Abstract
Microscopic-coprological examination of Asian Elephant (Elephas maximus L., 1798) dung piles (n = 55) in South Wayanad Forest Division from March to August, 2017 revealed 74.5% prevalence of parasites in elephants. Ancylostoma sp. Anoplocephala sp., Strongyle type egg and Strongyloides sp. were the major parasites recorded. Strongyloides sp. and Strongyle type egg were observed more frequently (58.1%). Ancylostoma sp. and Anoplocephala sp. were constituted 1.8% each; mixed parasitic species infections were recorded. The frequency distribution of parasitic load in elephants showed skewed distribution of propagules. Centrifugal sedimentation and floatation methods of fecal examination of outer and inner regions of dung did not show significant difference in number of propagules. The highest number of parasitic propagules was recorded in floatation method. The number of propagules varied among dung samples of different herds collected from different localities. There were no relation between the parasitic load and age of elephants. The mean density of parasite eggs was higher in solitary animals (214.3 ± 155.4 epg) than herd elephants (147.78 ± 111.1 epg). Though parasitic load was higher in solitary males, based on the occurrence of parasites using logistic regression it was found that females had 1.83 times higher occurrence for parasitic infection than males. Both length and width of parasite egg size classes were used to classify into different taxonomic groups using discriminate function analysis. Three distinct size clusters were identified. Nematode and Cestode eggs were classified correctly with 95.7% accuracy. Since, the egg size was similar in nematode group separation into genus was difficult. Further, inclusion of stages of development of egg and larvae enable better separation.
Keywords: Asian elephant, Elephas maximus, South Wayanad Forest Division, Gasterointestinal parasites, Strongyloides sp., Anoplocephala
Introduction
As per the IUCN Red List of Threatened Species, Asian Elephant (Elephas maximus) is ‘endangered’ and the estimated population to be 30,000–50,000 with approximately 60% of the population in India. Elephant population is threatened by many factors such as poaching, habitat loss and fragmentation, and epidemic disease outbreaks (Riddle et al. 2010). Further, parasitism affects both evolution and ecology of host species through sexual selection (Hamilton and Zuk 1982) and parasite mediated competition results in reduced population size or extinction (Price et al. 1986). Parasite also alters host behaviour, health, fertility and facilitates parasite transmission. There are only few studies on Asian elephant parasitism in natural forest area (Vidya and Sukumar 2002; Dharmarajan et al. 2005; Nishanth et al. 2012; Pechimuthu 2014; Vimalraj and Jayathangaraj 2015) and in captive elephants (Suresh et al. 2001; Saseendran et al. 2004; Kashid et al. 2002; Arunachalam et al. 2007; Thawait et al. 2014; Pandit et al. 2015).
South Wayanad Forest Division (SWFD) is contiguous forest of about 947.44 km2 with North Wayanad forest division and Nilambur forest division in South. Status of land is in reserve forest category, where people can access area for non-timber forest produce collection. This area is connected to Nilgiri Biosphere Reserve (NBR) and it plays a crucial role in the conservation of the elephant in this region since it offers the habitat contiguity for long ranging elephants (Desai and Baskaran 1996).
Parasitic infections can cause diseases and death in wild animals and can become a source of infection for domestic animals and vise-versa. Epidemiological studies are essential to know the status and transmission of such diseases. Parasitic diseases in domestic animals in the vicinity of wildlife are best controlled by preventing contact between wild and domestic animals and by manipulating the factors involved in disease transmission. Further, anthropogenic influence on habitat results in increased parasite prevalence in Bornean elephants (Hing et al. 2013). Thus present study was designed to assess gastrointestinal parasites of elephants of SWFD with objectives: to document different endoparasites, to find out age-sex class influence on parasitic load and to evaluate feasibility of using parasite egg morphology to identify parasite species.
Study area
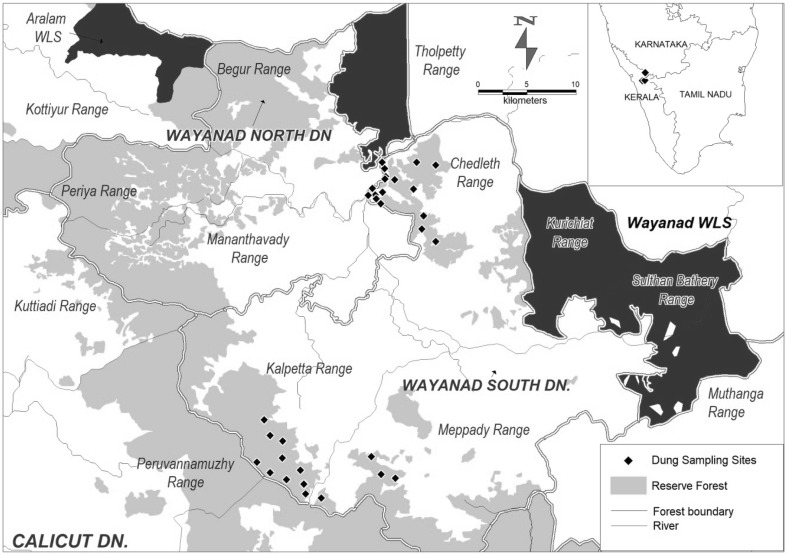
SWFD is a part of Wayanad Elephant Reserve. The total area of the division is 325.3 km2 and lies between North latitude 11°26′ to 12°00′ and East longitude 75°75′ to 76°56′. The altitude varies between 600 and 2100 m with average elevation of 730 m (MSL). The evergreen forests of Wayanad are considered as the highest rainfall regions of Kerala and South India. The annual rainfall ranges from 3000 to 4000 mm. The area is characterized by high relative humidity 95% during the South West Monsoon. Administrative divisions of the SWFD are Kalpetta, Mepady and Chedeleth ranges (Fig. 1). The area is located in the Western Ghats, which is one of the 34 Biodiversity hotspots of the world (Mittermeier et al. 2008). This division is dominated by pockets of evergreen and semi evergreen vegetation and moist deciduous forest, with the high elevation areas characterized by shola-grassland ecosystem. The mid-level plateau of the district is the most densely populated area which covers a major part of the land and the natural vegetation of these areas have been replaced by coffee, tea, eucalyptus, banana and rubber plantations.
Fig. 1.
Map showing the locations of dung sample collection sites in South Wayanad Forest Division, Kerala, India
Field sampling and analysis
Elephant herds and solitary male elephants were observed until defecate. Freshly voided dung samples of about 25–30 g were collected from the inner and outer surface of dung piles separately in 20 ml sample collection tubes with 10% formalin from March 2017 to August 2017. Elephant herd and individual animals (males) were identified based on the tail, ear shape, size and de-pigmentation. Photographs of individual animal were taken for identification by means of dung sample collection from same individual was avoided. A total of 55 elephant dung samples (12 solitary males and 43 individuals from herd) were collected and stored in refrigerator (− 10 °C) for analysis at a later stage (Lynsdale et al. 2015). The approximate age-class of elephant was estimated by measuring circumference of dung bolus which was intact (Reilly 2002; Daniel et al. 2008). Centrifugal sedimentation and fecal flotation techniques were used to determine helminth eggs (Soulsby 1982). Parasite eggs of helminth vary in size and weight. Sedimentation technique was used to isolate larger and heavy trematode eggs and floatation technique was used for nematode and cestode eggs. The number of eggs was counted using Light microscope (Zeiss-Axio Lab-1), and images were captured with computer interface (Progress Capture Version-2.8.8) with scale. The length and width of propagules were measured using measure tool. Intestinal parasites are best identified by examining adult worms, but eggs are the only available stages in the dung on which identification of parasite is based. Propagules were identified following standard references keys (Zajac and Conboy 2012).
Egg-counting method
To determine the number of parasite eggs per gram of feces, 3.5 g of feces and 15 mL of flotation solution were used. The following formula was used to find out number of eggs per gram for both floatation and sedimentation method based on modified Stoll Test (Zajac and Conboy 2012). Eggs/g = [No. of eggs counted × (T/V)]/F. Where T = Total volume of feces/floatation solution mixture − 1500 μL, V = Volume of aliquot examined in one slide (50 μL × 2) = 100 μL, and F = grams of feces used (3.5 g).
Statistical analysis
Non-parametric tests were performed since parasite loads were not normally distributed. The analysis was done using Windows based statistical package viz. Microsoft Excel and IBM SPSS statistics version 21. The difference in the number of propagules between inner and outer regions of dung piles was tested using Wilcoxon-Matched Pair Test. The difference among parasite species, herd/individual, among different places and methods of examination (floatation and sedimentation) were compared using Kruskal–Wallis ANOVA with randomization for 1000 times using Monte Carlo simulation. Binary logistic regression analysis was carried out to test the significance of occurrence of parasites (presence and absence of parasites in dung sample-dependent variable) and independent predictor variables i.e., individual and herd elephant, age classes and places of collection of dung piles. All factors were included initially and least significant was removed in turn, until only significant predictor remained. Within the family Strongylidae, eggs are similar in shape, colour and surface ornamentation, but egg size can be used to classify many Strongyles to the generic level (Bhalerao 1935). Discriminate function analysis was performed using egg dimension (egg length and width) to assign Strongyle eggs to different operational taxonomic units (OTUs). For hypothesis testing, p < 0.05 and p < 0.01 were considered and the level of significance were indicated at appropriate places (Zar 2003).
Results
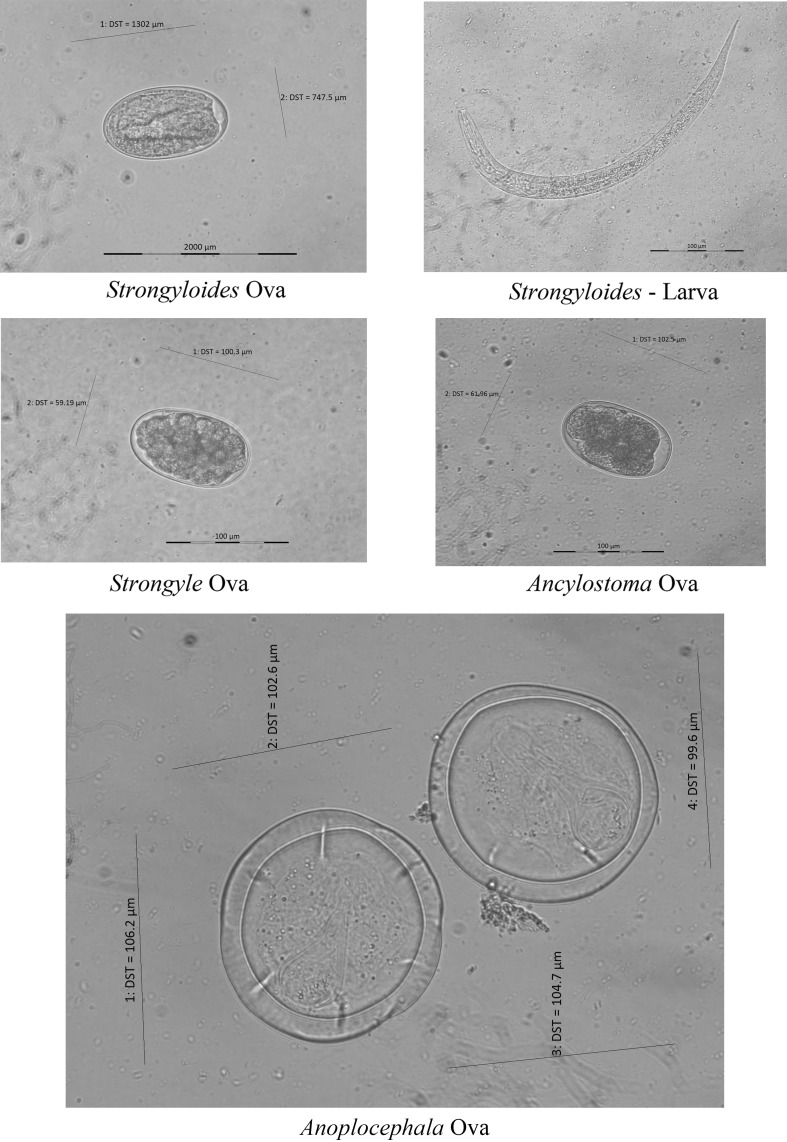
On microscopic examination of slides (n = 55), 74.5% of dung samples harboured parasite propagules and nematode larvae. Totally there were four kinds of parasite propagules/larvae recorded, i.e., Ancylostoma sp. Anoplocephala sp., Strongyle type egg and Strongyloides sp. (Fig. 2). There were more than one parasite observed in the dung. Strongyloides sp. and Strongyle type egg were the more frequently observed (58.1%) than other parasites. Anoplocephala sp. alone represented 3.6% with Strongyloides constituted 5.5%. The intensity of infection inferred from eggs per gram of feces varied from 128.6 to 1757.1/epg (Table 1).
Fig. 2.
Gastrointestinal parasites recorded in elephants (Micrographs with scale and length and width measurements)
Table 1.
Prevalence of parasite eggs and eggs per gram (n = 55) of elephant dung collected from South Wayanad Forest Division (Positive detection of parasites in 41 samples out of 55 samples analysed)
| Parasite egg identified | n | Category | Prevalence (%) | Eggs/g ± SE |
|---|---|---|---|---|
| Ancylostoma sp. | 1 | Nematode | 1.82 | 128.6 ± 0.0 |
| Anoplocephala sp. | 1 | Cestode | 1.82 | 342.9 ± 0.0 |
| Anoplocephala sp. + Strongyloides | 2 | Nematode | 3.64 | 364.3 ± 21.4 |
| Strongyloides sp. | 29 | Nematode | 52.73 | 121.2 ± 83.5 |
| Strongyloides sp. + Strongyle type egg | 3 | Nematode | 5.45 | 257.1 ± 212.9 |
| Strongyloides sp. + Strongyle type egg + Ancylostoma sp. | 2 | Nematode | 3.64 | 1757.1 ± 1457.1 |
| Strongyloides sp. +Ancylostoma sp. | 3 | Nematode | 5.45 | 171.4 ± 60.6 |
Parasite load within dung pile variation
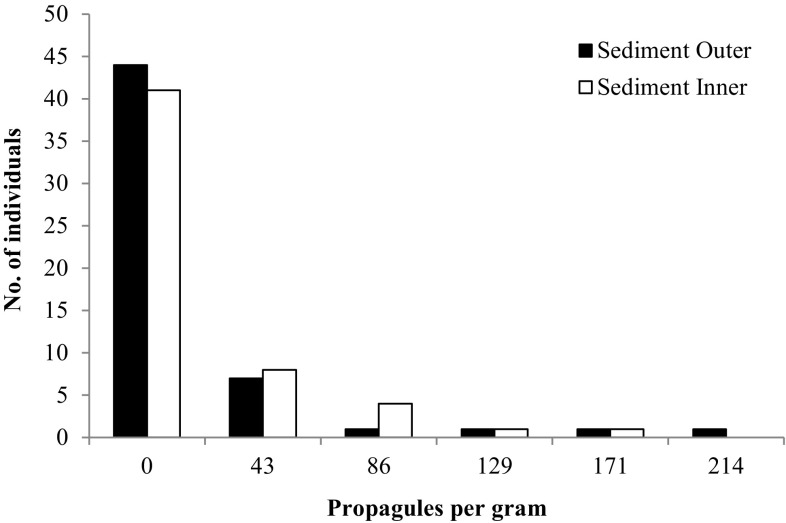
The frequency distribution of parasite load in individual elephants, samples from outer surface and inner region of dung showed skewed distribution of propagules, with few individuals having higher number of propagules. Comparison of centrifugal sedimentation method of outer surface and inner region of dung did not show significant difference in number of propagules (Wilcoxon Matched Paired Test Z = − 0.272; p > 0.05; Fig. 3). Number of propagules in fecal flotation method also did not vary between outer surface and inner region of dung (Wilcoxon Matched Paired Test Z = − 0.184; p > 0.05).
Fig. 3.
Frequency distribution of parasitic load in the individual elephants based on outer surface and inner region of dung based on centrifugal sedimentation method (n = 55)
Flotation vs Sedimentation
In total, irrespective of parasite species, there was no significant difference between the centrifugal sedimentation and flotation methods (Wilcoxon Signed Test Z = − 1.249; df = 1; p > 0.05). The number of propagules varied according to parasite species and method of microscopical examination. There was significant difference in the number of propagules of specific parasites in the floatation method (Kruskal–Wallis Test- χ2 = 9.88; df = 5; p < 0.05). For example, the eggs per gram was relatively higher for Anoplocephala (150/epg) in floatation method than that by sedimentation (42.8/epg). Similarly, Stronglye, Strongyloides and Ancylostoma were 1639/epg in floatation method and it was only 85/epg by sedimentation method. The mean number of propagules did not vary significantly among different species of parasites by sedimentation method (Kruskal–Wallis Test- χ2 = 4.28; df = 5; p > 0.05).
Inter-herd variation
The number of propagules varied among different herd dung samples collected from different localities. The epg was higher in the Kuruva (432.8 epg), followed by Srambi-Padiri, Padiri and Amba regions with 407, 214 and 171.4 epg, respectively. The number of parasite propagules were lowest in Anapara (85.7). Though there were differences in the number of propagules among different herds, it was not statistically significant (Kruskal–Wallis Test- χ2 = 6.27; df = 6; p > 0.05).
Age class versus number of propagules
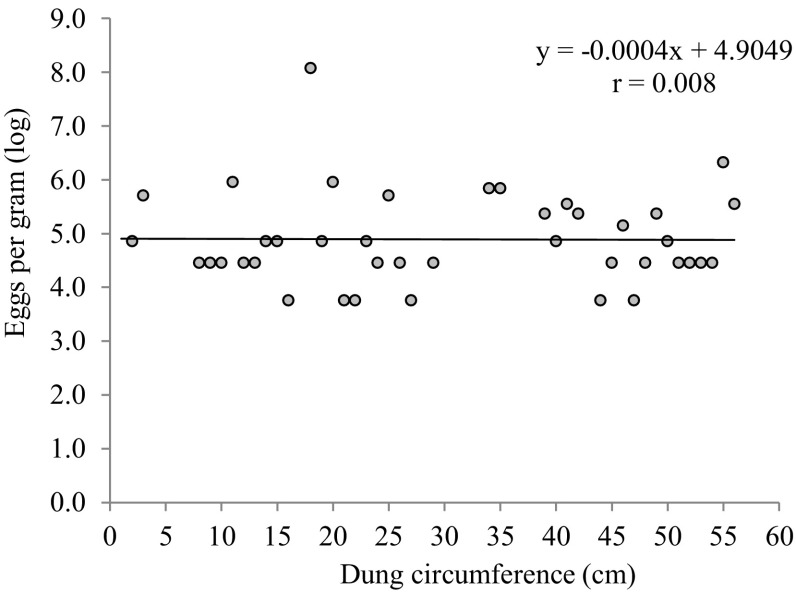
The relation between the dung size (indicative of age of elephant) and propagule density per gram were investigated. There were no relation between the parasite load and age of elephants (Fig. 4).
Fig. 4.
Parasite propagule density in relation to dung size of elephant in South Wayanad Forest Division (n = 55)
Individual versus herd
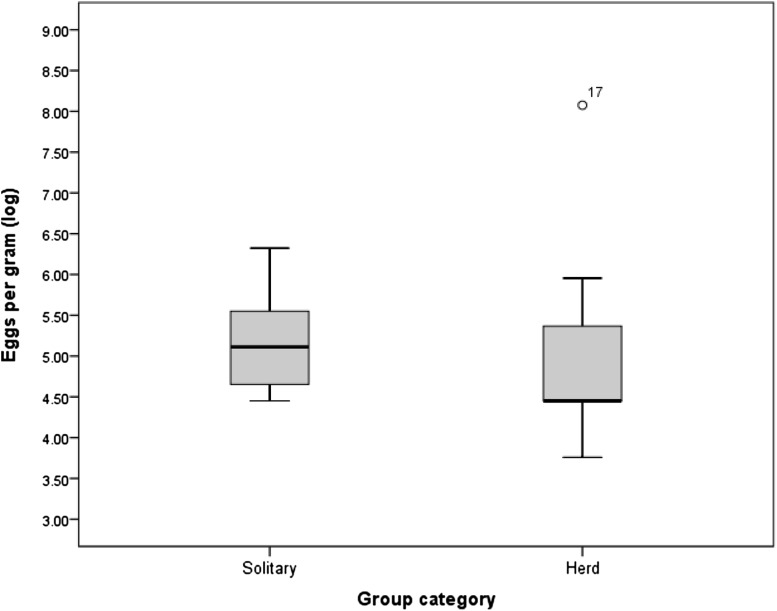
The mean density of parasite eggs was higher in the individual animals (214.3 ± 155.4 epg) than herd elephants (147.78 ± 111.1 epg). In the herd one calf had very high prevalence of parasitic eggs (878 epg) and it was shown as outlier. Since the variation in the mean eggs per gram of dung varied greatly, the data was log transformed (Fig. 5).
Fig. 5.
Parasite propagule density (log) between solitary and herd of elephant in South Wayanad Forest Division (n = 55)
Though male elephants harbour higher parasite load, the occurrence of parasites (number of individuals with parasitic infection) was more in herd (female) elephants (Table 2). Among the 12 individuals and 43 herd sampled the prevalence of parasitic infection was higher in females (78.2%) than in males (21.8%). Females are more likely, i.e. 1.83 times higher susceptible for parasitic infection than males (Wald = 12.3; df = 1; p < 0.00; Table 2). The other variables such as place of collection, age classes were not statistically significant.
Table 2.
Results of logistic binary regression analysis carried out to test the significance of parasitic prevalence (based on occurrence) between individual and herd elephant
| Predictor | β ± SE | Wald | df | p | Odds ratio |
|---|---|---|---|---|---|
| Herd | 0.605 ± 0.17 | 12.13 | 1 | 0.00 | 1.831 |
Operational taxonomic units based on size class
The parasite egg size classes were used to classify the parasite eggs into different taxonomic groups using discriminate function analysis (DFA). Three distinct size clusters were identified. Anoplocephala eggs were distinct from that of other groups with mean value of 26,845 ± 4059 μm with 95.7% correctly classified. Among nematode eggs, there were two distinct clusters formed with egg size between 12,923 ± 1064 and 17,369 ± 1750. Second group was Stronglyloid eggs (88.3%) that could not be distinguished from Ancylostoma (5%) and Stronglye type eggs (6.7%). The third group was composed of Strongyloid eggs (96%) and remaining were Strongyle and Ancylostoma eggs (Table 3). Hence, nematode and cestode eggs were classified correctly with 95.7%. Whereas, the egg size was similar within nematode group and separation into genus was difficult. Further, inclusion of stages of development of egg and larval stages could enable the better separation.
Table 3.
Classification of eggs based on elliptical area using discriminate function analysis (values in bracket indicate actual number and outside values are per cent)
| Clusters | Ancylostoma | Anoplocephala | Strongyle | Strongyloid |
|---|---|---|---|---|
| Anoplocephala | (22) 95.7% | (1) 4.3% | ||
| Ancylostoma | (3) 5% | (4) 6.7% | (53) 88.3% | |
| Strongyloid | (3) 3% | (2) 2% | (108) 96% |
Discussion
Examination of elephant dung samples (n = 55) collected from South Wayanad Forest Division revealed that 74.5% of dung samples harboured parasite propagules and nematode lavae such as Ancylostoma sp. Anoplocephala sp., Strongyle type egg and Strongyloides sp. Mixed infections were also recorded. Strongyloides sp. and Strongyle type egg were recorded to be the most prevalent parasites with 58.1% (32/55 samples) testing positive. In general macroparasites, where parasite species reproduction usually occurs via transmission of free-living infective stages that passes from one host to next aggregate across their host population with most individuals harbouring low number of parasites but a few individuals of host with higher parasitic burden (Shaw and Dobson 1995). Such heterogeneity is due to variation between individuals in their exposure to infective stages and differences in their susceptibility (Wilson et al. 2002). The prevalence of parasite load was lower than adjacent forest areas (Vimalraj and Jayathangaraj 2015), where 100% infection had reported. The lower prevalence of parasite in the study area could be due to low population density of elephants and other large herbivores. Similar prevalence were reported by Hing et al. (2013) and Heinrich (2016) in Bornean Elephants (E. m. borneensis) and in Sri Lankan elephant (E.m.maximus) respectively.
Highly skewed distribution of parasites in any given population of animals, also known as over disposal, is highly acknowledged (Gulland 1995). Many studies state that in a given population of free ranging animals, large number of individuals carry few parasites and few individuals carry large numbers of parasites could be due to the infection process, egg shedding methods and habitat (Shaw and Dobson 1995; Watve and Sukumar 1997; Wilson et al. 2002).
Elephant Stongyles are presumably similar to those of domestic livestock (Fowler and Mikota 2006). Higher prevalence of Strongyles indicates that there is high potential for fecal transmission of parasites. In the natural forest high host densities and transmission of parasite propagules through water may play an important role since elephants often defecate near or in water, obligate drinking behaviour and congregate at the limited water sources during summer (Watve 1992). Further, seasonal movement of elephants in the landscape from adjacent forest of Bandipur and Mudumalai Tiger Reserves during dry season might increase number of elephants and chances for transmission of parasites. Seasonal variation in the prevalence of parasitic infection has also reported in elephants (Vidya and Sukumar 2002; Pechimuthu 2014). Further, monitoring of elephants across different seasons could reveal seasonal changes in parasite prevalence.
In South Wayanad, the samples revealed the eggs of Strongyle, Strongyloides sp, Anoplocephala sp, Ancylostoma sp. mixed infection of Strongyle + Strongyloides sp, Strongyle + Strongyloides sp + Ancylostoma sp. and Strongyle + Anoplocephala sp., and this was in agreement with the findings of Vidya and Sukumar (2002) and Fowler and Mikota (2006). Nematode parasites were higher in the present population represented by three or more species when compared with platyhelminthes represented by a single species. Anoplocephala manubriata with orbatid mites in soil as intermediate host has been reported in elephant (McAloon 2004). Mixed species parasite infections are significantly higher in continuous habitats possibly due to higher biodiversity or greater freedom of animal movement in continuous compared to fragmented habitats (Hing et al. 2013). Mixed parasitic species infections in South Wayanad may be associated with the free movement of elephants between landscapes.
Both floatation and centrifugal sedimentation methods did not show any variation in the number of propagules between the inner region and outer surface of dung piles. Similar findings were also reported by Vidya and Sukumar (2002). Since the parasite has been found to be homogenously distributed in the dung pile within and across dung piles sampling method should not be a problem.
The number of propagules varied according to parasite species and method of microscopical examination. Highest number of parasitic propagules was recorded with floatation method when compared with sedimentation method. Flotation method appeared to enhance the detection of propagules and this method can be applied for screening parasites of Strongyles in future research.
The total parasitic load varied among different localities, with Kuruva-Padiri populations showing higher prevalence of endoparasites (432.8407 epg). Vidya and Sukumar (2002) reported lower average count of 1.44 epg to 2.50 epg in the wet season to 4.64–5.45 epg in the dry season in Nilgiri landscape. A study on 200 captive and wild Indian elephants revealed that a mean total of 8 epg was seen in the wet season to 25 epg was seen in the dry season (Watve 1992). Hing et al. (2013) reported higher total parasite prevalence (100%) and mean total parasite load (350.2 epg) in Bornean Elephant. The present study was carried out during dry season, which also may have contributed to the recording of higher parasitic loads. Chronic stress in animals which are associated with anthropogenic pressure, is known to predispose to parasites and disease (Schwitzer 2010). Sampling during dry season, fragmented forest area and individual variation in parasite prevalence might result in higher parasite abundance.
No significant variation was observed in the density of propagules in relation to the age of the elephants. However, a calf within a herd was recorded with highest parasitic load (878 epg), which may suggest the higher susceptibility of younger animals to parasitic infestation than adult individuals. Previous studies by Vidya and Sukumar (2002), Vanitha et al. (2011) and Heinrich (2016) also suggested that there was no significant relation between parasitic infestation and age class of elephants. Higher parasitic load in individual animals may also be attributed to their health conditions. Watve (1992), Vidya and Sukumar (2002) and Hing et al. (2013) reported that individuals being under pressure, factors like diet and body condition may determine parasite loads and very high parasite loads might result in impoverished body condition. Deficiencies in dietary components, particularly protein and energy, also influence susceptibility to nematodes (Chapman et al. 2006). Obanda et al. (2011) reported that dietary stress and parasitism had a synergistic effect leading to mass mortalities in African Elephants in Kenya, which further supports this view.
Though the parasitic loads in solitary animals and herds did not show significant variation, it was found to be higher in solitary animals (epg 214) than herds (epg 147). Solitary animals recorded during the study were adult males. Adult male elephants have higher foraging ranges when compared to females and the interaction of these animals with multiple herds or populations in different landscapes might have contributed to the higher parasitic load in males. Further male elephant tend to use degraded areas than female herds (Desai and Baskaran 1996). The factors such as higher foraging ranges, interaction with multiple herds and stress could have resulted in higher parasitic load in solitary males.
Though male elephants harbour higher parasitic load, the occurrence of parasites in the elephant dung was more in female elephants. The occurrence of parasitic infection was significantly higher in females (78.2%) than in males (21.8%). Females are more likely, i.e. 1.83 times higher susceptible for parasitic infection than males. The other variables such as place of collection, age classes were not statistically significant. Since female elephants lives in groups with higher interaction among each other and resulted in higher occurrence of parasitic infection.
Size classes were used to classify the parasitic eggs into different taxonomic groups using discriminate function analysis. Nematode and cestode eggs were separated easily with high accuracy (95.7%). However, the similarity in sizes within nematode groups made the identification of eggs difficult and the taxa were identified only up to genus level. Documentation of egg developmental stages and rearing of larval forms would enable better classification of parasites.
Acknowledgement
We thank the Chief Wildlife Warden of Kerala Forest Department for granting permission to conduct research within South Wayanad Forest Division of Kerala (Ref No. WLW-47755/2016 dated 17-10-2016) and District forest officer for his support at the time of sampling. We thank Dr. Reghu Ravindran for his help in identification of parasite species.
Author Contributions
TVA: this work is part of Abijith master dissertation of MS (Wildlife Studies). He conceived idea and collected field samples and microscopic examination of samples. MA: developed theory and performed computation and prepared manuscript. RTD: assisted in the field sample collection and preparation of manuscript. GC: Supervised the work and revision of manuscript. All authors discussed the results and contributed to the final manuscript
References
- Arunachalam K, Raman M, Harikrishnan TJ. Incidence of helminth ova in Indian Elephants (Elephas maximus) at Theppakadu, Nilgiris, Tamilnadu. Zoos Print J. 2007;22:2898–2899. doi: 10.11609/JoTT.ZPJ.1585.2898-9. [DOI] [Google Scholar]
- Bhalerao GD. Helminth parasites of the Indian elephant from the Andamans and Burma. Indian J Vet Sci Anim Husb. 1935;5:35–45. [Google Scholar]
- Chapman CA, Speirs ML, Gillespie TR, Holland T, Austad KM. Life on the edge: gastrointestinal parasites from the forest edge and interior primate groups. Am J Primatol. 2006;68:397–409. doi: 10.1002/ajp.20233. [DOI] [PubMed] [Google Scholar]
- Daniel JC, Desai AA, Mohanraj N, Ashokkumar M, Sakthivel C (2008) Evaluation of population enumeration methods and human elephant conflict mitigation methods in Mudumalai Wildlife Sanctuary and National Park, Tamil Nadu. Report, BNHS, Mumbai
- Desai AA, Baskaran N. Impact of human activities on the ranging behaviour of elephants in the Nilgiris Biosphere Reserve, South India. J Bombay Nat Hist Soc. 1996;93:145–156. [Google Scholar]
- Dharmarajan G, Raman M, John MC. Effect of season on helminth loads of wild herbivores and cattle in the Mudumalai Wildlife Sanctuary, Southern India. Zoos’ Print J. 2005;20:1766–1769. doi: 10.11609/JoTT.ZPJ.784.1766-9. [DOI] [Google Scholar]
- Fowler ME, Mikota SK. Biology, medicine, and surgery of elephants. London: Blackwell Publishing; 2006. [Google Scholar]
- Gulland FMD (1995) Impact of infectious diseases on wild animal population- a review. In: Grenfell BT, Dobson AP (eds) Ecology of infectious diseases in natural populations (Publications of the Newton Institute). Cambridge University Press, Cambridge, pp 20–51
- Hamilton DG, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Heinrich L. Prevalence and molecular identification of helminthes in wild and captive Sri Lankan elephant (Elephas maximus maximus) London: Bachelor of Veterinary Medicine- Research Project, Royal Veterinary College; 2016. [Google Scholar]
- Hing S, Othman N, Nathan SKSS, Fox M, Fisher M, Gossens B. First parasitological survey of endangered Bornean Elephants (Elephas maximus borneensis) Endang Species Res. 2013;21:223–230. doi: 10.3354/esr00527. [DOI] [Google Scholar]
- Kashid KP, Shrikhande GB, Bhojne GR. Incidence of gastrointestinal helminths in captive wild animals at different locations. Zoos’ Print J. 2002;18:1053–1054. doi: 10.11609/JoTT.ZPJ.18.3.1053-4. [DOI] [Google Scholar]
- Lynsdale CL, Francodossantos DJ, Haywart AD, Mar KU, Htut W, Aung HH, Soe AT, Lummaa V. A standardised faecal collection protocol for intestinal helminth egg counts in Asian elephants (Elephas maximus) Int J Parasitol Parasites Wildl. 2015;4(3):307–315. doi: 10.1016/j.ijppaw.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAloon M. Oribatid mites as intermediate hosts of Anoplocephala manubriata, cestode of the Asian elephant in India. Exp Appl Acarol. 2004;32:181–185. doi: 10.1023/B:APPA.0000021795.02103.d0. [DOI] [PubMed] [Google Scholar]
- Mittermeier RA, Myers N, Thomsen JB, Fonseca GAB, Oliver S. Biodiversity hotspots and major tropical wilderness areas: approaches to setting conservation priorities. Conserv Biol. 2008;12:516–520. doi: 10.1046/j.1523-1739.1998.012003516.x. [DOI] [Google Scholar]
- Nishanth B, Srinivasan SR, Jayathangaraj MG, Sridhar R. Incidence of endoparasitism in free-ranging elephants of Tamilnadu state. J Vet Anim Sci. 2012;8:332–335. [Google Scholar]
- Obanda V, Iwaki T, Mutinda NM, Gakuya F. Gastrointestinal parasites and associated pathological lesions in starving free-ranging African elephants. S Afr J Wildl Res. 2011;41:167–172. doi: 10.3957/056.041.0203. [DOI] [Google Scholar]
- Pandit A, Dhakal IP, Gairhe KP. Prevalence of endoparasitic diseases in private elephants of buffer zone of Chitwan National Park, Nepal. Int J Recent Sci Res. 2015;6(8):5768–5771. [Google Scholar]
- Pechimuthu D. Seasonal variation in prevalence of helminthic infection in captive Asian Elephant (Elephas maximus) Appl Biol Biotech. 2014;2:8–14. [Google Scholar]
- Price PW, Westoby M, Rice B, Atsatt PR, Fritz RS, Thompson JN, Mobley K. Parasite Mediation in Ecological Interactions. Annu Rev Ecol Syst. 1986;17(1):487–505. doi: 10.1146/annurev.es.17.110186.002415. [DOI] [Google Scholar]
- Reilly J. Growth in the Sumatran elephant (Elephas maximus sumatranus) and age estimation based on dung diameter. J Zool (Lond) 2002;258:205–213. doi: 10.1017/S0952836902001322. [DOI] [Google Scholar]
- Riddle HS, Schulte BA, Desai AA, Meer LVD. Elephants: a conservation overview. J Threat Taxa. 2010;2:653–661. doi: 10.11609/JoTT.o2024.653-61. [DOI] [Google Scholar]
- Saseendran PC, Rajendran S, Subramanian H, Sasikumar M, Vivek G, Anil KS. Incidence of helminthic infection among annually dewormed captive elephants. Zoos' Print J. 2004;19:1422. doi: 10.11609/JoTT.ZPJ.19.3.1422. [DOI] [Google Scholar]
- Schwitzer N. Parasite prevalence in blue-eyed black lemurs (Eulemur flavifrons) in differently degraded forest fragments. Endang Species Res. 2010;12:215–225. doi: 10.3354/esr00304. [DOI] [Google Scholar]
- Shaw DJ, Dobson AP. Patterns of macro-parasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology. 1995;111:111–133. doi: 10.1017/S0031182000075855. [DOI] [PubMed] [Google Scholar]
- Soulsby EJ. Helminths. ELBS, Bareilly Tindall, London: Arthropods and Protozoa of domestic animals; 1982. [Google Scholar]
- Suresh K, Choudhuri PC, Kumari KN, Hamza PA. Epidemiological and clinico-therapeutic studies of strongylosis in elephants. Zoos’ Print J. 2001;16:539–540. doi: 10.11609/JoTT.ZPJ.16.7.539-40. [DOI] [Google Scholar]
- Thawait VK, Maiti SK, Aditi AD. Prevalence of gastro-intestinal parasites in captive wild animals of Nandan Van Zoo, Raipur, Chhattisgarh. Vet World. 2014;7:448–451. doi: 10.14202/vetworld.2014.448-451. [DOI] [Google Scholar]
- Vanitha V, Thiyagesan K, Baskaran N. Prevalence of intestinal parasites among captive Asian elephants (Elephas maximus): effect of season, host demography, and management systems in Tamil Nadu, India. J Threat Taxa. 2011;3:1527–1534. doi: 10.11609/JoTT.o2488.1527-34. [DOI] [Google Scholar]
- Vidya TNC, Sukumar R. The effect of some ecological factors on the intestinal parasite loads of the Asian elephant (Elephas Maximus) in Southern India. J Biosci. 2002;27:521–528. doi: 10.1007/BF02705050. [DOI] [PubMed] [Google Scholar]
- Vimalraj PG, Jayathangaraj MG. Endoparasitic Infections in free-ranging Asiatic elephants of Mudumalai and Anamalai Wildlife Sanctuary. J Parasit Dis. 2015;39:474–476. doi: 10.1007/s12639-013-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watve MG (1992) Ecology of host-parasite interactions in a wild mammalian host community in Mudumalai, Southern India. Ph.D. thesis, Indian Institute of Science, Bangalore
- Watve MG, Sukumar R. Asian elephants with longer tusks have lower parasite loads. Curr Sci. 1997;72:885–888. [Google Scholar]
- Wilson K, Bjornstad ON, Dobson AP, Merler S, Poglayen G, Randolph SE, Read AF, Skorping A. Heterogeneities in macro-parasite infections: patterns and processes. In: Hudson P, Rizzoli A, Grenfell B, Heesterbeek H, Dobson A, editors. The ecology of wildlife diseases. Oxford: Oxford University Press; 2002. pp. 6–44. [Google Scholar]
- Zar JH. Biostatistical analysis. Singapore: Pearson Education Pvt. Ltd.; 2003. [Google Scholar]
- Zajac AM, Conboy GA (2012) Veterinary clinical parasitology. Wiley-Blackwell Publishing, London