Abstract
Cutaneous leishmaniasis is still a health problem worldwide, especially in tropical and subtropical areas. Currently, pentavalent antimony compounds are used to treat leishmaniasis. These compounds cause various side effects in the body. Therefore, there is a need to discover new drugs with less toxicity and more therapeutic effects. In this study, we encapsulated the meglumine antimonate into the albumin as a drug carrier and evaluated the anti-leishmanial effect of the prepared nanoparticles. The precipitation method was used for this purpose by applying different concentrations of glutaraldehyde and N-(3-Dimethylaminopropyl)-N-ethyl carbodiimide hydro chloride Ethyl (DEC) and then, field emission test was performed using Scanning Electron Microscopy for evaluating the morphology and size particles. The cytotoxicity and inhibitory of drugs were evaluated on J774 macrophages and Leishmania major promastigotes, respectively. Nanodrugs prepared using glutaraldehyde (10 μl/ml) and DEC (13 mg/ml) had the smallest and largest size, respectively. The highest anti-leishmanial activity was observed in the drugs prepared with glutaraldehyde (10 μl/ml). Also this nanodrug had the lowest cytotoxicity against macrophages. Given that meglumine antimonate loaded albumin nanoparticles prepared with glutaraldehyde (10 μg/ml), can improve the anti-leishmanial effects of this old drug, it can be a good option as a drug delivery system.
Keywords: Meglumine antimonate, Albumin, Glutaraldehyde, Encapsulation, Anti-leishmanial activity
Introduction
Leishmaniasis is one of the most important parasitic diseases caused by about 20 different species of Leishmania spp. At present, the disease causes 20,000 deaths every year in the world and nearly, 350 million people from 98 countries are at risk (Sabur and Ali 2015). It is estimated that around 2 million children and adults show clinical symptoms of leishmaniasis annually which is about 0.5 millions of these are visceral and 1.5 millions are cutaneous forms (Desjeux 1992; Nejati et al. 2014).
Leishmaniasis is considered as neglected diseases and less supported by public funds, governments and pharmaceutical companies and this is while the disease is ever-expanding in the world, So further research for the prevention and control of the disease is inevitable (Reithinger et al. 2007).
Selective drugs for treatment of leishmaniasis is pentavalent antimony compounds such as glucantime and pentostame (Fouladvand et al. 2013). Antimony compounds were first described in 1945 and have since been used as effective agents for some forms of leishmaniasis. However, the long-term administration of these drugs, variable effects and most importantly, the emergence and increase of parasite resistance are some factors that limit the effective use of these drugs (Borborema et al. 2011). Moreover, the side effects of these drugs are significant on different parts of the body, particularly in the liver and kidneys. On the other hand, there is still no effective vaccine to be used in endemic areas to control the disease (Noazin et al. 2009). Other alternative drugs for the treatment of leishmaniasis that can be mentioned are pentamidine, amphotericin-B and miltefosine (Fouladvand et al. 2011). A recent study conducted in the Bihar region in India (the most infected area with visceral leishmaniasis in the world) has shown that there are 10,000 unresponsive in one million patients to common types of anti-leishmanial drugs (Sundar et al. 2000; WHO 1995; Chowdhary et al. 2016).
In the current situation, the injection of these drugs and their distribution into the body causes side effects to other non-target areas. Also, a significant part of injected drugs are removed from the body before reaching the target site (Chowdhary et al. 2016). Therefore, due to the high prevalence of leishmaniasis in different parts of the world and the emergence of resistance to current common drugs, discovering new drugs with less toxicity and more therapeutic effects seems to be necessary (Santos et al. 2008). Even though, over the past decade, a number of alternative drugs have been introduced with new formulations and some drugs are still at the clinical trial stage, this problem is still critical.
Leishmania is an obligate intracellular parasite and resides and reproduces into the macrophages. After macrophage phagocytosis, the parasites enter the phagolysosomal vacuoles which it not only causes a restriction in drug access to the parasite, but also a relatively high dose of infusion will be needed to treat the disease and consequently, the side effects of the drug will increase, so, it seems the choice of macrophages as a drug target can minimize the cross-drug effects of non-target areas and solve many of the problems associated with these conventional drugs (Veerareddy et al. 2004). In this regard, drug delivery system can be used to send the drug to a specific target where the parasite is present (Gour et al. 2009) and it is obvious that such conditions can reduce the resistance of the parasite, increase the effect and minimize the side effects of the drug.
Nano and micro polymeric particles have been used as drug carriers in various studies (Venier-Julienne et al. 1995; Paul et al. 1998; Date et al. 2007) but one of the drawbacks of this group of compounds is their toxicity (Wissing et al. 2004). To overcome this problem, it is better to use degradable and non-toxic particles in drug delivery systems (Gupta and Hung 1989; Ahsan et al. 2002; Sanchez-Brunete et al. 2005). In 1972, albumin was approved by the US Food and Drug Administration for clinical use as a micro and nanospheric particle (Schafer et al. 1994) and subsequently, it found many diagnostic and therapeutic applications, as there are currently over 100 types of albumin-based diagnostic and therapeutic products available (Dandagi et al. 2006). Albumin is a degradable, non-toxic and inexpensive polymer that is available in pure form (Grinberg et al. 2007) and one of the important characteristics of this protein is its preferential removal by inflammatory tissues, so that is a good reason for choosing it as a drug carrier. In recent years, albumin nanoparticles have also been used as one of the most promising nanoparticle formulations for the treatment of cancers (Benita 2006).
Considering these issues, in this study, we are going to use different methods to load the meglumine antimonate onto the albumin as a drug carrier and compare their anti-leishmanial effects with the free drug.
Materials and methods
Ethical approval
This study was approved by the ethics committee of the Shiraz University of Medical Sciences (Ethics committee code: IR.SUMS.REC.1396.S473).
Chemicals
Chemicals were obtained as follows: Meglumine from Sigma-Aldrich Inc (St. Louis, MO, USA), Antimonate from Merck Chemicals (Darmstadt, Germany), Glutaraldehyde (labeled 25% aqueous solution) from Daejung Chemicals and Metals Co., Ltd (Seohaean-ro, Korea), N-(3-Dimethylaminopropyl)-N-ethyl carbodiimide hydro chloride from Merck Schuchardt OHG (DEC, Hohenbrunn, Germany), Acetone from Scharlab S.L (Sentmenat, Spain), Dimethyl Sulfoxide from Scharlab S.L (DMSO, Sentmenat, Spain). [3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl tetrazolium bromide)] from Sigma-Aldrich Inc (MTT, St. Louis, MO, USA), RPMI-1640 medium (with l-Glutamine and HEPES) from Caisson Laboratories Inc (North Logan, USA), Glucantime from Sanofi-Aventis (France), Penicillin–Streptomycin (labeled solution 100X) from Caisson Laboratories Inc (North Logan, USA), Bovine Serum Albumin from Sigma-Aldrich Inc (BSA, St. Louis, MO, USA), Fetal Bovine Serum from Sigma-Aldrich Inc (FBS, St. Louis, MO, USA), Other chemicals used in the tests were of laboratory reagents.
Preparation of meglumine antimonate-loaded albumin nanoparticles
The precipitation method was used for this purpose by applying different concentrations of glutaraldehyde and DEC. Briefly, meglumine carbohydrate was dissolved in distilled water. Then, by optimising the reaction conditions using various temperature and pH changes, antimonate was added to the meglumine solution at 55 °C and pH7. In the next step, albumin solution (0.2 gr/ml) was added to the above compound. In order to obtain a drug with a high level of anti-leishmanial activity, drug precipitation was performed in different ways using different concentrations of glutaraldehyde (0–10 µg/ml), with no using glutaraldehyde, and also using DEC (13 mg/ml). To enhance the drug loading and to achieve a higher amount of MA in the albumin nanoparticles, the acetone was gradually added during sonication with an Ultrasonic Sonicator (Bandelin, Germany) based on the method used previously to load Tamoxifen in the human serum albumin nanoparticles (Kouchakzadeh et al. 2014).
Evaluation of the surface morphology and particle size determination
From each of the products obtained, smears were prepared and after being coated with gold, a field emission test was performed using Scanning Electron Microscopy (SEM, Tescan Mira3-XMU, Kohoutovice, Czech Republic).
Parasite culture
The promastigote form of the standard strain of Leishmania major (MRHO/IR/75/ER) was taken from the Liquid Nitrogen Tank and initially transferred to a two-phase medium NNN. For mass cultivation, complete RPMI-1640 medium with L-Glutamin, HEPES and containing penicillin–streptomycin (100 IU/ml) supplemented with 10% heat-inactivated FBS was used as previously described (Fouladvand et al. 2013). The promastigotes in the stationary phase, also subcultured for achieving more parasites.
Macrophage culture
Strains of J774 mouse macrophages were taken from the Liquid Nitrogen Tank which has been provided before by the Department of Immunology, Shiraz University of Medical Sciences, Iran, and then cultured in complete RPMI-1640 medium at 37 °C and CO2 (5%) conditions.
Evaluation of the cytotoxicity of processed nanoparticles on macrophages
A suspension (1 × 105 cells/ml) was prepared from cultured macrophages and 100 µl was poured into each well of 96-well flat bottom microplates. After 24 h of incubation at 37 °C with CO2 (5%), supernatant was removed and 100 μl of drugs in serial dilutions (90, 30, 10, 3.3, 1.1, 0.34 and 0.12 μg/ml) were added to the wells and again incubated for 48 h at 37 °C.
The MTT assay technique was used to measure macrophage proliferation. First, 10 μl of MTT solution (5 mg/ml) was added to each well, and after 4 h of incubation at room temperature and in a dark place, DMSO was added in the wells to dissolve the formazan crystals formed. After being shaken for 20 min, the ODs were read at a wavelength of 570 nm and background absorption of 630 nm.
Evaluation of the inhibitory of processed nanoparticles on promastigote forms of parasites
A suspension (1 × 106 parasites/ml) from promastigotes in stationary phase was prepared and 100 µl was poured into each well of 96-well flat bottom microplates. Then, 100 μl of drugs (prepared at the same serial dilutions mentioned above) were added to the wells and incubated for 48 h at 25 °C. The next step was to add the MTT solution and continue the process as described above. All tests were performed as triplicate.
Statistical analysis
The results were analysed using statistical software (GraphPad Prism 6 Demo) and an ANOVA test.
Results
Evaluation of the surface morphology and determination of particle size
Images taken by the SEM in order to evaluate the surface morphology and the size of the drugs prepared by different methods (with different concentrations of glutaraldehyde, with no using glutaraldehyde, with EDC) are shown in Fig. 1. The evaluation of the images indicated that the drug prepared by 10 μl/ml glutaraldehyde has the best condition in terms of size and morphology compared with drugs treated with glutaraldehyde (5 μl/ml), non-treated with glutaraldehyde, and also treated with DEC.
Fig. 1.
Images taken by SEM from nanoparticles. a Treated with glutaraldehyde (10 μl/ml), b treated with glutaraldehyde (5 μl/ml), c non-treated with glutaraldehyde, d treated with EDC (13 mg/ml)
Evaluation of the cytotoxicity and inhibitory of processed nanoparticles
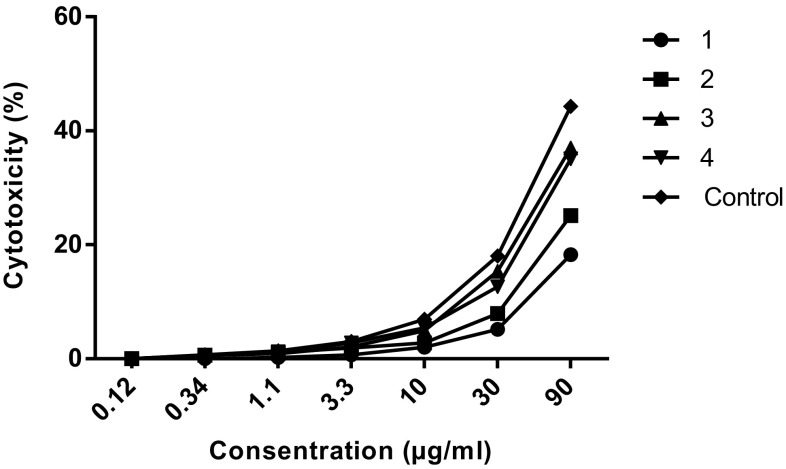
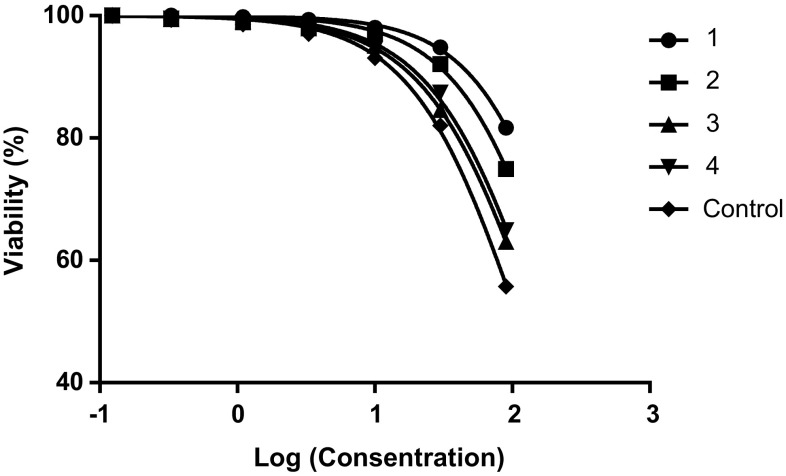
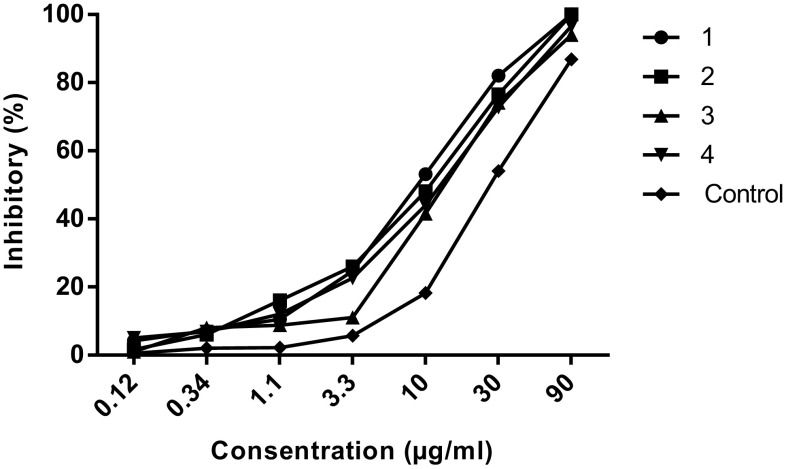
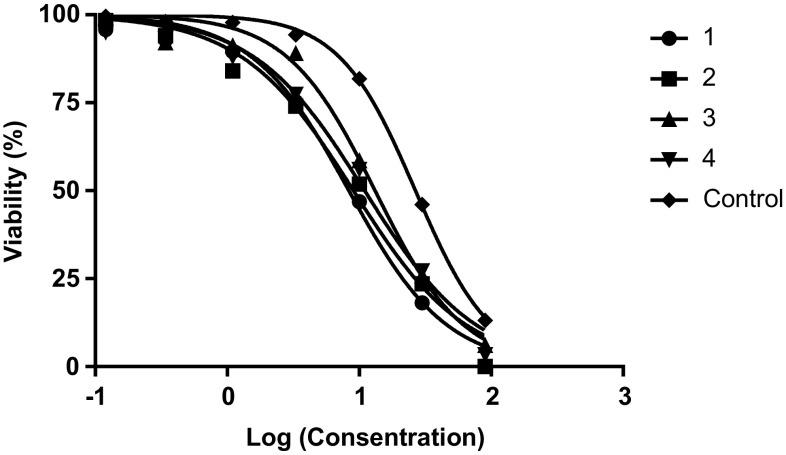
The cytotoxicity and inhibitory of the nanoparticles on macrophages and promastigotes were calculated using the formula [Viability (%) = ODTest − ODBlank/ODCont − ODBlank × 100] and [Cytotoxicity(%) = 100− Viability (%)]. Graphs were drawn using statistical software (GraphPad Prism 6 Demo) (Figs. 2, 3, 4, 5).
Fig. 2.
Evaluation of the cytotoxicity of nanoparicles on macrophages. 1: Nanoparticles treated with glutaraldehyde (10 µg/ml). 2: Nanoparticles treated with glutaraldehyde (5 µg/ml). 3: Nanoparticles non-treated with glutaraldehyde. 4: Nanoparticles treated with EDC. Control: Glucantime
Fig. 3.
Evaluation of the CC50 of nanoparticles on macrophages. 1: Nanoparticles treated with glutaraldehyde (10 µg/ml). 2: Nanoparticles treated with glutaraldehyde (5 µg/ml). 3: Nanoparticles non-treated with glutaraldehyde. 4: Nanoparticles treated with EDC. Control: Glucantime
Fig. 4.
Evaluation of the inhibitory of nanoparticles on promastigotes. 1: Nanoparticles treated with glutaraldehyde (10 µg/ml). 2: Nanoparticles treated with glutaraldehyde (5 µg/ml). 3: Nanoparticles non-treated with glutaraldehyde. 4: Nanoparticles treated with EDC. Control: Glucantime
Fig. 5.
Evaluation of the IC50 of nanoparticles on promastigotes. 1: Nanoparticles treated with glutaraldehyde (10 µg/ml). 2: Nanoparticles treated with glutaraldehyde (5 µg/ml). 3: Nanoparticles non-treated with glutaraldehyde. 4: Nanoparticles treated with EDC. Control: Glucantime
As shown in Table 1, the lowest IC50 and highest CC50 values were associated with nanoparticles treated with glutaraldehyde (10 µg/ml) (Table 1).
Table 1.
CC50 and IC50 values of nanoparticles on J774 macrophages and promastigotes, respectively
| Particles | CC50* | IC50** | SI*** |
|---|---|---|---|
| 1MA-ALB + GA (10 µg/ml) | 309.7 ± 2.49 | 8.35 ± 0.92 | 37.08 |
| 2MA-ALB + GA (5 µg/ml) | 232.8 ± 2.37 | 9.05 ± 0.96 | 25.73 |
| 3MA-ALB | 148.1 ± 2.17 | 13.18 ± 1.12 | 11.24 |
| 4MA-ALB + EDC (13 mg/ml) | 160.4 ± 2.20 | 10.99 ± 1.04 | 14.59 |
| Glucantime (Control) | 112.1 ± 2.05 | 26.67 ± 1.42 | 6.73 |
1Meglumine antimonate-loaded albumin nanoparticles treated with glutaraldehyde (10 µg/ml)
2Meglumine antimonate-loaded albumin nanoparticles treated with glutaraldehyde (5 µg/ml)
3Meglumine antimonate-loaded albumin nanoparticles non-treated with glutaraldehyde
4Meglumine antimonate-loaded albumin nanoparticles treated with EDC (13 mg/ml)
*Concentration of particles which has 50% cytotoxicity in macrophages
**Concentration of particles which has 50% inhibitory in promastigote forms
***Selectivity index
Disscusion
Leishmaniasis is one of the most important zoonotic diseases and is responsible for various species of Leishmania parasites. Cutaneous leishmaniasis (CL) is the most common form of the disease and is still a health problem worldwide, especially in tropical and subtropical areas. Currently, pentavalent antimony compounds such as glucantime and pentostame, are used to treat leishmaniasis as the first drugs of choice and amphotericin-B, paramomycin, pentamidine and miltefosine as second choices. These compounds cause various side effects in the body, but there are also other problems such as increased parasite resistance to these drugs. Therefore, there is a need to discover new drugs with less toxicity and more therapeutic effects (Santos et al. 2008).
In recent years, there has been a focus on providing nanoparticles as carriers for drug delivery. For some reasons, such as changes in the pharmacokinetics of a drug, these nanostructures can control drug release and protect the molecules of drugs. Also, because they are smaller in size than cells, they are able to cross biological barriers to deliver the drug to the target site and have a long life in the bloodstream. Therefore, they are very good options as a drug delivery system (Benita 2006).
In the preparation of these nanoparticles, various materials such as polymers, metal particles and lipids are used, out of which, depending on the production method, different shapes and sizes of particles can be produced. In recent years, protein nanoparticles have been used as an important carrier in the drug delivery system (Elzoghby et al. 2012; Peters 1995). In this study, albumin was used to encapsulate meglumine antimonate and, as already mentioned, the results achieved were satisfactory. Albumin is a plasma protein and acts as a natural carrier of many molecules, including fatty acids, eicosanoids, bile acids, steroid hormones, vitamin D, vitamin C, folate, copper, zinc, calcium and magnesium, as well as many drugs in the bloodstream such as penicillins, sulfanamides, benzodiazepines and indole compounds. Another important feature of albumin is its preferential removal by inflammatory as well as tumour tissues, which makes it a good candidate to be selected as a drug carrier. On the other hand, the naturaly presence of high amounts of albumin in the body makes it possible to inject significant amounts of it without or with very less complications (Peters 1995; Miele et al. 2009; Kratz 2008). In some similar studies, albumin has also been used to prepare nano-drugs. In a study conducted by Samir Das et al. (2011) to evaluate the anti-leishmanial effect of miltefosine-loaded albumin in invitro, the toxicity of the drug was reduced on the RAW264.7 macrophages, and the drug was able to target macrophages very well. In another study, the intake of paramomycin-loaded albumin by RAW264.7 macrophages was evaluated in invitro conditions and the authors mentioned that the rate of drug intake was significant, which is considered appropriate for the treatment of visceral leishmaniasis (Khan and Kumar 2011).
In the present study, various methods were used to synthesize meglumine antimonate-loaded albumin nanoparticles to compare final products in terms of morphology, particle size as well as anti-leishmanial activity and to select the best form for further studies. As shown in the figures of the results, the drug prepared by 10 μl/ml glutaraldehyde has the best condition in terms of size and morphology. In the evaluation of anti-leishmanial activities, this form of drug had the highest effect (IC50 = 8.35). On the other hand, in studying the cytotoxicity of these products on J774 macrophages, we observed the lowest toxicity of this drug compared to the other three prepared forms (CC50 = 309.7). In an experimental study on Hamsters infected with visceral leishmaniasis, Sanchez-Brunete et al. (2004) indicated that the amphotericin-B loaded albumin could reduce the toxicity of the drug to eight times and interestingly, after injection of the drug, its accumulation in the liver and spleen tissues was significantly higher, so that’s why the authors have introduced this form of the drug as a good alternative to amphotericin-B dezoxy culate (AMB-Doc). Jithan et al. (2011) encapsulated curcumin with albumin using glutaraldehyde and evaluated its therapeutic effects on breast cancer. In another study, Prabhakar Reddy Veerareddy et al. developed amphotericin-B as nanospheric lipids and after manosylation, evaluated its anti-leishmanial effects in BALB/c mice infected with Leishmania donovani. Researchers have used glutaraldehyde as an important ingredient in binding sufficient amounts of glycoside to aminophosphatidyl ethanolamine groups (Veerareddy et al. 2009). In a study, the anti-leishmanial effect of the liposomal meglumine antimonate on Leishmania-infected macrophages was evaluated and the results showed a significant inhibitory of the drug on parasite as well as its low toxicity on infected macrophages compared to the free drug (Borborema et al. 2011).
Conclusion
Considering that encapsulation of glucantime with albumin as a non-toxic carrier using glutaraldehyde at a concentration of 10 μg/ml, can improve and optimize the anti-leishmanial effects of this old drug, so, this form of drug can be a good alternative to the current drug and it is suggested that further studies be carried out in invivo studies.
Acknowledgements
This research is a part of the project approved by the Vice-Chancellor for Research of Shiraz University of Medical Sciences at Grant Number: 95-01-01-12795. Therefore, the authors express their gratitude and thanks to the approval and financial support of this plan.
Author contributions
Designed and performed the study: MHM; Nanoparticles syntesis: AB, NS, MHM; Performed the cell culture, anti-leishmanial assays and data analysis: AB, SR; Participated in writing the final paper: A B, MHM.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
Contributor Information
Afshin Barazesh, Phone: +98 (917) 186 0724, Email: afshin914@gmail.com.
Mohammad Hossein Motazedian, Phone: +98 (71) 32305291, Email: motazedian33@yahoo.com.
References
- Ahsan F, Rivas IP, Khan MA, Torres Suarez AI. Targeting to macrophages: role of physicochemical properties of particulate carriers—liposomes and microspheres—on the phagocytosis by macrophages. J Control Release. 2002;79:29–40. doi: 10.1016/S0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- Benita S. Microencapsulation methods and industrial applications. Boca Raton: CRC Press; 2006. [Google Scholar]
- Borborema SE, Schwendener RA, Osso JA, Jr, de Andrade HF, Jr, do Nascimento N. Uptake and antileishmanial activity of meglumine antimoniate-containing liposomes in Leishmania (Leishmania) major-infected macrophages. Int J Antimicrob Agents. 2011;38:341–347. doi: 10.1016/j.ijantimicag.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Chowdhary SJ, Chowdhary A, Kashaw S. Macrophage targeting: a strategy for leishmaniasis specific delivery. Int J Pharm Pharm Sci. 2016;8:16–26. [Google Scholar]
- Dandagi PM, Mastiholimath VS, Patil MB, Gupta MK. Biodegradable microparticulate system of captopril. Int J Pharm. 2006;307:83–88. doi: 10.1016/j.ijpharm.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Das S, Khan W, Mohsin Sh, Kumar N. Miltefosine loaded albumin microparticles for treatment of visceral leishmaniasis: formulation development and in vitro evaluation. Polym Adv Technol. 2011;22:172–179. doi: 10.1002/pat.1710. [DOI] [Google Scholar]
- Date AA, Joshi MD, Patravale VB. Parasitic diseases: liposomes and polymeric nanoparticles versus lipid nanoparticles. Adv Drug Deliv Rev. 2007;59:505–521. doi: 10.1016/j.addr.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Desjeux P. Human leishmaniases: epidemiology and public health aspects. World Health Stat Q. 1992;45:267–275. [PubMed] [Google Scholar]
- Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157:168–182. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Fouladvand M, Barazesh A, Farokhzad F, Malekizadeh H, Sartavi K. Evaluation of invitro anti-leishmanial activity of some brown, green and red algae from the Persian Gulf. Eur Rev Med Pharmacol Sci. 2011;15:597–600. [PubMed] [Google Scholar]
- Fouladvand M, Barazesh A, Tahmasebi R. Evaluation of in vitro antileishmanial activity of curcumin and its derivatives “gallium curcumin, Indium curcumin and diacetyle curcumin”. Eur Rev Med Pharmacol Sci. 2013;17:3306–3308. [PubMed] [Google Scholar]
- Gour JK, Srivastava A, Kumar V, Bajpai S, Kumar H, Mishra M. Nanomedicine and leishmaniasis: future prospects. Dig J Nanomater Biostruct. 2009;4:495–499. [Google Scholar]
- Grinberg O, Hayun M, Sredni B, Gedanken A. Characterization and activity of sonochemically-prepared BSA microspheres containing Taxol–an anticancer drug. Ultrason Sonochem. 2007;14:661–666. doi: 10.1016/j.ultsonch.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Gupta PK, Hung CT. Albumin microspheres: applications in drug delivery. J Microencapsul. 1989;6:463–472. doi: 10.3109/02652048909031166. [DOI] [PubMed] [Google Scholar]
- Jithan A, Madhavi K, Madhavi M, Prabhakar K. Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int J Pharm Invest. 2011;1:119–125. doi: 10.4103/2230-973X.82432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W, Kumar N. Drug targeting to macrophages using paromomycin-loaded albumin microspheres for treatment of visceral leishmaniasis: an in vitro evaluation. J Drug Target. 2011;19:239–250. doi: 10.3109/1061186X.2010.492524. [DOI] [PubMed] [Google Scholar]
- Kouchakzadeh H, Shojaosadati SA, Shokri F. Efficient loading and entrapment of tamoxifen in human serum albumin based nanoparticulate delivery system by a modified desolvation technique. Chem Eng Res Des. 2014;92:1681–1692. doi: 10.1016/j.cherd.2013.11.024. [DOI] [Google Scholar]
- Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int J Nanomedicine. 2009;4:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejati J, Mojadam M, Hanafi Bojd AA, Keyhani A, Habibi Nodeh F. An epidemiological study of cutaneous leishmaniasis in Andimeshk (2005–2010) SJIMU. 2014;21:94–101. [Google Scholar]
- Noazin S, Khamesipour A, Moulton LH, Tanner M, Nasseri K, Modabber F. Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis: a meta-analysis. Vaccine. 2009;27:4747–4753. doi: 10.1016/j.vaccine.2009.05.084. [DOI] [PubMed] [Google Scholar]
- Paul M, Durand R, Boulard Y, Fusaï T, Fernandez C, Rivollet D, Deniau M, Astier A. Physicochemical characteristics of pentamidine-loaded polymethacrylate nanoparticles: implication in the intracellular drug release in Leishmania major infected mice. J Drug Target. 1998;5:481–490. doi: 10.3109/10611869808997874. [DOI] [PubMed] [Google Scholar]
- Peters T. All about albumin. Biochemistry, genetics and medical applications. San Diego: Academic press; 1995. [Google Scholar]
- Reithinger R, Dujardin J, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- Sabur A, Ali N. Vaccine biology of Leishmania infection. In: Adak S, Datta R, editors. Leishmania, current biology and control. Norfolk: Caister Academic Press; 2015. pp. 167–192. [Google Scholar]
- Sanchez-Brunete JA, Dea MA, Rama S, Bolas F, Alunda JM, Raposo R, Mendez MT, Torrado-Santiago S, Torrado JJ. Treatment of experimental visceral leishmaniasis with amphotericin-B in stable albumin microspheres. Antimicrob Agents Chemother. 2004;48:3246–3252. doi: 10.1128/AAC.48.9.3246-3252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Brunete JA, Dea MA, Rama S, Bolas F, Alunda JM, Torrado-Santiago S, Torrado JJ. Influence of the vehicle on the properties and efficacy of microparticles containing amphotericin-B. J Drug Target. 2005;13:225–233. doi: 10.1080/10611860500097107. [DOI] [PubMed] [Google Scholar]
- Santos DO, Coutinho CER, Madeira MF, Bottino CG, Vieira RT, Nascimento SB, Bernardino A, Bourguignon SC, Corte-Real S, Pinho RT, Rodrigues CR, Castro HC. Leishmaniasis treatment—a challenge that remains: a review. Parasitol Res. 2008;103:1–10. doi: 10.1007/s00436-008-0943-2. [DOI] [PubMed] [Google Scholar]
- Schafer V, von Briesen H, Rubsamen-Waigmann H, Steffan AM, Royer C, Kreuter J. Phagocytosis and degradation of human serum albumin microspheres and nanoparticles in human macrophages. J Microencapsul. 1994;11:261–269. doi: 10.3109/02652049409040455. [DOI] [PubMed] [Google Scholar]
- Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the indian epidemic. Clin Infect Dis. 2000;31:1104–1107. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- Veerareddy PR, Vobalaboina V, Nahid A. Formulation and evaluation of oil-in-water emulsions of piperine in visceral leishmaniasis. Pharmazie. 2004;59:194–197. [PubMed] [Google Scholar]
- Veerareddy PR, Vobalaboina V, Ali N. Antileishmanial activity, pharmacokinetics and tissue distribution studies of mannosegrafted amphotericin-B lipid nanospheres. J Drug Target. 2009;17:140–147. doi: 10.1080/10611860802528833. [DOI] [PubMed] [Google Scholar]
- Venier-Julienne MC, Vouldoukis I, Monjour L, Benoit JP. In vitro study of the anti-leishmanial activity of biodegradable nanoparticles. J Drug Target. 1995;3:23–29. doi: 10.3109/10611869509015929. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Kayser O, Muller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–1272. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1995) WHO model prescribing information: drugs used in parasitic diseases, 2nd edn. World Health Organization, Geneva. http://www.who.int/iris/handle/10665/41765