Abstract
Significance: All cellular metabolic processes are tied to the cellular redox environment. Therefore, maintaining redox homeostasis is critically important for normal cell function. Indeed, redox stress contributes to the pathobiology of many human diseases. The cellular redox response system is composed of numerous interconnected components, including free radicals, redox couples, protein thiols, enzymes, metabolites, and transcription factors. Moreover, interactions between and among these factors are regulated in time and space. Owing to their complexity, systems biology approaches to the characterization of the cellular redox response system may provide insights into novel homeostatic mechanisms and methods of therapeutic reprogramming.
Recent Advances: The emergence and development of systems biology has brought forth a set of innovative technologies that provide new avenues for studying redox metabolism. This article will review these systems biology approaches and their potential application to the study of redox metabolism in stress and disease states.
Critical Issues: Clarifying the scope of biological intermediaries affected by dysregulated redox metabolism requires methods that are suitable for analyzing big datasets as classical methods that do not account for multiple interactions are unlikely to portray the totality of perturbed metabolic systems.
Future Directions: Given the diverse redox microenvironments within cells, it will be important to improve the spatial resolution of omic approaches. Futures studies on the integration of multiple systems-based methods and heterogeneous omics data for redox metabolism are required to accelerate the development of the field of redox systems biology. Antioxid. Redox Signal. 29, 953–972.
Keywords: : systems biology, redox metabolism, hypoxia, thiol
Introduction
Redox metabolism is regulated by a complex set of redox metabolites and enzymes that form a network with redox-sensitive transcription factors and signaling pathways. This network consists of a series of oxidation-reduction (redox) reactions that are involved in the synthesis and removal of reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide (H2O2), and other toxic nucleophiles produced from different metabolic reactions. ROS play critical roles in cell signaling and homeostasis; however, excessive ROS accumulation during various environmental stresses may cause progressive oxidative damage to DNA, lipids, and proteins.
Under oxidative or reductive stress (redox stress), several enzymatic and non-enzymatic antioxidants are activated in cells to protect them from redox injury through a redox-sensitive network. Within this network, the glutathione (GSH), peroxiredoxin (Prx), and thioredoxin (Trx) systems play important roles in redox homeostasis, antioxidant defense, and redox signaling (30). Dysfunction of antioxidant enzymes and perturbations in redox homeostasis are associated with many pathological disorders, such as cancer, cardiovascular diseases, and aging (56).
Redox metabolism and the redox-sensitive network in higher eukaryotic cells are much larger and more complex than in prokaryotes (59). Many cellular processes are involved in redox metabolism and regulated by redox states. Clarifying the scope of biological reaction intermediates that are affected by dysregulated redox metabolism requires computational and quantitative modeling methods that are suitable for analyzing large datasets, as conventional reductionist methods that do not account for multiple interactions among redox components are unlikely to predictably portray the totality of perturbed metabolic systems.
The emergence and development of systems biology has brought forth a set of innovative technologies that provide new avenues for studying redox metabolism from an integrated systems perspective (80). For example, top-down and bottom-up systems-level approaches have been developed to model redox systems and permit characterizing the dynamics of these systems from a network perspective (117). Redox proteomics and metabolomics data can be integrated together to investigate complex mechanisms of human disease (52).
In this review, we first provide an overview of the cellular redox environment and then introduce various systems biology approaches that have been or potentially could be applied to characterizing redox metabolism. We also discuss diseases and pathological disorders associated with dysfunctional redox metabolism and the areas in need of further investigation.
Redox Environment
Electron transfer reactions drive all aspects of cellular metabolism. Bioenergetic metabolism relies on electron transfer from substrates to the electron transport chain for adenosine triphosphate (ATP) synthesis by oxidative phosphorylation. Biosynthetic metabolic pathways incorporate electrons during carbohydrate, amino acid, fatty acid, and nucleic acid synthesis. Moreover, reactive oxygen, nitrogen, and sulfur species play critical physiological roles in a variety of processes, including post-translational protein modifications, cellular signaling pathways, and immune responses. By contrast, an excess of these mediators can damage all major cellular constituents, particularly lipids, proteins, and nucleic acids, thereby contributing to disease pathobiology. Thus, maintaining redox homeostasis is of fundamental importance to all aspects of cellular metabolism. For the purposes of this review, we will focus on the determinants of redox homeostasis, cellular responses to redox stress, and implications for disease pathobiology as characterized by utilizing the tools of system biology. For further detailed discussions of the biochemistry and signaling functions of ROS, the reader is directed to several recent reviews (102, 123, 130).
The redox environment of a cell or tissue is formally defined by the summation of the products of the reduction potential and reducing capacity of the linked redox couples present (126). Given the impracticality of measuring all linked redox couples accurately in a biological sample, the redox state of a representative redox couple is typically used as an indicator of changes in the overall cellular redox environment. This redox state is best defined by the half-cell reduction potential and reducing capacity of the redox couple. For the [NAD]/[NADH] and [NADP]/[NADPH] couples, the ratio of oxidized and reduced forms provides a reasonable estimate of the reduction potential. By contrast, for the glutathione disulfide [GSSG]/[GSH] couple, the absolute [GSH] concentration must be accounted for when estimating reducing potential (126). For example, benzyl isothiocyanate treatment increases [GSSG]/[GSH] by 3.6-fold in control colon cancer cells and by 3.7-fold in colon cancer cells pre-treated with sodium butyrate. However, this is associated with 16 mV oxidation in control cells and 40 mV oxidation in butyrate-treated cells. This 26 mV difference is due to a much lower [GSH] in sodium butyrate-treated cells (79).
Attempts to quantify the redox environment of the cell typically focus on measuring the relative ratios of the main intracellular redox couples, NAD/NADH, NADP/NADPH, and GSSG/GSH, as perturbations in these ratios can have marked impacts on cellular metabolism. For example, the NAD/NADH ratio plays an important role in coupling metabolism to energy demand (127, 141, 143, 148). Low-energy demand requires less ATP production, resulting in a decreased NAD/NADH ratio in the mitochondria and feedback inhibition on NADH-generating metabolic pathways. Conversely, high-energy demand consumes NADH, thereby favoring continued substrate oxidation. Indeed, many of the disease states reviewed here can be characterized by pathologic changes in the cellular redox environment.
Historically, redox ratios were estimated based on the ratio of a coupled enzymatic substrate and product. For example, lactate dehydrogenase (LDH) catalyzes the reversible reduction of pyruvate to lactate, which is coupled to the oxidation of NADH to NAD. Since pyruvate and lactate are easier to measure biochemically, the pyruvate/lactate ratio has been used as a surrogate for the NAD/NADH ratio (156). Cycling enzymatic assays and mass spectrometry (MS)-based methods have since been developed to measure these redox couples directly. Owing to their lability, however, careful attention must be paid to prevent oxidation or enzymatic degradation of these compounds, which can have a marked impact on the estimated ratio. With the advent of genetically encoded fluorescence-based biosensors, redox ratios can be determined in living cells in vitro, often with the ability to monitor experimental effects in a subcellular compartment, such as the mitochondrion (11, 12, 169).
Redox compartmentalization
Importantly, different subcellular compartments have different redox environments (74). For example, the steady-state redox potential of GSSG/GSH of the mitochondrial matrix is about −280 mV, which is more reducing than the cytoplasmic values of −200 mV, which is more reducing than the endoplasmic reticulum values of −190 mV (49). In addition, the relative pool sizes of the key redox couples differ among cellular compartments. Cytoplasmic NAD(H) concentrations range between 200 and 500 μM, with an NAD/NADH ratio between 200 and 800 (22, 121, 136, 146, 160, 167, 172, 173). Mitochondrial NAD(H) concentrations up to 800 μM have been reported, whereas the mitochondrial NAD/NADH ratio is ∼100-fold less than the cytoplasm ratio (NAD/NADH between 2 and 10) (22, 107, 146, 147, 156, 163). Whole-cell NADP concentrations are ∼80 μM with a mitochondrial concentration of 20 μM (22). Moreover, the ratio of NADP/NADPH is ∼100,000-fold lower than the NAD/NADH ratio [i.e., the majority of the NADP(H) pool is in the reduced state] (146). GSSG/GSH is the most abundant intracellular redox couple, with concentrations ranging from 1 to 10 mM (47). Similar to the NADP/NADPH couple, the GSSG/GSH pool is predominantly reduced with a ratio of 0.01 in the cytosol (32). Approximately 80–85% of intracellular GSH is in the cytosol, 10–15% in the mitochondria, and a few percent in the endoplasmic reticulum (66, 99, 168).
Redox couples in various compartments also appear to be isolated from one another. For example, the ratio of GSSG/GSH is much higher in the endoplasmic reticulum (0.3–1) than in the cytosol or mitochondrion (66). Similarly, treatment with the GSH synthesis inhibitor buthionine sulfoximine reduces the cytoplasmic pool of GSH whereas the nuclear pool resists depletion (70). Nuclear GSH maintains the redox state of critical protein sulfhydryls necessary for DNA repair and gene expression (5). Indeed, protein S-thiolation/dethiolation reactions are critically important for normal protein function and cellular responses to redox stress. Similarly, the mitochondrial pool of NAD is protected from depletion by FK866, a potent inhibitor of nicotinamide phosphoribosyltransferase (NAMPT) that decreases cytoplasmic NAD to 50% of control levels in HeLa cells treated for 24 h without affecting mitochondrial levels (118).
Protein thiols
Although GSH is considered the “cellular redox buffer,” protein thiols constitute a larger active redox pool (60). The nucleophilicity of the free electrons of the sulfur atom permits a range of enzyme-independent redox reactions involving thiols (Fig. 1). Perturbations to the redox potential of cells that alters the oxidation state of functionally essential protein cysteinyl thiols have been linked to dysregulated cell survival, growth, proliferation, senescence, aging, and phenotype switches (25, 157) that underpin the pathogenesis of cardiovascular disease (97, 175), dementia and neurodegenerative disorders (133), and solid tumor malignancy (142, 155), among many other major human diseases.
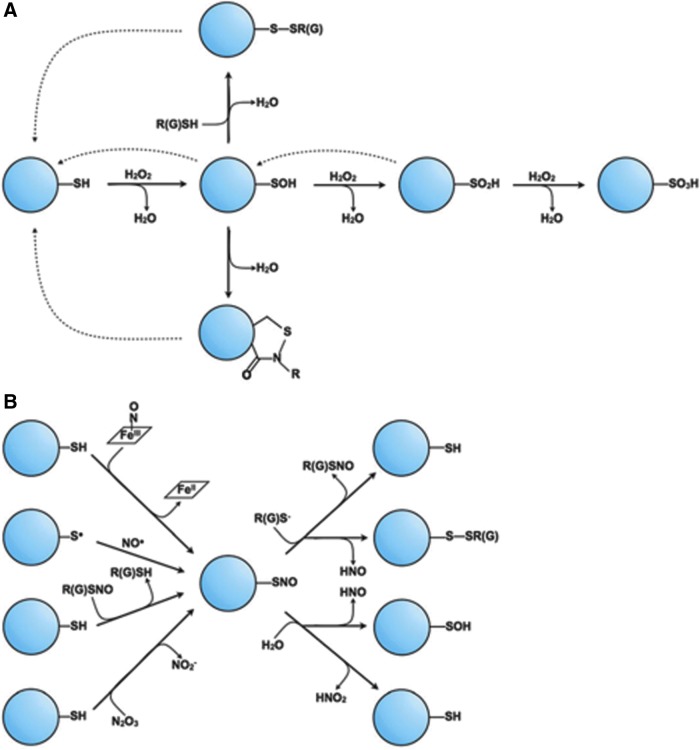
FIG. 1.
The range of oxidative/nitrosative cysteine post-translational modifications. (A) Oxidative modifications of cysteine can be characterized by its sequential redox reactions with H2O2 beginning with free thiolate. As additional equivalents of H2O2 react with cysteine, higher-order oxidations occur, from sulfenic acid (-SOH), to sulfinic acid (-SO2H), and finally sulfonic acid (-SO3H). Both sulfenic and sulfinic acids can be reduced through enzymatic reactions (dashed arrows). Sulfenic acids can also undergo further reactions with peptide backbone nitrogens to form sulfenamide adducts or with other protein or small-molecule thiols to form disulfides, which are also enzymatically reversible. (B) Nitrosative modifications occur through a number of distinct mechanisms that all involve NO or its derivatives (such as ONOO−). The fate of this SNO modification depends on the relative reactivity of the nitrogen and sulfur electrophiles; reaction with water can lead to either a sulfenic acid or a free thiolate, whereas reaction with a free thiol can result in either disulfide formation or transnitrosation. GSH, glutathione; H2O2, hydrogen peroxide; NO, nitric oxide; ONOO−, peroxynitrite; SNO, S-nitrosothiol. [Reproduced with some modifications with permission from Bak and Weerapana (7).]
Reduced thiols tautomerize to form thiolate anion (S−), which, in turn, may react with oxidants such as H2O2, peroxynitrite (ONOO−), and other hydroperoxides to form higher oxidative intermediates, including sulfenic acid (R-SOH), sulfinic acid (R-SO2H), and sulfonic acid (R-SO3H), as well as the disulfide form (R-S-S-H). The formation of higher oxidative states may exert a functional effect on proteins through different molecular mechanisms, including via conformational changes to the tertiary structure of proteins, particularly in the context of disulfide bond formation. These post-translational modifications can alter the kinetics or accessibility of ligand-receptor binding, such as G-protein subunit coupling. For example, under pathological conditions in vascular endothelial cells, increased ROS due to activation of NADPH oxidase type 4 is associated with disulfide bond formation involving Cys405 of the endothelin type-B (ETB) receptor, leading to impaired Gi subunit coupling and impaired ETB-dependent nitric oxide (NO) synthesis (97, 111) (Fig. 2).
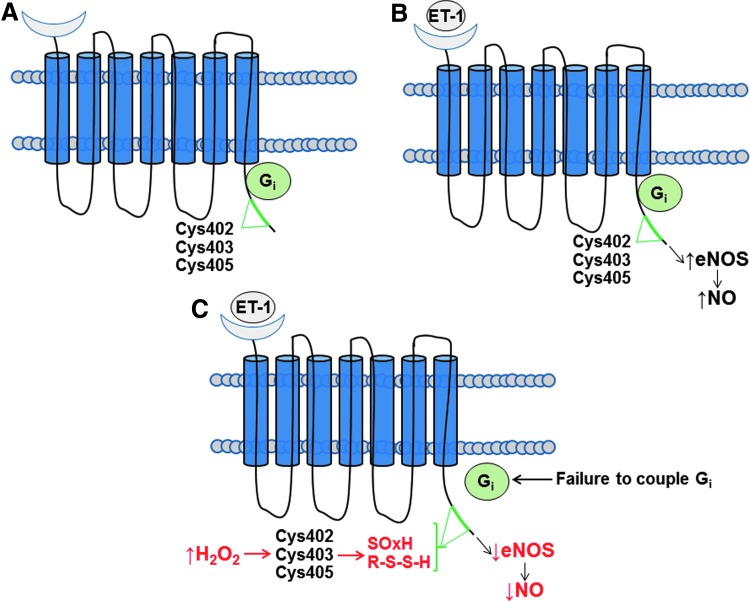
FIG. 2.
ETB receptor cysteinyl thiol redox switch inhibits NO synthesis. (A, B) Under normal conditions in endothelial cells, stimulation of the G protein-coupled receptor ETB by ET-1 activates coupling of the receptor C-terminus with the Gi subunit. This modification is required for ETB-dependent stimulation of eNOS and subsequent synthesis of the vasodilator and anti-mitogenic molecule NO. Importantly, the C-terminus contains a cysteine-rich region, including Cys402, Cys403, and Cys405. Site-directed substitution of serine at Cys403 and Cys405 prevents ETB-Gi coupling and normal ETB signal transduction. (C) In human pulmonary artery endothelial cells, increased H2O2 accumulation oxidizes Cys405 to sulfenic acid and induces the formation of intermolecular disulfides involving Cys405, which, collectively, decrease ETB-dependent activation of eNOS and synthesis of NO. This ETB cysteinyl thiol redox switch is implicated as a pathobiological mechanism underlying adverse vascular remodeling of distal pulmonary arterioles in pulmonary arterial hypertension. eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; ETB, endothelin-B.
Disulfide bond formation may also occur through thiol/disulfide exchange reactions. In this case, redox equivalents are exchanged between different thiol/disulfide pairs to promote oxidation of one reduced thiol and reduction of a disulfide bond (R1-SH + R2SSR2 ↔ R1S-SR2 + R2SH) (95). Protein disulfide isomerases (PDIs) and GSH are well-described redox buffers that may participate in thiol/disulfide exchange reactions. The functional effects of the thiol/disulfide exchange reaction are well characterized, and include cell surface disulfide bond formation that regulate protein folding and trafficking (164), intracellular delivery of NO (169), and cell structure (108) in the case of PDIs; and thrombosis by deactivating platelets through disulfides involving platelet integrin αIIbβ3 in the case of GSH (100, 161, 162).
Additional thiol modifications that are influenced by redox reactions and functionally relevant in biological systems include S-nitrosation (135), sulfhydration through endogenously produced H2S (98), and S-glutathiolation, as recently reviewed in Maron et al. (95). As one example, S-glutathionylation affects redox metabolism by inhibiting various intermediates of the tricarboxylic acid cycle. Incubation of subsarcolemmal mitochondria from rat heart with H2O2 causes reversible deactivation of α-ketoglutarate dehydrogenase (KGDH), which can be reactivated by glutaredoxin, an efficient catalyst of protein deglutathionylation (110). Although the teleological reason for this effect is not known precisely, some have speculated that S-glutathionylation may protect protein sulfhydryls, and, therefore, protein function, from irreversible oxidative damage (9). Similar inhibitory effects of S-glutathionlyation are reported for aconitase, isocitrate dehydrogenase, succinyl-CoA transferase, and various electron transport chain protein complexes (166).
These thiol reactions, collectively, provide a critical link between fluctuations in the cellular milieu and biological activity of cells. In this way, functionally essential and redox-sensitive thiols have been described as an “adaptive interface” between pre-programmed signaling pathways and environmental stressors (e.g., toxins, nutritional changes, exposures) (48, 50). Although the use of sophisticated computational models to predict the redox sensitivity of specific thiols in biological systems has been reported, the number of thiols that are susceptible to redox disequilibrium in mammalian cells is vast. Therefore, it is increasingly recognized that reductionist methods are insufficient to understand and accurately portray the global consequences of ROS accumulation on the thiol proteome. Systems-based analyses, however, permit the illustration of simultaneous changes to the redox status of multiple thiols within a protein and across proteins under similar conditions. These approaches have shed important light on understanding the coordinated functional ramifications of stressors on thiol oxidation as discussed in the next section.
ROS defense systems
Protein thiol reactions drive critical cellular redox defense systems. Most of these reactions ultimately rely on NADPH as a source of electrons to fully reduce partially oxidized ROS. Catalase, which reduces H2O2 to water and oxygen, utilizes electrons donated from NADPH. GSH reductase similarly utilizes electrons from NADPH to reduce GSSG to GSH. GSH provides reducing equivalents for glutathione peroxidase (Gpx) enzymes to reduce hydrogen and lipid peroxides. Trx proteins require NADPH-dependent recycling to reduce protein disulfides. In turn, Trx reduces Prx, which also plays a critical role in antioxidant defense (Fig. 3) (87). Prx contains a disulfide bond that is reduced under basal conditions, owing to the reductive potential of cells in “normal” steady-state conditions. This characteristic permits Prx to reduce H2O2, ONOO−, and lipid peroxides, and to function as a key regulator of cellular redox potential (18, 124). The importance of “reducing power” provided by NADPH is demonstrated in the setting of methylene tetrahydrofolate dehydrogenase-1 (MTHFD) deficiency, where low cellular NADPH/NADP is associated with decreased GSH/GSSG and increased sensitivity to oxidative stress (38).
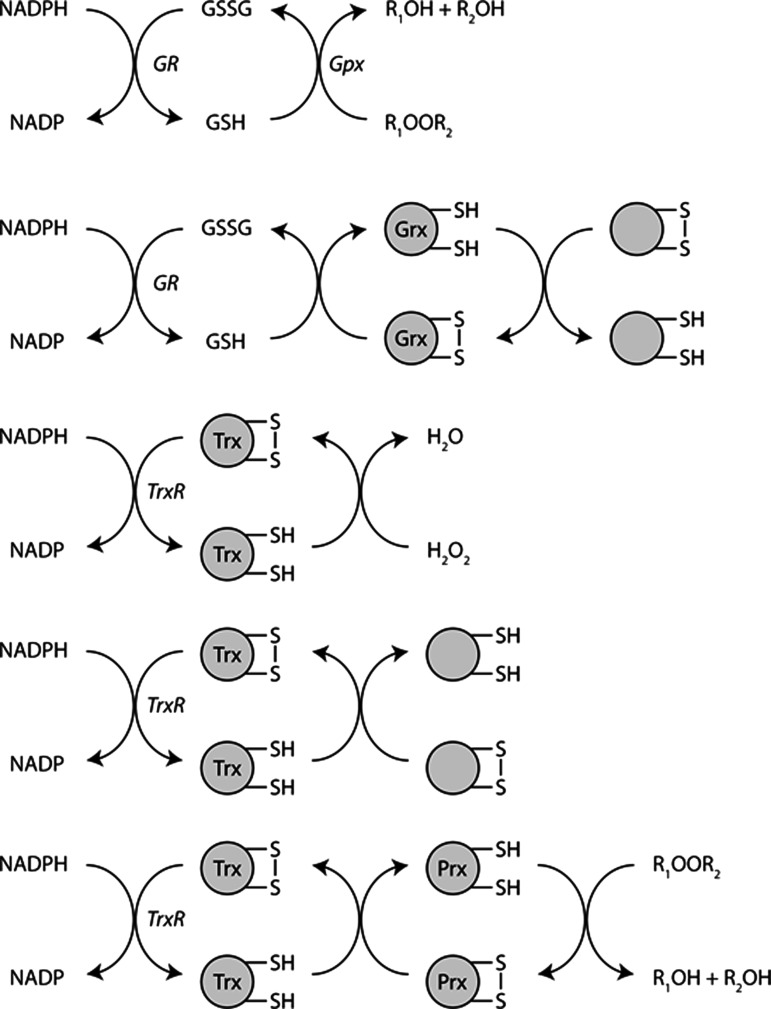
FIG. 3.
ROS defense systems. NADPH provides the reducing power to combat oxidative modifications through several mechanisms. GR reduces GSSG to GSH, which is re-oxidized by Gpx in the enzymatic reduction of hydrogen and lipid peroxides. Similarly, GSH reduces Grx, which can reduce a variety of protein thiols. NADPH is also required for TrxR to recharge Trx, a family of enzymes that plays a critical role in protein disulfide redox metabolism. Trx can reduce H2O2 and GSSG directly as well as a variety of protein disulfides, including Grx, PDI, and Prx. Prx proteins also detoxify hydrogen and lipid peroxides. Gpx, glutathione peroxidase; GR, glutathione reductase; Grx, glutaredoxin; GSSG, glutathione disulfide; PDI, protein disulfide isomerases; Prx, peroxiredoxins; ROS, reactive oxygen species; Trx, thioredoxin; TrxR, thioredoxin reductase.
Systems-Based Approaches to Defining the Cellular Redox Response System
Systems biology focuses on the study of biological systems as a whole, whose behavior cannot be reduced to the sum of its parts (80). The emergence and development of systems biology has brought forth a new set of innovative technologies that generate large-scale “omics” data and facilitate the study of biological systems on a global level through quantitative modeling. These biotechnologies not only allow quantification of biomolecules at different levels but, perhaps more importantly, also permit analysis of complex interactions among them on a massive scale. Systems biology approaches have been applied to different areas of biology and medicine (8, 132, 150). Large-scale data analysis and quantitative modeling are beginning to play significant roles in redox biology as well, and a subfield of “redox systems biology” is gaining increasing attention in particular (34) (Fig. 4). In the following sections, we review recent advances in the identification of novel components or interactions that form key components of the cellular redox response system utilizing “omics” and complementary systems approaches.
FIG. 4.
The emergence of redox systems biology. Integration of systems-level approaches and omics data generated by innovative techniques provides new avenues for studying redox biology.
Redox transcriptome
The transcriptome influenced by the cellular redox state is relatively well characterized at the level of messenger RNA (mRNA) abundance in bacteria and higher eukaryotes, but progress has been made in mammals as well. For example, in response to oxidant stress, mammalian cells trigger signaling cascades that result in expression of a wide range of protein-coding genes that are involved in the regulation of the cellular redox state (88). In addition to protein-coding genes, noncoding RNAs also contribute to redox regulation of gene expression. In a recent study, Giannakakis et al. investigated the effect of oxidant stress on the transcriptome of human fibroblasts by comparing the expression patterns of coding and noncoding parts of the human genome and found that oxidant stress causes rapid, transient, and dynamic alterations to the bioactivity of thousands of promoter-associated antisense long noncoding RNAs in the cell nucleus and cytosol (46).
Genome-wide transcriptional profiling can be helpful for predicting the biomarkers of oxidative stress or associated diseases. For example, the scope and relevance of NAMPT, which is the rate-limiting step in NAD synthesis, to redox metabolism abnormalities associated with cancer was recently assessed. Gujar et al. inferred a transcriptional regulatory network by integrating 169 RNA-Seq datasets from a glioblastoma line and then constructing NAMPT-regulated subnetworks by extracting network connections containing significantly altered transcripts in glioblastoma cells treated with an NAMPT inhibitor (58). Using gene expression microarrays and a real-time polymerase chain reaction, Houessinon et al. identified metallothionein-1 (MT1) as a biomarker of altered redox metabolism in hepatocellular carcinoma (HCC) cells exposed to sorafenib (63).
In addition, chromatin immunoprecipitation (ChIP)-Seq combines ChIP with massively parallel DNA sequencing and is able to integrate detection of genome-wide protein–DNA binding events (transcription factor-gene interaction) with chemical modifications of histone proteins (44). Utilizing a combination of ChIP-Seq and microarray analyses provides a powerful tool to identify upstream regulatory factors controlling gene transcription. With this approach, novel targets of the NRF2 antioxidant transcription factor have been identified, providing a picture of a global transcriptional network regulated by NRF2 (28, 90). Similarly, overlaying ChIP-Seq and RNA-Seq profiles, Tao et al. identified that PITX2 is an important gene regulating redox balance in neonatal myocardium (138).
Redox proteome
Although changes in protein expression and activity may be inferred from changes in gene expression patterns, correlation between the two is not guaranteed. Moreover, cellular RNA signatures provide no information on the key factors that regulate protein function, including protein concentration and post-translational modifications. Direct assessment of the proteome provides this information. Redox proteomics is a branch of proteomics that aims to detect oxidized proteins and quantify the status of oxidative modifications in the proteomes of interest. Such quantitative information is essential to build mathematical models for deciphering the dynamics of redox-related biochemical pathways and networks. Recently, there have been studies focusing on the development of proteomics techniques to identify all redox-regulated proteins and redox active compounds and to elucidate redox control networks in cellular systems (14, 140).
Some techniques have been developed to identify proteins with various post-translational modifications. For example, the biotin switch assay is a semi-quantitative method originally introduced by Jaffrey and Snyder to identify S-nitrosylated proteins and expanded subsequently to detect numerous specific cysteine modifications in redox-sensitive pathways (69, 104, 164). The assay first uses a replacement strategy to block free cysteines in complex protein mixtures by using NEM, MMTS, or iodoacetamide, after which S-nitrosyl modifications are then reduced with ascorbate and switched for a thiol-reactive biotin. The biotin-tagged proteins can be easily detected or enriched by affinity chromatography or Western blots. The biotin switch technique has been combined with quantitative isobaric tags for relative and absolute quantification (iTRAQ) to compare nitrosylation profiles in various samples (42).
Sulfenic acids are the first oxoforms of the family of organosulfur oxoacids and play a central role in many redox-regulated processes. Sulfenic acid modifications, known as sulfenylation, are reversible. A number of proteomic methodologies have been developed to detect proteins with sulfenic acid formation. An important advancement in this area is the oxidative isotope coded affinity tag (OxICAT) technique in which the ICAT reagent is used to monitor oxidative modification on individual Cys by measuring the difference in labeling between the “heavy” and “light” ICAT forms at the modified sites (84). OxICAT allows the precise quantification of oxidative thiol modifications in hundreds of different proteins in a single experiment. It can also identify physiologically important target proteins of oxidative stress and determine their redox-sensitive cysteine(s) (84).
S-acylation, S-glutathionylation, S-nitrosylation, and S-sulfenylation are prominent redox-cysteine modifications that regulate redox signaling. In a recent study, Gould et al. used MS-based proteomics technologies to identify the sites of the four modifications under normal physiological conditions and integrated them in the proteome (55). Analysis of this comprehensive redox proteome of endogenous cysteine residues indicates that redox-dependent post-translational modifications are surprisingly selective and largely non-overlapping in cysteine specificity in the proteome. Finding a single rule or property for cysteine post-translational modification on a proteome-wide scale is not, however, possible at this time (55). More proteomic tools for the large-scale analysis of the different oxidative cysteine modifications can be found in a recent review (104).
A successful study using large-scale omics data in redox biology was conducted by Fedorova and colleagues, who applied a multi-omics approach to characterize cross-talk signaling between lipid and protein carbonylation in rat cardiomyocytes (CM) by using a dynamic model of nitrosative stress (57). Systems biology integration of lipidomics and proteomics allowed the identification of more than 167 unique proteins, with 332 sites modified by reactive lipid peroxidation products. Although redox proteomics is currently in its early stage, MS-based biomarker discovery in diseases linked to oxidative and nitrosative stress is a promising strategy. Redox proteomics is becoming a key tool for obtaining novel insights into various protein modifications that are relevant to disease. Clinical research, in turn, may benefit from redox proteomics by identifying new drug targets and diagnostic markers.
Redox metabolome
The redox metabolome is a redox-active subset of the metabolome. Two leading analytical approaches for global metabolomic profiling are nuclear magnetic resonance (NMR) and liquid chromatography (LC) or gas chromatography coupled with MS, which can be used to measure the composition and concentration of both targeted and untargeted metabolites in disease diagnosis and analysis of biochemical pathways. These techniques can be used for redox metabolic profiling to identify metabolic biomarkers and reveal redox-regulated signaling mechanisms.
Suh et al. used thiol/redox metabolomics to identify metabolic perturbations associated with early preclinical and clinical stages of graft-versus host disease (GVHD) and found that early dysregulation of the host GSH-regulated redox system is associated with GVHD pathogenesis (137). In a recent study, Chen et al. conducted a metabolic profiling of aristolochic acid I-treated rats by using LC-MS to identify sensitive biomarkers for early detection and progression of tubulo-interstitial nephropathy, and found that chronic kidney disease progression is associated with activated redox signaling (21). Similarly, combining MS-based ionomics and targeted lipidomics of fatty acid metabolites, Huang et al. found that the levels of redox-active metal ions significantly decreased in patients with periodontitis compared with nonsmoker control subjects (64), which indicates that local redox alterations significantly contribute to periodontitis in response to oxidative stress. In a recent study, ex vivo 1H NMR spectroscopy has been used to simultaneously quantify the oxidized and reduced forms of coenzymes of cellular redox reactions (106). In another study, NMR combined with direct-infusion electrospray ionization MS was used to identify unique metabolic profile changes in the energy/redox metabolome in response to environmental and mitochondrial toxins (83).
Limitations of omic technologies
Although omic technologies have a broad range of applications in different areas, it is important to highlight current challenges and technique limitations. The sensitivity and resolution of omics instruments have continually been an issue in the field. For example, some proteomics techniques can only detect highly abundant proteins. There is currently insufficient sensitivity for proteins of low abundance. Given the specificity of redox modifications, the measurement and monitoring of the redox proteome has also not achieved high resolution. In addition, single-cell RNA-Seq examines the mRNA sequence information from individual cells and performs accurate quantitative transcriptome measurements with a higher resolution of cellular differences than current proteomics methods. We can imagine that this new technique will revise the redox transcriptome, given the heterogeneity of responses among different cells.
For proteomic and metabolomics studies, sample preparation often eliminates the ability to detect differences in protein or metabolite levels within various subcellular compartments. Attempts to overcome this hurdle have focused on subcellular fractionation of samples before analysis by using differential centrifugation (35). Recently, Chen et al. report an immunoprecipitation method to isolate intact mitochondria rapidly for metabolomics analyses (22). As instrument sensitivity continues to improve, single-cell and subcellular analyses will become more feasible.
Nevertheless, advances in the innovative omics technologies have resulted in progress in generating the redox transcriptome, proteome, and metabolome, and large-scale data are accumulating rapidly. How to integrate such heterogeneous and large-scale omics data to obtain a better understanding of the mechanisms underlying redox metabolism and their implications in human diseases is critical for the global analysis of redox biology. Systems-level approaches offered by systems biology are, in turn, indispensable for this purpose.
Systems-Level Approaches to Analyzing Redox Metabolism
The omics data generated by the technologies mentioned earlier provide quantitative information on redox metabolism for further modeling and analysis by systems biology approaches. Systems biology not only studies the functions and properties of biological components but also explores how they interact with each other in the system of interest. Systems biology has developed a wide spectrum of models and tools for analyzing large-scale data in redox metabolism.
Biological network analysis
Biological molecules do not function in isolation; rather, molecules and their dynamic interactions form different types of molecular networks that are indispensable for understanding the function and mechanisms of a complex biological system at a global level. In these networks, nodes represent biomolecules and edges denote certain types of interactions, for example, transcriptional regulation, microRNA–mRNA regulation, physical protein–protein interaction binding, and kinase–substrate interactions, among others (Fig. 5A). Network representation of interactome data not only simplifies the complexity of biological systems to focus on the molecules and their interactions, but also enables the use of various tools from network science and graph theory for systems-level analyses. For example, the antioxidant enzymes superoxide dismutase, Gpx, and catalase cooperate to defend against excessive ROS. The physical interactions among these antioxidant enzymes form a network (module) shown in Figure 5B. Network analysis can, then, provide an important approach in studying redox metabolism by exploring the static relationships among relevant molecular determinants of redox state and their dynamic consequences for redox state.
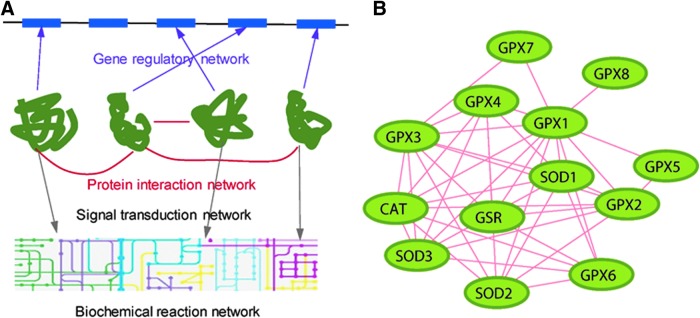
FIG. 5.
Molecular interaction networks. (A) Different types of molecular interaction networks. The interactions among different proteins and molecules across different levels in cellular systems form networks with characteristic topology: gene regulatory networks, protein–protein interactions, biochemical research networks, and signal transduction networks. (B) The protein–protein interaction network formed by antioxidant enzymes.
Mitochondria play an important role in numerous cellular processes including cell redox homeostasis. Mitochondria generate ROS that drive redox-sensitive signaling events and also respond to ROS-mediated alterations in cellular redox status. Protein interactions and complexes facilitate mitochondrial function, but the proteome and interactome in mitochondria are not well understood. In a recent study, Floyd et al. assessed condition-specific protein–protein interactions for 50 human mitochondrial proteins by using affinity-enrichment MS (40). This approach validated C17orf89 as a complex I assembly factor, discovered that LYRM5 deflavinates the electron-transferring flavoprotein that shuttles electrons to coenzyme Q (CoQ), and identified a CoQ biosynthetic complex and its components. In another study, Schweppe et al. used chemical cross-linking MS to identify 2427 cross-linked peptide pairs from 327 mitochondrial proteins in whole respiring murine mitochondria (128). This study provided evidence for the assembly of the Complex I–III respirasome and identified in situ interfacial regions of the complexes. The authors provide a database to allow observed cross-links to be utilized as molecular probes of mitochondrial protein–protein interactions for the study of in situ protein complex assembly. Thus, these mitochondrial interactomes enable novel insights into mitochondrial function and provide new avenues for studying mechanisms involved in redox metabolism.
The function of biological networks is closely related to their topological structures. Therefore, network and graph theoretical methods have been widely used for analyzing the topological features of biological networks and identifying important biomolecules. A path-finding approach incorporating reaction stoichiometry has been developed to detect carbon flux paths (CFPs) in metabolic networks (115). CFPs are essentially the shortest paths between a pair of source and target metabolites, but they satisfy two additional properties: (i) an ability to function in sustained steady-state conditions, and (ii) carbon exchange in each of its intermediate steps. This path-finding approach was achieved by integer linear programming based on the flux balance principle, and it has been used to predict key enzymes accounting for metabolite abundance alternations in disease phenotypes (116). Flux balance analysis is a widely used mathematical approach for analyzing the flow of metabolites in biochemical networks (Fig. 6A) (165). Elementary flux modes (EFMs) are non-decomposable steady-state metabolic pathways that are determined by flux balance analysis. The EFM approach has been applied to a metabolic network describing NAD biosynthesis and degradation to find the potential routes for NAD generation (33). With advances in the construction of human genome-scale metabolic network models, flux-based metabolic network modeling will become an essential tool to study redox metabolism and other aspects of metabolism.
FIG. 6.
Dynamic mathematical modeling approaches. (A) Continuous dynamic modeling. A set of ordinary differential equations can be formulated based on the biochemical reactions. A dynamic model based on kinetic analysis can then be used to output temporal quantitative concentration profiles. Alternatively, flux balance analysis of the steady-state condition based on optimization models can predict reaction flux profiles. (B) Discrete dynamic modeling; a Boolean network is an example of discrete dynamic models and consists of Boolean logical functions, network structure, and the state transition table.
Biological network inference
High-throughput techniques can generate abundant profiles of genes, proteins, and metabolites in a steady or transient state of the cell. These measurements reflect the quantitative activities of biological components in certain conditions, which are the results of the system-level regulation of biomolecules. Reverse engineering of biological networks, or biological network inference, aims at recovering the underlying regulatory relationships or signaling pathways by capturing the dependency of the abundance profiles of biomolecules through quantitative models. The Algorithm for the Reconstruction of Accurate Cellular Networks (ARACNE) is one gene regulatory network inference method based on the concept of mutual information theory that can eliminate the majority of indirect interactions inferred by co-expression methods (92). ARACNE defines an edge as an irreducible statistical dependency between gene expression profiles that cannot be explained as an artifact of other statistical dependencies in the network. Therefore, such irreducible statistical dependencies likely represent direct regulatory interactions between a transcription factor and a target gene. Context Likelihood of Relatedness (CLR), an extension of the relevance networks approach, also uses mutual information for scoring the similarity between the expression levels of two genes in a set of microarrays (15, 37).
Many diseases have been associated with oxidative stress, such as cardiovascular diseases, neurological diseases, and neoplasms. During oxidative stress, levels of ROS are elevated over significant periods, which trigger oxidative activation of a redox-sensitive gene regulatory network that is mediated by antioxidant transcription factors, such as NRF2. NRF2 regulates the transcription of a number of genes involved in response to oxidative stress, but the transcriptional regulatory network underlying the mechanisms in response to oxidative stress is far from complete. Systems biology provides data and tools for computationally predicting such a regulatory network. Based on microarray gene expression, ARACNE and CLR were used to capture dependencies in the gene expression profiles of the mouse lung from which the regulatory effect of NRF2 in response to oxidative stress was inferred (139). Machine-learning algorithms, such as the Support Vector Machine, have also been used to characterize the promoter sequences of NRF2 regulatory targets. These computational algorithms identified many known and novel targets of NRF2 transcriptional activation in the mouse lung under oxidative stress (139).
Recently, an NRF2-mediated redox-sensitive gene regulatory network was built from a systems perspective, which consisted of different functional modules: transducer (NRF2-regulated network), controller (antioxidant enzymes), actuator (GPX, SOD, CAT), and plant (redox state). This network can achieve specific performance objectives by utilizing nested feedback loops, feedforward control, and ultrasensitive signaling motifs (171). Such a system provides insights into the mechanisms underlying the interplay between adaptive antioxidant responses and ROS signaling. Gujar et al. applied ARACNE to the integration of the 169 RNA-Seq glioblastoma datasets from the Cancer Genome Atlas (TCGA) to build a transcriptional network and then construct NAMPT-regulated subnetworks (58). This network analysis identified transcription factor E2F2 as the center of a transcriptional hub in the NAD-dependent network. The network inference methods mentioned earlier can be applied to infer the regulatory networks of other redox-related transcription factors.
Dynamic mathematical modeling
Biological processes that drive the function of a biological system are condition-specific and highly dynamic. Dynamic mathematical modeling is, therefore, an important quantitative approach in systems biology for simulating the behaviors of the system and mimicking various perturbations to the system (103). These models determine how interacting elements achieve the temporal behavioral patterns of biological systems. Such simulations are very helpful for generating testable hypotheses and deriving mechanistic insights about the system. This class of methods usually originates with the quantitative activity data pertaining to biological components coupled with observational interaction networks assembled from individual small-scale experiments. Continuous dynamic modeling, usually realized by ordinary differential equations, can explain quantitative behaviors of a system (Fig. 6A); however, the construction of these models may be hampered by a lack of temporal quantitative data (e.g., the concentration or phosphorylation level of proteins at each time point) and sufficient in situ kinetic parameters, such as reaction rates, enzymatic capacity, and compartment information. When utilized, many of these parameters are necessarily based on determinations in simplified systems (i.e., biochemical assays of purified enzymes) rather than parameters determined in the most relevant biological context (i.e., whole cell or tissue enzyme activities).
Discrete dynamic modeling, such as Boolean network and Petri net approaches, offers two methods to overcome limitations related to parameter estimation. These simplify biological complexity, ignore quantitative kinetic parameters, and, thus, can make qualitative predictions of system behaviors (Fig. 6B) (152). Continuous modeling and discrete modeling complement each other. The choice between them depends on the availability of kinetic information and quantitative data, the size of the system, and the scientific question to be addressed.
Dynamic modeling has been used to investigate redox metabolism and oxidative stress. A Boolean dynamic network model integrating prior knowledge on oxidative stress response pathways has been developed to simulate the normal signaling present in the response mechanisms of oxidative stress and determine possible signaling alterations leading to disease states (134). Similarly, a Petri net-based model of the process of oxidative stress in atherosclerosis was developed to provide some testable biological predictions (41). Presnell et al. discussed mathematical models of GSH participation in oxidative stress constructed from mass balance principles and kinetic reactions (122). These models provide useful insights into the role of GSH as a key determinant of the cellular redox state and demonstrate that GSH decreases ROS levels and activates cellular oxidative stress responses by several mechanisms. GSH is also involved in inhibiting apoptosis and DNA damage in cells after oxidative stress and helps to maintain cellular sulfhydryl groups in their reduced form (122). A computational model accounting for the kinetics of GSH/Trx systems was developed to simulate the consequences of increases in H2O2 fluxes from mitochondria and to assess the relative contribution of the two major antioxidant systems in the mitochondrial matrix. The results show that GSH/Trx scavenging systems act in concert and are both essential for minimizing levels of H2O2 flux (4, 122).
A mathematical model of the redox regulatory network, including the pseudo-enzymatic oxidative turnover of protein thiols, the actions of redox enzymes, and the redox reactions of Trx and GSH, was originally developed and optimized for wild-type Jurkat cells (1, 78). This model can simulate the peroxide clearance dynamics associated with the remodeling of the redox network by targeted reduction of redox enzymes. The dynamic analysis indicates that perturbing Trx1, glutaredoxin 1 (Grx1), and G6PD have major consequences on antioxidant capacity. A detailed introduction of kinetic modeling to thiol/disulfide redox systems can be found in a recent review (76).
Kinetic modeling approaches have also been applied to understand the dynamics of the mitochondrial redox environment utilizing two-compartment models. Kembro et al. developed a two-compartment computational model incorporating the four main redox couples [NAD/NADH, NADP/NADPH, GSSG/GSH, and Trx(SS)/Trx(SH)2], ROS scavenging systems, and ROS (superoxide and H2O2) (75). The model successfully simulated the time course and magnitude of H2O2 production in response to the inhibition of ROS scavenging systems, as well as the effects of respiratory chain substrates and uncouplers on mitochondrial membrane potential and NADH concentrations compared with experimental determinations from isolated heart mitochondria. Similarly, Cortassa et al. utilized a two-compartment model to study the effects of long-chain fatty acid β-oxidation (29). Their model successfully simulated the relationship between oxygen consumption and H2O2 production as a function of lipid concentration as validated by experimental studies of isolated heart mitochondria from streptozotocin-treated guinea pigs and controls. This study demonstrated that high-dose palmitoyl-CoA uncouples mitochondrial respiration, thereby triggering excess generation of H2O2 while impairing antioxidant systems.
Applying Systems-Level Approaches to the Analysis of Stress and Disease States
Hypoxia and reductive stress
Inadequate oxygen supply, or hypoxia, is a valuable model for reductive redox stress that can provide important insights into the pathobiology of many other disease states, including cardiovascular disease, metabolic syndrome, and cancer, among others. Although substantial progress has been made in our understanding of cellular adaptations to hypoxia as described later, important questions remain, such as how cells dispose of excess electrons, how macromolecular biosynthesis for cell proliferation continues, and how the hypoxia program prepares cells for subsequent reoxygenation. Systems approaches to these questions allow the development of an integrated view of the hypoxia response and enable the discovery of novel adaptive mechanisms.
As the terminal electron acceptor in the electron transport chain, molecular oxygen plays a crucial role as an electron sink maintaining the forward flow of electrons through the respiratory complexes. In the absence of adequate oxygen, electron transport dysfunction ensues, resulting in decreased mitochondrial NAD/NADH, hyperpolarization of the mitochondrial membrane, uncoupled electron leak, and increased superoxide production (20, 77, 170). Glycolytic flux increases to meet cellular ATP demands, also resulting in decreases in cytoplasmic NAD/NADH. As NADH accumulates in cells, reductive enzymatic reactions, including those that are catalyzed by the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH), are inhibited (141).
To mitigate these adverse effects, metazoan species have developed a sophisticated response mechanism that is designed to mitigate reductive stress and to restore the NAD/NADH for bioenergetic and biosynthetic processes to continue. Low oxygen tension activates the heterodimeric nuclear factors, hypoxia inducible factors 1 and 2 (HIF1 and HIF2). The HIF transcriptional program increases the expression of glycolytic genes, including LDH A, enhances flux through the pentose phosphate pathway, inhibits pyruvate entry into the tricarboxylic acid cycle, and activates mitophagy (129). In addition, HIF-mediated expression of miR-210 decreases the expression of the iron-sulfur cluster assembly proteins ISCU1/2, thereby decreasing the activity of iron-sulfur containing enzymes such as aconitase in the tricarboxylic acid cycle and Complex I of the electron transport chain (19). Subunits of Complexes II and III also contain iron-sulfur clusters that may be affected by downregulation of ISCU1/2.
Transcriptional profiling has been invaluable in describing the full extent of the hypoxia transcriptional program. For example, pulmonary artery endothelial cells exposed to hypoxia upregulate a wide range of transcripts, including oxidoreductases, collagens and collagen biosynthetic enzymes, a variety of growth factors and cytokines, cell surface receptors and signal transduction intermediates, and a variety of transcription factors. Hypoxia primarily decreases the expression of proteins that are required for cell cycling and DNA replication (91). Notably, the functional role of many of these proteins implicated in the adaptive response to hypoxia is unknown.
Given the role of HIFs as the master regulators of the metabolic response to hypoxia, much of the research on the metabolic adaptations to hypoxia has centered around these transcription factors, their direct targets, and their impact on major bioenergetic metabolic pathways, such as glycolysis and the tricarboxylic acid cycle. To identify novel pathways associated with the metabolic response to hypoxia, we performed metabolomic profiling of cultured pulmonary vascular cells exposed to hypoxia (112). Using metabolite set enrichment and pathway analyses (158), we identified wide-ranging effects on all major bioenergetic and biosynthetic metabolic pathways, as well as on pathways involved in cellular redox homeostasis.
The tricarboxylic acid cycle was among the most strongly modulated pathways in hypoxia, where the levels of most intermediates decreased in hypoxia with the notable exception of 2-oxoglutarate (2OG). Interestingly, we and others identified related cellular increases specifically in the S-stereo-isomer L-2-hydroxyglutarate (L2HG) (67, 112). 2OG and L2HG form a redox couple, and the 2OG/L2HG ratio decreases in hypoxia, consistent with the overall reducing environment. 2OG is reduced to L2HG by malate dehydrogenase and LDH (61, 105, 112, 125). This interconversion may provide another mechanism for cells to transfer reducing equivalents from the NADH pool, which has broad metabolic effects due to the potential feedback inhibition of ∼75 enzymes (120), to the L2HG pool, which is a substrate for comparatively fewer (∼5) enzymes. Importantly, L2HG can be secreted from cells to clear reducing equivalents. Interestingly, we observed that L2HG accumulation slowed carbon flux through glycolysis as measured by extracellular acidification rate, suggesting a broader role of this metabolite in coordinating the response to reductive stress (112). Moreover, this example illustrates the value of a systems-based approach to the investigation of metabolic changes as it is unlikely that hypoxia-mediated increases in L2HG would have been identified through another approach.
The biological link between the hypoxia transcriptional program and myocardial ischemia, as well as other cardiovascular diseases, was investigated by us using a systems-based approach mapping hypoxia genes and cardiovascular disease genes onto the human interactome (151). Notably, hypoxia genes localize near cardiovascular disease genes in the human interactome and play significant roles linking network modules associated with different cardiovascular diseases (e.g., myocardial ischemia, cerebrovascular disease, and pulmonary hypertension). Moreover, this approach identified several hypoxia genes that may make novel contributions to the pathobiology of cardiovascular disease.
Cancer metabolism
In 1924, Otto Warburg described increase glucose uptake and lactate fermentation in normoxic cancer cells compared with normal tissues (153, 154). This so-called “Warburg effect” has since been attributed to abnormal aerobic stabilization of HIF1α in malignant cells and has since been recognized as a normal finding in other proliferating cells (144). Inhibition of LDH in the setting of Warburg metabolism increases ROS generation and inhibits cell proliferation (82). Presumably LDH inhibition in hypoxia would have similar consequences, although this has not been tested directly. Although less efficient for ATP production, Warburg metabolism provides carbon units and electrons for macromolecular biosynthesis required for cellular synthesis of nucleic acid, protein, and lipid species for cell division (144). As in hypoxia, this metabolic program is associated with a decrease in NAD/NADH, suggesting that increasing the NAD/NADH ratio may have therapeutic benefit in the treatment of cancer.
SoNar, a genetically encoded fluorescent biosensor of the NAD/NADH ratio, was used in a high-throughput screening assay to identify compounds that increased the NAD/NADH ratio in H1299 lung adenocarcinoma cells (173). Treating mice implanted with H1299 xenografts with one of the compounds identified in the screen, KP372-1, induced NADH oxidation and impaired tumor growth in vivo, in support of this hypothesis. How cancer cells thrive under reducing conditions that cause substantial damage in other settings (e.g., ischemic myocardium or pancreatic islet cells) remains of critical interest to the oncology community as a source of potential therapeutic targets. Indeed, KP372-1 has little adverse effects on primary cells culture or on treated mice, indicating that tumor cells have adapted to the reducing stress associated with Warburg metabolism.
Increased glucose uptake with secretion of lactate under aerobic conditions (i.e., the Warburg effect) and high glutamine uptake to sustain cell growth and proliferation are two principal metabolic mechanisms of current interest in oncology research (145). However, the factors underlying integration of dysfunctional cellular metabolism in cancer, particularly with respect to redox signaling and energy utilization, remain unclear. Zielinski et al. recently addressed this issue by performing a flux balance analysis to calculate metabolic flux states across a panel of 60 cancer cell lines (176). They observed that biosynthetic demand is the chief driver of metabolic phenotype. However, this group also observed that unprovoked elevation in glucose and glutamine uptake is a key feature of cancer cell metabolism that is associated with drug resistance and redox excess. This logic has been previously shown in studies in which enhanced glutamine-derived glutamate upregulates anti-oxidants, such as the enzymes G6PD and GSH, which, in turn, is associated with chemotherapeutic resistance (16, 17).
Dysregulated redox metabolism is also a pathogenic mechanism linking bacterial or viral infection with the development of various cancers, such as HCC or lymphoma from hepatitis B and C (68, 113). In one cross-sectional study of 40 untreated HCV patients, mitochondrial DNA (mtDNA) levels were decreased by 19% in liver tissue compared with controls, which parallels findings from experimental models of HCV indicating that infection disrupts mitochondrial respiration and the antioxidant enzyme heme oxygenase-1 to promote ROS accumulation in hepatocytes (43). Specifically, the HCV core proteins E1 and E2, among other proteins (NSR3, NS4B, NS5A) (53), impairs Complex I function to promote decreased NADPH content and an increase in the GSSG/GSH ratio (81). Other triggers of oxidant stress under these conditions include upregulation of pro-inflammatory cytokines by HCV, such as TNF-α and IL-6, which stimulates NOX-dependent O2•− and, in the case of NOX4, two-electron transfer to generate H2O2 (directly) from molecular oxygen (26).
Increased oxidant stress induced by HCV may convert liver tissue from a normal to oncogenic pathophenotype through various mechanisms. As discussed earlier for redox metabolism in cancer cells in general, increased ROS may affect cell survival pathways by altering the genetic code directly via double-stranded DNA breaks, inhibiting DNA repair mechanisms, or inducing proto-oncogene mutations, including p53 and VHL (27). The redox mechanisms by which to account for this effect are not fully resolved; however, inhibition of HCV-induced inducible NO synthase activity prevents p53/VHL mutagenesis, and suggests that oxidation of NO may be responsible for the oncogenic phenotype switch of HCV-infected liver cells in vitro (89). More recently, a lipidomic approach was used to discover an inverse association between levels of the mitochondrial lipid electrophile cardiolipin and HCC tumor progression. As a consequence of mitochondrial cardiolipin peroxidation, a decrease in bioactivity of the lipid electrophile 4-hydroxy-nonenal (4-HNE), usually pro-apoptotic (73), was observed compared with normal tissue, which was associated with apoptosis resistance in cancer cells (174).
Toxin and nutritional regulation of the thiol proteome
The interaction between environmental or nutritional factors and thiol-based signaling mechanisms is an important contributor to the pathogenesis of human disease (72). Take, for example, the consequences of cadmium toxicity that may occur due to dietary consumption or, more commonly, through tobacco use (3), and its association with hepatic, renal, and pulmonary injury (52). Indeed, the inflammatory effects of cadmium at low concentrations perturb the redox status of the cysteine proteome in murine hepatocytes, which stimulates translocation of Trx1 to the cell nucleus and dysregulates the organization of actin (51). Recently, Go et al. used isotope coded affinity tag-MS to determine that 46% of Cys-proteins in liver mitochondria were oxidized by treatment with cadmium by >1.5-fold compared with control (52). Further bioinformatics analysis indicated that Cys-regulating branched chain amino acids and fatty acid metabolism were principally affected, particularly carnitine metabolites, thereby providing important mechanistic insight into cadmium-induced hepatotoxicity.
Dietary intake may also influence the redox potential of cells directionally, which in the digestive system has particular functionality. For example, extracellular GSH in the stomach and intestinal lumen is an important defense mechanism against gastric acid and dietary toxins (101), respectively, and is synthesized primarily from foodstuffs such as asparagus, avocado, and other green leafy vegetables, as well as nuts and some red meats (71). The redox potential varies by tissue type and within cell types across the digestive tract, however, which is due, in part, to differences in the ratio of GSSG/GSH and its influences on cell function (109, 114). The GSH content of the intestinal lumen is regulated by biliary flow, and has numerous protective functions against free radical formation induced by the immune response to xenobiotics (119), as well as detoxification of peroxidized lipids and hydroperoxide elimination (6).
Dietary contributors to the thiol pool in plasma are determined by the intake of sulfur-containing amino acids directly, and the bioavailability of amino acids, nutrients, and vitamins that are antioxidant enzyme cofactors, such as glutamine, selenium, niacin, or flavin (131). Selenium, in particular, is a key micronutrient that is important for a wide range of biological processes in humans, including favorable effects on thyroid metabolism, thrombosis and immunogenicity via deiodination, anti-oxidant effects on suppressed platelet activation, and facilitation of the oxidative burst in phagocytic cells (65). Selenium is a constituent of several redox-associated proteins, including GPx1 through GPx6, Trx reductase, and the deiodinases. Acquired (nutritional) selenium deficiency is uncommon, but it increases the risk for specific cardiovascular diseases including Keshan diseases (24). In this form of cardiomyopathy, selenium deficiency augments the pro-oxidative environment of CM after infection from the Coxsackie virus, which, in turn, induces a mutation in the virus to a more virulent form (85). By contrast, reports in the late 1980s showed that plasma selenium levels in Dechang County, in which the Keshan prevalence was 20–40 cases/100,000 population, were 3.1-fold lower than Mianning and 9.1-fold lower than Beijing, and were directionally similar to plasma or Gpx activity (159).
Hyperhomocysteinemia is another clinically relevant, thiol-based disorder that may be precipitated by nutritional deficiency. Homocysteine is a sulfhydryl-containing amino acid synthesized from the metabolism of methionine in a two-step reaction involving S-adenosylmethionine and S-adenosylhomocysteine. Conversion of homocysteine to cystathionine requires vitamin-B6 as a cofactor for cystathionine β-synthase, or remethylation of homocysteine to methionine in a reaction that requires vitamin B12 and folate (94). When added to plasma, homocysteine may auto-oxidize to form homocystine, homocysteine-mixed disulfides, and homocysteine thiolactone. Oxidation of the sulfhydryl group promotes the formation of superoxide anion (O2−), hydroxyl radical (OH•), and H2O2 that initiates lipid peroxidation, decreases NO bioavailability, and induces endothelial dysfunction, atherothrombosis, and end-clinical cardiovascular pathophenotypes, such as myocardial infarction, stroke, and peripheral arterial disease (86).
Despite these sound mechanistic data and wide-ranging clinical reports indicating a positive association between increased plasma homocysteine and incident coronary heart or venothromboembolic disease (62) in at-risk populations, multi-center clinical trials have not shown a decisive benefit of B-vitamin supplementation-induced homocysteine lowering on clinical outcome. These findings have raised speculation that intermediates involved in homocysteine metabolism, rather than homocysteine per se, may be important in modulating the clinical risk observed in epidemiological studies in association with hyperhomocysteinemia. Our group's recent work demonstrating the role of S-adenosylhomocysteine in altering redox signaling and endothelial function supports this view (10). In addition, folic acid and other B-vitamins exert pro-proliferative effects, and increase levels of asymmetrical dimethylarginine (ADMA), which, in turn, is associated with a decrease in bioavailable NO in vascular endothelial cells (13).
These observations underscore the importance of systems-based methods that can account for multiple effects simultaneously (93). Methods aimed at addressing this issue in homocysteine biology are emerging. Pey et al. utilized a network stoichiometry method based on CFPs to stratify the importance of enzymes that may ultimately be responsible for homocysteine accumulation (115). This method, as described in detail earlier in this review, permits an in silico analysis of flux between reactants and products across a group of enzymes for a particular pathway. Their findings identify phosphatidylethanolamine N-methyltransferase, S-adenosyl-L-methionine (reversible) transport (mitochondrial), phosphatidylserine decarboxylase, and phosphatidylserine flippase as the top-ranked reactions underlying homocysteine accumulation (116).
A similar approach has also been used to clarify enzymatic control of important thiol-containing proteins regulated by mitochondrial oxidant stress. In particular, a systems pharmacology approach has recently been reported to clarify the key enzymes that regulate bioactivity of soluble guanylyl cyclase (sGC), which is a heterodimeric enzyme stimulated by NO• that catalyzes the conversion of guanosine-5′-triphosphate (GTP) to cyclic guanosine 3′,5′-monophosphate (cGMP) to promote blood vessel relaxation and inhibit platelet activation (31, 96). Impaired NO-sGC signaling, which may occur due to oxidation of α1- or β2-subunit thiol(s) (39, 96), is implicated in the pathogenesis of highly morbid clinical diseases, including myocardial infarction, stroke, and heart failure; however, the optimal targets within this pathway for pharmacotherapeutic development remain unresolved. Our group recently studied 12 molecular species and 13 rate constants for reactions bearing on the NO-sGC pathway to analyze the effect of oxidation on single reactions, reaction pairs, or reaction trios in pulmonary artery smooth muscle cells. From this approach, sGC oxidation, NO dissociation from sGC, and phosphodiesterase-mediated hydrolysis of cGMP were identified as the principal enzymatic regulators of NO bioactivity in vitro (45). These data taken together with systems data on homocysteine illustrate the importance of unbiased methods for shedding new light on the mechanisms that directly and indirectly regulate key intermediates involved in the development of cardiopulmonary disease, and they set the stage for a systems pharmacology approach to rational polypharmacy (43).
Identifying protein thiols as treatment targets
Fragment-based ligand discovery was used to study the interplay between functionality and druggability of cysteines within the mammalian proteome. Coupling this technology with an innovative method, isotopic tandem orthogonal proteolysis-activity-based protein profiling (149), allows large-scale quantitative analysis of cysteine-reactive small-molecule fragments in proteins. Briefly, cells are pre-treated with a vehicle control or an electrophilic small-molecule fragment (e.g., chloroacetamide or acrylamide) and then exposed to iodoacetamide-alkyne 1, which is a broad-spectrum cysteine-reactive probe. Tagged proteins are then conjugated with a heavy or light azide-biotin tag, and LC-MS is used to analyze the ratio of heavy:light fragments. Liganded cysteines are inferred by increased reduction in alkyne labeling. Backus and colleagues coupled this sophisticated approach with computational modeling of covalent docking to characterize the relationship between ligandable and druggable cysteines in MDA-MB-231 breast cancer cells (6a). Their findings suggest that key intermediates that regulate mitochondrial metabolism, particularly caspase-8 and caspase-10, are amenable to drug compounds targeting interactions in disease-relevant biological systems. These data may have important implications for pharmacotherapeutics that inhibit dysregulated caspase-8 signaling, such as for Barth syndrome (54) and other inborn errors of metabolism that are characterized by similar pathobiology (2).
Conclusions
Redox metabolism involves a complex series of reactions in which oxygen radicals influence the utilization of molecular oxygen and affect ATP synthesis. Disruptions to the normally reductive potential of the cell under conditions of increased oxidant stress exert important changes to the homeostatic equilibrium of redox metabolism, which, in turn, has important ramifications on health and human disease. Understanding the scope, scale, and complexity of effectors and targets of redox metabolism is beyond the framework of reductionist methodologies. To this end, systems-level models that permit interpretation of big data, particularly those that account for multiple interactions between metabolic intermediates, are increasingly utilized to unravel the molecular underpinnings of important diseases associated with ROS accumulation, mitochondrial dysfunction, and thiol oxidation. However, much opportunity remains for systems biology to clarify the mechanisms by which hypoxia and oxidant stress, in particular, promote diseases of neurodegeneration, cardiovascular dysfunction, and cancer, as well factors that are protective against these and other disorders.
Abbreviations Used
- 2OG
2-oxoglutarate
- ARACNE
Algorithm for the Reconstruction of Accurate Cellular Networks
- ATP
adenosine triphosphate
- CFP
carbon flux path
- cGMP
cyclic guanosine 3′,5′-monophosphate
- ChIP
chromatin immunoprecipitation
- CLR
Context Likelihood of Relatedness
- CM
cardiomyocytes
- CoQ
coenzyme Q
- EFM
elementary flux mode
- ETB
endothelin-B
- Gpx
glutathione peroxidase
- GSH
glutathione
- GSSG
glutathione disulfide
- GVHD
graft-versus host disease
- H2O2
hydrogen peroxide
- HCC
hepatocellular carcinoma
- HIF
hypoxia inducible factor
- L2HG
L-2-hydroxyglutarate
- LC
liquid chromatography
- LDH
lactate dehydrogenase
- mRNA
messenger RNA
- MS
mass spectrometry
- NAMPT
nicotinamide phosphoribosyltransferase
- NIH
National Institutes of Health
- NMR
nuclear magnetic resonance
- NO
nitric oxide
- ONOO−
peroxynitrite
- OxICAT
oxidative isotope coded affinity tag
- PDI
protein disulfide isomerase
- Prx
peroxiredoxin
- ROS
reactive oxygen species
- sGC
soluble guanylyl cyclase
- Trx
thioredoxin
Acknowledgments
The authors wish to thank Stephanie Tribuna for expert technical assistance. B.A.M.: (National Institutes of Health [NIH]) 1K08HL11207-01A1, American Heart Association (AHA 15GRNT25080016), Pulmonary Hypertension Association, Cardiovascular Medical Research and Education Fund (CMREF), and Klarman Foundation at Brigham and Women's Hospital. W.M.O.: (NIH) 1K08HL128802-01A1, Pulmonary Hypertension Association, American Thoracic Society Foundation, Inc., and the American Lung Association. J.L.: (NIH) R37HL61795 and GM107618. Research reported in this article was supported by the NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Director under Award Number U01 HG007690 (to J.L.). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH.
References
- 1.Adimora NJ, Jones DP, and Kemp ML. A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses. Antioxid Redox Signal 13: 731–743, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja S, Knudsen L, Chillappagari S, Henneke I, Ruppert C, Korfei M, Gochuico BR, Bellusci S, Seeger W, Ochs M, Guenther A, and Mahavadi P. MAP1LC3B overexpression protects against Hermansky-Pudlak syndrome type-1-induced defective autophagy in vitro. Am J Physiol Lung Cell Mol Physiol 310: L519–L531, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alloway BJ, Jackson AP, and Morgan H. The accumulation of cadmium by vegetables grown on soils contaminated from a variety of sources. Sci Total Environ 91: 223–236, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Aon MA, Stanley BA, Sivakumaran V, Kembro JM, O'Rourke B, Paolocci N, and Cortassa S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. J Gen Physiol 139: 479–491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrigo AP. Gene expression and the thiol redox state. Free Radic Biol Med 27: 936–944, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Aw TY. Biliary glutathione promotes the mucosal metabolism of luminal peroxidized lipids by rat small intestine in vivo. J Clin Invest 94: 1218–1225, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Backus KM, Correia BE, Lum KM, Forli S, Horning BD, Gonzalez-Paez GE, Chatterjee S, Lanning BR, Teijaro JR, Olson AJ, Wolan DW, and Cravatt BF. Proteome-wide covalent ligand discovery in biological systems. Nature 534: 570–574, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bak DW. and Weerapana E. Cysteine-mediated redox signalling in the mitochondria. Mol Biosyst 11: 678–697, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Barabasi AL. and Oltvai ZN. Network biology: understanding the cell's functional organization. Nat Rev Genet 5: 101–113, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, and Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 38: 6699–6705, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Barroso M, Kao D, Blom HJ, Tavares de Almeida I, Castro R, Loscalzo J, and Handy DE. S-adenosylhomocysteine induces inflammation through NFkB: a possible role for EZH2 in endothelial cell activation. Biochim Biophys Acta 1862: 82–92, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilan DS. and Belousov VV. Genetically encoded probes for NAD+/NADH monitoring. Free Radic Biol Med 100: 32–42, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Bilan DS, Matlashov ME, Gorokhovatsky AY, Schultz C, Enikolopov G, and Belousov VV. Genetically encoded fluorescent indicator for imaging NAD(+)/NADH ratio changes in different cellular compartments. Biochim Biophys Acta 1840: 951–957, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, and Bode-Boger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res 87: 99–105, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Buettner GR, Wagner BA, and Rodgers VG. Quantitative redox biology: an approach to understand the role of reactive species in defining the cellular redox environment. Cell Biochem Biophys 67: 477–483, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butte AJ, Tamayo P, Slonim D, Golub TR, and Kohane IS. Discovering functional relationships between RNA expression and chemotherapeutic susceptibility using relevance networks. Proc Natl Acad Sci U S A 97: 12182–12186, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byun S, Kim S, Choi H, Lee C, and Lee E. Augmentation of cisplatin sensitivity in cisplatin-resistant human bladder cancer cells by modulating glutathione concentrations and glutathione-related enzyme activities. BJU Int 95: 1086–1090, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Catanzaro D, Gaude E, Orso G, Giordano C, Guzzo G, Rasola A, Ragazzi E, Caparrotta L, Frezza C, and Montopoli M. Inhibition of glucose-6-phosphate dehydrogenase sensitizes cisplatin-resistant cells to death. Oncotarget 6: 30102–30114, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chae HZ, Chung SJ, and Rhee SG. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem 269: 27670–27678, 1994 [PubMed] [Google Scholar]
- 19.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, and Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab 10: 273–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, and Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 95: 11715–11720, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Cao G, Chen DQ, Wang M, Vaziri ND, Zhang ZH, Mao JR, Bai X, and Zhao YY. Metabolomics insights into activated redox signaling and lipid metabolism dysfunction in chronic kidney disease progression. Redox Biol 10: 168–178, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen WW, Freinkman E, Wang T, Birsoy K, and Sabatini DM. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166: 1324–1337.e11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.This reference has been deleted
- 24.Cheng Y. Epidemiologic studies on Keshan disease in Sichuan Province (in Chinese). In: Yu W, ed. The Study on Prevention and Treatment for Keshan Disease in China. Beijing: Publishing House of Chinese Environmental Science, 1987, p. 81 [Google Scholar]
- 25.Chio IIC, Jafarnejad SM, Ponz-Sarvise M, Park Y, Rivera K, Palm W, Wilson J, Sangar V, Hao Y, Ohlund D, Wright K, Filippini D, Lee EJ, Da Silva B, Schoepfer C, Wilkinson JE, Buscaglia JM, DeNicola GM, Tiriac H, Hammell M, Crawford HC, Schmidt EE, Thompson CB, Pappin DJ, Sonenberg N, and Tuveson DA. NRF2 promotes tumor maintenance by modulating mRNA translation in pancreatic cancer. Cell 166: 963–976, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi J, Corder NL, Koduru B, and Wang Y. Oxidative stress and hepatic Nox proteins in chronic hepatitis C and hepatocellular carcinoma. Free Radic Biol Med 72: 267–284, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J. and Ou JH. Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am J Physiol Gastrointest Liver Physiol 290: G847–G851, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, Xue P, Pi J, Kleeberger SR, and Bell DA. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res 40: 7416–7429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortassa S, Sollott SJ, and Aon MA. Mitochondrial respiration and ROS emission during beta-oxidation in the heart: an experimental-computational study. PLoS Comput Biol 13: e1005588, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Autreaux B. and Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8: 813–824, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Daiber A, Di Lisa F, Oelze M, Kroller-Schon S, Steven S, Schulz E, and Munzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol 174: 1670–1689, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, and Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal 10: 445–473, 2008 [DOI] [PubMed] [Google Scholar]
- 33.de Figueiredo LF, Gossmann TI, Ziegler M, and Schuster S. Pathway analysis of NAD+ metabolism. Biochem J 439: 341–348, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Dorrestein PC. and Carroll KS. “Omics” of natural products and redox biology. Curr Opin Chem Biol 15: 3–4, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Drissi R, Dubois ML, and Boisvert FM. Proteomics methods for subcellular proteome analysis. FEBS J 280: 5626–5634, 2013 [DOI] [PubMed] [Google Scholar]
- 36.This reference has been deleted
- 37.Faith JJ, Hayete B, Thaden JT, Mogno I, Wierzbowski J, Cottarel G, Kasif S, Collins JJ, and Gardner TS. Large-scale mapping and validation of Escherichia coli transcriptional regulation from a compendium of expression profiles. PLoS Biol 5: e8, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, and Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510: 298–302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernhoff NB, Derbyshire ER, and Marletta MA. A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. Proc Natl Acad Sci U S A 106: 21602–21607, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Floyd BJ, Wilkerson EM, Veling MT, Minogue CE, Xia C, Beebe ET, Wrobel RL, Cho H, Kremer LS, Alston CL, Gromek KA, Dolan BK, Ulbrich A, Stefely JA, Bohl SL, Werner KM, Jochem A, Westphall MS, Rensvold JW, Taylor RW, Prokisch H, Kim JJ, Coon JJ, and Pagliarini DJ. Mitochondrial protein interaction mapping identifies regulators of respiratory chain function. Mol Cell 63: 621–632, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Formanowicz D, Kozak A, and Formanowicz P. A Petri net based model of oxidative stress in atherosclerosis. FCDS 37: 59–78, 2012 [Google Scholar]
- 42.Forrester MT, Thompson JW, Foster MW, Nogueira L, Moseley MA, and Stamler JS. Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat Biotechnol 27: 557–559, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujinaga H, Tsutsumi T, Yotsuyanagi H, Moriya K, and Koike K. Hepatocarcinogenesis in hepatitis C: HCV shrewdly exacerbates oxidative stress by modulating both production and scavenging of reactive oxygen species. Oncology 81 Suppl 1: 11–17, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Furey TS. ChIP-Seq and beyond: new and improved methodologies to detect and characterize protein-DNA interactions. Nat Rev Genet 13: 840–852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garmaroudi FS, Handy DE, Liu YY, and Loscalzo J. Systems pharmacology and rational polypharmacy: nitric oxide-cyclic GMP signaling pathway as an illustrative example and derivation of the general case. PLoS Comput Biol 12: e1004822, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giannakakis A, Zhang J, Jenjaroenpun P, Nama S, Zainolabidin N, Aau MY, Yarmishyn AA, Vaz C, Ivshina AV, Grinchuk OV, Voorhoeve M, Vardy LA, Sampath P, Kuznetsov VA, Kurochkin IV, and Guccione E. Contrasting expression patterns of coding and noncoding parts of the human genome upon oxidative stress. Sci Rep 5: 9737, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giustarini D, Colombo G, Garavaglia ML, Astori E, Portinaro NM, Reggiani F, Badalamenti S, Aloisi AM, Santucci A, Rossi R, Milzani A, and Dalle-Donne I. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic Biol Med 112: 360–375, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Go YM, Chandler JD, and Jones DP. The cysteine proteome. Free Radic Biol Med 84: 227–245, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Go YM. and Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta 1780: 1273–1290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Go YM. and Jones DP. Redox biology: interface of the exposome with the proteome, epigenome and genome. Redox Biol 2: 358–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Go YM, Orr M, and Jones DP. Increased nuclear thioredoxin-1 potentiates cadmium-induced cytotoxicity. Toxicol Sci 131: 84–94, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Go YM, Roede JR, Orr M, Liang Y, and Jones DP. Integrated redox proteomics and metabolomics of mitochondria to identify mechanisms of cd toxicity. Toxicol Sci 139: 59–73, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong G, Waris G, Tanveer R, and Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci U S A 98: 9599–9604, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonzalvez F, D'Aurelio M, Boutant M, Moustapha A, Puech JP, Landes T, Arnaune-Pelloquin L, Vial G, Taleux N, Slomianny C, Wanders RJ, Houtkooper RH, Bellenguer P, Moller IM, Gottlieb E, Vaz FM, Manfredi G, and Petit PX. Barth syndrome: cellular compensation of mitochondrial dysfunction and apoptosis inhibition due to changes in cardiolipin remodeling linked to tafazzin (TAZ) gene mutation. Biochim Biophys Acta 1832: 1194–1206, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Gould NS, Evans P, Martinez-Acedo P, Marino SM, Gladyshev VN, Carroll KS, and Ischiropoulos H. Site-specific proteomic mapping identifies selectively modified regulatory cysteine residues in functionally distinct protein networks. Chem Biol 22: 965–975, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grek CL. and Tew KD. Redox metabolism and malignancy. Curr Opin Pharmacol 10: 362–368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Griesser E, Vemula V, Raulien N, Wagner U, Reeg S, Grune T, and Fedorova M. Cross-talk between lipid and protein carbonylation in a dynamic cardiomyocyte model of mild nitroxidative stress. Redox Biol 11: 438–455, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gujar AD, Le S, Mao DD, Dadey DY, Turski A, Sasaki Y, Aum D, Luo J, Dahiya S, Yuan L, Rich KM, Milbrandt J, Hallahan DE, Yano H, Tran DD, and Kim AH. An NAD+-dependent transcriptional program governs self-renewal and radiation resistance in glioblastoma. Proc Natl Acad Sci U S A 113: E8247–E8256, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Handy DE. and Loscalzo J. Redox regulation of mitochondrial function. Antioxid Redox Signal 16: 1323–1367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansen RE, Roth D, and Winther JR. Quantifying the global cellular thiol-disulfide status. Proc Natl Acad Sci U S A 106: 422–427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris AL. A new hydroxy metabolite of 2-oxoglutarate regulates metabolism in hypoxia. Cell Metab 22: 198–200, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 288: 2015–2022, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Houessinon A, Francois C, Sauzay C, Louandre C, Mongelard G, Godin C, Bodeau S, Takahashi S, Saidak Z, Gutierrez L, Regimbeau JM, Barget N, Barbare JC, Ganne N, Chauffert B, Coriat R, and Galmiche A. Metallothionein-1 as a biomarker of altered redox metabolism in hepatocellular carcinoma cells exposed to sorafenib. Mol Cancer 15: 38, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang Y, Zhu M, Li Z, Sa R, Chu Q, Zhang Q, Zhang H, Tang W, Zhang M, and Yin H. Mass spectrometry-based metabolomic profiling identifies alterations in salivary redox status and fatty acid metabolism in response to inflammation and oxidative stress in periodontal disease. Free Radic Biol Med 70: 223–232, 2014 [DOI] [PubMed] [Google Scholar]
- 65.Huang Z, Rose AH, and Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16: 705–743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwang C, Sinskey AJ, and Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257: 1496–1502, 1992 [DOI] [PubMed] [Google Scholar]
- 67.Intlekofer AM, Dematteo RG, Venneti S, Finley LW, Lu C, Judkins AR, Rustenburg AS, Grinaway PB, Chodera JD, Cross JR, and Thompson CB. Hypoxia induces production of L-2-hydroxyglutarate. Cell Metab 22: 304–311, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ivanov AV, Valuev-Elliston VT, Tyurina DA, Ivanova ON, Kochetkov SN, Bartosch B, and Isaguliants MG. Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget 8: 3895–3932, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaffrey SR. and Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001: pl1, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Jevtovic-Todorovic V. and Guenthner TM. Depletion of a discrete nuclear glutathione pool by oxidative stress, but not by buthionine sulfoximine. Correlation with enhanced alkylating agent cytotoxicity to human melanoma cells in vitro. Biochem Pharmacol 44: 1383–1393, 1992 [DOI] [PubMed] [Google Scholar]
- 71.Jones DP, Coates RJ, Flagg EW, Eley JW, Block G, Greenberg RS, Gunter EW, and Jackson B. Glutathione in foods listed in the National Cancer Institute's Health Habits and History Food Frequency Questionnaire. Nutr Cancer 17: 57–75, 1992 [DOI] [PubMed] [Google Scholar]
- 72.Jozefczak M, Remans T, Vangronsveld J, and Cuypers A. Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13: 3145–3175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kagan VE, Tyurina YY, Tyurin VA, Mohammadyani D, Angeli JP, Baranov SV, Klein-Seetharaman J, Friedlander RM, Mallampalli RK, Conrad M, and Bayir H. Cardiolipin signaling mechanisms: collapse of asymmetry and oxidation. Antioxid Redox Signal 22: 1667–1680, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaludercic N, Deshwal S, and Di Lisa F. Reactive oxygen species and redox compartmentalization. Front Physiol 5: 285, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kembro JM, Aon MA, Winslow RL, O'Rourke B, and Cortassa S. Integrating mitochondrial energetics, redox and ROS metabolic networks: a two-compartment model. Biophys J 104: 332–343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kemp M, Go YM, and Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med 44: 921–937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim JW, Tchernyshyov I, Semenza GL, and Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Kippner LE, Finn NA, Shukla S, and Kemp ML. Systemic remodeling of the redox regulatory network due to RNAi perturbations of glutaredoxin 1, thioredoxin 1, and glucose-6-phosphate dehydrogenase. BMC Syst Biol 5: 164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, and Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med 27: 1208–1218, 1999 [DOI] [PubMed] [Google Scholar]
- 80.Kitano H. Computational systems biology. Nature 420: 206–210, 2002 [DOI] [PubMed] [Google Scholar]
- 81.Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, and Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem 280: 37481–37488, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, and Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A 107: 2037–2042, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lei S, Zavala-Flores L, Garcia-Garcia A, Nandakumar R, Huang Y, Madayiputhiya N, Stanton RC, Dodds ED, Powers R, and Franco R. Alterations in energy/redox metabolism induced by mitochondrial and environmental toxins: a specific role for glucose-6-phosphate-dehydrogenase and the pentose phosphate pathway in paraquat toxicity. ACS Chem Biol 9: 2032–2048, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, Walker AK, Strahler JR, Andrews PC, and Jakob U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A 105: 8197–8202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levander OA. and Beck MA. Selenium and viral virulence. Br Med Bull 55: 528–533, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest 98: 5–7, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu J. and Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med 66: 75–87, 2014 [DOI] [PubMed] [Google Scholar]
- 88.Ma Q. Transcriptional responses to oxidative stress: pathological and toxicological implications. Pharmacol Ther 125: 376–393, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Machida K, Cheng KT, Sung VM, Lee KJ, Levine AM, and Lai MM. Hepatitis C virus infection activates the immunologic (type II) isoform of nitric oxide synthase and thereby enhances DNA damage and mutations of cellular genes. J Virol 78: 8835–8843, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, and Biswal S. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 38: 5718–5734, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, and Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105: 659–669, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Margolin AA, Nemenman I, Basso K, Wiggins C, Stolovitzky G, Dalla Favera R, and Califano A. ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinformatics 7 Suppl 1: S7, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maron BA. and Leopold JA. Systems biology: an emerging strategy for discovering novel pathogenetic mechanisms that promote cardiovascular disease. Global Cardiol Sci Prac e201627, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maron BA. and Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med 60: 39–54, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maron BA, Tang SS, and Loscalzo J. S-nitrosothiols and the S-nitrosoproteome of the cardiovascular system. Antioxid Redox Signal 18: 270–287, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J, and Leopold JA. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem 284: 7665–7672, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, Loscalzo J, and Leopold JA. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation 126: 963–974, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meng G, Xiao Y, Ma Y, Tang X, Xie L, Liu J, Gu Y, Yu Y, Park CM, Xian M, Wang X, Ferro A, Wang R, Moore PK, Zhang Z, Wang H, Han Y, and Ji Y. Hydrogen sulfide regulates kruppel-like factor 5 transcription activity via specificity protein 1 S-sulfhydration at Cys664 to prevent myocardial hypertrophy. J Am Heart Assoc 5: e004160, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meredith MJ. and Reed DJ. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem 257: 3747–3753, 1982 [PubMed] [Google Scholar]
- 100.Mor-Cohen R. Disulfide bonds as regulators of integrin function in thrombosis and hemostasis. Antioxid Redox Signal 24: 16–31, 2016 [DOI] [PubMed] [Google Scholar]
- 101.Moriarty-Craige SE. and Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr 24: 481–509, 2004 [DOI] [PubMed] [Google Scholar]
- 102.Moris D, Spartalis M, Tzatzaki E, Spartalis E, Karachaliou GS, Triantafyllis AS, Karaolanis GI, Tsilimigras DI, and Theocharis S. The role of reactive oxygen species in myocardial redox signaling and regulation. Ann Transl Med 5: 324, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Motta S. and Pappalardo F. Mathematical modeling of biological systems. Brief Bioinform 14: 411–422, 2013 [DOI] [PubMed] [Google Scholar]
- 104.Murray CI. and Van Eyk JE. Chasing cysteine oxidative modifications: proteomic tools for characterizing cysteine redox status. Circ Cardiovasc Genet 5: 591, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nadtochiy SM, Schafer X, Fu D, Nehrke K, Munger J, and Brookes PS. Acidic pH is a metabolic switch for 2-hydroxyglutarate generation and signaling. J Biol Chem 291: 20188–20979, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nagana Gowda GA, Abell L, Lee CF, Tian R, and Raftery D. Simultaneous analysis of major coenzymes of cellular redox reactions and energy using ex vivo (1)H NMR spectroscopy. Anal Chem 88: 4817–4824, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakagawa T, Lomb DJ, Haigis MC, and Guarente L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137: 560–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Narayan M. Disulfide bonds: protein folding and subcellular protein trafficking. FEBS J 279: 2272–2282, 2012 [DOI] [PubMed] [Google Scholar]
- 109.Nkabyo YS, Ziegler TR, Gu LH, Watson WH, and Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am J Physiol Gastrointest Liver Physiol 283: G1352–G1359, 2002 [DOI] [PubMed] [Google Scholar]
- 110.Nulton-Persson AC, Starke DW, Mieyal JJ, and Szweda LI. Reversible inactivation of alpha-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry 42: 4235–4242, 2003 [DOI] [PubMed] [Google Scholar]
- 111.Okamoto Y, Ninomiya H, Tanioka M, Sakamoto A, Miwa S, and Masaki T. Palmitoylation of human endothelinB. Its critical role in G protein coupling and a differential requirement for the cytoplasmic tail by G protein subtypes. J Biol Chem 272: 21589–21596, 1997 [DOI] [PubMed] [Google Scholar]
- 112.Oldham WM, Clish CB, Yang Y, and Loscalzo J. Hypoxia-mediated increases in L-2-hydroxyglutarate coordinate the metabolic response to reductive stress. Cell Metab 22: 291–303, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Omland LH, Jepsen P, Krarup H, Christensen PB, Weis N, Nielsen L, Obel N, Sorensen HT, and Stuver SO; DANVIR cohort study. Liver cancer and non-Hodgkin lymphoma in hepatitis C virus-infected patients: results from the DANVIR cohort study. Int J Cancer 130: 2310–2317, 2012 [DOI] [PubMed] [Google Scholar]
- 114.Perez S, Talens-Visconti R, Rius-Perez S, Finamor I, and Sastre J. Redox signaling in the gastrointestinal tract. Free Radic Biol Med 104: 75–103, 2017 [DOI] [PubMed] [Google Scholar]
- 115.Pey J, Prada J, Beasley JE, and Planes FJ. Path finding methods accounting for stoichiometry in metabolic networks. Genome Biol 12: R49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pey J, Tobalina L, de Cisneros JP, and Planes FJ. A network-based approach for predicting key enzymes explaining metabolite abundance alterations in a disease phenotype. BMC Syst Biol 7: 62, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pillay CS, Hofmeyr JH, Mashamaite LN, and Rohwer JM. From top-down to bottom-up: computational modeling approaches for cellular redoxin networks. Antioxid Redox Signal 18: 2075–2086, 2013 [DOI] [PubMed] [Google Scholar]
- 118.Pittelli M, Formentini L, Faraco G, Lapucci A, Rapizzi E, Cialdai F, Romano G, Moneti G, Moroni F, and Chiarugi A. Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J Biol Chem 285: 34106–34114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Plaa GL. and Witschi H. Chemicals, drugs, and lipid peroxidation. Annu Rev Pharmacol Toxicol 16: 125–141, 1976 [DOI] [PubMed] [Google Scholar]
- 120.Placzek S, Schomburg I, Chang A, Jeske L, Ulbrich M, Tillack J, and Schomburg D. BRENDA in 2017: new perspectives and new tools in BRENDA. Nucleic Acids Res 45: D380–D388, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Posakony JW, England JM, and Attardi G. Mitochondrial growth and division during the cell cycle in HeLa cells. J Cell Biol 74: 468–491, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Presnell CE, Bhatti G, Numan LS, Lerche M, Alkhateeb SK, Ghalib M, Shammaa M, and Kavdia M. Computational insights into the role of glutathione in oxidative stress. Curr Neurovasc Res 10: 185–194, 2013 [DOI] [PubMed] [Google Scholar]
- 123.Reczek CR. and Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol 33: 8–13, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rhee SG, Woo HA, Kil IS, and Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem 287: 4403–4410, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rzem R, Vincent MF, Van Schaftingen E, and Veiga-da-Cunha M. L-2-hydroxyglutaric aciduria, a defect of metabolite repair. J Inherit Metab Dis 30: 681–689, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Schafer FQ. and Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212, 2001 [DOI] [PubMed] [Google Scholar]
- 127.Schulz H. Regulation of fatty acid oxidation in heart. J Nutr 124: 165–171, 1994 [DOI] [PubMed] [Google Scholar]
- 128.Schweppe DK, Chavez JD, Lee CF, Caudal A, Kruse SE, Stuppard R, Marcinek DJ, Shadel GS, Tian R, and Bruce JE. Mitochondrial protein interactome elucidated by chemical cross-linking mass spectrometry. Proc Natl Acad Sci U S A 114: 1732–1737, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci 33: 207–214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shadel GS. and Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell 163: 560–569, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sinha I, Karagoz K, Fogle RL, Hollenbeak CS, Zea AH, Arga KY, Stanley AE, Hawkes WC, and Sinha R. “Omics” of selenium biology: a prospective study of plasma proteome network before and after selenized-yeast supplementation in healthy men. OMICS 20: 202–213, 2016 [DOI] [PubMed] [Google Scholar]
- 132.Sobie EA, Lee YS, Jenkins SL, and Iyengar R. Systems biology—biomedical modeling. Sci Signal 4: tr2, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spencer JP, Jenner P, Daniel SE, Lees AJ, Marsden DC, and Halliwell B. Conjugates of catecholamines with cysteine and GSH in Parkinson's disease: possible mechanisms of formation involving reactive oxygen species. J Neurochem 71: 2112–2122, 1998 [DOI] [PubMed] [Google Scholar]
- 134.Sridharan S, Layek R, Datta A, and Venkatraj J. Boolean modeling and fault diagnosis in oxidative stress response. BMC Genomics 13 Suppl 6: S4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stamler JS, Osborne JA, Jaraki O, Rabbani LE, Mullins M, Singel D, and Loscalzo J. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest 91: 308–318, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stubbs M, Veech RL, and Krebs HA. Control of the redox state of the nicotinamide-adenine dinucleotide couple in rat liver cytoplasm. Biochem J 126: 59–65, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suh JH, Kanathezhath B, Shenvi S, Guo H, Zhou A, Tiwana A, Kuypers F, Ames BN, and Walters MC. Thiol/redox metabolomic profiling implicates GSH dysregulation in early experimental graft versus host disease (GVHD). PLoS One 9: e88868, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tao G, Kahr PC, Morikawa Y, Zhang M, Rahmani M, Heallen TR, Li L, Sun Z, Olson EN, Amendt BA, and Martin JF. Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury. Nature 534: 119–123, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Taylor RC, Acquaah-Mensah G, Singhal M, Malhotra D, and Biswal S. Network inference algorithms elucidate Nrf2 regulation of mouse lung oxidative stress. PLoS Comput Biol 4: e1000166, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thamsen M. and Jakob U. The redoxome: proteomic analysis of cellular redox networks. Curr Opin Chem Biol 15: 113–119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tilton WM, Seaman C, Carriero D, and Piomelli S. Regulation of glycolysis in the erythrocyte: role of the lactate/pyruvate and NAD/NADH ratios. J Lab Clin Med 118: 146–152, 1991 [PubMed] [Google Scholar]
- 142.Tong Q, Zhu Y, Galaske JW, Kosmacek EA, Chatterjee A, Dickinson BC, and Oberley-Deegan RE. MnTE-2-PyP modulates thiol oxidation in a hydrogen peroxide-mediated manner in a human prostate cancer cell. Free Radic Biol Med 101: 32–43, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tsai CS, Burgett MW, and Reed LJ. Alpha-keto acid dehydrogenase complexes. XX. A kinetic study of the pyruvate dehydrogenase complex from bovine kidney. J Biol Chem 248: 8348–8352, 1973 [PubMed] [Google Scholar]
- 144.Vander Heiden MG, Cantley LC, and Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vander Heiden MG. and DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell 168: 657–669, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Veech RL, Eggleston LV, and Krebs HA. The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem J 115: 609–619, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Veech RL, Guynn R, and Veloso D. The time-course of the effects of ethanol on the redox and phosphorylation states of rat liver. Biochem J 127: 387–397, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Voet D. and Voet JG. Biochemistry, 4th ed. Hoboken, NJ: John Wiley & Sons, 2011 [Google Scholar]
- 149.Wang C, Weerapana E, Blewett MM, and Cravatt BF. A chemoproteomic platform to quantitatively map targets of lipid-derived electrophiles. Nat Methods 11: 79–85, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang RS, Maron BA, and Loscalzo J. Systems medicine: evolution of systems biology from bench to bedside. Wiley Interdiscip Rev Syst Biol Med 7: 141–161, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang RS, Oldham WM, and Loscalzo J. Network-based association of hypoxia-responsive genes with cardiovascular diseases. New J Phys 16: 105014, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang RS, Saadatpour A, and Albert R. Boolean modeling in systems biology: an overview of methodology and applications. Phys Biol 9: 055001, 2012 [DOI] [PubMed] [Google Scholar]
- 153.Warburg O. On respiratory impairment in cancer cells. Science 124: 269–270, 1956 [PubMed] [Google Scholar]
- 154.Warburg O. On the origin of cancer cells. Science 123: 309–314, 1956 [DOI] [PubMed] [Google Scholar]
- 155.Watanabe R, Nakamura H, Masutani H, and Yodoi J. Anti-oxidative, anti-cancer and anti-inflammatory actions by thioredoxin 1 and thioredoxin-binding protein-2. Pharmacol Ther 127: 261–270, 2010 [DOI] [PubMed] [Google Scholar]
- 156.Williamson DH, Lund P, and Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103: 514–527, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wobbe L, Blifernez O, Schwarz C, Mussgnug JH, Nickelsen J, and Kruse O. Cysteine modification of a specific repressor protein controls the translational status of nucleus-encoded LHCII mRNAs in Chlamydomonas. Proc Natl Acad Sci U S A 106: 13290–13295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xia J, Mandal R, Sinelnikov IV, Broadhurst D, and Wishart DS. MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Res 40: W127–W133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xia YM, Hill KE, and Burk RF. Biochemical studies of a selenium-deficient population in China: measurement of selenium, glutathione peroxidase and other oxidant defense indices in blood. J Nutr 119: 1318–1326, 1989 [DOI] [PubMed] [Google Scholar]
- 160.Yamada K, Hara N, Shibata T, Osago H, and Tsuchiya M. The simultaneous measurement of nicotinamide adenine dinucleotide and related compounds by liquid chromatography/electrospray ionization tandem mass spectrometry. Anal Biochem 352: 282–285, 2006 [DOI] [PubMed] [Google Scholar]
- 161.Yan B. and Smith JW. A redox site involved in integrin activation. J Biol Chem 275: 39964–39972, 2000 [DOI] [PubMed] [Google Scholar]
- 162.Yan B. and Smith JW. Mechanism of integrin activation by disulfide bond reduction. Biochemistry 40: 8861–8867, 2001 [DOI] [PubMed] [Google Scholar]
- 163.Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, and Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130: 1095–1107, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang Y, Song Y, and Loscalzo J. Regulation of the protein disulfide proteome by mitochondria in mammalian cells. Proc Natl Acad Sci U S A 104: 10813–10817, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yilmaz LS. and Walhout AJ. Metabolic network modeling with model organisms. Curr Opin Chem Biol 36: 32–39, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yin F, Sancheti H, and Cadenas E. Mitochondrial thiols in the regulation of cell death pathways. Antioxid Redox Signal 17: 1714–1727, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 10: 179–206, 2008 [DOI] [PubMed] [Google Scholar]
- 168.Yuan L. and Kaplowitz N. Glutathione in liver diseases and hepatotoxicity. Mol Aspects Med 30: 29–41, 2009 [DOI] [PubMed] [Google Scholar]
- 169.Zai A, Rudd MA, Scribner AW, and Loscalzo J. Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide. J Clin Invest 103: 393–399, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, and Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283: 10892–10903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 171.Zhang Q, Pi J, Woods CG, and Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol 244: 84–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang Q, Piston DW, and Goodman RH. Regulation of corepressor function by nuclear NADH. Science 295: 1895–1897, 2002 [DOI] [PubMed] [Google Scholar]
- 173.Zhao Y, Hu Q, Cheng F, Su N, Wang A, Zou Y, Hu H, Chen X, Zhou HM, Huang X, Yang K, Zhu Q, Wang X, Yi J, Zhu L, Qian X, Chen L, Tang Y, Loscalzo J, and Yang Y. SoNar, a highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents. Cell Metab 21: 777–789, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhong H, Xiao M, Zarkovic K, Zhu M, Sa R, Lu J, Tao Y, Chen Q, Xia L, Cheng S, Waeg G, Zarkovic N, and Yin H. Mitochondrial control of apoptosis through modulation of cardiolipin oxidation in hepatocellular carcinoma: a novel link between oxidative stress and cancer. Free Radic Biol Med 102: 67–76, 2017 [DOI] [PubMed] [Google Scholar]
- 175.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Broughton Pipkin F, and Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature 468: 108–111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zielinski DC, Jamshidi N, Corbett AJ, Bordbar A, Thomas A, and Palsson BO. Systems biology analysis of drivers underlying hallmarks of cancer cell metabolism. Sci Rep 7: 41241, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]