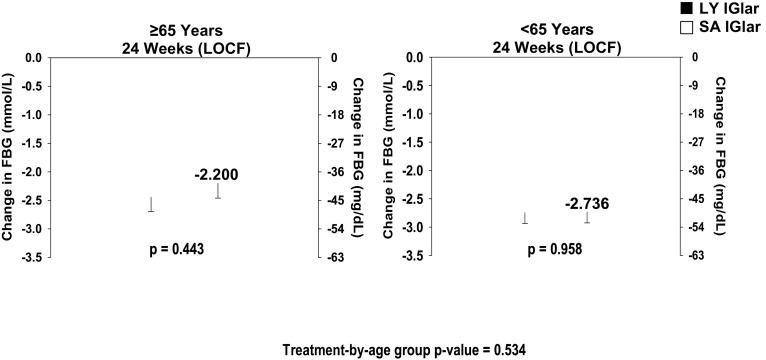Abstract
Introduction
To compare efficacy and safety of Basaglar® [insulin glargine 100 units/mL; LY insulin glargine (LY IGlar)] to Lantus® [insulin glargine 100 units/mL; SA insulin glargine (SA IGlar)] in older (≥ 65 years) or younger (< 65 years) patients with type 2 diabetes (T2D).
Methods
This subgroup analysis of a phase 3, randomized, double-blind, multinational, 24-week study compared LY IGlar and SA IGlar on several clinical efficacy (change in glycated hemoglobin (A1c), basal insulin dose, weight) and safety outcomes (incidence of adverse events, insulin antibodies, hypoglycemia incidence and rates) in patients either ≥ 65 or < 65 years.
Results
Compared with patients aged < 65 years (N = 542), patients aged ≥ 65 years (N = 214) had a significantly longer duration of diabetes; lower baseline A1c and body weight; and body mass index; and were more likely to report prestudy SA IGlar use. Compared to patients < 65 years, patients ≥ 65 years needed a lower basal insulin dose and experienced lower body weight gain. There were no significant treatment-by-age interactions for the clinical efficacy and safety outcomes, indicating that there was no differential treatment effect (LY IGlar vs SA IGlar) for patients ≥ 65 years vs those < 65 years. Moreover, within each age subgroup, LY IGlar and SA IGlar were similar for all clinical efficacy and safety outcomes.
Conclusions
LY IGlar and SA IGlar exhibit similar efficacy and safety in patients with T2D who are ≥ 65 years and in those < 65 years.
Trial Registration
ClinicalTrials.gov trial registration: NCT01421459.
Funding
Eli Lilly and Company and Boehringer-Ingelheim.
Keywords: Age, Efficacy, Insulin, Safety, Type 2 diabetes
Plain Language Summary
Plain language summary available for this article.
The aim of this phase 3 clinical study was to compare the efficacy and safety of two drugs, Basaglar® (LY IGlar) and Lantus (SA IGlar), in patients with type 2 diabetes that were either 65 years of age and/or older or younger than 65 years of age. This study ran for 24 weeks. The factors used to measure efficacy were changes in glycated hemoglobin (A1c), insulin dose, and weight. The safety outcomes were incidence of adverse events, incidence and levels of insulin antibodies, and the incidence and rate of low blood sugar. Compared with patients less than 65 years of age (N = 542), patients 65 years of age and older (N = 214) had diabetes for a significantly longer time period; had a lower baseline A1c, body weight, and body mass index; and were more likely to report that they used SA IGlar prestudy. Compared to patients less than 65 years of age, patients equal to or older than 65 years of age showed significantly smaller increases in insulin dose and body weight. There were no significant treatment-by-age interactions for the efficacy and safety outcomes, indicating that there was no difference in treatment effect (LY IGlar vs SA IGlar) for patients equal to or older than 65 years of age vs those less than 65 years of age. Moreover, within each age subgroup, LY IGlar and SA IGlar were similar for all clinical efficacy and safety outcomes. LY IGlar and SA IGlar have similar efficacy and safety in patients with T2D who are equal to or older than 65 years of age and in those less than 65 years of age.
Introduction
It is anticipated that from 2000 to 2030, the prevalence of diabetes among adults older than 64 years is expected to increase with estimates ranging from 48 million in developed countries to more than 82 million in developing countries [1]. Older adults with diabetes often present with other comorbid conditions that limit self-care abilities and impact health outcomes and quality of life [2]. Maintaining glycemic control can be challenging in this population because of cognitive deficits and increased functional decline, which may impact the ability to provide self-care [3]. Additionally, older adults are more likely to take multiple medications, which may contribute to increased risks, such as urinary incontinence, falls, and fractures [2]. Once diagnosed, many older adults with diabetes may remain under the care of a primary care provider who should customize treatment on the basis of the clinical and functional heterogeneity of this population [2, 4–6].
Insulin glargine is an initial insulin treatment option and part of basal-bolus therapy in patients with type 2 diabetes (T2D) who are not achieving glycemic control with their current treatment [7]. Older adults with T2D may benefit from insulin glargine treatment because of prolonged duration of action allowing for once-daily dosing and lower risk of hypoglycemia relative to neutral protamine Hagedorn (NPH) [8–10] or other comparators [10]. Basaglar® [insulin glargine 100 units/mL, LY insulin glargine (LY IGlar); Eli Lilly and Company, Indianapolis, IN, USA] is the first authorized biosimilar insulin in the European Union [11]. LY IGlar has an identical primary amino acid sequence to that of Lantus® [insulin glargine 100 units/mL, SA insulin glargine (SA IGlar); Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany] [11]. Both LY IGlar and SA IGlar have highly similar preclinical, efficacy, safety, and immunogenicity profiles in patients with type 1 diabetes and T2D [11–14]. To determine whether these similarities in efficacy and safety profiles of LY IGlar and SA IGlar are also true for older adults (≥ 65 years) with T2D, this subgroup analysis compared the efficacy and safety of LY IGlar to SA IGlar in patients with T2D from the ELEMENT-2 study on the basis of age (≥ or < 65 years) at study entry.
Methods
Study Design
The ELEMENT-2 study was a phase 3, multinational, randomized, double-blind, 24-week study in patients with T2D. Details of the ELEMENT-2 study have been previously reported [11], and a post hoc study from ELEMENT-2 is reported here. The study conduct conformed to the ethical principles described in the Declaration of Helsinki [15] and written informed consent was obtained from all patients. The ELEMENT-2 study was registered at ClinicalTrials.gov (NCT01421459).
Adult patients (≥ 18 years) with T2D were included in the study if they were either insulin-naïve or reported prior SA IGlar treatment, received at least two oral antihyperglycemic medications (OAMs) with or without SA IGlar during the 12 weeks before screening, had a body mass index (BMI) ≤ 45 kg/m2, and had glycated hemoglobin (A1c) levels ≥ 7.0% and ≤ 11.0% (if insulin-naïve) or ≤ 11.0% (patients with prior SA IGlar experience). Patients were excluded if they reported prestudy treatment with pramlintide or insulin other than SA IGlar within the previous 30 days, received basal-bolus therapy, required a total daily insulin dose of at least 1.5 U/kg, or experienced more than one severe hypoglycemic episode within the prior 6 months [11].
On the basis of their randomization allocation, patients who reported prestudy SA IGlar at study entry received an initial dose of LY IGlar or SA IGlar that was the same as their prestudy SA IGlar dose. Patients who were insulin-naïve at randomization received an initial 10 U/day dose of LY IGlar or SA IGlar [11]. During the 12-week titration period, all patients followed a patient-driven titration schedule where 1 unit of basal insulin per day was added until fasting plasma glucose (FPG) levels ≤ 5.6 mmol/L (100 mg/dL) were attained [16]. Covered vials and insulin syringes were used to administer the assigned study treatment in order to maintain blinding [11].
Statistical Analysis
The efficacy and safety of LY IGlar and SA IGlar were evaluated in patients aged ≥ 65 years and in patients aged < 65 years. Analyses were based on the full analysis set, which included all randomized patients who received at least one dose of study drug [11]. For insulin antibody level assessment, the analysis population was defined as all randomized patients who received at least one dose of study drug and had a baseline and at least one post-baseline insulin antibody level assessment [14]. Prespecified analyses comparing LY IGlar to SA IGlar in both age subgroups included change from baseline in A1c at 24 weeks, the primary efficacy outcome, change in body weight, hypoglycemia (total, severe, and nocturnal), serious adverse events (SAEs), treatment-emergent adverse events, adverse events (AEs) leading to discontinuation, allergic events, injection site reactions, and insulin antibodies as a categorical outcome [treatment-emergent antibody response (TEAR)]. Post hoc analyses included the proportion of patients achieving A1c targets, basal insulin dose, FPG, insulin antibody levels and documented symptomatic hypoglycemia. Daily mean blood glucose was derived from the
7-point self-monitored blood glucose (SMBG) as an average across all the time points in the daily SMBG profile (i.e., pre-meal for each meal, post-meal of breakfast and lunch, bedtime, and 3 A.M.). SMBG profiles were collected three times in the 2 weeks before each clinic visit and measured using study-provided glucometers. TEAR was defined as having a percentage antibody binding of at least 1.26% for patients with nondetectable antibodies at baseline, or at least 1% (absolute) and 30% (relative) above baseline values for patients with detectable antibodies at baseline [14]. Hypoglycemia was defined as having a blood glucose ≤ 3.9 mmol/L (70 mg/dL), consistent with European Medicine Agency [17] and American Diabetes Association [18] guidelines.
An analysis of covariance (ANCOVA) model was used to analyze continuous data (A1c change, weight change); the ANCOVA model included baseline A1c, country, sulfonylurea use, time of basal insulin injection [A.M., (P.M. or bedtime)], and treatment as fixed effects, baseline value of response variable as a covariate, and subgroup (≥ 65 years, < 65 years) and subgroup-by-treatment interaction. The Mantel–Haenszel test was used to analyze categorical data. The Wilcoxon test was used to analyze treatment comparisons for insulin antibodies and hypoglycemia rate. SAS Version 9.1.3 (SAS Drug Development, Cary, NC, USA) was used to analyze data.
Results
Baseline Characteristics
Of the 756 patients enrolled, 214 (28.3%) were ≥ 65 years old and 542 (71.7%) were < 65 years old. Patients aged ≥ 65 years were more likely to be white, more likely to report prior SA IGlar use, have a significantly longer duration of diabetes, and have significantly lower A1c, body weight, and body mass index than patients < 65 years. Significantly fewer patients 65 years or older had normal renal function status. Other baseline characteristics were similar between both age subgroups (Table 1).
Table 1.
Baseline demographics and patient characteristics
| Variable | ≥ 65 years (N = 214) | < 65 years (N = 542) | p value |
|---|---|---|---|
| Age, years | 70.42 (4.35) | 54.25 (7.77) | < 0.001 |
| Age, LY IGlar/SA IGlar, years (%) | 29.8/26.8 | 70.2/73.2 | 0.376 |
| Sex, male, n (%) | 103 (48.1) | 275 (50.7) | 0.572 |
| Race, n (%) | < 0.001 | ||
| American Indian or Alaska Native | 2 (0.9) | 36 (6.6) | |
| Asian | 14 (6.5) | 50 (9.2) | |
| Black or African American | 9 (4.2) | 49 (9.0) | |
| Multiple | 0 (0.0) | 3 (0.6) | |
| White | 189 (88.3) | 404 (74.5) | |
| Duration of diabetes, years | 14.42 (7.42) | 10.28 (6.17) | < 0.001 |
| Weight (kg) | 85.95 (18.60) | 91.72 (19.80) | < 0.001 |
| BMI (kg/m2) | 30.70 (5.35) | 32.37 (5.44) | < 0.001 |
| Glycated hemoglobin (%) | 8.06 (0.99) | 8.43 (1.09) | < 0.001 |
| Sulfonylurea use (yes), n (%) | 183 (85.5) | 447 (82.5) | 0.332 |
| Time of basal insulin injection [AM/(PM or bedtime)], % | 47.2/52.8 | 50.6/49.4 | 0.420 |
| Renal function status, n (%) | < 0.001 | ||
| Normal GFR (> 90 mL/min/1.73 m2) | 70 (32.7) | 440 (81.2) | |
| Mild reduction in GFR (60–89 mL/min/1.73 m2) | 112 (52.3) | 88 (16.2) | |
| Moderate reduction in GFR (30–59 mL/min/1.73 m2) | 31 (14.5) | 13 (2.4) | |
| Basal insulin (%), SA IGlar/none | 45.3/54.7 | 37.3/62.7 | 0.047 |
Data are mean (SD) unless otherwise indicated
BMI body mass index, GFR glomerular filtration rate, LY IGlar LY2963016 insulin glargine, N total number of patients, SA IGlar insulin glargine, SD standard deviation
Efficacy
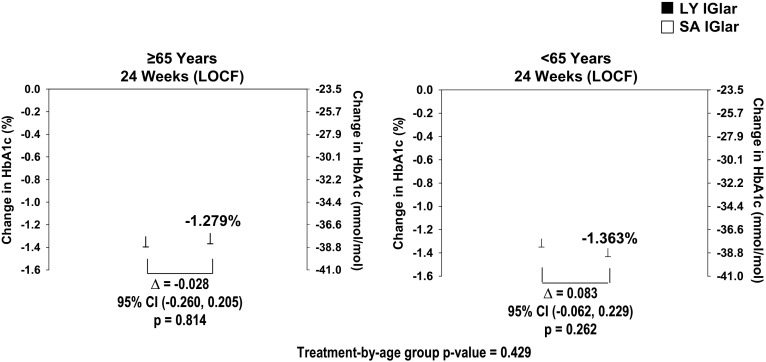
Older (≥ 65 years) and younger (< 65 years) patients in both treatment groups showed similar reductions in A1c at the 24-week endpoint [last observation carried forward (LOCF), ≥ 65 years: least squares mean (LSM) ± standard error [SE] LY IGlar: − 5.6 ± 2.2%, SA IGlar − 5.6 ± 2.2%, p = 0.814; < 65 years: LY IGlar: − 5.6 ± 2.2%; SA IGlar: − 5.7 ± 2.2%, p = 0.262) (Fig. 1). No statistically significant age subgroup difference was observed for A1c (p = 0.700). The percentage of patients achieving their glycemic targets (A1c < 7%) was similar for both LY IGlar- and SA IGlar-treated patients in patients aged ≥ 65 years [LY IGlar: 55 (50.0%), SA IGlar: 55 (53.9%), p = 0.569] and in those aged < 65 years [LY IGlar: 125 (48.3%), SA IGlar: 142 (52.0%), p = 0.387].
Fig. 1.
Baseline-to-endpoint changes in A1c in patients with type 2 diabetes ≥ 65 and < 65 years. Data are least squares mean ± standard error. CI confidence interval, LOCF last observation carried forward, LY IGlar LY2963016 insulin glargine, SA IGlar insulin glargine
Patients ≥ 65 years who were treated with LY IGlar or SA IGlar showed similar decreases in daily mean blood glucose (LSM ± SE, LY IGlar: − 2.074 ± 0.25 mmol/L, SA IGlar: − 1.919 ± 0.26 mmol/L, p = 0.617). Similar findings were observed in patients aged < 65 years (LY IGlar: − 2.250 ± 0.19 mmol/L, SA IGlar: − 2.382 ± 0.19 mmol/L, p = 0.487). There was no statistically significant effect of age for daily mean blood glucose (p = 0.086). No treatment differences between LY IGlar and SA IGlar were observed for FBG by SMBG in either age subgroup (Fig. 2).
Fig. 2.
Baseline-to-endpoint changes in FBG by SMBG in patients with type 2 diabetes ≥ 65 and < 65 years. Data are least squares mean ± standard error. FBG fasting blood glucose, LOCF last observation carried forward, LY IGlar LY2963016 insulin glargine, SA IGlar insulin glargine, SMBG self-monitored blood glucose
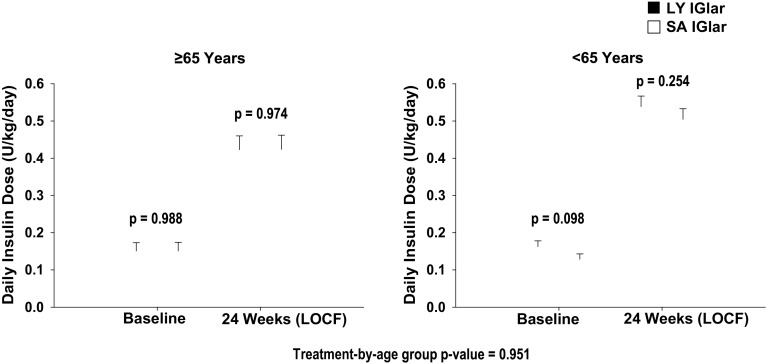
Similar increases in basal insulin dose were observed in LY IGlar- and SA IGlar-treated patients across both age subgroups (Fig. 3). Basal insulin dose increased in both age subgroups at the 24-week endpoint (LOCF); however, the increase was significantly smaller in patients ≥ 65 years old (age group p < 0.001). Both treatment groups showed similar increases in body weight in patients ≥ 65 years old (LY IGlar: 1.412 ± 0.372 kg, SA IGlar: 1.397 ± 0.382 kg, p = 0.975) and < 65 years old (LY IGlar: 1.978 ± 0.281 kg, SA IGlar: 2.298 ± 0.279 kg, p = 0.282). However, patients aged ≥ 65 years exhibited statistically significantly smaller increases in body weight than patients under 65 years at the 24-week endpoint (LOCF) (age group p = 0.012).
Fig. 3.
Baseline-to-endpoint changes in basal insulin dose in patients with type 2 diabetes ≥ 65 and < 65 years. Data are least squares mean ± standard error. FBG fasting blood glucose, LOCF last observation carried forward, LY IGlar LY2963016 insulin glargine, SA IGlar insulin glargine
Safety
The incidence and 1-year adjusted rates of total, documented symptomatic, and nocturnal hypoglycemia were similar for both LY IGlar and SA IGlar, regardless of age subgroup (Fig. 4). Too few patients (≤ 10) experienced severe hypoglycemia for valid statistical analysis as prespecified in the Statistical Analysis Plan (LY IGlar: 3 patients; SA IGlar: 2 patients).
Fig. 4.
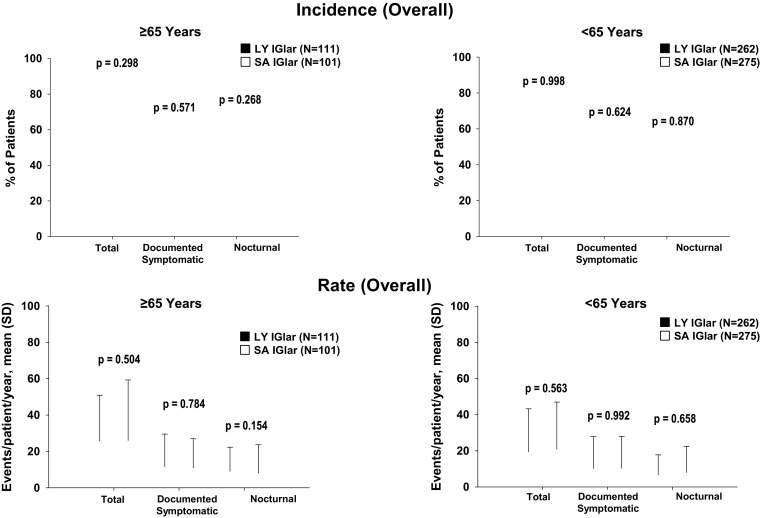
Overall incidence and rate of total, documented symptomatic, and nocturnal hypoglycemia in patients with type 2 diabetes ≥ 65 and < 65 years. Data for overall rate are presented as mean and SD. Hypoglycemia is defined as BG ≤ 3.9 mmol/L (70 mg/dL) or signs or symptoms of hypoglycemia. Overall refers to any time during the post-randomization visits. The treatment-by-age subgroup interaction values for the incidence of total, documented symptomatic, and nocturnal hypoglycemia are 0.362, 0.459, and 0.389, respectively. The treatment-by-age subgroup interaction values for rates of total, documented symptomatic, and nocturnal hypoglycemia are 0.737, 0.769, and 0.310, respectively. BG blood glucose, LY IGlar LY2963016 insulin glargine, SA IGlar insulin glargine, SD standard deviation
The overall proportion of patients with TEAR was similar in both treatment groups regardless of age subgroup [≥ 65 years, LY IGlar: 3 (2.8%), SA IGlar: 1 (1.0%), p = 0.363; < 65 years, LY IGlar: 11 (4.3%), SA IGlar: 13 (4.9%), p = 0.747]. Median insulin antibody levels (percent binding) were similar for LY IGlar- and SA IGlar-treated patients in both age subgroups (Table 2). Likewise, both treatment groups in each age subgroup showed similar incidences of AEs and SAEs (Table 3). Two patients (1 LY IGlar, 68 years and 1 SA IGlar, 67 years) died during the study. Neither death was considered by the investigator to be related to study drug.
Table 2.
Insulin antibodies in patients
| ≥ 65 years | p value | < 65 years | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LY IGlar | SA IGlar | LY IGlar | SA IGlar | |||||||
| N | n (%) | N | n (%) | N | N (%) | N | N (%) | |||
| Proportion of patients with detectable antibodies | ||||||||||
| Baseline | 107 | 5 (4.7) | 97 | 1 (1.0) | 0.215 | 258 | 15 (5.8) | 268 | 12 (4.5) | 0.556 |
| 24-week endpoint (LOCF) | 107 | 3 (2.8) | 97 | 3 (3.1) | > 0.999 | 258 | 27 (10.5) | 268 | 19 (7.1) | 0.217 |
| Overalla | 107 | 12 (11.2) | 97 | 4 (4.1) | 0.071 | 258 | 44 (17.1) | 268 | 36 (13.4) | 0.275 |
| N | Median (Q1, Q3) | N | Median (Q1, Q3) | p value | N | Median (Q1, Q3) | N | Median (Q1, Q3) | p value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Endpoint median insulin antibody levels | ||||||||||
| Baseline | 5 | 2.32 (0.44–2.97) | 1 | 0.96 (0.96–0.96) | > 0.999 | 15 | 0.71 (0.46–1.54) | 12 | 0.44 (0.34–0.78) | 0.251 |
| 24-week endpoint (LOCF) | 3 | 1.96 (1.11–5.66) | 3 | 0.45 (0.26–4.38) | 0.383 | 27 | 0.99 (0.38–5.14) | 19 | 0.65 (0.36–2.76) | 0.616 |
Values for N included in the analysis comprised only patients with detected or non-detected insulin antibody levels at baseline and post-baseline
The unit of measurement for insulin antibodies is percent binding
IQR interquartile range, LOCF last observation carried forward, LY IGlar LY2963016 insulin glargine, Q1 25th percentile, Q3 75th percentile, SA IGlar insulin glargine
aOverall refers to measurements taken during the 24-week treatment period and not at any specific visit or at endpoint (LOCF)
Table 3.
Adverse events summary for patients ≥ 65 and < 65 years
| Adverse events, n (%) | ≥ 65 years | p value | < 65 years | p value | Treatment-by-age subgroup interaction | ||
|---|---|---|---|---|---|---|---|
| LY IGlar N = 112 | SA IGlar N = 102 | LY IGlar N = 264 | SA IGlar N = 278 | ||||
| Patients with ≥ 1 TEAE | 63 (56.3) | 56 (54.9) | 0.843 | 133 (50.4) | 128 (46.0) | 0.313 | 0.714 |
| Special topic assessmenta | 5 (4.5) | 7 (6.9) | 0.447 | 16 (6.1) | 20 (7.2) | 0.597 | 0.695 |
| Injection site reactions | 4 (3.6) | 1 (1.0) | 0.211 | 9 (3.4) | 8 (2.9) | 0.723 | 0.337 |
| Patients with ≥ 1 SAE | 8 (7.1) | 11 (10.8) | 0.351 | 7 (2.7) | 7 (2.5) | 0.922 | 0.487 |
INT interaction, LY IGlar LY2963016 insulin glargine, N number of evaluable patients, n number of patients with TEAE, SAE serious adverse event, SA IGlar insulin glargine, TEAE treatment-emergent adverse event
aCategories of adverse events also include special topic assessment of adverse (allergic) events, injection site reactions, and SAEs though overall events are less than 5%
Relationship Between Age and Clinical Outcomes
The change from baseline to endpoint (LOCF) for the clinical efficacy (Figs. 1–3 and p > 0.05 for weight) and safety (Fig. 4 and Tables 2 and 3) outcome measures was similar for each treatment group regardless of age. No statistically significant treatment-by-age interaction was observed for patients in either age subgroup.
Discussion
The results of these subgroup analyses demonstrate similar clinical efficacy and safety outcomes within each age group for patients who receive LY IGlar or SA IGlar. Moreover, no effect of age was observed for any of the clinical efficacy and safety outcomes, except for basal insulin dose and body weight change. Older patients (≥ 65 years) required a lower basal insulin dose and gained less weight than younger patients (< 65 years). The effects of age on insulin dose and weight are consistent with previous reports of randomized controlled studies that evaluated insulin glargine in older (≥ 65 years) and younger (< 65 years) adults with T2D [9, 10].
This subgroup analysis of elderly patients (≥ 65 years) enrolled in the double-blind, phase 3 study showed similar hypoglycemic rates to patients under 65 years, which are consistent with hypoglycemia results seen in other studies comparing insulin glargine and NPH in older adults with T2D [9, 10]. In our subgroup analysis, 2 patients (1 LY IGlar, 1 SA IGlar) ≥ 65 years and 3 patients (2 LY IGlar, 1 SA IGlar) < 65 years reported severe hypoglycemic events.
The risk of hypoglycemia is an important consideration when treating older adults with T2D. Older adults may not recognize the signs of hypoglycemia, particularly if they have cognitive deficits or comorbid diseases that make self-monitoring of glucose challenging [5, 8, 19, 20]. In addition, with hypoglycemic events, there is an additional concern of related complications, such as injuries from falls [21]. Treatment guidelines recommend a glycemic-improving medicine with a lower risk of hypoglycemia for older patients at moderate risk of hypoglycemia [5]. Therefore, insulin glargine may be a useful treatment option in older patients because of its lower risk of hypoglycemia vs other comparators (e.g., NPH) [8–10, 19]. Combining insulin glargine with OAM, such as metformin or glimepiride, compared with premixed insulins has been effective in reducing A1c with a lower risk of hypoglycemia when OAMs are no longer effective in achieving glycemic targets [22].
In our subgroup analysis, AEs in the LY IGlar and SA IGlar groups were similar. Four LY IGlar patients ≥ 65 years reported injection site reactions (Table 3), which were characterized by rash or redness, or pain at the injection site, and were mild to moderate in severity, and the patients recovered from the event. One SA IGlar patient ≥ 65 years reported an injection site reaction, which was severe in intensity, but not characterized by rash or redness at the injection site and the patient recovered from the event.
Elderly patients with diabetes often have more comorbidities [2]; however, patients with significant cardiac disease and active cancers were excluded from our study. Therefore, the older age (≥ 65 years) subgroup including 34 (15.9%) patients (≥ 75 years) may have been more representative of an older population that has fewer comorbid health problems. Considering this study’s limitation, it is important to remember, as experts and professional organizations recommend, that health care providers need to customize treatment on the basis of a patient’s lifestyle, health status, risk factors, cognitive function, medical history, and social support [2, 4, 5].
Conclusions
Our results demonstrate that LY IGlar and SA IGlar exhibit similar efficacy and safety in patients with T2D who are aged ≥ 65 years and in those who are aged < 65 years. For adult patients with T2D who require basal insulin as part of their treatment regimen, LY IGlar is an alternative basal insulin glargine that may be used with the same dose titration as SA IGlar.
Acknowledgements
Funding
This study, article processing charges, and editorial assistance were funded by Eli Lilly and Company (Indianapolis, IN, USA) and Boehringer-Ingelheim (Ingelheim am Rhein, Germany). The funding organization participated in study design, data collection and analysis, and manuscript preparation.
Medical Writing and Editorial Assistance
The authors acknowledge Eileen Girten, MS, and Barbara Nambu, PhD, of Syneos Health (formerly INC Research/inVentiv Health), for assistance with the preparation of this manuscript. Support for this assistance was funded by Eli Lilly and Company and Boehringer-Ingelheim.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Robyn K. Pollom participated in the interpretation of the research and in the critical revision of the manuscript. Timothy Costigan participated in the conception, design, analysis, and interpretation of the research and the critical revision of the manuscript. Lyndon B. Lacaya participated in the conception, design, acquisition of data for, the analysis, and interpretation of the research and in the writing and critical revision of the manuscript. Liza L. Ilag participated in the conception, design, acquisition of data for, and interpretation of the research and in the writing and critical revision of the manuscript. Prisicilla A. Hollander participated in the analysis and interpretation of the research and the critical revision of the manuscript. All authors have given final approval of the manuscript.
Prior Presentation
This work was previously published as an abstract presented at the American Diabetes Association’s 76th Scientific Sessions, New Orleans, Louisiana, June 2016 (964-P).
Disclosures
Robyn K. Pollom is an employee of and holds stock in Eli Lilly and Company. Timothy Costigan is an employee of and holds stock in Eli Lilly and Company. Lyndon B. Lacaya is an employee of and holds stock in Eli Lilly and Company. Liza L. Ilag is an employee of and holds stock in Eli Lilly and Company. Priscilla A. Hollander has served on scientific advisory panels for Johnson & Johnson, Merck, Novo Nordisk, and Roche Pharmaceuticals.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1964 Declaration of Helsinki, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to 10.6084/m9.figshare.5951947.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes, older adults. Diabetes Care. 2016;39(Suppl 1):S81–S85. doi: 10.2337/dc16-S013. [DOI] [PubMed] [Google Scholar]
- 3.Ligthelm RJ, Kaiser M, Vora J, Yale JF. Insulin use in elderly adults: risk of hypoglycemia and strategies for care. J Am Geriatr Soc. 2012;60:1564–1570. doi: 10.1111/j.1532-5415.2012.04055.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. Diabetes Care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Diabetes Federation. IDF global guideline for managing older people with type 2 diabetes. 2013. https://www.idf.org/our-activities/advocacy-awareness/resources-and-tools/78:global-guideline-for-managing-older-people-with-type-2-diabetes.html. Accessed 29 Nov 2017.
- 6.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Meneilley GS, Knip A, Tessier D. Diabetes in the elderly. Can J Diabetes. 2013;37(Suppl 1):S184–S190. doi: 10.1016/j.jcjd.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 8.Kim KS, Kim SK, Sung KM, Cho YW, Park SW. Management of type 2 diabetes mellitus in older adults. Diabetes Metab J. 2012;36:336–344. doi: 10.4093/dmj.2012.36.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee P, Chang A, Blaum C, Vlajnic A, Gao L, Halter J. Comparison of safety and efficacy of insulin glargine and neutral protamine Hagedorn insulin in older adults with type 2 diabetes mellitus: results from a pooled analysis. J Am Geriatr Soc. 2012;60:51–59. doi: 10.1111/j.1532-5415.2011.03773.x. [DOI] [PubMed] [Google Scholar]
- 10.Pandya N, DiGenio A, Gao L, Patel M. Efficacy and safety of insulin glargine compared to other interventions in younger and older adults: a pooled analysis of nine open-label, randomized controlled trials in patients with type 2 diabetes. Drugs Aging. 2013;30:429–438. doi: 10.1007/s40266-013-0069-9. [DOI] [PubMed] [Google Scholar]
- 11.Rosenstock J, Hollander P, Bhargava A, et al. Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin-naïve or previously treated with insulin glargine: a randomized, double-blind controlled trial (the ELEMENT 2 study) Diabetes Obes Metab. 2015;17:734–741. doi: 10.1111/dom.12482. [DOI] [PubMed] [Google Scholar]
- 12.Linnebjerg H, Lam EC, Seger ME, et al. Comparison of the pharmacokinetics and pharmacodynamics of LY2963016 insulin glargine and EU- and US-approved versions of Lantus insulin glargine in healthy subjects: three randomized euglycemic clamp studies. Diabetes Care. 2015;38:2226–2233. doi: 10.2337/dc14-2623. [DOI] [PubMed] [Google Scholar]
- 13.Blevins TC, Dahl D, Rosenstock J, et al. Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus®) in patients with type 1 diabetes in a randomized controlled trial: the ELEMENT 1 study. Diabetes Obes Metab. 2015;17:726–733. doi: 10.1111/dom.12496. [DOI] [PubMed] [Google Scholar]
- 14.Ilag LL, Deeg MA, Costigan T, et al. Evaluation of immunogenicity of LY2963016 insulin glargine compared with Lantus® insulin glargine in patients with type 1 or type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18:159–168. doi: 10.1111/dom.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association Declaration of Helsinki Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. doi: 10.1001/jama.1997.03540350075038. [DOI] [PubMed] [Google Scholar]
- 16.Gerstein HC, Yale JF, Harris SB, et al. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet Med. 2006;23:736–742. doi: 10.1111/j.1464-5491.2006.01881.x. [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf. Accessed 9 Mar 2016.
- 18.American Diabetes Association Workgroup on Hypoglycemia Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–1249. doi: 10.2337/diacare.28.5.1245. [DOI] [PubMed] [Google Scholar]
- 19.Janka HU. Insulin therapy in elderly patients with type 2 diabetes: the role of insulin glargine. Diabetes Obes Metab. 2008;10(Suppl 2):35–41. doi: 10.1111/j.1463-1326.2008.00843.x. [DOI] [PubMed] [Google Scholar]
- 20.Mooradian AD. Special considerations with insulin therapy in older adults with diabetes mellitus. Drugs Aging. 2011;28:429–438. doi: 10.2165/11590570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Moghissi E. Management of type 2 diabetes mellitus in older patients: current and emerging treatment options. Diabetes Ther. 2013;4:239–256. doi: 10.1007/s13300-013-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manucci E, Cremasco F, Romoli E, Rossi A. The use of insulin in elderly patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2011;12:2865–2881. doi: 10.1517/14656566.2011.633512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.