Abstract
Significance: Reductionist studies have contributed greatly to our understanding of the basic biology of aging in recent years but we still do not understand fundamental mechanisms for many identified drugs and pathways. Use of systems approaches will help us move forward in our understanding of aging.
Recent Advances: Recent work described here has illustrated the power of systems biology to inform our understanding of aging through the study of (i) diet restriction, (ii) neurodegenerative disease, and (iii) biomarkers of aging.
Critical Issues: Although we do not understand all of the individual genes and pathways that affect aging, as we continue to uncover more of them, we have now also begun to synthesize existing data using systems-level approaches, often to great effect. The three examples noted here all benefit from computational approaches that were unknown a few years ago, and from biological insights gleaned from multiple model systems, from aging laboratories as well as many other areas of biology.
Future Directions: Many new technologies, such as single-cell sequencing, advances in epigenetics beyond the methylome (specifically, assay for transposase-accessible chromatin with high throughput sequencing ), and multiomic network studies, will increase the reach of systems biologists. This suggests that approaches similar to those described here will continue to lead to striking findings, and to interventions that may allow us to delay some of the many age-associated diseases in humans; perhaps sooner that we expect. Antioxid. Redox Signal. 29, 973–984.
Keywords: : aging, systems biology, diet restriction, neurodegenerative disease, Alzheimer's, biomarkers
Introduction
Aging is the single greatest risk factor for much of the mortality and morbidity in the developed world, leading to dramatic increases in rates of cancer, cardiovascular disease, and neurodegenerative disease (12, 13, 49). As our global population structure becomes more aged, the cost of these diseases, in human suffering and in economic terms, will continue to increase (86). It has been pointed out that work to slow aging itself has the potential to simultaneously delay all of these diseases (51, 55).
In recent decades, work in laboratory-based genetic model organisms—the nematode Caenorhabditis elegans, the budding yeast Saccharomyces cerevisiae, the fruit fly Drosophila melanogaster, and the mouse Mus spp.—has demonstrated very large changes in lifespan from single mutations, or from combinations of small number of different mutations. This work illustrates the extreme plasticity of this phenotype in the laboratory in response to genetic alterations (3, 54, 56, 60, 76, 95, 101). Interestingly, in some cases, mutations of the same gene in all these species have extended lifespan, not only in invertebrate models but also in mammals (9, 39, 67, 68), suggesting conservation of phenotype over many hundreds of millions of years.
Obviously, if any of these interventions were shown to have a beneficial impact on human healthspan, the biological and social impact would be considerable. In fact, some pharmacological interventions are now moving into the testing phase in companion dogs (50, 104) and humans (7, 73).
The very nature of aging lends itself to a systems biology approach. Perhaps more than just about any other biological phenomenon, aging is notable for its diversity. Despite some common and evolutionarily conserved hallmarks of aging (72), we see tremendous variation in the consequences of aging, among tissues within a single individual, and among individuals and species. And one of the clear messages from the past 25 years of molecular studies of aging is that the molecular causes of aging are great in number and show highly complex interactions.
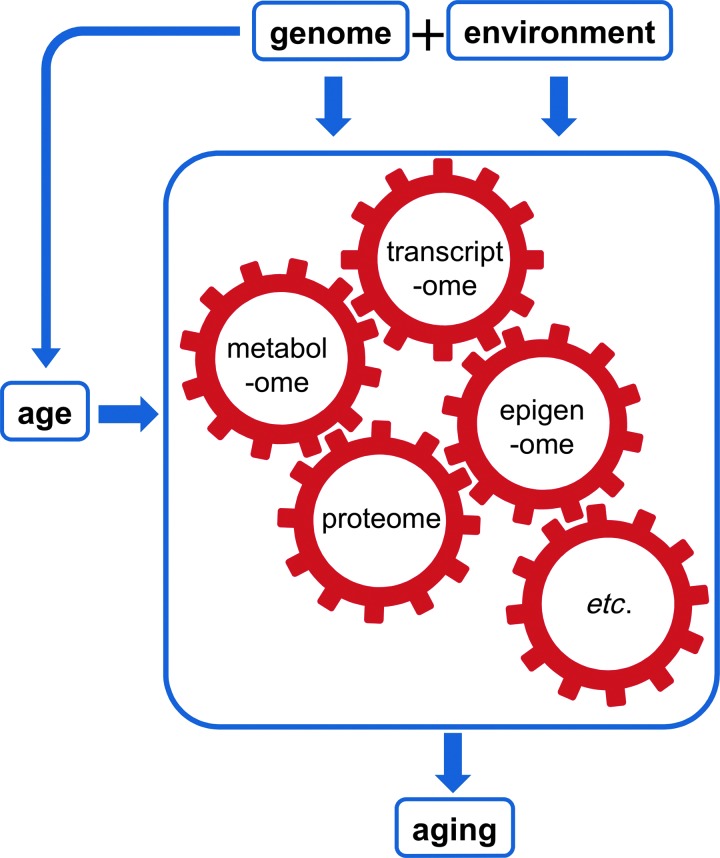
Our view of the causes and consequences of aging is illustrated in Figure 1. Some individuals are healthier agers than others. At a population level, variation in the risk of different age-related traits (the phenotypes, P) is shaped by variation in genes (G), the environment (E), and the interaction between those two factors, as well as age itself. We can illustrate this relationship using the classic quantitative genetic equation:
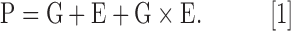 |
FIG. 1.
The standard quantitative genetics equation, P = G + E, illustrates that phenotypic variation is determined by genetic and environmental variance components. This simple equation belies a much more complex pipeline, whereby genetic and environmental factors influence high-dimensional and highly interactive “omic” endophenotypes, which then affect downstream aging phenotypes. Moreover, age itself also affects these endophenotypes, in ways partly modulated by genetic and environmental factors. E, environment; G, genes; P, phenotypes.
From an aging perspective, as we illustrate in Figure 1, we would add that age influences phenotypes, and does so in a way unique to each individual, determined by genetic and environmental factors. However, Equation [1] is agnostic about the mechanisms by which G and E shape P. We hypothesize that this gap between G and E and P can be bridged by omic analysis (in particular, the epigenome, transcriptome, proteome, metabolome, and microbiome). This conceptual model has, in turn, led us to recognize the power of a systems biology approach, one that embraces all domains, from genes and environment, to downstream aging-related phenotypes, and the intermediate omic levels that bridge the two.
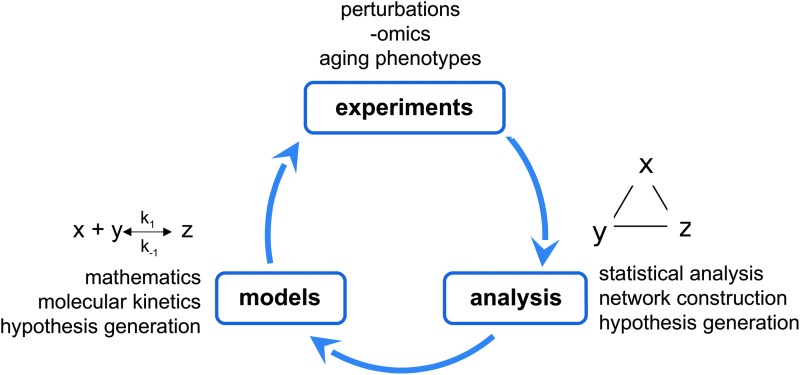
Here we explore ways that further progress in aging research can be made through systems biology. Systems biology, broadly, is defined by an integrated set of approaches that can include large-scale data sets, analysis at multiple levels of biological organization, computational models (often focused on construction and analysis of networks), and mathematical models. These approaches can be used in an attempt to integrate and connect findings from preceding reductionist work (40), as well as to generate novel mechanistic hypotheses in an iterative process (Fig. 2).
FIG. 2.
Schematic of systems biology pipeline for aging research.
The tools applied in this endeavor range from mathematical approaches with a long history of success in other branches of science, now being applied to biology (1, 38, 110), to computational approaches that rely on relatively recent advances in computing power (36), among others. As aging, or any biological question, becomes more intensively studied, the number of interesting and potentially interconnected findings that may be incorporated into systems-level analyses increases. We may also find an increase in the number of questions that have proven difficult to answer using other, more reductionist, approaches. Aging research may now have reached a stage where it is very well positioned for fruitful application of systems biology techniques and approaches.
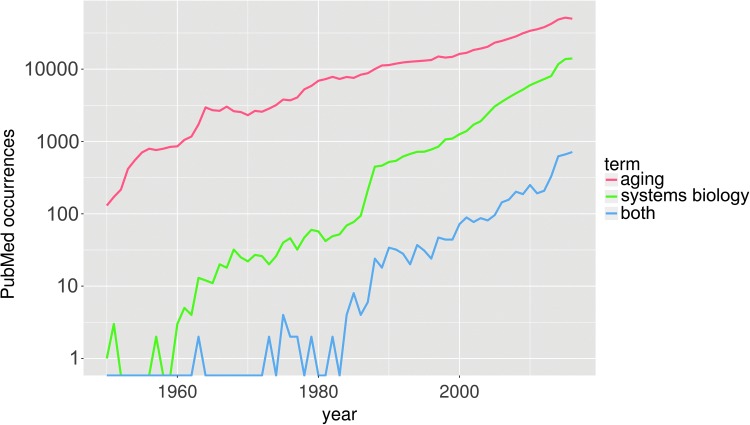
Both aging (6, 57) and systems biology (53, 96) are large and rapidly expanding fields, and reviewing each exhaustively would require a textbook-length treatment. Although systems biology as a discipline is newer than the biology of aging, it has been around since at least the 1960s (79). Widespread increases in interest in systems approaches were ushered in by an explosion of biological data that arose from enormous improvements in the throughput of DNA sequencing technology in the early 2000s, and aging has been considered a fruitful hypothetical target for systems approaches since at least that time (40). This is illustrated by the increasing number of systems biology/aging studies, as shown in Figure 3, which compares the number of PubMed entries by year for “aging/ageing,” “systems biology,” and both in combination, from 1950 to 2016. It clearly demonstrates a striking recent increase in systems biology-related publications, which is roughly proportionally reflected in the number of combined aging and systems biology-related publications.
FIG. 3.
Incidence of the terms “aging/ageing,” “systems biology,” or the two together, in PubMed article listings from 1950 to present, plotted on a log-linear scale. Similar slopes suggest a common rate of increase in number of publications over time for the various categories shown here.
To highlight the power and potential of systems biology of aging, instead of providing a comprehensive analysis of the many approaches that have been used, we have chosen instead to present a few very recent illustrative examples. These select studies serve as strong examples of what can be accomplished when we integrate systems biology and aging. In particular, we discuss three case studies that illustrate the power of systems biology to inform our understanding of aging through the study of (i) diet restriction, (ii) neurodegenerative disease, and (iii) biomarkers of aging.
Although the studies we present here are highly illustrative of the power of systems biology, we necessarily leave out much important work in this short review. In particular, although mathematical modeling plays a critical role in systems biology, the studies we describe here include computational models but not formal mathematical models. For reviews of more mathematical approaches to systems biology studies of aging, especially with respect to models of biochemical pathways, readers are encouraged to read reviews by Kirkwood (58) and, more recently, McAuley et al. (74). After our discussion of these examples, we briefly survey some of the state-of-the-art methods currently being applied by systems biologists generally, which might be worth applying to aging-related questions in the near future.
Aging Biology Is Poised to Benefit from Systems-Level Approaches
We have long had a strong conceptual framework to understand why aging evolved (35, 59, 61, 78, 113). Over the past two decades, researchers have also recognized that the mechanistic causes (19, 24, 56, 60, 69, 77, 98, 112) and consequences (72) of aging appear to be deeply evolutionarily conserved. Nevertheless, attempts to move from any of these pathways or genes in isolation to a fuller mechanistic understanding of aging and how it can be modulated have proved difficult. In this light, a systems-level approach, one that incorporates a large number of pathways into a single framework, could move us closer to a mechanistic understanding of aging.
Although we suggest here that the biology of aging may now be particularly well poised to benefit from systems approaches, applications of systems biology approaches to the study of aging are not new. Systems approaches have been used to investigate molecular networks associated with aging (63, 103, 115). Systems level thinking has also been used to dissect several specific facets of aging biology, such as telomere length (93), bioenergetics (105), proteotoxic stress (82), inflammation (14), epigenetic changes (88), mTOR signaling (21, 22, 102), and cellular senescence (28, 100). Several previous reviews also offer additional perspectives on the intersection of aging biology and systems biology at multiple stages during its development (16, 29, 58, 62, 81, 116).
Some Notable Recent Applications of Systems Approaches to Aging Biology
Dietary restriction
Caloric or dietary restriction is one of the earliest interventions shown to significantly increase lifespan (75), and subsequent work has shown multiple regimes of altered food intake that have dramatic effects on lifespan and healthspan in invertebrates (52) as well as mammals (2). Although many genetically identified nutrient sensing pathways appear to play a role in lifespan extension by dietary restriction (57), none of them appear to be completely responsible for these effects.
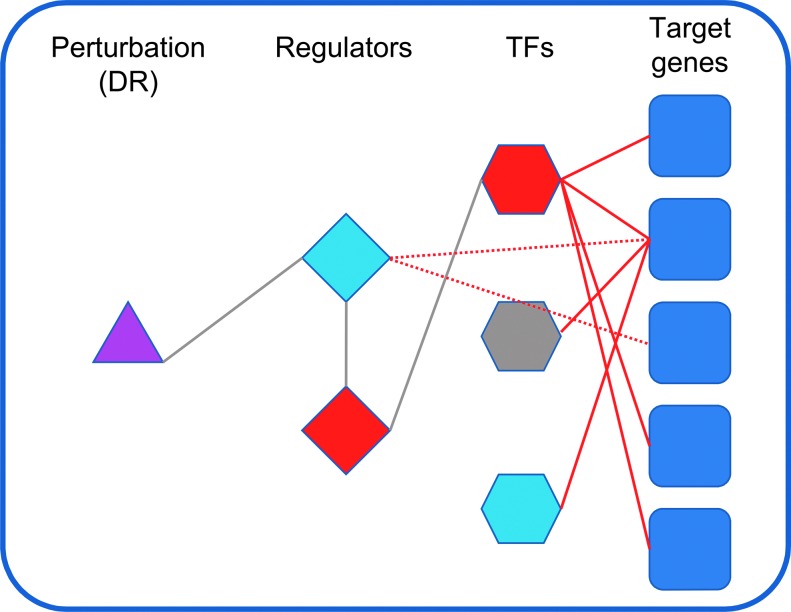
From this starting point, Hou et al. have recently used a careful systems approach to add greatly to our understanding of dietary restriction (43) (Fig. 4). They measured the transcriptomes of cohorts of the nematode C. elegans, under ad libitum fed conditions, as well as under diet restricted (DR) and intermittent fasting (IF) conditions, both of which extend lifespan significantly. These transcriptomic measurements were repeated periodically, generating a transcriptomic time course for each treatment throughout the adult life of these organisms. These time courses were then used to cluster all transcripts (genes) using Bayesian Information Criterion-Super K Means clustering (117). They note an over-representation of known prolongevity factors among genes they observe to be upregulated by DR or IF, and of known antilongevity factors among those downregulated by DR or IF, consistent with the notion that DR and IF lead to extended lifespan through these genetic pathways.
FIG. 4.
Modified schematic representation of findings, after Hou et al. (43). The authors sought transcriptomic changes due to diet restriction in Caenorhabditis elegans that were similar to transcriptomic changes arising from genetic perturbations. In this way, they were able to identify a “regulator-target-cluster” network of interacting genes associated with the lifespan-extending effects of diet restriction. DR, diet restricted.
To ask which genes might be expected to mediate the observed DR and IF responses, the authors used extended Deletion Mutant Bayesian Network (eDM_BN) analysis, an adaptation of their previous Deletion Mutant Bayesian Network analysis (66). They compared 73 genetic perturbations with their measurements of calorically restricted and IF worms, identifying nine genes whose perturbation closely mirrored the effects of DR or IF on the worm transcriptome: rheb-1, aak-2, xbp-1, tax-6, glp-1, daf-16, dpy-10, gas-1, and ogt-1.
Several of these genes have previously been shown to have some relationship with dietary restriction, and some have been shown to have relationships with one another (e.g., glp-1 and daf-16) (44). These genes were grouped into three clusters, and additional analysis based on eDM_BN incorporating modENCODE TF chromatin immunoprecipitation sequencing data (17) (TF-expanded eDM_BN), as well as CoCiter (89), also resulted in the same three clusters. These implicate three pathways—rheb-1/let-363, aak-2/tax-6/xbp-1, and daf-16/glp-1, as distinct modules involved in the transcriptome level changes observed upon DR or IF.
The authors then constructed aak-2(o/e), daf-2(e1370), and rsks-1(ok1255) worms, and used these, as well as daf-2(e1370), rsks-1(ok1255), and tax-6(RNAi) worms, to ask what happened when all three of the modules they identified were perturbed simultaneously. In general, as they moved from single genetic perturbations to double and then triple, the transcriptomes of these worms began to more closely resemble those of DR or IF worms. They modeled the kinetics and steady states of their network model and concluded that the triple module-perturbed animals should show a shift in the network steady state toward a longevity state. This model predicts that making a small perturbation in all three modules should have a larger effect on lifespan than a much larger perturbation in a single module, as was observed.
As the triple-perturbed worms were insensitive to dietary restriction, they conclude that perturbing these three modules recapitulates essentially all of the DR phenotypes. Finally, they asked whether new individual genetic phenotypes might be uncovered by their approach. For example, they predicted a lifespan interaction between aak-2(o/e) and xbp-1(RNAi) as suggested by their placement into the same module, and found that xbp-1 is necessary for the extended lifespan of aak-2(o/e).
This work has important implications for attempts to phenocopy lifespan and healthspan extension by DR using drugs or genetic manipulation. Based on these findings that perturbation of three distinct modules is needed to recover most of the lifespan extension caused by caloric restriction or intermittent fasting, we might need to simultaneously modulate multiple modules to recover the full phenotype of this intervention in the absence of caloric restriction. These findings of multiple necessary modules and their identities would be hard to arrive at by a nonsystems approach. This study provides a compelling example of the kind of progress that systems approaches will continue to bring to aging biology.
Neurodegenerative disease
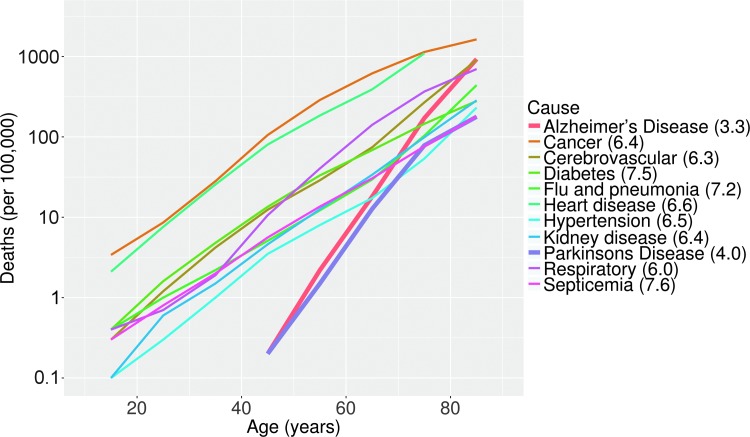
Aging is the single greatest risk factor for a wide range of diseases, but the effect is seen most dramatically for neurodegenerative diseases (Fig. 5). This has led to a sustained interest among aging researchers in neurodegenerative diseases. The most commonly diagnosed and most intensively studied of these is Alzheimer's disease (AD) (34). Total annual healthcare costs for AD and related diseases are now estimated to exceed $200 billion in the United States, and could rise to $1 trillion by 2050 (23). We have now identified multiple human genes associated with both early onset (genetic or familial) and late onset (sporadic) Alzheimer's disease (LOAD), often using genome-wide association studies (GWASs) (20, 32, 64, 85), but a complete mechanistic understanding of this disease has yet to emerge. The need for systems approaches in AD has been recognized, and ongoing systems-level National Institute on Aging-supported initiatives such as Accelerating Medicines Partnership-Alzheimer's Disease target discovery and preclinical validation project (8, 83) and Model Organism Development and Evaluation for Late-onset AD have been funded with this in mind.
FIG. 5.
Log-linear plot of annual deaths per 100,000, by disease. Numbers in legend refer to doubling time of risk, in years. Doubling time for AD and PD (thicker red and purple lines) is ∼2 × faster than other major age-related disease. Data from 2013 CDC National Vital Statistics Report. AD, Alzheimer's disease; PD, Parkinson's disease.
Mukherjee and colleagues (84) recently used a versatile gene-based test for genome-wide association study (VEGAS) analysis (70), as well as a dense module search (DMS) approach (47) to synthesize human protein–protein interaction (PPI) data and GWAS data. Briefly, the VEGAS approach uses a weighted sum of single nucleotide polymorphism chi-squared tests, corrected for linkage disequilibrium. Per-gene empirical p-values are determined by simulation-based permutations, stopping when a value of 1 × 10−6 is reached, corresponding to a genome-wide false discovery rate of 0.05. In the DMS approach, the authors mined human interactome PPI data, and used DMS GWASs to identify statistically significant (improbable) networks of genes by exhaustively examining the combined effect of multiple genes in this interactome. They then tested the resulting predictions using both transgenic C. elegans models of Aβ toxicity, and human brain expression level data. This multimethod, multiorganism systems biology approach proved fruitful and may illustrate the types of approaches that will become increasingly prevalent. A schematic diagram of their approach is shown in Figure 6.
FIG. 6.
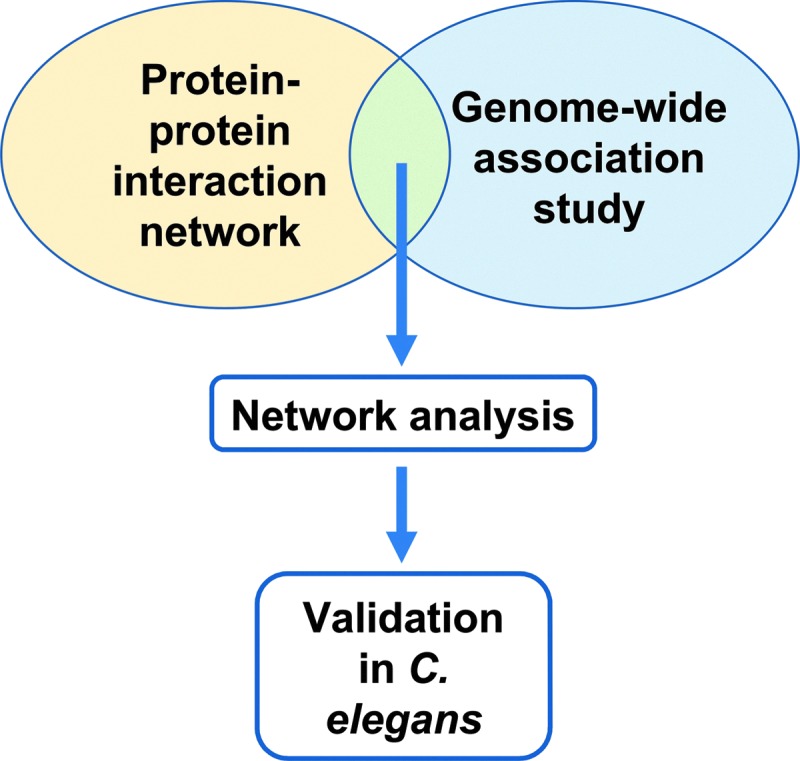
Mukherjee et al. (84) combined genome-wide association data for late onset AD with protein–protein interaction data in a cross-platform network analysis. This led to a set of candidate loci, which were tested in C. elegans for their ability to ameliorate the effects of Aβ expression.
The VEGAS approach corresponded well with previously published GWAS analyses and yielded an additional three genes and four pseudogenes. The DMS approach, which integrates additional information from human PPI data, provided a top module with 33 genes, 16 of which were on chromosome 19, leaving open the possibility of linkage disequilibrium with APOE, the strongest known genetic determinant of Alzheimer's risk. Of the remaining 17 genes, four had previously been associated with increased risk of LOAD in GWAS analyses. The authors focused on the 13 novel genes not on chromosome 19: ALB, EGR1, HLA-DRA, CHRNA2, MYC, NDUFS3, UBC, SLC25A11, C1QBP, KRT14, ICT1, ATP5H, and APP.
Of these, four had predicted C. elegans orthologs, indicated in parentheses: NDUFS3 (nuo-2), UBC (ubq-1 and ubq-2), ATP5H (atp-5), and EGR-1 (egrh-1). Ribonucleic acid interference knockdown of all five of these predicted orthologs had significant effects on Aβ toxicity phenotypes. Knockdown of ubq-1 and ubq-2 significantly accelerated Aβ toxicity, whereas knockdown of nuo-2, atp-5, and egrh-1 delayed it, in various transgenic worm models. In examining human transcriptomic data comparing diagnosed LOAD patients with patients with other neurological diseases, reliable transcript levels were available for 11 of the 13 novel genes (excluding CHRNA2 and KRT14). Of these, four or 36% (NDUFS3, SLC25A11, ATP5H, and APP) were differentially expressed in the temporal cortex of LOAD patients, compared with 14% of all measured probes, and two more (UBC and C1Q8P) were differentially expressed in the cerebellum.
This work demonstrates the ability of systems approaches to generate and validate entirely novel genes of interest and leverage existing large data sets. The results expand our understanding of the biology of AD and of targets for possible intervention, and similar approaches should be expected to continue to add to our understanding of this complex and important disease.
These strengthened connections between known C. elegans genes and AD point out an interesting future direction for this work. C. elegans has been used extensively as a model of aging more generally, and many genes and pathways have been identified that alter aging in worms, hif-1, hsf-1, etc. Similarly powerful models to study the impact of Aβ and tau also exist in the fruit fly, Drosophila melanogaster (11, 114). Much could potentially be learned from both of these invertebrate models by studying how these pathways alter Aβ toxicity phenotypes and interact with the Alzheimer's related genes described in this study.
Biomarkers of aging
Finally, a longstanding interest among aging researchers has been the reliable generation of biomarkers of aging. These could, for example, more rapidly suggest whether or not an intervention appears to be slowing aging, without waiting for a cohort of experimental organisms or human patients to live out their entire lifespans. Thirty years ago, the National Institutes of Health created an initiative to identify biomarkers of aging (4). Such efforts have often been criticized (18), and at least until recently, these initiatives showed little success. With the advent of high-dimensional and relatively inexpensive molecular phenotypes, systems biology approaches may at last be able to provide us with meaningful biomarkers of aging (so-called biological clocks), measures that could predict future risk morbidity and mortality even in apparently healthy individuals. For example, a series of recent studies suggest that epigenetic marks (specifically DNA methylation) have promise as a predictor of biological age (10, 37, 42).
In humans, wherein the largest differences in lifespan among modern cohorts are modest relative to the changes in survival we can demonstrate in model organisms, it can be difficult to distinguish a chronological clock (which measures years lived so far) from a biological clock (which would predict years still to live). To clearly discern the two requires that we consider individuals that have been alive for the same amount of time (same chronological age) but have a very different remaining lifespan and healthspan (biological age). Recent work from Wang et al. has applied systems approaches to demonstrate epigenetic signatures that differ between control mice and mice that age more slowly (107).
The authors analyzed 107 mouse liver methylomes from mice aged 0.2–26 months, and used an ElasticNet statistical regression approach (118) to train an age predictor, using a subset of 148 CpG sites, regions in the genome that can be highly methylated, and whose methylation status can influence rates of transcription. Four-way cross-validation using the original data set, and application of the predictor to 50 novel methylome samples of either 2- or 22-month-old wild-type mice, both showed excellent correlation between predicted age and chronological age. The resulting model was then applied to the methylomes of three cases of mice with increased lifespan and delayed aging. These included Prop1df/df dwarf mice (5), as well as rapamycin-treated mice and calorically restricted mice (80).
The methylome-based model predicted a lower chronological age for all three of these groups of mice, and in all three cases the difference between the predicted epigenetic lifespan of the long-lived mice and their chronological lifespan was statistically significant, whereas epigenetic lifespan and chronological lifespan were not significantly different for the control mice that were not long lived (Fig. 7). Looking at the first principal component from a principal components analysis of long-lived and control methylomes, or at the trends of the most significantly changed CpG islands selected in their model, led to the conclusion that changes in methylation over time were generally less pronounced in long-lived mice than control mice that were not long lived, as might be expected. In humans, Steve Horvath's epigenetic clock analysis based on the methylome indicates that tissues do not age at identical rates (42), a finding supported by recent analysis of molecular biomarkers in different tissues of an annual killifish, Notobranchus guentheri (25). Based on Horvath's findings, Wang et al. (107) note that extension of their current liver-specific epigenetic model will eventually allow us to ask whether some tissues or cell types have an epigenetic age that diverges more or less from their chronological age than others, in long-lived mouse models. This and other similar recent work suggests that the time is near when we will be able to assay the effect on meaningful biomarkers of interventions that delay aging rapidly and cheaply, which would promise to greatly enhance the pace of this type of research.
FIG. 7.
Epigenetic clocks purport to identify epigenetic profiles that are predictive of future life expectancy. The figure shows the isometric (dashed) line, where the epigenetic age is identical to the chronological age, and findings from two mouse populations. Wang et al. (107) examined wild-type and rapamycin-treated mice. In wild-type mice (solid blue line), the epigenetic clock matches chronological age early on, but at late ages, it tends to underestimate age. Treatment with rapamycin (solid red line) significantly extends lifespan and makes mice appear younger than their chronological age across the lifespan.
Systems Biology Includes a Powerful and Rapidly Evolving Set of Tools that can be Applied to Questions in Aging Biology
Systems biology approaches are often characterized by the analysis of very large, multidimensional data sets, with thousands to millions of data points. Moreover, these data might represent highly disparate traits—genome, epigenome, transcriptome, proteome, microbiome, and metabolome—each with different ways of being described. Typically, the number of traits and trait interactions greatly outnumbers the samples collected, creating tremendous challenges in the collection, curation, and analysis of data.
To address the challenges of understanding the statistical and biological significance of systems biology data, we need methods that can incorporate standardization on open formats (97). Systems biologists have embraced this concern and proposed or adopted multiple open formats for the exchange of data and models, for example, the systems biology markup language (SBML) (46), as well as CellML (71) and NeuroML (33). Multiple searchable repositories now use SBML, including the BioModels database of quantitative models (48) and the Reactome pathway database (27).
These open formats for describing data are equally important when it comes to software for analyzing data. It is critical that we have openly available software that allows biologists to apply modern computational techniques to their data. Several useful stand-alone systems such as COPASI (41), the complex pathway simulator, have been developed for systems biology (31). As with file formats, many advantages accrue to users of open source software that allows contributors to freely improve, add, edit, and modify the tools that they use (92), and most systems biology tools fall into this category. The Cytoscape framework is a powerful general-purpose platform emphasizing network-based approaches, with a large and growing set of add-ons that greatly extend its core functionality (94). GeneMANIA (108), which exists as a stand-alone tool or a Cytoscape App, allows users to easily construct and explore networks based on multiple biological features, whereas STRING (99) focuses specifically on PPI.
Perhaps the most rapidly evolving set of tools for computational work by biologists centers around the R programming language (90). Like Cytoscape, R seamlessly utilizes user-contributed packages that add to its core functionality, and there are many R packages that are specific to systems biology as well as many others covering multiple statistical and machine-learning techniques of interest to the systems biologist. Many of these packages are hosted at the Comprehensive R Archive Network. Among these are systems biology packages implementing Bayesian active learning strategy (87) and modeling and simulation of stochastic kinetic biochemical network models (111). Another centralized repository of biology-specific R packages is hosted by the Bioconductor project (45), and Bioconductor hosts several systems biology-related R packages. These include a metagenomic pipeline for systems biology (15), a graphical user interface for exploratory analysis of systems biology data (65), metaheuristics for global optimization in systems biology (26), flexible identification of differentially expressed subpathways using RNA-seq experiments (106), constraint-based modeling using metabolic reconstruction databases (30), and a SBML interface to R (91), among many others. Although users may understandably be drawn to the powerful mathematical methodologies or aging-specific use cases, the most important tools to master to most effectively utilize a framework like the R programming language are probably the bread-and-butter data transformation, collation, visualization, and import functions. These tools quickly make large and unwieldy data sets useful and interpretable. Readers new to R are strongly encouraged to familiarize themselves with this type of essential R package sooner rather than later (109). All of the R packages already mentioned are summarized in Table 1.
Table 1.
Some Systems Biology Packages in R
| Package | Description | URL |
|---|---|---|
| pauwels2014 | Bayesian active learning for sequential experimental design | https://cran.r-project.org/web/packages/pauwels2014/index.html |
| smfsb | Modeling and simulation of stochastic kinetic biochemical network models | https://cran.r-project.org/web/packages/smfsb/index.html |
| mmnet | Metagenomics pipeline for systems biology | http://bioconductor.org/packages/release/bioc/html/mmnet.html |
| explorase | Graphical user interface for exploratory analysis of systems biology data | http://bioconductor.org/packages/devel/bioc/html/explorase.html |
| MEIGOR | Metaheuristics for global optimization in systems biology | http://bioconductor.org/packages/release/bioc/html/MEIGOR.html |
| DEsubs | Flexible identification of differentially expressed subpathways | http://bioconductor.org/packages/release/bioc/html/DEsubs.html |
| BiGGR | Constraint-based modeling using metabolic reconstruction databases | http://bioconductor.org/packages/release/bioc/html/BiGGR.html |
| SBMLR | Systems biology markup language interface to R | http://bioconductor.org/packages/release/bioc/html/SBMLR.html |
Conclusions
In the past two decades or so, we have moved from a study of aging based largely in physiology, comparative biology, and evolutionary theory, which are still very strong components of ongoing research, to one that now includes multiple, diverse genetic, and pharmacological interventions, many with very large effects on lifespan and healthspan. These interventions are often broadly conserved genetically, suggesting further utility in pointing toward additional human interventions in time. Although we do not understand all of the individual genes and pathways that affect aging, as we continue to uncover more of them, we have now also begun to synthesize existing data using systems-level approaches, often to great effect. The three examples noted here all benefit from computational approaches that were unknown a few years ago, and from biological insights gleaned from multiple model systems, from aging laboratories as well as many other areas of biology. These data sets and computational tools are both improving at a rapid and accelerating rate. Many new technologies, such as single-cell sequencing, advances in epigenetics beyond the methylome (specifically, assay for transposase-accessible chromatin with high throughput sequencing), and multiomic network studies, will also increase the reach of systems biologists. This suggests that approaches similar to those described here will continue to lead to striking findings, and to interventions that may allow us to delay some of the many age-associated diseases in humans, perhaps sooner that we expect.
Abbreviations Used
- AD
Alzheimer's disease
- DM_BN
Deletion Mutant Bayesian Network
- DMS
dense module search
- DR
diet restricted
- E
environment
- eDM_BN
extended Deletion Mutant Bayesian Network
- G
genes
- GWASs
genome-wide association studies
- IF
intermittently fasted
- LOAD
late onset (sporadic) Alzheimer's disease
- P
phenotypes
- PPI
protein–protein interaction
- SBML
systems biology markup language
- VEGAS
versatile gene-based test for genome-wide association study
Acknowledgments
DELP was supported in part by funding from the NIH (AG049494 and CA211160) and the National Science Foundation (DMS1561814).
References
- 1.Alon U, Camarena L, Surette MG, Aguera y Arcas B, Liu Y, Leibler S, and Stock JB. Response regulator output in bacterial chemotaxis. EMBO J 17: 4238–4248, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RM, Shanmuganayagam D, and Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol 37: 47–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arantes-Oliveira N, Berman JR, and Kenyon C. Healthy animals with extreme longevity. Science 302: 611, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Baker GT., 3rd and Sprott RL. Biomarkers of aging. Exp Gerontol 23: 223–239, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Bartke A. and Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol 63: 189–225, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Barzilai N, Crandall JP, Kritchevsky SB, and Espeland MA. Metformin as a tool to target aging. Cell Metab 23: 1060–1065, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzilai N, Guarente L, Kirkwood TBL, Partridge L, Rando TA, and Slagboom PE. The place of genetics in ageing research. Nat Rev Genet 13: 589–594, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Bennett DA, Yu L, and De Jager PL. Building a pipeline to discover and validate novel therapeutic targets and lead compounds for Alzheimer's disease. Biochem Pharmacol 88: 617–630, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blüher M, Kahn BB, and Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299: 572–574, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Bocklandt S, Lin W, Sehl ME, Sánchez FJ, Sinsheimer JS, Horvath S, and Vilain E. Epigenetic predictor of age. PLoS One 6: e14821, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonini NM. and Fortini ME. Human neurodegenerative disease modeling using Drosophila. Annu Rev Neurosci 26: 627–656, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Butler RN, Miller RA, Perry D, Carnes BA, Williams TF, Cassel C, Brody J, Bernard MA, Partridge L, Kirkwood T, Martin GM, and Olshansky SJ. New model of health promotion and disease prevention for the 21st century. BMJ 337: a399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler RN, Warner HR, Williams TF, Austad SN, Brody JA, Campisi J, Cerami A, Cohen G, Cristofalo VJ, Drachman DA, Finch CE, Fridovich I, Harley CB, Havlik RJ, Martin GM, Miller RA, Olshansky SJ, Pereira-Smith OM, Smith JR, Sprott RL, West MD, Wilmoth JR, and Wright WE. The aging factor in health and disease: the promise of basic research on aging. Aging Clin Exp Res 16: 104–112, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Calçada D, Vianello D, Giampieri E, Sala C, Castellani G, de Graaf A, Kremer B, van Ommen B, Feskens E, Santoro A, Franceschi C, and Bouwman J. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: a systems biology approach. Mech Ageing Dev 136–137: 138–147, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Zheng X, Li F, and Bo X. mmnet: an R package for metagenomics systems biology analysis. BioMed Res Int 2015: 167249, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chauhan A, Liebal UW, Vera J, Baltrusch S, Junghanß C, Tiedge M, Fuellen G, Wolkenhauer O, and Köhling R. Systems biology approaches in aging research. Interdiscip Top Gerontol 40: 155–176, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Contrino S, Smith RN, Butano D, Carr A, Hu F, Lyne R, Rutherford K, Kalderimis A, Sullivan J, Carbon S, Kephart ET, Lloyd P, Stinson EO, Washington NL, Perry MD, Ruzanov P, Zha Z, Lewis SE, Stein LD, and Micklem G. modMine: flexible access to modENCODE data. Nucleic Acids Res 40: D1082–D1088, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa PT. and McCrae RR. Measures and markers of biological aging: “a great clamoring … of fleeting significance.” Arch Gerontol Geriatr 7: 211–214, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Cui H-J, Liu X-G, McCormick M, Wasko BM, Zhao W, He X, Yuan Y, Fang B-X, Sun X-R, Kennedy BK, Suh Y, Zhou ZJ, Kaeberlein M, and Feng WL. PMT1 deficiency enhances basal UPR activity and extends replicative lifespan of Saccharomyces cerevisiae. Age (Dordr) 37: 9788, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuyvers E. and Sleegers K. Genetic variations underlying Alzheimer's disease: evidence from genome-wide association studies and beyond. Lancet Neurol 15: 857–868, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Dalle Pezze P, Ruf S, Sonntag AG, Langelaar-Makkinje M, Hall P, Heberle AM, Razquin Navas P, van Eunen K, Tölle RC, Schwarz JJ, Wiese H, Warscheid B, Deitersen J, Stork B, Fäßler E, Schäuble S, Hahn U, Horvatovich P, Shanley DP, and Thedieck K. A systems study reveals concurrent activation of AMPK and mTOR by amino acids. Nat Commun 7: 13254, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalle Pezze P, Sonntag AG, Thien A, Prentzell MT, Gödel M, Fischer S, Neumann-Haefelin E, Huber TB, Baumeister R, Shanley DP, and Thedieck K. A dynamic network model of mTOR signaling reveals TSC-independent mTORC2 regulation. Sci Signal 5: 217, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Deb A, Thornton JD, Sambamoorthi U, and Innes K. Direct and indirect cost of managing Alzheimer's disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res 17: 189–202, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, and Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science 298: 2398–2401, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Dong Y, Cui P, Li Z, and Zhang S. Aging asymmetry: systematic survey of changes in age-related biomarkers in the annual fish Nothobranchius guentheri. Fish Physiol Biochem 43: 309–319, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Egea JA, Henriques D, Cokelaer T, Villaverde AF, MacNamara A, Danciu D-P, Banga JR, and Saez-Rodriguez J. MEIGO: an open-source software suite based on metaheuristics for global optimization in systems biology and bioinformatics. BMC Bioinformatics 15: 136, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, Jassal B, Jupe S, Korninger F, McKay S, Matthews L, May B, Milacic M, Rothfels K, Shamovsky V, Webber M, Weiser J, Williams M, Wu G, Stein L, Hermjakob H, and D'Eustachio P. The reactome pathway knowledgebase. Nucleic Acids Res 44: D481–D487, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figueredo GP, Siebers P-O, Aickelin U, Whitbrook A, and Garibaldi JM. Juxtaposition of system dynamics and agent-based simulation for a case study in immunosenescence. PLoS One 10: e0118359, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuellen G, Dengjel J, Hoeflich A, Hoeijemakers J, Kestler HA, Kowald A, Priebe S, Rebholz-Schuhmann D, Schmeck B, Schmitz U, Stolzing A, Sühnel J, Wuttke D, and Vera J. Systems biology and bioinformatics in aging research: a workshop report. Rejuvenation Res 15: 631–641, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Gavai AK, Supandi F, Hettling H, Murrell P, Leunissen JAM, and van Beek JHGM. Using bioconductor package BiGGR for metabolic flux estimation based on gene expression changes in brain. PLoS One 10: e0119016, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh S, Matsuoka Y, Asai Y, Hsin K-Y, and Kitano H. Software for systems biology: from tools to integrated platforms. Nat Rev Genet 12: 821–832, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Giri M, Zhang M, and Lü Y. Genes associated with Alzheimer's disease: an overview and current status. Clin Interv Aging 11: 665–681, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleeson P, Crook S, Cannon RC, Hines ML, Billings GO, Farinella M, Morse TM, Davison AP, Ray S, Bhalla US, Barnes SR, Dimitrova YD, and Silver R. NeuroML: a language for describing data driven models of neurons and networks with a high degree of biological detail. PLoS Comput Biol 6: e1000815, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goedert M. Neurodegeneration. Alzheimer's and Parkinson's diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 349: 1255555, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Haldane JBS. New Paths in Genetics. London, United Kingdom: Harper, 1942 [Google Scholar]
- 36.Halevy A, Norvig P, and Pereira F. The unreasonable effectiveness of data. IEEE Intell Syst 24: 8–12, 2009 [Google Scholar]
- 37.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R, Chen M, Rajapakse I, Friend S, Ideker T, and Zhang K. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 49: 359–367, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holy TE. and Leibler S. Dynamic instability of microtubules as an efficient way to search in space. Proc Natl Acad Sci U S A 91: 5682–5685, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, and Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421: 182–187, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Hood L. Systems biology: integrating technology, biology, and computation. Mech Ageing Dev 124: 9–16, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Hoops S, Sahle S, Gauges R, Lee C, Pahle J, Simus N, Singhal M, Xu L, Mendes P, and Kummer U. COPASI—a COmplex PAthway SImulator. Bioinformatics 22: 3067–3074, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol 14: R115, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou L, Wang D, Chen D, Liu Y, Zhang Y, Cheng H, Xu C, Sun N, McDermott J, Mair WB, and Han JD. A systems approach to reverse engineer lifespan extension by dietary restriction. Cell Metab 23: 529–540, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsin H. and Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399: 362–366, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, Gottardo R, Hahne F, Hansen KD, Irizarry RA, Lawrence M, Love MI, MacDonald J, Obenchain V, Oleś AK, Pagès H, Reyes A, Shannon P, Smyth GK, Tenenbaum D, Waldron L, and Morgan M. Orchestrating high-throughput genomic analysis with bioconductor. Nat Methods 12: 115–121, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hucka M, Finney A, Sauro HM, Bolouri H, Doyle JC, Kitano H, Arkin AP, Bornstein BJ, Bray D, Cornish-Bowden A, Cuellar AA, Dronov S, Gilles ED, Ginkel M, Gor V, Goryanin II, Hedley WJ, Hodgman TC, Hofmeyr JH, Hunter PJ, Juty NS, Kasberger JL, Kremling A, Kummer U, Le Novère N, Loew LM, Lucio D, Mendes P, Minch E, Mjolsness ED, Nakayama Y, Nelson MR, Nielsen PF, Sakurada T, Schaff JC, Shapiro BE, Shimizu TS, Spence HD, Stelling J, Takahashi K, Tomita M, Wagner J, Wang J, and SBML Forum. The systems biology markup language (SBML): a medium for representation and exchange of biochemical network models. Bioinformatics 19: 524–531, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Jia P, Zheng S, Long J, Zheng W, and Zhao Z. dmGWAS: dense module searching for genome-wide association studies in protein–protein interaction networks. Bioinformatics 27: 95–102, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juty N, Ali R, Glont M, Keating S, Rodriguez N, Swat MJ, Wimalaratne SM, Hermjakob H, Le Novère N, Laibe C, and Chelliah V. BioModels: content, features, functionality, and use. CPT Pharmacometrics Syst Pharmacol 4: e3, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaeberlein M. Longevity and aging. F1000prime Rep 5: 5, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaeberlein M, Creevy KE, and Promislow DEL. The dog aging project: translational geroscience in companion animals. Mamm Genome 27: 279–288, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaeberlein M, Rabinovitch PS, and Martin GM. Healthy aging: the ultimate preventative medicine. Science 350: 1191–1193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kapahi P, Kaeberlein M, and Hansen M. Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res Rev 39: 3–14, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karahalil B. Overview of systems biology and omics technologies. Curr Med Chem 23: 4221–4230, 2016 [DOI] [PubMed] [Google Scholar]
- 54.Kennedy BK, Austriaco NR, Jr, Zhang J, and Guarente L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell 80: 485–496, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Kenyon C. The genetics of ageing. Nature 464: 504–512, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Kenyon C, Chang J, Gensch E, Rudner A, and Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature 366: 461–464, 1993 [DOI] [PubMed] [Google Scholar]
- 57.Kenyon CJ. The genetics of ageing. Nature 464: 504–512, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Kirkwood TBL. Systems biology of ageing and longevity. Philos Trans R Soc Lond B Biol Sci 366: 64–70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirkwood TBL. and Holliday R. The evolution of ageing and longevity. Proc R Soc Lond B Biol Sci 205: 531–546, 1979 [DOI] [PubMed] [Google Scholar]
- 60.Klass MR. A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech Ageing Dev 22: 279–286, 1983 [DOI] [PubMed] [Google Scholar]
- 61.Kowald A. and Kirkwood TB. Towards a network theory of ageing: a model combining the free radical theory and the protein error theory. J Theor Biol 168: 75–94, 1994 [DOI] [PubMed] [Google Scholar]
- 62.Kriete A, Lechner M, Clearfield D, and Bohmann D. Computational systems biology of aging. Wiley Interdiscip Rev Syst Biol Med 3: 414–428, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Kriete A, Sokhansanj BA, Coppock DL, and West GB. Systems approaches to the networks of aging. Ageing Res Rev 5: 434–448, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Morón FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fiévet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossù P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, European Alzheimer's Disease Initiative (EADI), Genetic and Environmental Risk in Alzheimer's Disease; Alzheimer's Disease Genetic Consortium, Cohorts for Heart and Aging Research in Genomic Epidemiology, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O'Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Jr., Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nöthen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, and Amouyel P. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet 45: 1452–1458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lawrence M, Lee E-K, Cook D, Hofmann H, and Wurtele E. exploRase: exploratory data analysis of systems biology data. In International Conference on Coordinated and Multiple Views in Exploratory Visualization Proceedings, London, UK 14–20, 2006 [Google Scholar]
- 66.Li J, Liu Y, Liu M, and Han JD. Functional dissection of regulatory models using gene expression data of deletion mutants. PLoS Genet 9: e1003757, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li W, Li X, and Miller RA. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell 13: 1012–1018, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li W. and Miller RA. Elevated ATF4 function in fibroblasts and liver of slow-aging mutant mice. J Gerontol A Biol Sci Med Sci 70: 263–272, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin YJ, Seroude L, and Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science 282: 943–946, 1998 [DOI] [PubMed] [Google Scholar]
- 70.Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, Brown KM.AMFS Investigators, Hayward NK, Montgomery GW, Visscher PM, Martin NG, and Macgregor S. A versatile gene-based test for genome-wide association studies. Am J Hum Genet 87: 139–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lloyd CM, Halstead MDB, and Nielsen PF. CellML: its future, present and past. Prog Biophys Mol Biol 85: 433–450, 2004 [DOI] [PubMed] [Google Scholar]
- 72.López-Otín C, Blasco MA, Partridge L, Serrano M, and Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, and Klickstein LB. mTOR inhibition improves immune function in the elderly. Sci Transl Med 6: 268ra179, 2014 [DOI] [PubMed] [Google Scholar]
- 74.McAuley MT, Guimera AM, Hodgson D, Mcdonald N, Mooney KM, Morgan AE, and Proctor CJ. Modelling the molecular mechanisms of aging. Biosci Rep 37: pii:, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCay CM. and Crowell MF. Prolonging the life span. Sci Mon 39: 405–414, 1934 [Google Scholar]
- 76.McCormick MA, Delaney JR, Tsuchiya M, Tsuchiyama S, Shemorry A, Sim S, Chou AC, Ahmed U, Carr D, Murakami CJ, Schleit J, Sutphin GL, Wasko BM, Bennett CF, Wang AM, Olsen B, Beyer RP, Bammler TK, Prunkard D, Johnson SC, Pennypacker JK, An E, Anies A, Castanza AS, Choi E, Dang N, Enerio S, Fletcher M, Fox L, Goswami S, Higgins SA, Holmberg MA, Hu D, Hui J, Jelic M, Jeong KS, Johnston E, Kerr EO, Kim J, Kim D, Kirkland K, Klum S, Kotireddy S, Liao E, Lim M, Lin MS, Lo WC, Lockshon D, Miller HA, Moller RM, Muller B, Oakes J, Pak DN, Peng ZJ, Pham KM, Pollard TG, Pradeep P, Pruett D, Rai D, Robison B, Rodriguez AA, Ros B, Sage M, Singh MK, Smith ED, Snead K, Solanky A, Spector BL, Steffen KK, Tchao BN, Ting MK, Vander Wende H, Wang D, Welton KL, Westman EA, Brem RB, Liu XG, Suh Y, Zhou Z, Kaeberlein M, and Kennedy BK. A comprehensive analysis of replicative lifespan in 4,698 single-gene deletion strains uncovers conserved mechanisms of aging. Cell Metab 22: 895–906, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCormick MA, Mason AG, Guyenet SJ, Dang W, Garza RM, Ting MK, Moller RM, Berger SL, Kaeberlein M, Pillus L, La Spada AR, and Kennedy BK. The SAGA histone deubiquitinase module controls yeast replicative lifespan via Sir2 interaction. Cell Rep 8: 477–486, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Medawar PB. An Unsolved Problem of Biology. London, United Kingdom: H.K. Lewis and Co., 1952 [Google Scholar]
- 79.Mesarović MD. Systems Theory and Biology. Berlin, Heidelberg, Germany: Springer Berlin Heidelberg, 1968 [Google Scholar]
- 80.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, and Strong R. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13: 468–477, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mooney KM, Morgan AE, and McAuley MT. Aging and computational systems biology. Wiley Interdiscip Rev Syst Biol Med 8: 123–139, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Morimoto RI. The heat shock response: systems biology of proteotoxic stress in aging and disease. Cold Spring Harb Symp Quant Biol 76: 91–99, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Mukherjee S, Kim S, Ramanan VK, Gibbons LE, Nho K, Glymour MM, Ertekin-Taner N, Montine TJ, Saykin AJ, Crane PK, and Alzheimer's Disease Neuroimaging Initiative. Gene-based GWAS and biological pathway analysis of the resilience of executive functioning. Brain Imaging Behav 8: 110–118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mukherjee S, Russell JC, Carr DT, Burgess JD, Allen M, Serie DJ, Boehme KL, Kauwe JS, Naj AC, Fardo DW, Dickson DW, Montine TJ, Ertekin-Taner N, Kaeberlein MR, and Crane PK. Systems biology approach to late-onset Alzheimer's disease genome-wide association study identifies novel candidate genes validated using brain expression data and Caenorhabditis elegans experiments. Alzheimers Dement 13: 1133–1142, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Naj AC, Schellenberg GD, and Alzheimer's Disease Genetics Consortium (ADGC). Genomic variants, genes, and pathways of Alzheimer's disease: an overview. Am J Med Genet B Neuropsychiatr Genet 174: 5–26, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nikolich-Žugich J, Goldman DP, Cohen PR, Cortese D, Fontana L, Kennedy BK, Mohler MJ, Olshansky SJ, Perls T, Perry D, Richardson A, Ritchie C, Wertheimer AM, Faragher RG, and Fain MJ. Preparing for an aging world: engaging biogerontologists, geriatricians, and the society. J Gerontol A Biol Sci Med Sci 71: 435–444, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pauwels E, Lajaunie C, and Vert JP. A Bayesian active learning strategy for sequential experimental design in systems biology. BMC Syst Biol 8: 102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Przybilla J, Rohlf T, Loeffler M, and Galle J. Understanding epigenetic changes in aging stem cells—a computational model approach. Aging Cell 13: 320–328, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qiao N, Huang Y, Naveed H, Green CD, and Han JDJ. CoCiter: an efficient tool to infer gene function by assessing the significance of literature co-citation. PLoS One 8: e74074, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria, 2017 [Google Scholar]
- 91.Radivoyevitch T. and Venkateswaran V. SBMLR: SBML-R Interface and Analysis Tools. 2015
- 92.Raymond ES. The Cathedral and the Bazaar.O'Reilly Media, Sebastopol, CA, 2001 [Google Scholar]
- 93.Shachar R, Ungar L, Kupiec M, Ruppin E, and Sharan R. A systems-level approach to mapping the telomere length maintenance gene circuitry. Mol Syst Biol 4: 172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, and Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shmookler Reis RJ, Bharill P, Tazearslan C, and Ayyadevara S. Extreme-longevity mutations orchestrate silencing of multiple signaling pathways. Biochim Biophys Acta 1790: 1075–1083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simpson MR. Systems biology: impressions from a newcomer graduate student in 2016. Adv Physiol Educ 40: 443–445, 2016 [DOI] [PubMed] [Google Scholar]
- 97.Stallman R. Free community science and the free development of science. PLoS Med 2: e47, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steffen KK, MacKay VL, Kerr EO, Tsuchiya M, Hu D, Fox LA, Dang N, Johnston ED, Oakes JA, Tchao BN, Pak DN, Fields S, Kennedy BK, and Kaeberlein M. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell 133: 292–302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, and von Mering C. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43: D447–D452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tacutu R, Budovsky A, Yanai H, and Fraifeld VE. Molecular links between cellular senescence, longevity and age-related diseases—a systems biology perspective. Aging 3: 1178–1191, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tatar M, Bartke A, and Antebi A. The endocrine regulation of aging by insulin-like signals. Science 299: 1346–1351, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Thobe K, Sers C, and Siebert H. Unraveling the regulation of mTORC2 using logical modeling. Cell Commun Signal 15: 6, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tu CT. and Chen BS. On the increase in network robustness and decrease in network response ability during the aging process: a systems biology approach via microarray data. IEEE/ACM Trans Comput Biol Bioinform 10: 468–480, 2013 [DOI] [PubMed] [Google Scholar]
- 104.Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DEL, and Kaeberlein M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. GeroScience 39: 117–127, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Leeuwen IMM, Vera J, and Wolkenhauer O. Dynamic energy budget approaches for modelling organismal ageing. Philos Trans R Soc Lond B Biol Sci 365: 3443–3454, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vrahatis AG, Balomenos P, Tsakalidis AK, and Bezerianos A. DEsubs: an R package for flexible identification of differentially expressed subpathways using RNA-seq experiments. Bioinformatics 32: 3844–3846, 2016 [DOI] [PubMed] [Google Scholar]
- 107.Wang T, Tsui B, Kreisberg JF, Robertson NA, Gross AM, Yu MK, Carter H, Brown-Borg HM, Adams PD, and Ideker T. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol 18: 57, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, and Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 38: W214–W220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wickham H. and Grolemund G. R for Data Science: Import, Tidy, Transform, Visualize, and Model Data. Beijing, China: O'Reilly Media, 2017 [Google Scholar]
- 110.Wigner EP. The unreasonable effectiveness of mathematics in the natural sciences. Richard courant lecture in mathematical sciences delivered at New York University, May 11, 1959. Commun Pure Appl Math 13: 1–14, 1960 [Google Scholar]
- 111.Wilkinson DJ. Stochastic Modelling for Systems Biology, Second Edition. Boca Raton, FL: CRC Press, 2011 [Google Scholar]
- 112.Wilkinson JE, Burmeister L, Brooks SV, Chan C-C, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, and Miller RA. Rapamycin slows aging in mice. Aging Cell 11: 675–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11: 398–411, 1957 [Google Scholar]
- 114.Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, and Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293: 5530, 2001 [DOI] [PubMed] [Google Scholar]
- 115.Xue H, Xian B, Dong D, Xia K, Zhu S, Zhang Z, Hou L, Zhang Q, Zhang Y, and Han JD. A modular network model of aging. Mol Syst Biol 3: 147, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zahn JM. and Kim SK. Systems biology of aging in four species. Curr Opin Biotechnol 18: 355–359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang W, Liu Y, Sun N, Wang D, Boyd-Kirkup J, Dou X, and Han J-DJ. Integrating genomic, epigenomic, and transcriptomic features reveals modular signatures underlying poor prognosis in ovarian cancer. Cell Rep 4: 542–553, 2013 [DOI] [PubMed] [Google Scholar]
- 118.Zou H. and Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B 67: 301–320, 2005 [Google Scholar]