Abstract
Background
We aimed to identify the candidate prostate cancer patients suitable for neoadjuvant androgen-deprivation therapy (ADT) with radical prostatectomy (RP).
Materials and methods
This study included 711 Japanese patients with clinically localized prostate cancer who were treated with RP between 2000 and 2013. Patients were treated with or without neoadjuvant ADT before RP. The prognostic significance of neoadjuvant ADT on biochemical recurrence (BCR) was analyzed according to various clinicopathological characteristics.
Results
BCR occurred in 186 (26.2%) of 711 patients. The group treated with neoadjuvant ADT showed higher levels of prostate-specific antigen at diagnosis and advanced clinical T-stage, but suppressed pathological T-stage. Neoadjuvant ADT was not associated with the risk of BCR. In subgroup analysis, neoadjuvant ADT was significantly associated with increased BCR in patients aged >65 years [hazard ratio (95% confidence interval), 2.04 (1.13–3.43), P = 0.020]. Among the 53 patients with available serum testosterone levels, neoadjuvant ADT was associated with the risk of BCR according to serum testosterone levels.
Conclusion
This study demonstrated that neoadjuvant ADT showed potential deleterious effects in older patients and patients with lower serum testosterone levels, while a possible improved prognosis in patients with high serum testosterone levels treated with neoadjuvant ADT was suggested, warranting further exploration.
Keywords: Age, Neoadjuvant androgen-deprivation therapy, Prostate cancer, Radical prostatectomy, Testosterone
1. Introduction
Prostate cancer is the second most common cancer and the second leading cause of cancer-related mortality among patients in developed countries.1 Radical prostatectomy (RP) has been the gold standard treatment for clinically localized prostate cancer.2 Though most patients with prostate cancer are successfully treated with RP, approximately 17–53% of patients experience biochemical recurrence (BCR) within 10 years.3, 4 Therefore, multimodal approaches such as neoadjuvant or adjuvant therapy combined with RP have been investigated to improve the oncological outcome for high-risk prostate cancer.5 Among these approaches, adjuvant radiotherapy6 and adjuvant androgen-deprivation therapy (ADT)7 have been proven to improve overall survival (OS) in prostate cancer invasive to seminal vesicle and metastatic to regional lymph node, respectively. ADT reduces androgen production and inhibits androgen action; it is the standard therapy for metastatic prostate cancer.8 Although the hypothesis that neoadjuvant ADT may improve oncological outcome has been investigated, most randomized studies failed to show improved BCR-free survival as well as OS after RP, although pathological stage and surgical margin status have been improved by neoadjuvant ADT.9, 10, 11 Consistent with these findings, in systematic review and meta-analysis, OS was not improved by neoadjuvant ADT with RP.12 With the aim of improving outcome, several studies are investigating whether the combination of novel agents such as abiraterone acetate, enzalutamide, and taxanes with ADT before RP improves outcome; however, no results have been obtained showing improved prognosis.
The low impact of neoadjuvant ADT on BCR may partially be due to the delay before surgical intervention, which may result in cancer progression during ADT treatment in the subgroup of patients that may be less sensitive to ADT. Therefore, identifying the subgroup sensitive to neoadjuvant ADT would be useful. In this study, we performed subgroup analyses among patients who had undergone RP with or without neoadjuvant ADT on outcome.
2. Materials and methods
2.1. Patients
This study enrolled 711 patients who were histopathologically diagnosed with adenocarcinoma of the prostate and treated with open RP, laparoscopic RP, or robot-assisted laparoscopic RP as the primary treatment for prostate cancer at Kyushu University Hospital (Fukuoka, Japan) between 2000 and 2013. Patients diagnosed with metastasis by imaging modalities including computed tomography scan and bone scan and patients with a history of neoadjuvant/adjuvant radiotherapy or chemotherapy and adjuvant ADT against prostate cancer were excluded. Lymph node dissection for the bilateral regions along the external and internal iliac vessels and within the obturator fossa was performed according to the operation method, and the risk of lymph node involvement was determined by preoperative cancer risk.13, 14 This study was approved by the institutional review board.
2.2. Data collection
Available data for patients enrolled in this investigation included age, prostate-specific antigen (PSA) level at diagnosis, clinical and pathological stages, biopsy and pathological Gleason score, and serum testosterone levels within 3 months before treatment if available. Blood was obtained from patients between 8:00 AM and 10:00 AM, and serum testosterone level was measured by an electrochemiluminescence immunoassay method.15 Neoadjuvant ADT was performed mainly for the following reasons: (1) initiation in previous institutions; (2) high-risk prostate cancer; and (3) volume reduction for large prostate. Patients were treated by neoadjuvant ADT with surgical castration or medical castration using a luteinizing hormone-releasing hormone agonist (goserelin acetate or leuprorelin acetate)/antagonist (degarelix acetate) and/or an anti-androgen agent (bicalutamide, flutamide, or chlormadinone acetate). Of these, 49, 9, and 17 patients were treated with combined androgen blockade by castration with anti-androgen agent, castration alone, and anti-androgen agent alone, respectively. The median duration of neoadjuvant ADT was 4 months (interquartile range, 2–8 months). BCR was defined as absent for postoperative PSA levels <0.2 ng/mL, or present in the event of two consecutive postoperative PSA levels ≥0.2 ng/mL.
2.3. Statistical analysis
All statistical analyses were performed using the JMP11 software (SAS Institute, Cary, NC, USA). BCR-free survival was determined by the Kaplan–Meier method, and the log-rank test was used to compare survival durations across risk groups. Univariate and multivariate analyses were performed using the Cox proportional hazards regression model to estimate the hazard ratio. The predictive role of subgroup for recurrence-free survival (RFS) of neoadjuvant ADT was investigated by the interaction test. Correlations between parameters were examined by Pearson Chi-square test or Wilcoxon rank sum test. All P values were two-sided, and P values < 0.05 were considered significant.
3. Results
3.1. Patient characteristics
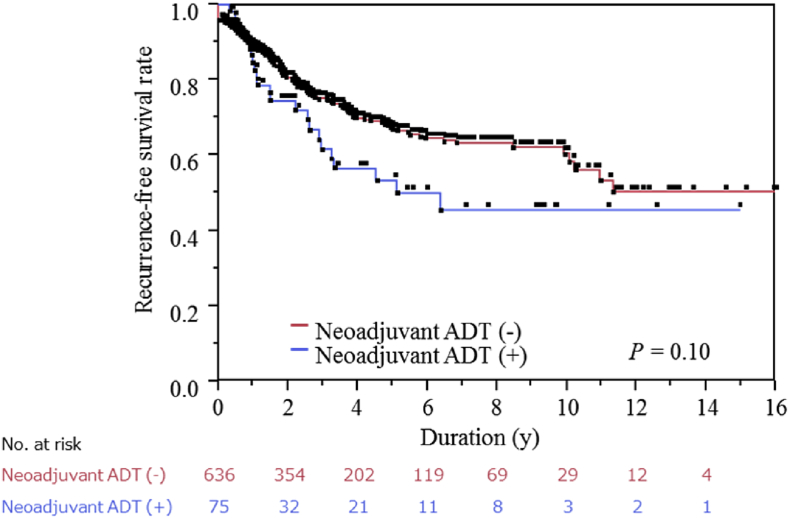
This study included 711 Japanese men who underwent RP for prostate cancer. During the median observation period of 2.2 years (interquartile range, 0.8–4.9 years), a total of 186 (26.2%) patients experienced BCR. As shown in Table 1, PSA levels at diagnosis were higher and advanced T-stage was more prevalent in the neoadjuvant group (75 patients), reflecting the preferred introduction of neoadjuvant ADT for high-risk prostate cancer. Pathological T-stage was lower in the neoadjuvant group, in which pT0 was observed in 8 (11.9%) of 75 patients who had undergone neoadjuvant ADT due to tumor regression by ADT. However, the BCR-free survival rate was lower in patients treated with neoadjuvant ADT (median RFS, 6.4 years) than in those treated without neoadjuvant ADT (median RFS, not yet reached), although the difference was statistically insignificant (Fig. 1, Table 2), reflecting the difference in the clinicopathological background between patients treated with and without neoadjuvant ADT.
Table 1.
Characteristics of patients who underwent radical prostatectomy with or without neoadjuvant androgen-deprivation therapy.
| Variable | All (n = 711) | Neoadjuvant ADT |
P | |
|---|---|---|---|---|
| Not performed (n = 636) | Performed (n = 75) | |||
| Median age, y (IQR) | 66 (61–70) | 66 (61–70) | 67 (63–70) | 0.25 |
| Median PSA at diagnosis, ng/mL (IQR) | 7.9 (5.6–12.2) | 7.8 (5.5–11.9) | 10.3 (6.7–15.5) | 0.0013a) |
| NA | 7 | 4 | 3 | |
| Biopsy Gleason score, n (%) | ||||
| <7 | 251 (37.1) | 231 (37.7) | 20 (31.7) | |
| 7 | 309 (45.7) | 281 (45.8) | 28 (44.4) | |
| >7 | 116 (17.2) | 101 (16.5) | 15 (23.8) | 0.31 |
| NA | 35 | 23 | 12 | |
| Clinical T-stage, n (%) | ||||
| cT1/2a | 536 (81.6) | 487 (82.1) | 49 (76.6) | |
| cT2b | 46 (7.0) | 44 (7.4) | 2 (3.1) | |
| cT2c/3 | 75 (11.4) | 62 (10.5) | 13 (20.3) | 0.0025a) |
| NA | 54 | 43 | 11 | |
| Surgical procedures, n (%) | ||||
| Open RP | 317 (44.6) | 278 (43.7) | 39 (52.0) | |
| Laparoscopic RP | 92 (12.9) | 83 (13.1) | 9 (12.0) | |
| Robot-assisted RP | 302 (42.5) | 275 (43.2) | 27 (36.0) | 0.38 |
| Pathological Gleason score, n (%) | ||||
| <7 | 131 (21.3) | 131 (21.3) | — | |
| 7 | 425 (69.1) | 425 (69.1) | — | |
| >7 | 59 (9.6) | 59 (9.6) | — | NA |
| NA | 96 | 21 | 75 | |
| Pathological T-stage, n (%) | ||||
| pT0 | 12 (1.7) | 4 (0.6) | 8 (11.9) | |
| pT2 | 473 (68.7) | 427 (68.7) | 46 (68.7) | |
| pT3 | 199 (28.9) | 186 (29.9) | 13 (19.4) | |
| pT4 | 5 (0.7) | 5 (0.8) | 0 (0.0) | <0.0001a) |
| NA | 22 | 14 | 8 | |
ADT, androgen-deprivation therapy; IQR, interquartile range; NA, not available; PSA, prostate-specific antigen; RP, radical prostatectomy.
Statistically significant.
Fig. 1.
Biochemical recurrence-free survival in patients treated with radical prostatectomy. Biochemical recurrence-free survival of all patients with or without neoadjuvant androgen-deprivation therapy. ADT, androgen-deprivation therapy.
Table 2.
Recurrence-free survival among patients treated with or without neoadjuvant androgen-deprivation therapy according to clinicopathological parameters.
| Variable | Neoadjuvant ADT (−)/(+) |
HR (95% CI)a) | P | Interaction | |
|---|---|---|---|---|---|
| No. of patients | Median RFS (y) | ||||
| All patients | 636/75 | NYR/6.4 | 1.43 (0.90–2.17) | 0.12 | — |
| Age, y | |||||
| ≤65 | 311/35 | NYR/NYR | 0.92 (0.41–1.78) | 0.81 | P = 0.066 |
| >65 | 325/40 | 11.3/3.3 | 2.04 (1.13–3.43) | 0.020b) | |
| PSA at diagnosis, ng/mL | |||||
| ≤10 | 420/35 | NYR/5.1 | 1.59 (0.71–3.10) | 0.24 | P = 0.51 |
| >10 | 212/37 | 9.9/4.5 | 1.11 (0.60–1.90) | 0.72 | |
| Biopsy Gleason score | |||||
| <7 | 231/20 | NYR/NYR | 1.37 (0.33–3.84) | 0.62 | P = 0.88 |
| 7 | 281/28 | NYR/6.4 | 1.16 (0.49–2.35) | 0.71 | |
| >7 | 101/15 | 3.6/1.5 | 1.24 (0.51–2.57) | 0.61 | |
| Clinical T-stage | |||||
| cT1/2a | 487/49 | 11.3/NYR | 1.24 (0.63–2.21) | 0.50 | P = 0.90 |
| cT2b | 44/2 | 2.7/NYR | 1.04 (0.057–5.12) | 0.97 | |
| cT2c/3 | 62/13 | 2.2/3.3 | 0.85 (0.29–1.99) | 0.73 | |
ADT, androgen-deprivation therapy; CI, confidence interval; HR, hazards ratio; NYR, not yet reached; PSA, prostate-specific antigen; RFS, recurrence-free survival.
Reference is neoadjuvant ADT (−).
Statistically significant.
3.2. Subgroup analysis for RFS
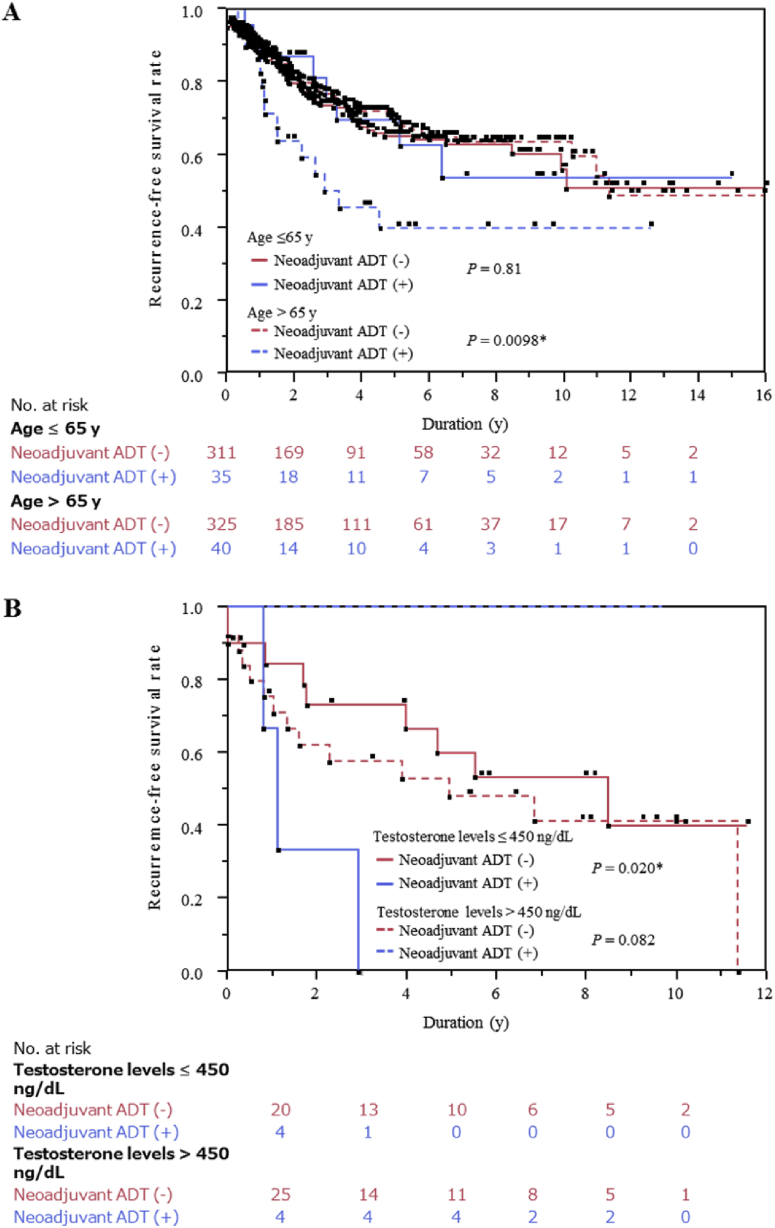
Next, we performed subgroup analyses for RFS using various clinicopathological parameters. As shown in Table 2, in patients aged > 65 years, RFS was lower in patients treated with neoadjuvant ADT than in those treated without neoadjuvant ADT [hazard ratio (95% confidence interval), 2.04 (1.13–3.43), P = 0.020]. Furthermore, among the patients aged >65 years, the Kaplan–Meier curve showed reduced RFS in patients treated with neoadjuvant ADT compared with those treated without neoadjuvant ADT (log-rank test, P = 0.0098, Fig. 2A), suggesting an adverse role of neoadjuvant ADT in older patients. The interaction between neoadjuvant ADT and age on RFS was statistically marginal (P = 0.066). There was no difference in clinicopathological parameters among patients aged >65 years between those treated with and without neoadjuvant ADT (Supplementary Table 1).
Fig. 2.
Biochemical recurrence-free (BCR) survival in patients treated with radical prostatectomy divided into subgroups. (A) BCR-free survival of patients aged ≤ 65 years or > 65 years. (B) BCR-free survival of patients with serum testosterone level before ADT ≤ 450 ng/dL or > 450 ng/dL, with or without neoadjuvant ADT. ADT, androgen-deprivation therapy.
3.3. RFS stratified by serum testosterone levels before treatment
To explore the possible reason of this differential RFS between patients aged >65 years with and without neoadjuvant ADT, we next investigated serum testosterone levels before treatment, which is well known to be adversely associated with age16 and a prognostic factor of ADT for metastatic prostate cancer.17, 18, 19, 20 Among the total 711 patients included in this study, serum testosterone levels before treatment were available in 53 patients only. Intriguingly, the Kaplan–Meier curve analysis in patients with serum testosterone levels ≤450 ng/dL showed significantly worse RFS when treated with neoadjuvant ADT than when not treated with neoadjuvant ADT (log-rank test, P = 0.020, Fig. 2B). In contrast, the Kaplan-Meier curve analysis in patients with serum testosterone levels >450 ng/dL showed marginally superior RFS when treated with neoadjuvant ADT compared with when treated without neoadjuvant ADT (log-rank test, P = 0.082, Fig. 2B). Furthermore, the interaction between neoadjuvant ADT and serum testosterone levels on RFS was statistically significant (P = 0.011), suggesting that the effect of neoadjuvant ADT may be dependent on serum testosterone levels before ADT.
4. Discussion
To date, no biomarker for identifying prostate cancer patients who are suitable for neoadjuvant ADT has been identified. Here we identified age as well as serum testosterone levels before treatment as possible biomarkers to identify candidates suitable for neoadjuvant ADT.
Intriguingly, both age and serum testosterone levels before treatment have been reported as being possible prognostic markers in ADT. Young age was associated with a worse prognosis in patients with metastatic prostate cancer treated with ADT in previous studies using a population-based database across different races and nations,21, 22, 23 in contrast with RP and radiotherapy,24 suggesting the uniqueness of age in therapeutic sensitivity to ADT. Low serum testosterone levels before treatment may represent a reduced androgen dependency of the prostate cancer, which would be less effectively repressed by ADT. Serum testosterone level has also repeatedly been shown to be prognostic in patients with metastatic prostate cancer treated with ADT,17, 18, 19, 20 which may be due to high malignant potential in patients with lower serum testosterone levels.25 Thus, both age and serum testosterone level may be common predictive markers for the efficacy of ADT and may serve as parameters for selection of candidates suitable for neoadjuvant ADT.
Since serum testosterone level is known to decline with age,16 age may affect the sensitivity to ADT through serum testosterone levels. In addition, because an improvement of RFS was suggested by neoadjuvant ADT in patients with serum testosterone levels >450 ng/dL and a clear inverse association between testosterone level and effect of neoadjuvant ADT was shown, serum testosterone levels would be an attractive biomarker to identify candidates suitable for neoadjuvant ADT. This suggests that a clinical trial including patients with high serum testosterone levels to compare outcome by RP with or without neoadjuvant ADT would be intriguing. However, the study has several limitations, and validation studies using existing data are thus required before initiating such clinical trials. In this study, the observation period was relatively short for observing BCR, the sample size was small, and the study design was retrospective. RP was performed by several surgeons, and neoadjuvant ADT used in a limited number of cases varied in mode and term. Moreover, serum testosterone levels before ADT were available in only a limited number of patients.
To the best of our knowledge, this study suggested for the first time that neoadjuvant ADT showed potential deleterious effects in older patients and patients with lower serum testosterone levels, while a possible improved prognosis was suggested for patients with high serum testosterone levels treated with neoadjuvant ADT. Further exploration of these parameters on prognosis in neoadjuvant ADT with RP is warranted.
Conflicts of interest
We declare no conflicts of interest.
Grant support
This work was supported by Kakenhi grants (17K11145) from The Ministry of Education, Culture, Sports, Science, and Technology of Japan, Research Promotion Grant from the Takeda Science Foundation, and Research Promotion Grant from Shin-Nihon Advanced Medical Research.
Acknowledgments
We would also like to thank Edanz Group Japan for their editorial assistance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.prnil.2017.10.002.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2015;2015(65):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Faria E.F., Chapin B.F., Muller R.L., Machado R.D., Reis R.B., Matin S.F. Radical prostatectomy for locally advanced prostate cancer: current status. Urology. 2015;86:10–15. doi: 10.1016/j.urology.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Pound C.R., Partin A.W., Eisenberger M.A., Pound C.R., Partin A.W., Eisenberger M.A. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Simmons M.N., Stephenson A.J., Klein E.A. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol. 2007;51:1175–1184. doi: 10.1016/j.eururo.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Bach C., Pisipati S., Daneshwar D., Wright M., Rowe E., Gillatt D. The status of surgery in the management of high-risk prostate cancer. Nat Rev Urol. 2014;11:342–351. doi: 10.1038/nrurol.2014.100. [DOI] [PubMed] [Google Scholar]
- 6.Thompson I.M., Tangen C.M., Paradelo J., Lucia M.S., Miller G., Troyer D. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messing E.M., Manola J., Yao J., Kiernan M., Crawford D., Wilding G. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 8.Huggins C., Hodges C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 9.Soloway M.S., Pareek K., Sharifi R., Wajsman Z., McLeod D., Wood D.P., Jr. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112–116. [PubMed] [Google Scholar]
- 10.Aus G., Abrahamsson P.A., Ahlgren G., Hugosson J., Lundberg S., Schain M. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int. 2002;90:561–566. doi: 10.1046/j.1464-410x.2002.02982.x. [DOI] [PubMed] [Google Scholar]
- 11.Klotz L.H., Goldenberg S.L., Jewett M.A., Fradet Y., Nam R., Barkin J. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170:791–794. doi: 10.1097/01.ju.0000081404.98273.fd. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S., Shelley M., Harrison C., Coles B., Wilt T.J., Mason M.D. Neo-adjuvant and adjuvant hormone therapy for localised and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006;4 doi: 10.1002/14651858.CD006019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Amico A.V., Whittington R., Malkowicz S.B., Schultz D., Blank K., Broderick G.A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 14.Naito S., Kuroiwa K., Kinukawa N., Goto K., Koga H., Ogawa O. Validation of Partin tables and development of a preoperative nomogram for Japanese patients with clinically localized prostate cancer using 2005 International Society of Urological Pathology consensus on Gleason grading: data from the Clinicopathological Research Group for Localized Prostate Cancer. J Urol. 2008;180:904–909. doi: 10.1016/j.juro.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 15.Shiota M., Fujimoto N., Yokomizo A., Takeuchi A., Kashiwagi E., Dejima T. The prognostic impact of serum testosterone during androgen-deprivation therapy in patients with metastatic prostate cancer and the SRD5A2 polymorphism. Prostate Cancer Prostatic Dis. 2016;19:191–196. doi: 10.1038/pcan.2016.2. [DOI] [PubMed] [Google Scholar]
- 16.Feldman H.A., Longcope C., Derby C.A., Johannes C.B., Araujo A.B., Coviello A.D. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 17.Chodak G.W., Vogelzang N.J., Caplan R.J., Soloway M., Smith J.A. Independent prognostic factors in patients with metastatic (stage D2) prostate cancer. The Zoladex Study Group. JAMA. 1991;265:618–621. [PubMed] [Google Scholar]
- 18.Matzkin H., Perito P.E., Soloway M.S. Prognostic factors in metastatic prostate cancer. Cancer. 1993;72:3788–3792. doi: 10.1002/1097-0142(19931215)72:12+<3788::aid-cncr2820721705>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Imamoto T., Suzuki H., Akakura K., Komiya A., Nakamachi H., Ichikawa T. Pretreatment serum level of testosterone as a prognostic factor in Japanese men with hormonally treated stage D2 prostate cancer. Endocr J. 2001;48:573–578. doi: 10.1507/endocrj.48.573. [DOI] [PubMed] [Google Scholar]
- 20.Shiota M., Takeuchi A., Sugimoto M., Kashiwagi E., Dejima T., Kiyoshima K. Prognostic impact of serum testosterone and body mass index before androgen-deprivation therapy in metastatic prostate cancer. Anticancer Res. 2015;35:6925–6932. [PubMed] [Google Scholar]
- 21.Ryan C.J., Elkin E.P., Cowan J., Carroll P.R. Initial treatment patterns and outcome of contemporary prostate cancer patients with bone metastases at initial presentation: data from CaPSURE. Cancer. 2007;110:81–86. doi: 10.1002/cncr.22736. [DOI] [PubMed] [Google Scholar]
- 22.Cetin K., Beebe-Dimmer J.L., Fryzek J.P., Markus R., Carducci M.A. Recent time trends in the epidemiology of stage IV prostate cancer in the United States: analysis of data from the surveillance, epidemiology, and end results program. Urology. 2010;75:1396–1404. doi: 10.1016/j.urology.2009.07.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T., Onozawa M., Miyazaki J., Matsuoka T., Joraku A., Kawai K. Prognostic impact of young age on stage IV prostate cancer treated with primary androgen deprivation therapy. Int J Urol. 2014;21:578–583. doi: 10.1111/iju.12389. [DOI] [PubMed] [Google Scholar]
- 24.Hotston M.R., Burden H., Thurairaja R., McFarlane J., Persad R.A. Young men with prostate cancer: are they different and how should they be managed? BJU Int. 2007;99:5–7. doi: 10.1111/j.1464-410X.2007.06534.x. [DOI] [PubMed] [Google Scholar]
- 25.Ide H., Yasuda M., Nishio K., Saito K., Isotani S., Kamiyama Y. Development of a nomogram for predicting high-grade prostate cancer on biopsy: the significance of serum testosterone levels. Anticancer Res. 2008;28:2487–2492. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.