Abstract
The emergence of resistance to the current available drugs used for treatment against Indian Kala-azar (KA) or Visceral Leishmaniasis makes the control strategy inadequate for the disease. This grave epidemiological situation directed researches towards alternative treatments including herbal therapy. In this background, the aim of the present study was to evaluate the antileishmanial activity of the leaves of Coccinia grandis (a tropical vine) against both the Sodium Stibo Gluconate (SSG) sensitive and resistant as well as Miltefosine (MIL) sensitive and resistant field isolates of Leishmania donovani. The cytotoxicity effect of ethanolic extract of leaves of C. grandis (Cg-LE) against the clinical isolates of L. donovani was checked both in promastigotes and intracellular amastigotes stages. In both sensitive and resistant promastigotes, Cg-LE stimulated reactive oxygen species generation and apoptosis. Parasites infected macrophages showing enhanced nitric oxide production after Cg-LE treatment suggested the leishmanicidal activity of the leaf extract. Furthermore, Cg-LE treatment led to mitochondrial membrane damage and DNA fragmentation in promastigotes. The present study is very encouraging for the fact that Cg-LE showed promising antileishmanial activity against both SSG and MIL drug resistant clinical isolates of Indian KA.
Keywords: Leishmania donovani, Drug resistant field isolates, Coccinia grandis, Antileishmanial activity, Apoptosis, DNA fragmentation
Introduction
Leishmaniasis is one of the neglected tropical diseases caused by the protozoan parasites belonging to the genus Leishmania. Among three different clinical forms, Visceral Leishmaniasis (VL) or Kala-azar (KA) is the most severe form, if left untreated (Sundar and Rai 2002). It is a systemic infection and most affected vital organs are spleen, liver, lymph nodes etc.
Around the World, 90% of VL cases occur in Indian subcontinent, Sudan, Ethiopia and Brazil (Desjeux 2004). Currently available drugs for the treatment of VL are not working efficaciously due to the emergence of resistance (Croft et al. 2006) and there is no potent vaccine (Kedzierski 2010). In this bleak scenario, alternative therapeutics is gaining impetus for obtaining antileishmanial compounds. Thus control measures have now focused mainly on alternative therapeutics using medicinal plants (De Carvalho and Ferreira 2001; Croft and Yardley 2002). World Health Organisation reported that almost 80% of the total population of developing countries depends on traditional medicines for their health care needs (WHO 2000). C. grandis is one of such medicinal plants with enormous medicinal values. Aqueous extract of leaves of C. grandis showed promising antibacterial activity against both gram positive and gram negative bacteria (Pekamwar et al. 2013) while root, fruit and leaves of C. grandis possess antioxidant activity (Mujumder et al. 2008). The aqueous extracts of C. grandis leaves and stem has the anti-inflammatory activity (Deshpande et al. 2011). Prostaglandin biosynthesis seems influenced by C. grandis achieving antipyretic activity (Aggarwal et al. 2011). It was also evaluated for its anticancer property (Bhattacharya et al. 2010) and alcoholic extract of the fruit of the plant showed hepatoprotective role (Vadivu et al. 2008). C. grandis has potent antihelminthic activity (Tamilselvan et al. 2011) and anti protozoan activity (Samanta et al. 2011; Sundaram et al. 2012). Secondary metabolites (quinones, alkaloids, saponins, terpenes, etc.) obtained from many plants are the potent sources of antileishmanial compounds (Chan-Bacab and Pena-Rodriguez 2001; Schinor et al. 2007; Soares et al. 2007). C. grandis leaf extract kills the Plasmodium falciparum and Plasmodium berghei (Samanta et al. 2011; Sundaram et al. 2012) and it has further been reported that leaf extract of C. grandis has serine protease inhibitor (Das et al. 2015).
In this background, the present study aims to evaluate the anti-leishmanial activities of C. grandis leaf extract on clinical isolates collected from confirmed Indian Kala-azar patients giving special emphasis on the drug resistant field isolates of KA. The rationale for the endeavor is to search medicinal plants which may have the capacity to kill those resistant isolates.
Materials and methods
Reagents and chemicals
M199 medium, RPMI 1640 medium, HEPES, sodium bicarbonate, sulfanilamide, N-(1-naphthyl) ethylene diamine dihydrochloride, Giemsa, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich. 5,5,6,6-Tetrachloro-1,1,3,3-tetraethylbenzimidazole carbocyanide iodide (JC-1) and FITC AnnexinV-PI apoptosis kits were purchased from molecular probes. Ethanol was purchased from Merck. Fetal bovine Serum (FBS) was purchased from Gibco, USA. Agarose was obtained from HIMEDIA and QIAamp DNA mini kit was purchased from Qiagen, Germany.
Ethical approval
Bone marrow aspirates or splenic aspirates were collected from KA patients (as a part our DBT Twinning Programme) with prior approval of the Ethical Committee of the Calcutta National Medical College, Kolkata. The written consent was taken from the patients. The presence of amastigotes in the aspirates was confirmed by Giemsa staining and transforming them into promastigotes form in M199 supplemented with 10% Fetal Bovine Serum (FBS). The isolates were further maintained in golden hamsters (Mesocricetus auratus) and transformed the parasites from infected spleen of hamsters. The hamsters were maintained in animal care facilities in CSIR-Indian Institute of Chemical Biology approved by Institutional animal Ethical Committee. Animal experiments were carried out the National Regulatory Guidelines issued by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India (Ac. Number. 147/1999/CPCSEA).
Field isolates of Indian KA
Three field isolates of Indian KA patients were taken for the present study (Table 1): A2, field isolate sensitive to both SSG and MIL; T8, field isolate resistant to SSG and T9, resistant to MIL. The resistance and sensitivity of the isolates to the respective drugs were established (Khanra et al. 2016, 2017). The underlying rationale to select these isolates for the present study was based on the understanding that we wanted to check the efficacy of the ethanolic leaf extract of C. grandis (Cg-LE) on Indian KA clinical isolates both sensitive and resistant to the current drugs of use: SSG and MIL.
Table 1.
Sources of the clinical isolates of Indian KA used for the present study
| Study codea | Ageb | Sexc | Clinical statusd | Countrye |
|---|---|---|---|---|
| A2 | 3 years 6 months | Female | VL | India |
| T8 | 11 years | Male | VL | India |
| T9 | 5 years | Female | VL | India |
aStudy codes given to the collected clinical isolates of KA for the present study
bAge of the KA patients
cSex of the KA patients
dClinical status of the patients
eCountry of the patients
Cell line
Murine macrophage cell line named RAW 264.7 used for the present study was obtained from Dr. Syamal Roy, INSA Senior Scientist and J. C. Bose Fellow, Indian Institute of Chemical Biology, Kolkata. The cell line was maintained in RPMI 1640 medium with 10% FBS under 5% CO2 incubator at 37°C.
Plant material
The leaves of C. grandis were collected during March, 2015 from Sodepur, Kolkata, West Bengal. The leaves were identified in the Department of Botany, Calcutta University. The specimen named as Lahiry 004 and accession number is 20032 (CUH). Specimen was kept in Calcutta University Herbarium (CUH).
Extract preparation
Leaves of C. grandis were dried in dark at the environmental temperatures (22–29°C) for 20–35 days and extracted with ethanol using Soxhlet apparatus (boiling point range 60–80°C) for 6–8 h. The ethanolic leaf extract of C. grandis (Cg-LE) was condensed at temperature ranges from − 8 to − 10°C in 100 mm Hg pressure. The remainder extract was stored at 4°C (Rahuman and Venkatesan 2008). One gram of residue extract was first dissolved in 100 ml of ethanol and served as a stock solution. From the stock solution, 500 µg/ml was used in the final test solution.
Toxicity assay of Cg-LE on macrophages
Macrophages were incubated with increasing concentrations of Cg-LE. After 24 h MTT was added and incubated for 4–6 h at room temperature at 37°C. The optical density (OD) was measured after 30 min in a plate reader at 570 nm (Verma and Dey 2004).
Anti-leishmanial activity by MTT assay
Log phase promastigotes (2 × 105/well) were incubated at different concentrations of Cg-LE (0–500 µg/ml) for 24 h. Promastigotes viability was measured by MTT assay as described elsewhere (Mosmann 1983). The optical density was measured using an ELISA plate reader at 570 nm.
In order to determine EC50, macrophages were plated in sterile freshly prepared 22 mm cover slips with different concentrations of Cg-LE for 24 h at 37°C and untreated macrophages received only medium and acted as control (Kremb et al. 2010). Intracellular amastigotes were counted under microscope and the 50% effective concentration (EC50) of Cg-LE was estimated by GraphPad Prism5 software (version 5.03) as described previously (Kremb et al. 2010; da Luz et al. 2009).
Measurement of nitric oxide production
Macrophages (1 × 105 macrophages /well) were plated in sterile tissue culture plate at 37°C in 5% CO2 incubator and provided 48 h as adherence time for macrophages. Infection was given by log phage promastigotes for 24 h and treatment was given by Cg-LE for 24 h at 37°C in 5% CO2 incubator. Nitrite was accumulated in cell free supernatants and measured by the Griess assay (Green et al. 1982) and the results are expressed in mM nitrite.
Measurement of reactive oxygen species production
The cell permeable, 2,7-dichlorofluorescein diacetate, was used to measure the reactive oxygen species (ROS) (Haldar et al. 2009). Adherent macrophages on tissue culture plate were infected with log phase promastigotes for 24 h and treatment was given by Cg-LE for another 24 h at 37°C in 5% CO2 incubator. Relative fluorescence was measured (LS50B spectrophotometer; Perkin-Elmer, USA) at an excitation wavelength of 510 nm and emission wavelength of 525 nm.
Mitochondrial membrane potential
Mitochondrial transmembrane potential depolarization is hallmark of early apoptotic events, was routinely measured by JC1 dye (Dolai et al. 2009), sensitive to the electrochemical potential of mitochondria. Untreated and Cg-LE treated Leishmania promastigotes were incubated JC1in dark for 20 min and flow cytometry analysis was carried out in a BDFACSAria II cell sorter and analyzed by FACSDIVA software (BD Biosciences, San Jose, CA, USA).
DNA fragmentation assay
Three sets of experiments were performed: 1 × 106 promastigotes of three types of clinical isolates were treated (1) with the drug, SSG, (2) with the drug, MIL and (3) with the leaf extract of C. grandis (Cg-LE) respectively. Genomic DNA was isolated from all groups by using QIAamp DNA mini kit as per manufacturer’s instruction. DNAs (900 ng) were loaded on a 1% agarose gel and run for 1 h at 100 V. The bands were visualized under UV light.
FITC AnnexinV-PI assay for apoptosis
Flipping of Phosphatidylserine (PS) of plasma membrane from inside to outside is another hallmark of apoptosis. In brief, untreated and Cg-LE treated promastigotes were washed in cold sterile PBS and stained with FITC conjugated AnnexinV and PI (Albanyan et al. 2009). Samples were analyzed immediately by flow cytometry at an excitation wavelength of 488 nm and emissions at 530 nm for green and 590 nm for red fluorescence.
Statistical analysis
Graph Pad prism software version V (San Diego, CA) was used for statistical analysis. Student’s t test was performed and statistical significance of differences between groups was determined by the unpaired two-tailed Student’s t test. Statistical significance was defined as P value of <0.05 and results were expressed as mean ± standard deviations of triplicate measurements. *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Antileishmanial activity
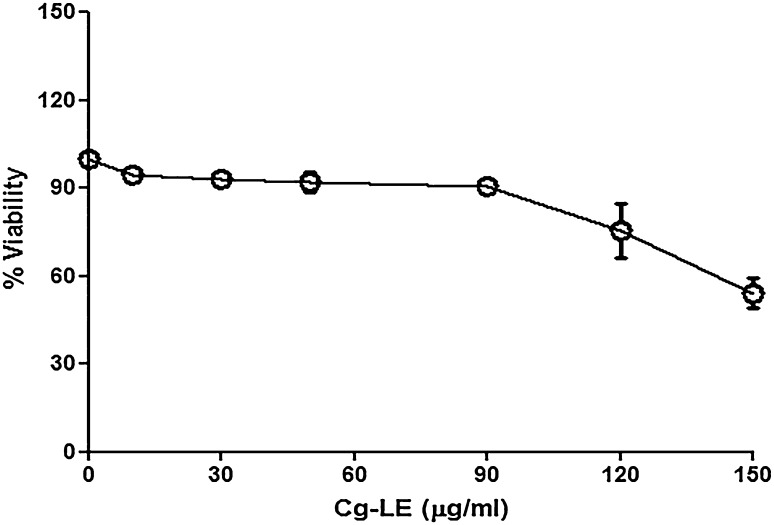
Both drug sensitive and resistant isolates of KA (A2, T8 and T9) were treated with Cg-LE in vitro conditions to measure the sensitivity of the isolates towards the extracts. RAW 264.7 cell line was used as host cell in the present study. Cg-LE extract have no cytotoxicity on host macrophages, it was observed that more than 90% macrophages remained viable up to 90 μg/ml Cg-LE concentrations (Fig. 1). The IC50 and EC50 values of each of the isolates (Table 2) were estimated against Cg-LE using Graph Pad Prism Software (San Diego, California, USA).
Fig. 1.
Effects of ethanolic leaf extracts of C. grandis on viability of macrophage cell line (RAW 264.7)
Table 2.
IC50 and EC50 value of Leishmania donovani clinical isolates
| Isolate code | Coccinia grandis leaf extract (μg/ml) | |
|---|---|---|
| IC50 | EC50 | |
| A2(SSG-S/MIL-S) | 5.12 ± 0.68 | 25.18 ± 0.95 |
| T8(SSG-R) | 5.86 ± 0.73 | 27.19 ± 0.99 |
| T9(MIL-R) | 5.39 ± 0.30 | 24.27 ± 1.02 |
When A2 was subjected to test the susceptibility towards Cg-LE, the EC50 value was 25.18 ± 0.95 μg/ml for the intracellular amastigotes and IC50 value of 5.12 ± 0.68 μg/ml for the promastigote stage of the isolate. The T8 showed the EC50 value, 27.19 ± 0.99 μg/ml and IC50value of 5.86 ± 0.73 μg/ml for Cg-LE while the T9 showed EC50 value of 24.27 ± 1.02 μg/ml and IC50 value of 5.39 ± 0.30 μg/ml respectively.
Nitric oxide production
Since the MTT assay showed clear antileishmanial effect of the C. grandis leaf extract, we carried out experiments to estimate the status of NO in our Cg-LE treated group. It was observed that macrophages infected with A2 isolate and treated with Cg-LE produced about 1.8 times higher (Fig. 2; bar 2) NO level compared to that of the control group (Fig. 2; bar 1). More interestingly, macrophages infected with T8 isolate treated with Cg-LE enhanced 2.4 times higher NO level (Fig. 2; bar 4) than that of the infected group (Fig. 2; bar 3). Similarly, the level of NO in macrophages infected with T9 isolate and then treated with Cg-LE was about 2.8 times higher NO level (Fig. 2; bar 6) than that of the infected macrophages group (Fig. 2; bar 5). It was interesting to observe that drug resistant isolates released more NO compared to that of the drug sensitive isolate of KA.
Fig. 2.
Effects of the C. grandis leaf extracts on the nitric oxide production from the macrophages infected with L. donovani isolates sensitive and resistant to Sodium Stibo Gluconate and Miltefosine. Infected macrophages were treated with leaf extracts for 24 h at 37 °C and then Griess assay were performed. Data represents mean ± SD of three independent experiments; unpaired two-tailed. Student’s t test was performed. P < 0.05 was considered significant. *P < 0.05, **P < 0.01, ***P < 0.001
Reactive oxygen species generation
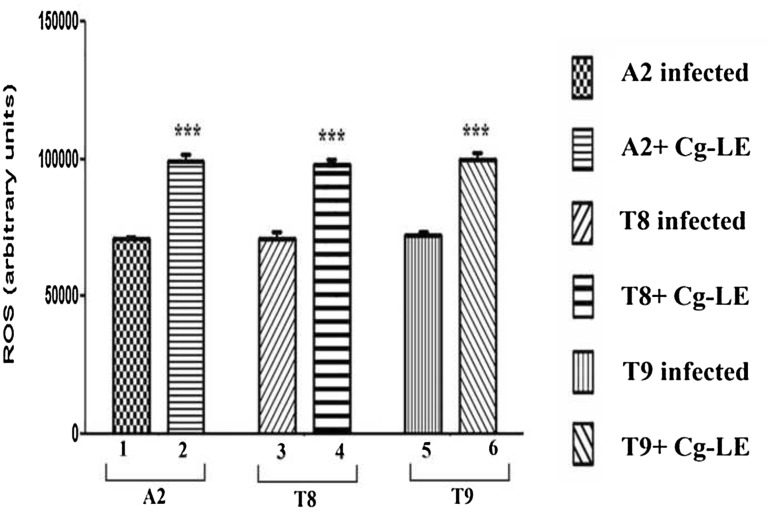
Result demonstrated that ROS production was enhanced in Cg-LE treated macrophages infected with both sensitive as well resistant parasites. The ROS generations were increased by 1.33 times in case of macrophages infected with A2 (Fig. 3; bar 2) and treated with Cg-LE than that of infected group (Fig. 3; bar 1). While the macrophages infected with T8 isolate and T9 and treated with the extract, the ROS generations had been seen increased about 1.4–1.5 times (Fig. 3; bar 4, 6) compared to ROS generation from infected group (Fig. 3; bar 3, 5).
Fig. 3.
Effect of the C. grandis leaf extracts on the superoxide production by the macrophages infected with both drug Sodium Stibo Gluconate, Miltefosine sensitive and resistant isolates. Superoxide anion productions were increased in all the extract treated macrophage groups. Infected macrophages were treated with of C. grandis leaf extracts and after 24 h of treatment, the assay were performed. Data represents mean ± SD of three independent experiments; unpaired two-tailed Student’s t test was performed. P < 0.05 was considered significant. *P < 0.05, **P < 0.01, ***P < 0.001
DNA fragmentation assay
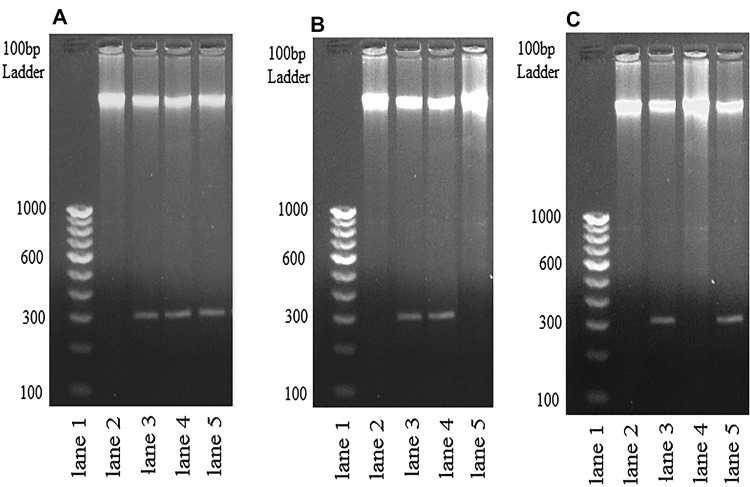
Mitochondrial membrane potential change and DNA fragmentation are linked phenomenon. Mitochondrial membrane potential depolarization leads to fragmentation of DNA, is another event leads to apoptotic like cell death. Result showed that, DNA of A2 showed fragmentation of its DNA in presence of leaf extract of C. grandis (Fig. 4; panel A, lane 3), MIL (panel A, lane 4) and SSG (Fig. 4; panel A, lane 5) while DNA of untreated promastigotes, UT (panel A, lane 2) showed no such fragmentation. The DNA of T8 was fragmented when incubated with MIL (Fig. 4; panel B, Lane 4) but not with SSG (Fig. 4; panel B, Lane 5). When DNA of T8 was incubated with Cg-LE, it has been cleaved (panel B, lane 3). As stated earlier, DNA of T9 was chewed by drug, SSG (panel C, lane 5) but not by drug, MIL (panel C, lane 4). Interestingly, Cg-LE could destroy its DNA as evidenced in Lane 3 of panel C, Fig. 3. So, agarose gel electrophoresis of DNA fragmentation assay clearly showed that Cg-LE has nuclease activity that is able to fragment the DNAs of both sensitive (A2) and resistant clinical isolates (T8 & T9) of L. donovani.
Fig. 4.
The picture depicts a pattern of genomic DNA fragmentation in both drug sensitive and resistant isolates after C. grandis leaf extract treatment. a Genomic DNA of A2 promastigotes get fragmented after extract treatment; lane 1, DNA ladder; lane 2, genomic DNA of untreated promastigotes; lane 3, genomic DNA of Cg-LE treated promastigotes; lane 4, genomic DNA of Miltefosine treated promastigotes; lane 5, genomic DNA of Sodium Stibo Gluconate treated promastigotes. b Genomic DNA of T8 promastigotes get fragmented after extract treatment; lane 1, DNA ladder; lane 2, genomic DNA of untreated promastigotes; lane 3, genomic DNA of Cg-LE treated promastigotes; lane 4, genomic DNA of Miltefosine treated promastigotes; lane 5, genomic DNA of Sodium Stibo Gluconate treated promastigotes. c Genomic DNA of T9 promastigotes get fragmented after extract treatment; lane 1, DNA ladder; lane 2, genomic DNA of untreated promastigotes; lane 3, genomic DNA of Cg-LE treated promastigotes; lane 4, genomic DNA of Miltefosine treated promastigotes; lane 5, genomic DNA of Sodium Stibo Gluconate treated promastigotes
Mitochondrial membrane potential
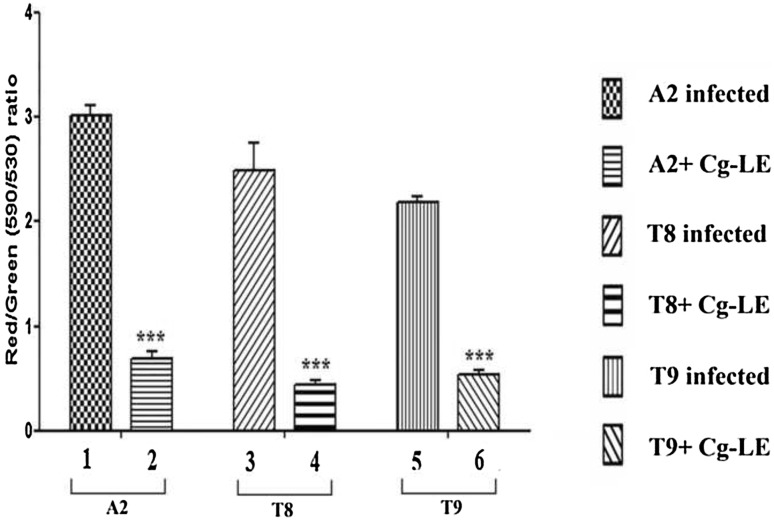
Increased production of free radicals (ROS, NO) generates oxidative stress inside the parasites and depolarizes mitochondrial membrane potential, early event of apoptosis. JC1 enter into the cell from cell membrane and then into mitochondria forms aggregates. In apoptotic cells, these aggregates pour out from mitochondria as monomer. Cg-LE treated both sensitive and resistant promastigotes revealed a shift in the fluorescence intensity from red (590 nm) to green (530 nm). The analysis of the data revealed that the red/green ratio for the Cg-LE treated A2 promastigotes was 4.4 fold lower (Fig. 5; bar 2) than control (Fig. 5; bar 1). Cg-LE induced mitochondrial membrane depolarization (red/green ratio 5.5 fold lower) in T8 promastigotes in respect to control (Fig. 5; bar 3, 4). The red/green ratio for the Cg-LE treated T9 promastigotes was fourfold lowers (Fig. 5; bar 6) than control (Fig. 5; bar 5).
Fig. 5.
Cg-LE induced alteration of mitochondrial transmembrane potential in both drug sensitive and resistant Leishmania donovani promastigotes. A2, T8 and T9 promastigotes were exposed to Cg-LE for 24 h and the mitochondrial transmembrane potential was determined using JC-1 fluorescent probe. Three independent experiments were performed and mean ± SE was presented. P < 0.05 was considered significant. *P < 0.05, **P < 0.01, ***P < 0.001
Apoptosis study
Externalization of PS from the inner side of the cell membrane to the outer cell surface is characteristic of apoptotic cells. FITC conjugated annexin-V will binds to exposed charged head group of phosphatidylserine. During necrosis or in late apoptosis, there is membrane damage that permits PI to diffuse inside the cell and stain the DNA.
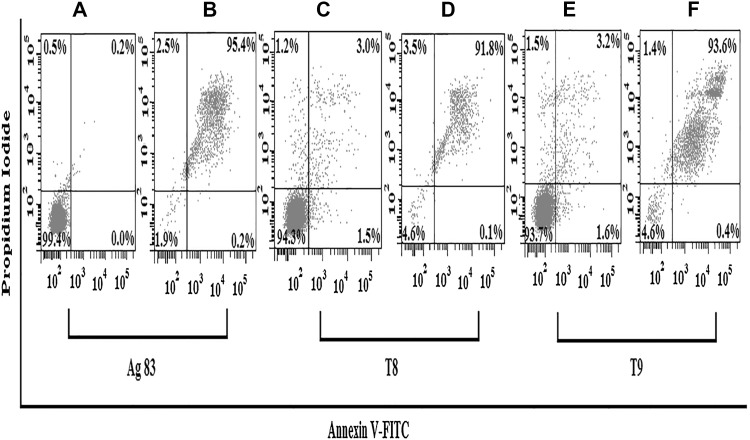
As shown earlier, Cg-LE induces ROS generation inside the Leishmania promastigotes. Because it is already reported by many researchers that intracellular ROS generation is a key regulator for inducing apoptosis (Sen et al. 2004; Roy et al. 2008). We investigated if Cg-LE causes apoptosis in promastigotes of both sensitive and resistant strain. A significant percent of promastigotes of both sensitive and resistant strain stained positive for both annexinV and PI. As shown in the Fig. 6B, 95.4% of Cg-LE treated A2 parasites are in the late apoptotic stage (upper right quadrant) compared with control parasites, where only 0.2% are positively stained (Fig. 6A). For T8 parasites, 91.8% of Cg-LE treated parasites are in the late apoptotic stage (upper right quadrant) (Fig. 6D) whereas only 3.0% were present in control (Fig. 6C). 93.6% of Cg-LE treated T9 parasites (Fig. 6F) are in late apoptotic (upper right quadrant) but in control only 3.2% cells are late apoptotic (Fig. 6E).
Fig. 6.
Cg-LE elicits apoptosis in Leishmania donovani promastigotes. (A) A2 Leishmania donovani promastigotes treated with 0.2% dimethyl sulfoxide (DMSO) for 24 h were double stained with propidium iodide (PI) and annexin V-FITC and used as control. (B) A2 Leishmania donovani promastigotes, treated with Cg-LE extract for 24 h, were double stained with both PI and annexin V-FITC. (C) T8 promastigotes treated with 0.2% dimethyl sulfoxide (DMSO) for 24 h were double stained with propidium iodide (PI) and annexin V-FITC and used as control. (D) T8 Leishmania donovani promastigotes, treated with Cg-LE extract for 24 h, were double stained with both PI and annexin V-FITC. (E) T9 promastigotes treated with 0.2% dimethyl sulfoxide (DMSO) for 24 h were double stained with propidium iodide (PI) and annexin V-FITC and used as control. (F) T9 Leishmania donovani promastigotes, treated with Cg-LE extract for 24 h, were double stained with both PI and annexin V-FITC. All data shown are representative experiments of at least three experiments which gave similar results
Discussion and conclusion
Lack of effective drugs, vaccine against leishmaniasis gave thrust to discover an effective treatment with reasonable cost and minimal side effects. Phytotherapy is emerged as an important tool in the search for potent antileishmanial compounds. Aloe vera leaf extract has shown leishmanicidal activity by activating reactive oxygen species against Leishmania (Dutta et al. 2007). Azadirachta indica leaf extract is an effective leishmanicidal compound having immunomodulatory activity seen in Leishmania donovani infection (Dayakar et al. 2015). Emblica officinalis extract is also very effective for treating visceral Leishmaniasis (Kaur et al. 2013). Methanolic leaf extracts of Acorus calamus, Alstonia scholaris and Berberis aristata and Polyalthia longifera showed significant antileishmanial activities (Pal et al. 2011; Sidana and Farooq 2015). Essential oils of Coriandrum sativum, Lippia sidoides and Copaifera reticulate have potent antileishmanial activity in vitro (Rondon et al. 2012). Andrographolide is a labdane diterpenoid that has been isolated from the stem and leaves of Andrographis paniculata, used to treat the experimental leishmaniasis in the hamster model and found to reduce the parasitic burden (Sinha et al. 2000). Curcuma longa cortex rich in turmerones and their liposomal formulations has antileishmanial activity and could represent good strategy for the development of new antileishmanial agent (Amaral et al. 2014).
In our present study, we have examined the antileishmanial activity of ethanolic leaf extract of Coccinia grandis (Cg-LE) against both drug sensitive as well as drug resistant isolates of VL. The rationale behind selection of both drug sensitive and resistant clinical isolates of Leishmania lies in the fact that so far, whatever chemotherapy has been employed for the killing of the parasites, after few years of their usage, resistance would appear making the control measure futile. Thus, if we come across any herbal preparations that could be effective for drug resistant isolates along with the sensitive counterparts, it would serve the best purpose of it.
The in vitro activity of Cg-LE against intracellular amastigotes as well as on promastigotes (Table 1) coupled with the absence of cytotoxicity on host macrophages formed the basis for advanced exploration of this promising lead (Evans and Croft 1994). We have observed that Cg-LE induces production of superoxide and nitric oxide in macrophages. Generations of nitrite and superoxides are the key weaponry of the macrophages for killing the Leishmania parasites inside the cell (Gottlieb et al. 2003). It has been seen that Cg-LE extract treated groups of infected macrophages showed increased level of both Nitric Oxide and Reactive Oxygen Species production compared to that of the infected group. It was interesting to observe that macrophages infected with T9 isolate showed more NO generation than that of the macrophages infected with A2 and T8 isolates respectively.
Our study showed that extract treatment leads to change in mitochondrial membrane potential at 24 h treatment. AnnexinV-PI double staining showed that Cg-LE treatment causes apoptosis of parasites both in drug sensitive and resistant strain. It is already reported that apoptosis and change in mitochondrial membrane potential are linked phenomenon (Ly et al. 2003; Matassov et al. 2004). DNA fragmentation is another hallmark of apoptotic cell death (Amer and Swanson 2002) and Cg-LE treatment was able to fragmented promastigotes’ DNA after 24 h of treatment.
Resistance to SSG is a major problem in Indian subcontinent. A number of cases of SSG unresponsiveness are reported every year throughout India. Present epidemiological study indicates that about 70% VL cases are not responding to the drug (Sundar et al. 2000). Amphotericin B, Pentamidine are used as second line effort to combat the deadly disease of the poor but the scenario turns bleak as these drugs have severe side effects (Sundar 2001a). Liposomal formulation of Amphotericin is less toxic and effective but very costly (Sundar 2001a). Anticancer drug Miltefosine was introduced in Indian subcontinent as an oral antileishmanial drug especially to treat the cases which are unresponsive to antimonials (Sundar 2001b; Sundar et al. 2002). After few years of use, it also started facing problem of emergence of resistance (WHO 2000; Das et al. 2013).
Traditional uses of herbal plant parts may be our last refuge to overcome the burden of the diseases. Our present study clearly showed that ethanolic leaf extract of medicinal plant Coccinia grandis exhibited potent antileishmanial activity against both drug sensitive as well as drug resistant isolates of L. donovani. The uniqueness of the observations was that this extract has all spectrum activities against any kind of clinical isolates of L. donovani either sensitive or resistant types. This is first such kind of report.
Acknowledgements
We are thankful to the Director, Indian Institute of Chemical Biology, Kolkata. We are grateful to Dr. Syamal Roy, INSA Senior Scientist and J. C. Bose Fellow and Dr. Debasish Bhattacharyya, Chief Scientist, Structural Biology and Bioinformatics, Indian Institute of Chemical Biology, Kolkata for their material help and valuable advices. The cooperation of the Principal, Barasat Government College, Kolkata and Director, Public Instructions, Higher Education Department of Government of West Bengal are duly acknowledged.
Author contribution
S.L. performed all laboratory tests; A.K.D. along with M.M. and S.L. collected the clinical isolates of the KA patients. S.N.D. helped in statistical analysis of the data along with some technical suggestions. M.M. designed the experiments, analyzed and interpreted the data. S.L. and M.M. wrote the manuscript.
Compliance with ethical standards
Conflict of interest
None.
Contributor Information
Anjan K. Das, Email: dranjan_nrspath@yahoo.co.in
Sachindra N. Das, Email: sdas@isc.jdvu.ac.in
Madhumita Manna, Email: madhumita.manna09@gmail.com.
References
- Aggarwal AS, Sural U, Chaudhari S, Desphande S, Garud A, Talele S. Analgesic and antipyretic activity of methanolic extract of Coccinia grandis L. Leaves in experimental animals. RJPBCS. 2011;2:175–182. [Google Scholar]
- Albanyan AM, Murphy MF, Rasmussen JT, Heegaard CW, Harrison P. Measurement of phosphatidylserine exposure during storage of platelet concentrates using the novel probe lactadherin: a comparison study with annexin V. Transfusion. 2009;49:99–107. doi: 10.1111/j.1537-2995.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- Amaral AC, Gomes LA, Silva JR, Ferreira JL, Ramos Ade S, Rosa Mdo S, Vermelho AB, Rodrigues IA. Liposomal formulation of turmerone-rich hexane fractions from Curcuma longa enhances their antileishmanial activity. Biomed Res Int. 2014;2014:1–8. doi: 10.1155/2014/694934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer AO, Swanson MS. A phagosome of one’s own: a microbial guide to life in the macrophage. Curr Opin Microbiol. 2002;5:56–61. doi: 10.1016/S1369-5274(02)00286-2. [DOI] [PubMed] [Google Scholar]
- Bhattacharya B, Samanta M, Pal P, Chakraborty S, Samanta A. In vitro evaluation of antifungal and Antibacterial activities of the plant Coccinia grandis (L.) Voigt. (Family-Cucurbitaceae) J Phytol. 2010;2(11):52–57. [Google Scholar]
- Chan-Bacab MJ, Pena-Rodriguez LM. Plant natural products with leishmanicidal activity. Nat Prod Rep. 2001;18:674–688. doi: 10.1039/b100455g. [DOI] [PubMed] [Google Scholar]
- Croft SL, Yardley V. Chemotherapy of leishmaniasis. Curr Pharm Des. 2002;8:319–342. doi: 10.2174/1381612023396258. [DOI] [PubMed] [Google Scholar]
- Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;10:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Luz RI, Vermeersch M, Dujardin JC, Cos P, Maes L. In vitro sensitivity testing of Leishmania clinical field isolates: preconditioning of promastigotes enhances infectivity for macrophage host cells. Antimicrob Agents Chemother. 2009;53:5197–5203. doi: 10.1128/AAC.00866-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Saudagar P, Sundar S, Dubey VK. Miltefosine-unresponsive Leishmania donovani has a greater ability than miltefosine-responsive L. donovani to resist reactive oxygen species. FEBS J. 2013;280:4807–4815. doi: 10.1111/febs.12449. [DOI] [PubMed] [Google Scholar]
- Das P, Paik D, Pramanik A, De T, Chakraborti T. Antiproteolytic and leishmanicidal activity of Coccinia grandis (L.) Voigt leaf extract against Leishmania donovani promastigotes. Indian J Exp Biol. 2015;53:740–746. [PubMed] [Google Scholar]
- Dayakar A, Chandrasekaran S, Veronica J, Sundar S, Maurya R. In vitro and in vivo evaluation of anti-leishmanial and immunomodulatory activity of Neem leaf extract in Leishmania donovani infection. Exp Parasitol. 2015;153:45–54. doi: 10.1016/j.exppara.2015.02.011. [DOI] [PubMed] [Google Scholar]
- De Carvalho PB, Ferreira EI. Leishmaniasis phytotherapy-Nature’s leadership against an ancient disease. Fitoterapia. 2001;72:599–618. doi: 10.1016/S0367-326X(01)00301-X. [DOI] [PubMed] [Google Scholar]
- Deshpande SV, Patil JM, Daswadkar CS, Telekone SR, Kale SR. In vitro antioxidant study of petroleum ether chloroform and ethyl acetate fractions of Coccinia grandis stems extract. Int J Chem Sci. 2011;9:80–86. [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Dolai S, Yadav RK, Pal S, Adak S. Overexpression of mitochondrial Leishmania major ascorbate peroxidase enhances tolerance to oxidative stress-induced programmed cell death and protein damage. Eukaryot Cell. 2009;8:1721–1731. doi: 10.1128/EC.00198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, Mandal G, Mandal C, Chatterjee M. In vitro antileishmanial activity of Aloe vera leaf exudate: a potential herbal therapy in leishmaniasis. Glycoconj J. 2007;24:81–86. doi: 10.1007/s10719-006-9014-z. [DOI] [PubMed] [Google Scholar]
- Evans AT, Croft SL. Antileishmanial actions of tricyclic neuroleptics appear to lack structural specificity. Biochem Pharmacol. 1994;48:613–616. doi: 10.1016/0006-2952(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Gottlieb E, Armour SM, Harris MH, Thompson CB. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10:709–717. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal Biochem. 1982;26:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Haldar AK, Banerjee S, Naskar K, Kalita D, Islam NS, Roy S. Sub-optimal dose of sodium antimony gluconate (SAG)-diperoxovanadate combination clears organ parasites from BALB/c mice infected with antimony resistant Leishmania donovani by expanding antileishmanial T-cell repertoire and increasing IFN-gamma to IL-10 ratio. Exp Parasitol. 2009;122:145–154. doi: 10.1016/j.exppara.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Kaur S, Kaur G, Sachdeva H, Kaur J. In vivo evaluation of the antileishmanial activity of two immunomodulatory plants, Emblica officinalis and Azadirachta indica in Balb/c mice. IJAHM. 2013;3:1066–1079. [Google Scholar]
- Kedzierski L. Leishmaniasis vaccine: where are we today? J Glob Infect Dis. 2010;2:177–185. doi: 10.4103/0974-777X.62881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanra S, Sarraf RN, Das S, Das KA, Roy S, Manna M. Genetic markers for antimony resistant clinical isolates differentiation from Indian Kala-azar. Acta Trop. 2016;164:177–184. doi: 10.1016/j.actatropica.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Khanra S, Sarraf RN, Das KA, Roy S, Manna M. Miltefosine resistant field isolate from Indian Kala-azar patient shows similar phenotype in experimental infection. Sci Rep. 2017 doi: 10.1038/s41598-017-09720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremb S, Helfer M, Heller W, Hoffmann D, Wolff H, Kleinschmidt A, Cepok S, Hemmer B, Durner J, Brack-Werner R. EASY EASY-HIT: HIV full-replication technology for broad discovery of multiple classes of HIV inhibitors. Antimicrob Agents Chemother. 2010;54:5257–5268. doi: 10.1128/AAC.00515-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly JD, Grubb DR, Lawer A. The mitochondrial membrane potential (Δψm) in apoptosis; an update. Apoptosis. 2003;8:115–128. doi: 10.1023/A:1022945107762. [DOI] [PubMed] [Google Scholar]
- Matassov P, Kagan T, Leblanc J, Sikorska M, Zakeri Z. Measurement of apoptosis by DNA fragmentation. Methods Mol Biol. 2004;282:1–17. doi: 10.1385/1-59259-812-9:001. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mujumder PM, Sasmal D, Nimbi RA. Antiulcerogenic and antioxidant effect of Coccinia grandis leaves on aspirin induced gastric ulcer in rat. Nat Prod Res. 2008;7:15–18. [Google Scholar]
- Pal D, Bhattacharya S, Baidya P, De KB, Pandey JN, Biswas M. Antileishmanial activity of Polyalthia longifolia Leaf extract on the in vitro growth of Leishmania donovani promastigotes. GJP. 2011;5:97–100. [Google Scholar]
- Pekamwar SS, Kalyankar TM, Kokate SS. Pharmacological activities of Coccinia grandis: review. J Appl Pharma Sci. 2013;3:114–119. [Google Scholar]
- Rahuman AA, Venkatesan P. Larvicidal efficacy of five cucurbitaceous plant leaf extracts against mosquito species. Parasitol Res. 2008;103:133–139. doi: 10.1007/s00436-008-0940-5. [DOI] [PubMed] [Google Scholar]
- Rondon MCF, Bevilaqua LMC, Accioly PM, Morais MS, Andrade-Júnior FH, Carvalho AC, Lima CJ, Magalhães RCH. In vitro efficacy of Coriandrum sativum, Lippia sidoides and Copaifera reticulata against Leishmania chagasi. Rev Bras Parasitol Vet. 2012;3:185–191. doi: 10.1590/S1984-29612012000300002. [DOI] [PubMed] [Google Scholar]
- Roy A, Ganguly A, BoseDasgupta S, Das BB, Pal C, Jaisankar P, Majumder HK. Mitochondria dependent ROS-mediated programmed cell death (PCD) induced by 3,3′-Diindolylmethane (DIM) through Inhibition of FoF1-ATP Synthase in unicellular protozoan parasite Leishmania donovani. Mol Pharm. 2008;74:1292–1307. doi: 10.1124/mol.108.050161. [DOI] [PubMed] [Google Scholar]
- Samanta A, Bhattacharya B, Ghosh S, Das G. In vivo antimalarial activity of the plant Coccinia grandis. Ijprd. 2011;3:73–79. [Google Scholar]
- Schinor EC, Salvador MJ, Pral EMF, Alfieri SC, Albuquerque S, Dias DA. Effect of extracts and isolated compounds from Chresta scapigera on viability of Leishmania amazonensis and Trypanosoma cruzi. Braz J Pharma Sci. 2007;43:295–300. [Google Scholar]
- Sen N, Das BB, Ganguly A, Tripathi G, Bandyopadhyay S, Rakshit S, Sen T, Majumder HK. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ. 2004;11:924–936. doi: 10.1038/sj.cdd.4401435. [DOI] [PubMed] [Google Scholar]
- Sidana A, Farooq U. Evaluation of antileishmanial activity of plants used in Indian traditional medicine Bangladesh. J Pharmacol. 2015;10:423–426. [Google Scholar]
- Sinha J, Mukhopadhyay S, Das N, Basu MK. Targeting of liposomal andrographolide to L. donovani infected macrophages in vivo. Drug Deliv. 2000;7:209–213. doi: 10.1080/107175400455137. [DOI] [PubMed] [Google Scholar]
- Soares DC, Pereira CG, Meireles MA, Saraiva EM. Leishmanicidal activity of supercritical fluid fraction from Tabernaemontana catharinensis. Parasitol Int. 2007;56:135–139. doi: 10.1016/j.parint.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Sundar S. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health. 2001;6:849–854. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- Sundar S. Treatment of visceral leishmaniasis. Med Microbiol Immunol. 2001;190:89–92. doi: 10.1007/s004300100088. [DOI] [PubMed] [Google Scholar]
- Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9:951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, Kumar KCP, Murray WH. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–1107. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, Junge K, Bryceson A, Berman J. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med. 2002;347:1739–1746. doi: 10.1056/NEJMoa021556. [DOI] [PubMed] [Google Scholar]
- Sundaram RK, Samuel JI, Palavesam S. In vitro antiplasmodial activity of ethanolic extracts of South Indian Medicinal plants against Plasmodium falciparum. Asian Pac J Trop Dis. 2012;2:180–183. doi: 10.1016/S2222-1808(12)60043-7. [DOI] [Google Scholar]
- Tamilselvan N, Thirumalai T, Elumalai EK, Balaji R, David E. Pharmacognosy of Coccinia grandis: a review. Asian Pac J Trop Biomed. 2011;1:299–302. doi: 10.1016/S2221-1691(11)60176-7. [DOI] [Google Scholar]
- Vadivu R, Krithika A, Biplab C, Dedeepya P, Shoeb N, Lakshmi KS. Evaluation of hepatoprotective activity of the fruits of Coccinia grandis Linn. Int J Health Sci Res. 2008;1:163–168. [Google Scholar]
- Verma NK, Dey CS. Possible mechanism of miltefosine-mediated death of Leishmania donovani. Antimicrob Agents Chemother. 2004;48:3010–3015. doi: 10.1128/AAC.48.8.3010-3015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (2000) A report of the consultation meeting on traditional and modern medicine. Harmonizing two approaches, Beijing, China, West Pacific Region