sito immobilization. J Clin Pathol. 1989;74:191–201. [Google Scholar]
- Emad A, Koshak M, Kamal J, Daghistani T, Tarel S, Jamal HY. Allergy workshop in Allergic Rhinitis in Jeddah, Saudi Arabia. Int J Health Serv. 2006;5(1):1–6. [Google Scholar]
- Erel F, Sarioglu N, Kose M, Kaymakci M, Gokcen M. Intradermal skin testing in allergic rhinitis and asthma with negative skin prick tests. Iran J Allergy Asthma Immunol. 2017;16(3):193–197. [PubMed] [Google Scholar]
- Gheith T, Almehdar H, Koshak E. Common environmental allergens in the Kingdom of Saudi Arabia and their use in the diagnosis of allergic diseases by skin test. Egypt J Med Microbiol. 2016;25(4):57–71. doi: 10.12816/0037022. [DOI] [Google Scholar]
- Haftenberger M, Laußmann D, Ellert U, Kacklösch M, Langen U, Schlaud M. Prävalenz von Sensibilisierungengegen Inhalations- und Nahrungsmittelallergene. Ergeb- nisse der Studiezur Gesundheit Erwachsener in Deutsch- land (DEGS1) Bundesgesundheitsb. 2013;56:687–697. doi: 10.1007/s00103-012-1658-1. [DOI] [PubMed] [Google Scholar]
- Hassan AA, Hagrass SA. Prevalence of bronchial asthma in primary school children. Am J Med Sci. 2017;7(2):67–73. [Google Scholar]
- Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, Darsow U, Gjomarkaj M, Haahtela T, Bom AT, Locke R. The skin prick test–European standards. Clin Trans Allergy. 2013;3(1):3. doi: 10.1186/2045-7022-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist L, Vesterberg O. Immunochromatographic direct sampling on filter testing for aeroallergens. J Biochem Biophys Methods. 2003;57(3):183–190. doi: 10.1016/S0165-022X(03)00107-6. [DOI] [PubMed] [Google Scholar]
- Hosny E, El-Sayed S, Abdul-Rahman N. Sensitivity to five types of house dust mite in a group of allergic Egyptian children. Pediatr Allergy Immunol Pulmonol. 2014;27(3):133–137. doi: 10.1089/ped.2014.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kwon JW, Lim YM, Yoon D, Seo JH, Chang WS, Kim HY, Park JW, Cho SH, Hong SJ, Lee JS. Assessment of total/specific IgE levels against 7 inhalant allergens in children aged 3 to 6 years in Seoul, Korea. Allergy Asthma Immunol Res. 2013;5(3):162–169. doi: 10.4168/aair.2013.5.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort HSM. Mites, dust lice, fungi and their interrelationships on damp walls and room partitions. In: Bruin J, editor. Proceedings of the section experimental and applied entomology of the Netherlands Entomological Society (NEV) Amsterdam: Elsevier; 1990. pp. 63–68. [Google Scholar]
- Krishnan JA, Diette GB, Skinner EA, Clark BD, Steinwachs D, Wu AW. Race and sex differences in consistency with national asthma guidelines in managed care organizations. Arch Intern Med. 2001;161(13):1660–1668. doi: 10.1001/archinte.161.13.1660. [DOI] [PubMed] [Google Scholar]
- Kubo T, Morita H, Sugita K, Akdis CA. Middleton's allergy essential, Chap. 1. China: Elsevier; 2017. Introduction to mechanisms of allergic diseases; pp. 1–27. [Google Scholar]
- Lin H, Lin R, Li N. Sensitization rates for various allergens in children with allergic rhinitis in Qingdao, China. Int J Environ Res Publ Health. 2015;12(9):10984–10994. doi: 10.3390/ijerph120910984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse MS, Granger Vallee A, Chiriac A, Dhivert-Donnadieu H, Bousquet-Rouanet L, Bousquet PJ. Comparison of five techniques of skin prick tests used routinely in Europe. Allergy. 2011;66(11):1415–1419. doi: 10.1111/j.1398-9995.2011.02679.x. [DOI] [PubMed] [Google Scholar]
- Mihrshahi S, Marks GB, Vanlaar C, ToveyE Peat J. Predictors of high house dust mite allergen concentrations in residential homes in Sydney. Allergy. 2002;57:137–142. doi: 10.1034/j.1398-9995.2002.5720999.x. [DOI] [PubMed] [Google Scholar]
- Minami T, Fukutomi Y, Taniguchi M. IgE to Der p 1 and Der p 2 as predictors of airway response to house dust mite. Allergy. 2013;68:306. [Google Scholar]
- Moghtaderi M, Hejrati Z, Kolahi N, Heidari B. Sensitization to aeroallergens in patients with allergic rhinitis, asthma, and atopic dermatitis in Shiraz, Southwestern Iran. Indian J Allergy Asthma Immunol. 2015;29:79–83. doi: 10.4103/0972-6691.178272. [DOI] [Google Scholar]
- Mosbech H, Korsgaard J, Lind P. Control of house dust mites by electrical heating blankets. J Allergy Clin Immunol. 1988;81(4):706–710. doi: 10.1016/0091-6749(88)91042-1. [DOI] [PubMed] [Google Scholar]
- Osterballe O, Weeke B. A new lancet for skin prick testing. Allergy. 1979;34:187–194. doi: 10.1111/j.1398-9995.1979.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Pittner G, Vrtala S, Thomas WR, Weghofer M, Kundi M, Horak F, Kraft D, Valenta R. Component-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens. Clin Exp Allergy. 2004;34(4):597–603. doi: 10.1111/j.1365-2222.2004.1930.x. [DOI] [PubMed] [Google Scholar]
- Sabry EY. Relation of perimenstrual asthma with disease severity and other allergic co-morbidities (The first report of perimenstrual asthma prevalence in Saudi Arabia) Allergol Immunopathol. 2011;39(1):23–36. doi: 10.1016/j.aller.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Sakaki I, Suto C. Multiple regression analysis of effect of housing conditions on the prevalence of domestic mites in wooden houses in Nagoya, Japan. Jpn J Sanit Zool. 1994;45:341–351. doi: 10.7601/mez.45.341. [DOI] [Google Scholar]
- Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L. Atopic dermatitis a practice parameter update. J Allergy Clin Immunol. 2013;131(2):295–299. doi: 10.1016/j.jaci.2012.12.672. [DOI] [PubMed] [Google Scholar]
- Sears MR, Herbison GP, Holdaway MD, Hewitt CJ, Flannery EM, Silva PA. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy. 1989;19(4):419–424. doi: 10.1111/j.1365-2222.1989.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Somani VK. A study of allergen-specific IgE antibodies in Indian patients of atopic dermatitis. Indian J Dermatol Venereol Leprol. 2008;4(2):100–104. doi: 10.4103/0378-6323.39689. [DOI] [PubMed] [Google Scholar]
- Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p 1) and the development of asthma in childhood: a prospective study. N Engl J Med. 1990;323(8):502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2):73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WR. Geography of house dust mite allergens. Asian Pac J Allergy Immunol. 2010;28(4):211–224. [PubMed] [Google Scholar]
- Thomas RA, Green RH, Brightling CE, Birring SS, Parker D, Wardlaw AJ. The influence of age on induced sputum differential cell counts in normal subjects. Chest. 2004;126(6):77–86. doi: 10.1016/S0012-3692(15)31427-6. [DOI] [PubMed] [Google Scholar]
- Tu YL, Chang SW, Tsai HJ, Chen LC, Lee WI, Hua MC, Yao TC. Total serum IgE in a population-based study of Asian children in Taiwan: reference value and significance in the diagnosis of allergy. PLoS ONE. 2013;8(11):e80996. doi: 10.1371/journal.pone.0080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee JS, de Groot H, van Swieten P, Jansen HM, Aalberse RC. Discrepancies between the skin test and IgE antibody assays: study of histamine release, complement activation in vitro, and occurrence of allergen-specific IgG. J Allergy Clin Immunol. 1988;82(2):270–281. doi: 10.1016/0091-6749(88)91011-1. [DOI] [PubMed] [Google Scholar]
- Vojta PJ, Randels SP, Stout J, Muilenberg M, Burge HA, Lynn H, Mitchell H, O’Connor GT, Zeldin DC. Effects of physical interventions on house dust mite allergen levels in carpet, bed, and upholstery dust in low-income, urban homes. Environ Health Perspect. 2001;109(8):815–819. doi: 10.1289/ehp.01109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JM, Luo QH, Sun JL, Shi CL, Yin J, Zhou YL, Yu Z, Chen M. Diversity of house dust mite species in Xishuangbanna Dai a tropical rainforest Region in Southwest China. Biomed Res Int. 2015;2015:421716. doi: 10.1155/2015/421716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunginger JW, Ahlstedt S, Eggleston PA. Quantitative IgE antibody assays in allergic diseases. J Allergy Clin Immunol. 2000;105(1):1077–1084. doi: 10.1067/mai.2000.107041. [DOI] [PubMed] [Google Scholar]