Abstract
A total 1862 goats from 12 stall fed goat farms under slatted floor system were examined for the presence flea infestation in six districts of Tamil Nadu. Overall prevalence of flea infestation on goat was 30.79% of which, 72.73 and 27.27% were Ctenocephalides orientis and C. felis respectively. Out of these 12 farms, heavy flea infestation was noticed on goats which were reared on slatted floor with dung on floor, where as the goats that were reared on the clean shed (without dung on the floor) were free from flea infestation. In human beings first time flea bite exposure showed erythematous patches and partially healed erythematous patch with irregular crusts. Efficacy of flumethrin (pour-on) and deltamethrin (dipping) was carried out in 30 kids. Of these, deltamethrin controlled the flea within a day after application whereas, flumethrin controlled the flea after 2–3 days of application.
Keywords: Flea, Goat, Occurrence, Control
Introduction
Goat farming are growing as industry in Tamil Nadu as well as in India due to the increased and steady market price for chevon and also due to increase in consumer preferences. Due to heavy demand for the meat of goats, these animals were reared under stall fed conditions on slatted floor system (Soundararajan et al. 2014). This system provides provision for partition for the dung and thereby ensures a clean environment within the stall. In situations when farmers are reluctant to remove the dung on the floor, the environment becomes conducive for breeding of ectoparasites, particularly the fleas. Fleas are blood sucking arthropods which affect mammals, poultry, reptiles and humans (Joseph et al. 1984; Halos et al. 2014). Goats are constantly attacked by fleas, Ctenocephalides felis orientis (Joseph 1981), C. canis (Obasaju and Otesile 1980; Opasina 1983; Kilonzo and Khama 1989), C.felis (Yeruham et al. 1989), C. felis strongylus (Fagbemi 1982; Kaal et al. 2006) and Pulex irritants (Christodoulopoulos 2003; Christodoulopoulos and Theodoropoulos 2003). Heavy flea infestation can cause severe anaemia and even death in lambs and kids (Obasaju and Otesile 1980, Yakobson et al. 1981; Fagbemi 1982; Opasina 1983). The present study was carried out to study the occurrence of fleas on goats and their larval stages in the dung of goats as well as to evaluate the effect of flumethrin and deltamethrin against flea infestation in organized farms of Tamil Nadu, India.
Materials and methods
A total of 1862 goats from 12 stall fed goat farms under wooden repaired slatted floor system was examined for the presence of flea infestation in 6 districts of Tamil Nadu namely Vellore, Thiruvanamalai, Kanchipuram, Salem, Namakkal and Perambalur. Fleas were collected from infested goats. They were preserved in 70% ethanol and brought to the laboratory for identification of species. Litter materials were also examined for the presence of eggs and larvae. Lesions on the workers and veterinarians were also recorded. The flea bite allergic dermatitis lesions were observed and photographed on the workers/Veterinarians with their full consent and cooperation. Hence, the ethical approval may not be necessary for this study. Collected fleas were identified for morphological characters (Soulsby 1982; Joseph 1981) under stereozoom microscope. Efficacy of flumethrin (pour-on) and deltamethrin (dipping) was carried out in 30 kids. In Group-I, 10 kids were given 0.1% flumethrin (pour-on) @ 1 mg/kg body weight, whereas in Group-II, 10 kids were dipped with 0.2% deltamethrin. The animals in Group- III were kept as control. Fleas were manually collected and counted (Table 2).
Table 2.
Efficacy of Flumethrin and Deltamethrin on flea infestation on goats
| Group | Avg. no. of animals | Avg. no. of fleas (pre-treatment) | Avg. no. of fleas (post-treatment) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st day | 2nd day | 3rd day | 4th day | 5th day | 6th day | 7th day | |||
| Group I (0.1% flumethrin) | 10 | 35.7 ± 1.16 | 8.4 ± 0.27 | 5.1 ± 1.00 | 0 | 0 | 0 | 0 | 0 |
| Group II (0.2% deltamethrin) | 10 | 34.8 ± 1.14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Group III (control) | 10 | 36.2 ± 0.87 | 36.2 ± 1.03 | 38.1 ± 0.74 | 35.3 ± 0.86 | 38.3 ± 082 | 34.8 ± 0.90 | 36.3 ± 0.98 | 35.2 ± 1.13 |
Results and discussion
Out of 1862 goats examined for the presence of flea, 30.29% (564/1862) goats were infested with flea (Table 1). On microscopic examination, based on the morphological features, 72.73% fleas were Ctenocephalides orientis (syn. C.felis orientis) (Fig. 1) and 27.27% were C.felis (Syn. C.felis felis). Kaal et al. (2006) recorded 0.77% of C. felis strongylus on goats within Libya Whereas Christodoulopoulos et al. (2006) reported P. irritans on dairy goats within Greece.
Table 1.
Prevalence of different species of flea
| District | Farms | No. of animals examined | No. of animals infested | No. of kids died | Removal of dung | Flea status | Species of the flea |
|---|---|---|---|---|---|---|---|
| Vellore | Ranipet | 132 | 0 | 0 | Daily | Absent | Nil |
| Kannamangalam | 150 | 78 | 2 | Weekly | Present (+) | C. orientis, C. felis | |
| Thiruvanamalai | Ginjee | 140 | 102 | 6 | Monthly | Present (++) | C. orientis, C. felis |
| Thiruvanamalai | 120 | 0 | 0 | Daily | Absent | Nil | |
| Kancheepuram | Katupakkam | 220 | 0 | 0 | Daily | Absent | Nil |
| Kalpakkam | 180 | 98 | 5 | Weekly | Present (+) | C. orientis, C. felis | |
| Salem | Sentharapatty | 270 | 0 | 0 | Daily | Absent | Nil |
| Panamarathupatty | 90 | 52 | 2 | Monthly | Present (++) | C. orientis, C. felis | |
| Namakkal | Paramathy velur | 180 | 162 | 13 | Yearly | Present (++++) | C. orientis, C. felis |
| Namakkal | 160 | 42 | 4 | Weekly | Present (+) | C. orientis, C. felis | |
| Perambalur | Malayalapatty | 140 | 30 | 3 | Weekly | Present (+) | C. orientis, C. felis |
| Malayalapatty | 80 | 0 | 0 | Daily | Absent | Nil | |
| Total | 1862 | 564 | 35 |
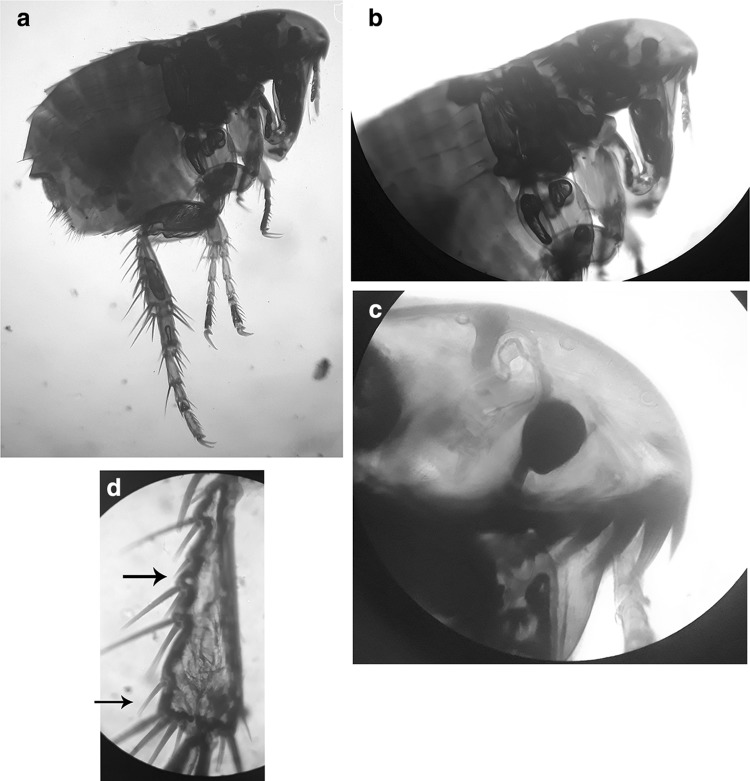
Fig. 1.
Ctenocephalides orientis adult flea (a), head end with genal and pronotal combs (b), shorter 1st genal spine than the 2nd genal spine (c), single brsistle on the 3rd and 6th notches (d)
The presence of fleas on the body of goats causes extreme annoyance, irritation, loss of hair, anemia and loss of body weight. Infested animals scratch their head, ears and body with their legs. Affected animals were weak, debilitated, with rough coat and lusterless skin). In heavy infestation, hairs become dark brown or reddish colour (due to the oozing of blood from the flea bite injury) and arched back due to heavy flea infestation on dorsal sternum (Fig. 2). Infested animals were dull and lie down by keeping their heads inside the abdomen and covered by legs. Christodoulopoulos et al. (2006) observed restlessness, rubbing, chewing, cut hairs, excoriations and redness in adult goats and alopecia and lichenification and with other sings in kids. Kaal et al. (2006) observed restlessness, self excoriation, alopecia, emaciation, weakness and hyperkeratinization of the lower limbs of sheep. A total of 35 kids were died due to heavy flea infestation and the mortality rate was 6.21%. Fagbemi (1982) reported the death of goats due to Ctenocephalides felis strongylus in West Arica, whereas Yeruham et al. (1989) reported mortality in calves, lambs and kids due to C. felis in Israel. Similar reports were observed in kids (Obasaju and Otesile 1980; Opasina 1983). Veterinarians and farmers were also get infested by fleas while handling flea infested animals. Persons exposed first time to the flea infestation showed erythymatous patches, partially healed erythematous patch with irregular crusts and severe irritation at the site of bite due to flea bite allergy (Fig. 3). Pulex irritans from goat were reported to infest the farmers while milking and feeding the dairy goats in Greece and produced intensive itching, redness and erythematous papules and pustules (Christodoulopoulos et al. 2006).
Fig. 2.

Severe flea infestation on a kid
Fig. 3.
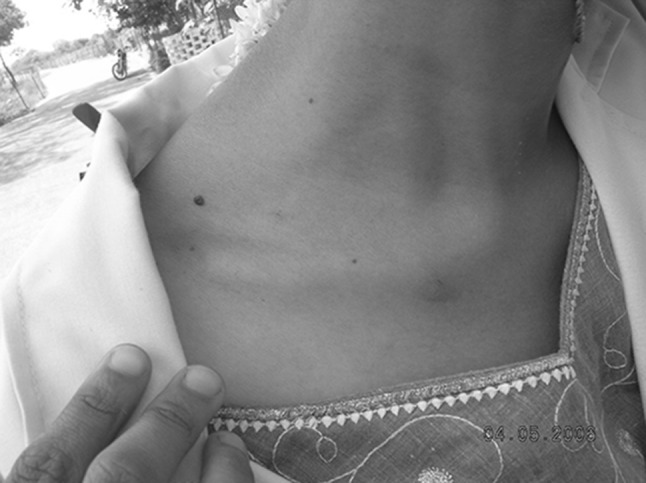
Erythematous patches on the neck near collar due to flea bite (first exposure) in a 22 year old girl
Out of 12 goat farms examined, goat’s dung were removed daily in 6 farms where as it accumulated on the floor for months together. Out of these 12 farms, heavy flea infestation was noticed on goats which were reared on slatted floor with dung on floor, where as the goatswhich were reared on the clean shed (without dung on the floor) were free from the flea infestation. In the present study, highest no. of flea infestation was observed in goats reared under slatted floor without cleaning the litter material for more than a month to years. Accumulation of litter material on the floor favors the development of eggs, hatching of larvae and pupae and proliferation of fleas. (Obasaju and Otesile 1980; Kaal et al. 2006). Kaal et al. (2006) reported significantly higher proportion of C. felis strongylus on animals reared under intensive system compared to animals in the semi-intensive system. In the present study, eggs and larvae were collected from the manure on the floor (Fig. 4).
Fig. 4.
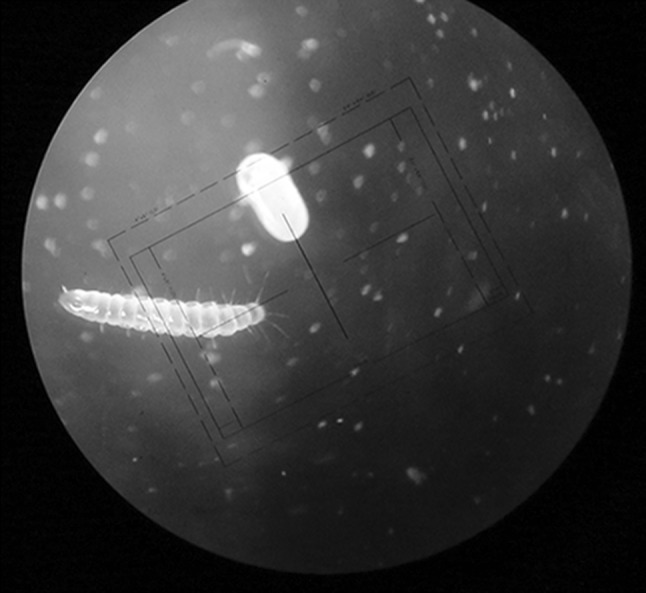
Egg and larval stage of flea
Efficacy of flumethrin (pour-on) and deltamethrin (dipping) was carried out in 30 kids. Of these, deltamethrin controlled the flea within a day after application whereas the flumethrin controlled the flea after 2–3 days of application. There is a statistically significant (P < 0.01) reduction in the flea numbers on treatment with flumethrin on the first day (Tables 2 and 3). Bouhsira et al. (2012) used novel spot-on formulation combining permethrin, pyriproxifen and dinotefuran (Vectra 3D™ spot-on solution for dogs) in adult Beagle dogs to control C.felis and observed 99.7% adulticidal efficacy on fleas within 48 h after treatment and controlled re-infestations for up to 30 days. Significant (P < 0.01) reduction in the flea count noticed on first day itself after the application of deltamethrin.
Table 3.
Paired ‘t’ Statistics
| Comparison | T-value | Df | P value |
|---|---|---|---|
| Flumethrin pre-day1 | 26.69** | 9 | P < 0.01 |
| Flumethrin pre-day2 | 30.53** | 9 | P < 0.01 |
| Deltamethrin pre-post | 30.44** | 9 | P < 0.01 |
| Control pre-day1 | 0.001NS | 9 | P > 0.05 |
| Control pre-day2 | − 1.95NS | 9 | P > 0.05 |
Conclusion
It is concluded that the dung on the floor is the main source for breeding of fleas. The farmers and farm owners were advised to remove the dung daily.
Author’s contribution
CS: Collection and identification of fleas, collection of literature and preparation of manuscript. KN: Assistance in literature collection, typing and preparation of manuscript. MAP: Assisted in collection of fleas and collection of literature
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Bouhsira E, Lienard E, Jacquiet P, Warin S, Kaltsatos V, Baduel L, Franc M. Efficacy of permethrin, dinotefuran and pyriproxyfen on adult fleas, flea eggs collection, and flea egg development following transplantation of mature female fleas (Ctenocephalides felis felis) from cats to dogs. Vet Parasitol. 2012;190:541–546. doi: 10.1016/j.vetpar.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Christodoulopoulos G. Infestation of dairy goats with Pulex irritans. Author reply. Vet Rec. 2003;153:128. [PubMed] [Google Scholar]
- Christodoulopoulos G, Theodoropoulos G. Infestation of dairy goats with the human flea, Pulex irritans, in central Greece. Vet Rec. 2003;152:371–372. doi: 10.1136/vr.152.12.371. [DOI] [PubMed] [Google Scholar]
- Christodoulopoulos G, Theodoropouluos G, Kominakis A, Theis JH. Biological, seasonal and environmental factors associated with Pulex irritans infestation of dairy goats of Greece. Vet Parasitol. 2006;137:137–143. doi: 10.1016/j.vetpar.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Fagbemi BO. Effect of Ctenocephalides felis Strongylus infestation on the performance of West African dwarf sheep and goats. Vet Q. 1982;4:92–95. doi: 10.1080/01652176.1982.9693846. [DOI] [PubMed] [Google Scholar]
- Halos L, Beugnet F, Cardoso L, Farkas R, Franc M, Guillot J, Pfister K, Wall R. Flea control failure? Myths and realities. Trends Parasitol. 2014;30:228–233. doi: 10.1016/j.pt.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Joseph SA. Studies on the binomics of Ctenocephalides felis orientis (Jordan) 1925. Cheiron. 1981;22:192–194. [Google Scholar]
- Joseph SA, Karunamoorthy G, Lalitha CM. Cat flea, Ctenocephalides felis felis (Bouche) infestation in a poultry farm of Tamil Nadu. Indian J Poult Sci. 1984;19:192–193. [Google Scholar]
- Kaal JF, Baker K, Torgerson PR. Epidemiology of flea infestation in ruminants in Libya. Vet Parasitol. 2006;141:313–318. doi: 10.1016/j.vetpar.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Kilonzo BS, Khama LRS. The effects of goats’ (Capra hircus) age and sex on flea infestation in Tanzania. Bulletin Anim Health Prod Afr. 1989;37:61–66. [Google Scholar]
- Obasaju MF, Otesile EB. Ctenocephalides canis infestation of sheep and goats. Trop Anim Health Prod. 1980;12:116–118. doi: 10.1007/BF02242620. [DOI] [PubMed] [Google Scholar]
- Opasina BA. Ctenocephalides canis infestation of goats. Trop Anim Health Prod. 1983;15:106. doi: 10.1007/BF02239805. [DOI] [PubMed] [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. London: ELBS and Bailliere Tindal; 1982. pp. 206–207. [Google Scholar]
- Soundararajan C, Arul Prakash M, Senthilkumar K (2014) Status of stallfed goat farms in different agroclimatic zones of Tamil Nadu. In: Paper presented at national seminar on “prospects and challenges in small ruminant production in India”, Sheep Breeding Research Station, Sandynallah, Nilgiris, Tamil Nadu, 11–12 Dec 2014, p 97
- Yakobson B, Perl S, Sklair A, David EH. Mortality in lambs associated with the dog flea (Ctenocephalides canis) infestation. Refuah Veterinarith. 1981;38:35–36. [Google Scholar]
- Yeruham I, Rosen S, Hadani A. Mortality in calves, lambs and kids caused by severe infestation with the cat flea, Ctenocephalides felis felis (Bouche, 1835) in Israel. Vet Parasitol. 1989;30:351–356. doi: 10.1016/0304-4017(89)90105-2. [DOI] [PubMed] [Google Scholar]