Abstract
Radiation therapy (RT) is a curative treatment option for localized prostate cancer. Prostate irradiation with focal dose escalation to the intraprostatic dominant nodule (IDN) is an emerging treatment option that involves the prophylactic irradiation of the whole prostate while increasing RT doses to the visible prostatic tumor. Because of the lack of large multicentre trials, a systematic review was performed in an attempt to get an overview on the feasibility and efficacy of focal dose escalation to the IDN.
A bibliographic search for articles in English, which were listed in MEDLINE from 2000 to 2016 to identify publications on RT with focal directed boost to the IDN, was performed. The review was completed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Twenty-two articles describing 1,378 patients treated with RT using focal boost were identified and fulfilled the selection criteria. Intensity-modulated radiation therapy (IMRT) was used in 720 patients (52.3%), volumetric modulated arc therapy was used in 45 patients (3.3%), stereotactic body radiation therapy (SBRT) in 113 patients (8.2%), and low–dose rate and high–dose rate brachytherapy (BT) were used in 305 patients (22.1%) and 195 patients (14.1%), respectively. Use of androgen deprivation therapy varied substantially among series. Biochemical disease-free survival at 5 years was reported for a cohort of 812 (58.9%) patients. The combined median biochemical disease-free survival for this group of patients was 85% (range: 78.8–100%; 95% confidence interval: 77.1–82.7%).
The average occurrence of grade III or worse gastrointestinal and genitourinary late toxicity was, respectively, 2.5% and 3.1% for intensity-modulated RT boost, 10% and 6% for stereotactic body RT, 6% and 2% for low–dose rate BT, and 4% and 4.3% for high–dose rate BT.
This review shows encouraging results for focal dose escalation to the IDN with acceptable short- to medium-term side effects and biochemical disease control rates. However, owing to the heterogeneity of patient population and the short follow-up, the results should be interpreted with caution. Considering that the clinical endpoint in the studies was biochemical recurrence, the use and duration of androgen deprivation therapy administration should be carefully considered before driving definitive conclusions. Randomized trials with long-term follow-up are needed before this technique can be generally recommended.
Keywords: Boost, Brachytherapy, Dominant intraprostatic lesion, Hypofractionated radiotherapy, Localized prostate cancer, Review, Stereotactic body radiation therapy
Abbreviations
- ADT
Androgen deprivation therapy
- ADC
Apparent diffusion coefficient
- BED
Biological equivalent dose
- bDFS
Biological disease-free survival
- BT
Brachytherapy
- CTCAE
Common Terminology Criteria for Adverse Events
- CBCT
Cone-beam Computed tomography
- DWI
Diffusion-weighted imaging
- DCE
Dynamics contrast enhancement
- DSS
Disease-specific survival
- EBRT
External beam radiotherapy
- ERC
Endorectal coil
- GI
Gastrointestinal
- GU
Genitourinary
- Gy
Gray
- GTV
Gross tumor volume
- HDR
High–dose rate brachytherapy
- IMRT
Intensity-modulated radiation therapy
- IGRT
Image-guided radiation therapy
- IDN
Intraprostatic dominant nodule
- KVCT
Kilovoltage Computed tomography
- LDR
Low dose rate
- LC
Local control
- MRSI
Magnetic resonance spectroscopy imaging
- MVCT
Megavoltage computed tomography
- mpMRI
Multiparametric Magnetic Resonance Imaging
- NCCN
National Comprehensive Cancer Network
- OAR
Organs at risk
- OS
Overall survival
- PET CT
Positron emission computed tomography
- PCa
Prostate cancer
- PSA
Prostatic Specific Antigen
- PTV
Planning Target Volume
- PTVb
PTV boost
- PTVpr
PTV prostate
- SIB
Simultaneous integrated boost
- SPECT
Single-Photon Emission Computed Tomography
- SUV
Standard Uptake Value
- SBRT
Stereotactic body radiation therapy
- T2W
T2-weighted sequence
- US
Ultrasound
- VMAT
Volumetric modulated arc therapy
1. Introduction
Prostate cancer (PCa) is among the third most common malignancy in Europe. An estimated 417,000 PCa cases were diagnosed in Europe in 20121 and 1.4 million cases of PCa worldwide with 293,000 deaths in 20132. Traditionally, PCa patients have been considered for active surveillance programs or radical whole-gland therapies such as prostatectomy, external beam radiotherapy (EBRT), or brachytherapy (BT)3. In the case of EBRT, the advent of more sophisticated treatment plans yields better dose conformity to the target, allowing for dose escalation and better biochemical disease control, although not without toxicity because of the close proximity of organs at risk (OARs), particularly bladder and rectum4, 5, 6, 7, 8, 9, 10.
Randomized data comparing different methods of dose escalation are sparse, with three randomized trials comparing EBRT plus whole prostate BT boost with EBRT alone. These trials have demonstrated improved biochemical disease-free survival (bDFS) using distinct BT boost regimens, but only the Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (ASCENDE-RT) trial has shown significantly greater urinary side effects11, 12, 13, 14.
Importantly, studies of patterns of failure after conventionally fractionated EBRT show that the area responsible for local recurrence is the intraprostatic dominant nodule (IDN) in 90% of cases15, 16, 17, 18. The IDN is defined as the largest nodule in a multifocal disease which harbors in more than 80% of the cases the most aggressive biological behavior and therefore dictates the overall clinical prognosis of PCa19.
Retrospective studies compared the site of the primary tumor on pre- and post-EBRT magnetic resonance images (MRIs), and by using large block pathology sections of the salvage radical prostatectomy specimen as the reference gold standard, they mapped the position of the recurrent tumor within the prostate showing that the pre-EBRT intraprostatic dominant nodule visible on the MRI was responsible for the local recurrence and could be specifically targeted to receive higher doses of radiation15, 16, 17, 18.
Intraprostatic dose escalation requires advanced imaging capabilities, which can detect intraprostatic tumor deposits with acceptable sensibility and specificity. Nowadays, it is possible to identify the IDN by using multiparametric magnetic resonance image (mpMRI), which uses various T1 and T2 sequences, dynamic contrast enhancement to assess perfusion, and diffusion-weighted imaging to calculate the different diffusion capability of PCa versus normal tissue20. Other imaging methods to detect the IDN such as 11C-choline-positron emission computed tomography (PET/CT), 68Ga-prostate-specific membrane antigen (PSMA) PET/CT, and newer generation ultrasound equipment are also under evaluation21, 22, 23, 24.
Furthermore, highly conformational EBRT techniques with improvement in patient positioning during treatment, such as image-guided radiation therapy (IGRT) and the use of fiducial markers to track prostate movements during a radiotherapy session, are needed for safe and effective treatment delivery25, 26, 27.
Because of the lack of large multicentric trials, a systematic review was performed in an attempt to get an overview on the feasibility and efficacy of focal dose escalation to the IDN, with special attention to gastrointestinal (GI) and genitourinary (GU) toxicity as well as clinical efficacy.
2. Materials and methods
2.1. Literature search strategy
The literature review included a search in MEDLINE from 2000 to 2016, using the terms “intraprostatic” OR “intra-prostatic” OR “dominant intraprostatic lesion” OR “intraprostatic lesion” OR “gross tumor volume (GTV)” OR “simultaneous integrated boost” AND “radiation” OR “radiation therapy” OR “brachytherapy” OR “stereotactic body radiation therapy (SBRT)” OR “intensity modulated radiation therapy (IMRT)” OR “volumetric arc therapy (VMAT)” AND “prostate cancer”.
2.2. Assessment of study quality and inclusion criteria
The search results were assessed on content before inclusion into the review. The selection criteria for inclusion in the systematic review were accessible fully published articles in English, which reported the treatment outcome of PCa patients who received a boost to the IDN either by BT or EBRT. The primary endpoint was treatment-related side effects and efficacy outcome.
Articles dealing with case reports, recurrent disease, or planning studies were not included. Reports from conference proceedings were excluded. All authors participated in the design of the search strategy and inclusion criteria.
The following data were extracted from each study: predefined eligibility criteria, year of report, sample size, type of treatment, histology Gleason score, TNM stage, National Comprehensive Cancer Network cancer risk classification, median prostate-specific antigen, median time of follow-up, pretreatment diagnostic tools, such as imaging techniques used to localize the disease, radiotherapy technique and dose, use of androgen deprivation therapy (ADT), follow-up duration, acute and late side effects, quality of life assessment, biochemical control, and when available disease-specific survival (DSS), overall survival (OS), and local control rate. The side effects were translated into the current classification of adverse events Common Terminology Criteria of Adverse Events version 4. Acute radiation effects are seen from day 1 through day 90, whereas late radiation effects are all adverse effects seen after 90 days from the beginning of RT.
2.3. Statistical considerations
The outcome was analyzed in terms of local control, DSS, and OS rates at 5 years. If available, estimates and 95% confidence intervals (CI), as reported in the articles, were used. To perform meta-analysis of median survival, we pooled the estimates as median survival and standard error. Ninety-five CIs were extrapolated and reported on variation, number of events, and/or median follow-up times using RStudio software, version 1.0.153.
Given the heterogeneous nature of the patient series reported, no formal attempt at a quantitation of bias or analysis of pooled results was attempted; however, qualitative appraisal of the relative strengths and weaknesses of the individual series was made, and qualitative statements are included in the results and discussion of the articles.
3. Results
In total, twenty-two articles describing 1,378 patients were identified for data extraction. A Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram of the search results is available in Fig. 1. Table 1 summarizes a patient's characteristics, type of radiotherapy delivery, and outcome.
Fig. 1.
Diagram showing the results from the literature search using PubMed and from the selection of articles, resulting in the withholding of 22 articles reporting on results of treatment using focal dose irradiation to the intraprostatic dominant nodule.
Table 1.
Literature summary of prostate irradiation with intraprostatic directed boost
| Author | N | IDN identification modality | Treatment technique | NCCN | Median PSA (μg/L) | Median follow-up time | Volume delineation and margins | Boost technique | Dose (Gy/fr) | ADT | 5-year bDFS (phoenix) | Survival (DSS, OS) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zelefsky et al28 | 4 | 1,5T ERC MRSI (elevated choline + elevated creatine-to-citrate ratio) | LDR B (I125) |
LR (2) IR (2) HR (0) |
4.5 | NR | PTVb = GTV PTVpr = prostate |
LDR B (I125) | PTVb = 150% of PTVpr PTVpr = 100–145 Gy |
No | NR | NR |
| DiBiase et al29 | 15 | 1,5T ERC MRSI (elevated choline + elevated creatine-to-citrate ratio) | LDR B (I125) |
LR (15) IR (0) HR (0) |
7.1 | NR | PTVb = GTV PTVpr = prostate + 2 mm | LDR B (I125) | PTVb = 188 Gy PTVpr = 145 Gy |
No | NR | NR |
| De Meerleer et al30 | 15 | 1,5T ERC MRI (T2W) + biopsy |
IMRT (3 field, Step and Shoot) |
LR (2) IR (8) HR (5) |
10.2 | NR | PTVb = GTV PTVpr= (prostate + SV)+ 7–10 mm | IMRT | PTVb = 80 Gy/37 PTVpr = 74 Gy/37 |
Yes (73%) neoadj + adj (6–36 mo) |
NR | NR |
| Singh et al31 | 3 | 3T ERC MRI (T2W + DCE + DWI) + biopsy | IMRT | NR | NR | 3–18 | PTVb = GTV + 3 mm PTVpr = prostate + 7 mm |
PTVb = 94,5 Gy/42 PTVpr = 75,6 Gy/42 |
No | NR | NR | |
| Fonteyne et al32 | 230 | 1,5T ERC MRI (T2W + T1W) or MRSI | IMRT (3 field, Step and Shoot) |
LR (17) IR (97) HR (116) |
11.2 | NR | PTVb = GTV + 4 mm PTVpr = (prostate ± SV) + 4 mm |
IMRT | PTVb = 80 Gy/39 PTVpr = 78 Gy/39 |
No | NR | NR |
| Ares et al33 | 77 | ERC MRI (T2W + DCE) +biopsy | 3DCRT + HDR B (Ir192) | LR (6) IR (25) HR (46) |
NR | 41.2 | PTVb = boost prostate volume PTVpr = prostate + VS PLN |
HDR B (Ir192) | PTVb = 85.6-99.2 Gy PTVpr = 64 Gy/32 |
Yes (>80%) neoadj/adj (18–24 mo) |
78.8% | 90% 5-year DSS |
| Miralbell et al34 | 50 | ERC MRI (T2W + DCE) +biopsy | 3DCRT/IMRT (Step and shoot, sliding window and VMAT) | LR (5) IR (12) HR (33) |
NR | NR | PTVb = GTV + 3 mm PTVpr = prostate + SV PLN |
SBRT | PTVb = 80-99 Gy PTVpr = 64 Gy/32 |
Yes (66%) neoadj + adj (6-30 mo) |
98% | 100% 5-year DSS |
| Schick et al35 | 77 | ERC MRI (T2W + DCE) +biopsy | 3DCRT + HDR B (Ir192) | LR (7) IR (9) HR (61) |
NR | 62–67 | PTVb = hemi prostate PTVpr = prostate + vs PLN |
HDR B (Ir192) | PTVb = 88-104 Gy PTVpr = 64.4 Gy/32 |
Yes (81%) neoadj + adj (18–24 mo) |
70.5–79.7% | NR |
| Ellis et al36, 37 | 239 | 111In-Capromab SPECT Imaging | LDR prostate +3DCRT in 37% | LR (116) IR (94) HR (29) |
7.6 | 84 | PTVb = GTV + 5 mm PTVpr = prostate + 2–5 mm ±PLN |
LDR prostate (Pd103 or I125) | PTVb = 150% of PTVpr PTVpr = 108–144 Gy (I125) PTVpr = 100–125 Gy (Pd103) |
Yes (21%) neoadj |
84.6% | 97.7% 10-year DSS 84.8% 10-year OS |
| Wong et al38 | 71 | 111In-Capromab SPECT Imaging | IMRT | LR (31) IR (30) HR (10) |
6.1 | 66 | PTVb = GTV PTVpr = prostate + SV (when involved) + 6 mm |
IMRT | PTVb = 82 Gy (SIB) PTVpr = 75.6 Gy/42 |
Yes (24%) adj (6–12 mo) |
94% | 93% 5-year OS |
| Pinkawa et al39 | 66 | 18F-Fluorocholine PET CT | IMRT | LR (23) IR (21) HR (22) |
14 | 19 | PTVb = GTV + 3-4 mm PTVpr = prostate + SV + 4–8 mm |
IMRT | PTVb = 80 Gy (SIB) PTVpr = 76 Gy/38 |
Yes (16%) (NR) |
NR | NR |
| Ippolito et al40 | 40 | 1,5T ERC MRI + biopsy | IMRT | LR (4) IR (17) HR (19) |
7 | 19 | PTVb= (GTV + 5 mm)+1 cm PTVpr = prostate + SV + 1 cm |
IMRT | PTVb = 80 Gy (SIB) PTVpr = 72 Gy/40 |
Yes (100%) neoadj + adj(24 mo) |
100% | NR |
| Myers et al41 | 26 | TRUS | IMRT+ HDR B (Ir192) |
LR (7) IR(19) HR (0) |
6.1 | 53 | PTVb = peripheral zone PTVpr = (prostate+1.5 cm + VS + 5 mm) + 5 mm PLN |
HDR B (Ir192) | PTVb = 9 Gy + 63 Gy/28 PTVpr = 6Gy + 63 Gy/28 |
Yes (73%) neoadj + adj(4 mo) |
100% | NR |
| Aluwini et al42 | 50 | 1.5 T MRI (T1W + T2W) | SBRT (Cyberknife) | LR (30) IR (20) HR (0) |
8.2 | 23 | PTVb = GTV PTVpr = prostate + 3 mm |
SBRT (Cyberknife) | PTVb = 44 Gy (SIB) PTVpr = 38 Gy/4, daily |
No | 100% 2-year bDFS | NR |
| Schild et al43 | 78 | 1.5 T MRI (T2W + DCE + DWI) | IMRT (sliding window and VMAT) | LR (18) IR (43) HR (17) |
6.7 | 36 | PTVb = GTV PTVpr = prostate + 3 mm |
IMRT (sliding window and VMAT) | PTVb = 81-83 Gy (SIB) PTVpr = 77,4 Gy/43 |
Yes (41%) adj(6–30 mo) |
92% 3-year bDFS | 95% 3-year OS |
| Gomez-Iturriaga et al44 | 15 | 1.5 T MRI (T2W + DCE + DWI) | IMRT + HDR B (Ir192) | LR (0) IR and HR: not specified |
9 | 18 | PTVb = GTV PTVpr = prostate |
HDR B (Ir192) | PTVb = 18.75 Gy + 37.5 Gy/15 PTVpr = 15 Gy + 37.5 Gy/15 |
No | NR | NR |
| King et al45 | 47 | MRSI (elevated choline + elevated creatine-to-citrate ratio) | LDR (I125or Pd103) + IMRT PLN for 1 patient | LR (35) IR (12) HR (0) |
5.1 | 86.4 | PTVb = GTV PTVpr = prostate PLN |
LDR (I125 or Pd103) |
PTVb = 150% of PTVpr PTVpr = 144 Gy (I125) or 140 Gy (Pd103) |
Yes (17%) neoadj |
98% 10-year bDFS | 84% 10-year OS |
| Sundahl et al46 | 225 | 1,5T ERC MRI or 3T MRI (T1W + T2W) | IMRT | LR (5) IR (97) HR (123) |
NR | 72 | PTVb = GTV PTVpr = prostate ± SV + 7 mm |
IMRT | PTV1 = 82 Gy (SIB) PTV2 = 78 Gy/38 |
No | 84% 6-year bDFS | NR |
| Kotecha et al47 | 24 | MRI (no specification) | SBRT (Cyberknife) | LR (0) IR (11) HR (13) |
NR | 25 | PTVb = GTV PTVpr = prostate + SV+3 mm(0 mm posteriorly) |
SBRT (Cyberknife) | PTVb = 50 Gy (SIB) PTVpr = 36.25 Gy/5 |
Yes (67%) Adj(4–30 mo) |
95.8% 2-year bDFS | NR |
| Uzan et al 48 | 11 | MRI (T2W + DCE + DWI) + biopsy | IMRT (VMAT) | NR | 15.9 | 36 | PTVb = GTV + 5 mm PTVpr 1 = prostate + SV + 9 mm PTVpr 2 = prostate and base of SV + 5 mm |
IMRT (VMAT) | PTVb = 85–105 Gy (SIB) PTVpr1 = 64 Gy/37 PTVpr2 = 74 Gy/37 |
Yes (100%) neoadj + adj (6–36 mo) |
NR | NR |
| Garibaldi et al49 | 15 | 1.5 ERC MRI (T2W + DWI + DCE) | IMRT (VMAT) | LR (0) IR (14) HR (1) |
6.5 | 16 | PTVb = GTV + 6 mm PTVpr 1 = prostate + 7 mm (5 mm posteriorly) PTVpr 2 = SV + 5–7 mm |
IMRT (VMAT) | PTVb = 83.2 Gy (SIB) PTVpr1 = 75.2 Gy/32 PTVpr2 = 67.2 Gy/32 |
Yes (80%) neoadj + adj (6–24 mo) |
100% | NR |
3DCRT, 3-D conformational radiation therapy; ADT, androgen deprivation therapy; Adj, adjuvant ADT; bDFS, biochemical disease-free survival; CTCAE v2, Common Terminology Criteria of Adverse Events version 2, CTCAE v4, Common Terminology Criteria of Adverse Events version 4; DCE, dynamic contrast enhancement; DSS, disease-specific survival; EORTC, European Organization for Research and Treatment for Cancer; DWI, diffusion-weighted imaging; EPIC, Expanded Prostate Cancer Index Composite questionnaire; ERC, endorectal coil; Fr, fraction; GI, gastrointestinal; GTV, Gross tumor volume (=dominant intraprostatic lesion); GU, genitourinary; Gy, gray; HDR, high–dose rate brachytherapy; HR, high risk; IDN, intraprostatic dominant nodule; IMRT, intensity-modulated radiation therapy; IR, intermediate risk; LDR B, low–dose rate brachytherapy; LR, low risk; MRI, magnetic resonance imaging; mpMRI, multiparametric MRI [T2 weighed + dynamic contrast enhancement (DCE) + diffusion-weighted imaging (DWI)]; MRSI, magnetic resonance spectroscopic imaging; NCCN, National Comprehensive Cancer Network; Neoadj, neoadjuvant ADT; NR, not reported; OS, overall survival; PET CT, positron emission tomography–computed tomography; PLN, pelvic lymph nodes irradiation; PLND, pelvic lymph nodes dissection; PSA, prostate-specific antigen; PTV, planning target volume; PTVb, boost; PTVpr, whole prostate; SBRT, stereotactic body radiation therapy; SPECT, single-photon emission computed tomography; SV, seminal vesicles; TRUS, transrectal ultrasound; VMAT, volumetric modulated arc therapy.
The level of the evidence is low to medium, with no study yielding a level of evidence >2b. This suggests that the results of this review should be interpreted with caution. Of particular importance is the fact that we were unable to retrieve the exact definition of IDN used in each study.
The median follow-up for the 1,378 patients was 36 months (range 3–86 months).
3.1. Patient's characteristics
The analyzed literature28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 included 323 (23%) patients with National Comprehensive Cancer Network low-risk disease, 509 (37%) patients with intermediate-risk disease, and 517 (38%) patients with high-risk disease. Three studies did not specify the risk group (29 patients, 2.1%).
3.2. Disease localization
The spatial location of the tumor within the prostate is essential for dose escalation of radiotherapy treatment. There is no accepted standard for disease localization for the purpose of delivering boost therapy.
In 976 patients (70.8%; n = 17 studies), an MRI was used to identify the IDN. From those series, five (488 patients, 35.4%) used magnetic resonance spectroscopy imaging (Choline/creatine-to-citrate ratios >1.4–2) to define the tumor28, 29, 32, 33, 45, three series (116 patients, 8.4%) used 3T MRI, and nine series (438 patients, 31.7%) used mpMRI (with sequences T2W + dynamic contrast enhancement + diffusion-weighted imaging)31, 33, 34, 35, 43, 44, 46, 49, 48, of which two studies used open MRI33, 35.
Six hundred ninety-eight patients (71.5%) (n = 15 studies) of 976 underwent 1.5 T magnetic resonance imaging with an endorectal coil to identify the IDN. Two series (162 patients, 16.6%) used MRI without an endorectal coil42, 46.
One study with 66 patients (4.8%) used 18-Fluorocholine PET/CT imaging39 [with a gross tumor volume (GTV) = standard uptake value (SUV) > 2 × background]. Three studies (310 patients, 22.5%) used 111In-Capromab single-photon emission computed tomography36, 37, 38 (with a GTV = SUV 3 × muscle SUV) for tumor localization.
Only one study (26 patients, 1.9%) used transrectal ultrasound (TRUS) to identify the tumor during the HDR BT treatment41. In 476 patients (49.9%) treated with BT, the procedure was delivered under US (n = 7 studies, 3 studies with transrectal and 4 studies with transabdominal US).
The mean percentage of IDN identified and irradiated was 80.4% (range 28–100%).
3.3. Radiotherapy planning
MRIs, PET CT, or single-photon emission computed tomography images used to identify the IDN were transferred to the radiotherapy planning computed tomography images through automatic rigid image registration (1079 patients, 78.3%, n = 16 studies)31, 32, 33, 34, 35, 38, 39, 40, 42, 43, 44, 45, 46, 47, 48, 49 or manual transfer (269 patients, 19.5%, n = 4 studies)29, 30, 36, 37. The visible tumor was considered as GTV by all the studies. Most series defined the planning target volume (PTV) as the GTV with an extension of 3- to 4-mm margins, excluding OARs. None of the studies used a margin for clinical target volume around the GTV, and thus, the boost PTV comprised the tumor with a margin of 1–3 mm. The prostate PTV definition varied among series. It most commonly included prostate + seminal vesicles when they were involved and an isotropic extension of 3–7 mm.
Deformable registration was used in only one study (4 patients, 0.3%)28. Fiducial markers were used for tracking intrafraction and interfraction tumor movement in five studies (168 patients, 12.2%)31, 41, 42, 43, 48.
In the case of BT, only one study (26 patients, 1.9%)41 used TRUS to define the IDN.
3.4. Protection of healthy tissue
OARs contouring guidelines varied among studies, and most of the studies considered rectum, bladder, and bowel as critical organs to be preserved from high doses of radiation. Efforts to spare the urethra were made in 14 studies (830 patients, 60.2%)
A total of 127 patients (9.2%) in two studies were treated with a rectal balloon to reduce internal organ immobilization33, 34.
3.5. Radiotherapy delivery
A total of 878 patients (63.7%) were treated with EBRT with focal boost using
-
1.
IMRT (720 patients, 52.3%, n = 8 studies)
-
2.
VMAT (34 patients, 2.5%, n = 3 studies)
-
3.
SBRT (124 patients, 8.9%, n = 3 studies). Fig. 2
Fig. 2.
Stereotactic body radiation therapy plan using Cyberknife. The patient is treated in the context of the HYPORT phase I/II trial (NCT02254746) that the authors of this review perform at the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland. The tumor is located in the right posterior prostate lobule. Fiducial markers are placed in the prostate for robotic-assisted tracking purposes. A rectal balloon spares the rectum from high doses of radiation. The prostate is treated with 36.25 Gy in five fractions of 7.25 Gy with a boost of 50 Gy to the intraprostatic dominant nodule. Red lines represent prescription isodose (80%).
A total of 500 patients (36.3%) were treated with BT with focal boost using
-
4.
Low–dose rate brachytherapy (LDR BT) (305 patients, 22.1%, n = 4 studies)
-
5.
HDR BT (195 patients, 14.1%, n = 4 studies).
3.6. External beam radiation therapy setup monitoring
Online corrections through IGRT to minimize patients' setup uncertainty were used at different frequencies in 941 patients (68.2%; n = 15 studies). Fifteen patients of 941 (1.5%) were monitored with daily megavoltage computed tomography (n = 1 study), 236 patients (25%) were monitored with daily cone-beam CT (n = 2), 152 patients (16.1%) were monitored with kilovoltage computed tomography (n = 3), 18 patients (1.9%) with daily portal images (n = 1), and 50 patients (5.3%) with daily monitoring of infrared skin-reflecting markers.
Fiducial markers were used for tracking intrafraction and interfraction tumor movement in five studies (168 patients, 12.2%)31, 41, 42, 43, 48.
3.7. Target doses and organs at risk dose constraints
For the purpose of this review, all doses were converted to EQD2 (equivalent dose in 2-Gy fractions) with α/β = 1.5 Gy for prostate50, 51 and α/β = 3 Gy for OARs.
Most of the studies using EBRT prescribed the radiation dose to isocenter to cover homogeneously 98–100% of the prostate PTV. For IMRT series, the mean doses delivered to the PTV boost (PTVb) were 89 Gy (range 80–130 Gy), and the mean dose to the prostate PTV was 74.7 Gy (range 67.9–82.7 Gy). The average differential dose [PTVb–PTV prostate (PTVpr)] was 14.8 Gy (range 3.2–29.9 Gy).
For the VMAT series, the mean dose to the PTVb and PTVpr were 104 Gy (range 92.2–130 Gy) and 78 Gy (range 74–82,7 Gy), respectively. The average differential dose was 26 Gy (range 17–35 Gy).
For the SBRT series, the PTVb and PTVpr mean doses were 136.4 Gy (range 89.7–164.3 Gy) and 91.4 Gy (range 64–119.4 Gy), respectively. The average differential dose was 45 Gy (range 25.7–73.5 Gy). The most common EBRT rectal dose constraint was V70 < 15–30% with rectal Dmax of 76–80 Gy. Bladder dose constraint was V70 < 15–30% and Dmax of 80 Gy. The urethra Dmax was ≤74–113 Gy when it was possible to spare, depending on the modality used.
For the BT series, I125 or Pd103 LDR was most commonly used with a mean PTVb and PTVpr dose of 177.5 Gy (range 150–217 Gy) and 123 Gy (range 100–145 Gy), respectively. The average differential dose was 61.8 Gy (range 43–72 Gy). The urethra Dmax was <85–150% of the PTVb dose and rectal Dmax was <120% of the PTVb.
For HDR BT the mean dose for PTVb and PTVpr were 106.3 Gy (range 89.7–151.3 Gy) and 80.5 Gy (range 64–113.6 Gy). The average differential dose was 31.7 Gy (range 25.7–43.4 Gy).
A total of 153 patients (11.1%) in five studies received pelvic lymph node irradiation with a total dose of 50.4 Gy in 28 fractions33, 34, 35, 41, 45.
3.8. Androgen deprivation therapy
ADT varied among series.
A total of 384 patients (27.8%) in 15 studies were treated with ADT30, 33, 34, 35, 36, 37, 38, 39, 40, 41, 43, 45, 47, 48, 49, 52. Three hundred twenty-three patients of 384 (84%) in 12 studies were treated with adjuvant ADT. Neoadjuvant ADT was used in 300 patients (78%; n = 10 studies) Usually, EBRT started 1–3 months after the first day of hormonal blockage. The ADT lasted 6 months for intermediate-risk patients and 2–3 years for high-risk patients.
3.9. Disease outcome
The median follow-up for the 1,378 patients was 36 months (range 3–86 months).
bDFS at 5 years was reported for a cohort of 812 (58.9%) patients in eight studies33, 34, 35, 37, 38, 41, 45, 46. The median bDFS for these series was 85% (range 78.8–100%; 95% CI: 77.1–82.7%). Fig. 3.
Fig. 3.
Forest plots presenting the 5-year biochemical disease-free survival with their calculated 95% confidence interval from the included articles where this could be retrieved.
Other survival outcomes included 5- to 10-year OS which was reported for 357 patients (25.9%) and had a median of 85% (range from 84% to 93%). DSS was also studied for 366 patients (26.5%) with a median of 97.7% at 5 years (range 90–100%).
3.10. Side effects
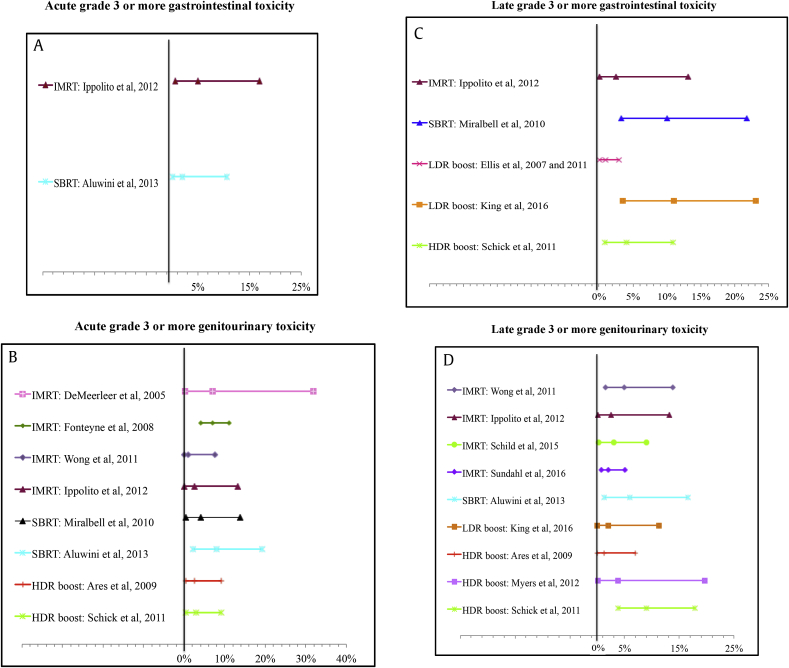
According to the RT technique, the median grade 3 or more acute and late GI toxicity were 5% and 2.5% for IMRT boost, respectively; 2% and 10% for SBRT, respectively; 0% and 6% (range 1–11%) for LDR BT, respectively; and 0% and 4% for HDR BT, respectively.
Grade 3 or more acute and late GU toxicity were 4.4% (range 1–7%) and 3.1% (range 2–5%) for IMRT boost, respectively; 6% (range 4–8%) and 6% for SBRT, respectively; 0% and 2% for LDR BT, respectively; and 2.8% and 4.7% for HDR BT, respectively. Fig. 4.
Fig. 4.
Forest plots presenting the grade 3 or more: acute gastrointestinal (A), acute genitourinary toxicity (B), late gastrointestinal (C), and late genitourinary toxicity (D) with their calculated 95% confidence interval from the included articles where this could be retrieved.
IMRT, intensity-modulated radiation therapy; SBRT, stereotactic body radiation therapy; LDR, low–dose rate brachytherapy; HDR, high–dose rate brachytherapy.
Grade 4 late GI toxicities was reported in four studies with a median of 2% (range 1–4%). One study reported a 1% GU late grade 4 toxicity35, 36, 37. In these reports, three patients had rectovesical fistulae, and one had hematuria. The studies reporting late grade 4 toxicity used the following technology: IMRT (n = 1 study), LDR BT (n = 2 studies), and HDR BT (n = 1 study).
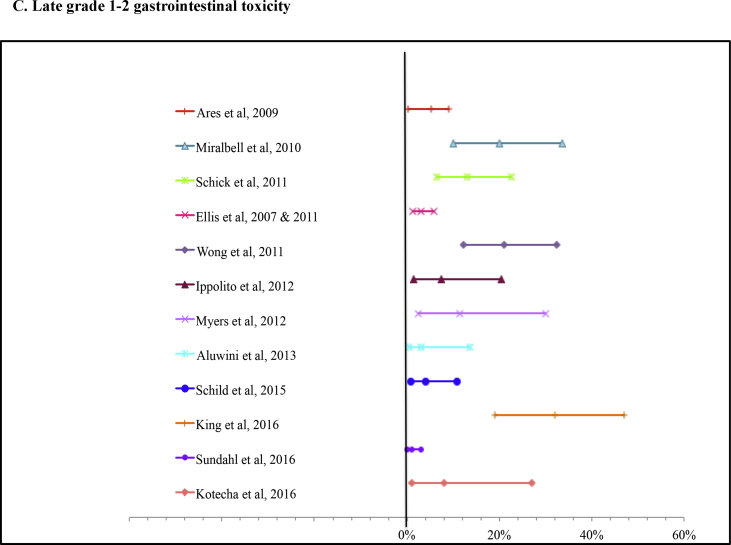
The acute and late grade 1–2 GI toxicity were 20.1% (range 6.6–45%) and 6.2% (range 0–21%) for IMRT boost, respectively; 6.7% (range 0–12%) and 7% (range 3–10%) for SBRT, respectively; 21.3% (range 0–60%) and 11.5% (range 2–21%) for LDR BT, respectively; and 6.6% (range 2.6–13.4%) and 6.14% (range 0–11.5%) for HDR BT, respectively.
The mean acute and late grade 1–2 GU toxicity were 39.2% (range 13.3–66%) and 18.8% (range 5–39%) for IMRT boost, respectively; 33% (range 15–46%) and 10% (range 8–12%) for SBRT, respectively; 28.5% (range 4–53%) and 13% for LDR BT, respectively; and 9.6% (range 3–20%) and 8.35% (range 6.7–11.5%) for HDR BT, respectively Supplementary Fig. 5.
4. Discussion
Multiple studies have confirmed the importance of delivering sufficiently high doses of radiotherapy to the prostate to cure patients4. First-class radiation technology and appropriate imaging technology are absolute prerequisites to safely deliver higher focal doses to prostate tumor/s.
In our review of the literature, we were able to identify 1,378 patients treated with whole prostate irradiation and dose escalation to the IDN.
We showed that the adoption of new technologies is strongly associated with an increase in the radiation doses delivered to the IDN. The average differential doses between the prostate and the boost increased with more complex technologies. Patients treated with IMRT had modest differential doses between PTVpr and PTVb of only 14.8 Gy compared with 25 and 45 Gy for those patients treated with VMAT and SBRT, respectively.
This systematic review highlights that when dose escalation to the dominant nodule is delivered either with IMRT, VMAT, SBRT, or BT, the functional and disease control outcomes are encouraging. We showed that at a short-to-medium follow-up time, the grade 3 or more GU and GI late toxicity were in the order of 3% to 11%. We recognize that these rates may underestimate the true toxicity rates that may develop with longer follow-up. Nevertheless, Fig. 4 and Supplementary Fig. 5 illustrate that the toxicities for the different modalities compare favorably with those observed with other radiation modalities depicted in Supplementary Table 2.
This is in striking contrast to studies of whole prostate dose escalation using SBRT delivered in five fractions of 45, 47.5, and 50 Gy which showed 10.6% late grade 3 or more GI toxicity at the highest dose level. In that study, late grade 3 or more rectal toxicity was strongly correlated with the volume of rectal wall receiving 50 Gy > 3 cm3 (P < 0.0001) and treatment of >35% circumference of rectal wall to 39 Gy (P = 0.003)53. This highlights the need for limiting the dose escalation to a defined area of the prostate while new methods are needed for minimizing rectal toxicity. The use of a rectal spacer has been proven in randomized trials to be an effective method to reduce GI side effects and maintain the patient's quality of life54. These methods should be implemented in future trials of prostate dose escalation.
It is important to note that in our review, the series with the highest boost differentials included 66 patients with reported late grade 3 or greater toxicities that ranged from 0% to 10% including one patient with fistula formation34, 35, 36, 37. This series used large (hemi prostate or bilateral prostate GTV) volumes with relatively high boost doses. More importantly, these series did not make any attempt to protect the rectum with the use of a rectal spacer or balloons to separate, as much as possible, the rectum from high radiation doses.
The side effects reported in our systemic review are not necessarily different compared with other forms of PCa treatment. Table 1, Fig. 3, Supplementary Table 2.
In general, GU toxicity has always remained a challenge for new radiation technology. For instance, patients treated with IMRT or 3DCRT in the Radiation Therapy Oncology Group protocol14 had 40% incidence of GU grade 3 late toxicity. Ma et al55 recently published a prospective study where 1,198 patients with different urological complications were admitted in an emergency service. Seventy-seven percentage of the admissions were elective and 23% were emergency. Thirty-three patients of 1,198 had grade 3 or more complications related to previous exposure to radiotherapy, representing the 1.4% and 7.2% of the elective and emergency admissions, respectively. From these 33 patients, 15 patients had PCa, and four of them were initially treated with radical prostatectomy followed by EBRT. The main mode of RT was EBRT (delivered in a median of 34.5 fractions of 2 Gy, median dose 70 Gy). Importantly, the median time from EBRT treatment to admission was 4 years (range 1–9 years). This study highlights that although radiotherapy complications represented a small proportion of the emergency admissions, the gravity of the side effects trigger a surgical intervention or invasive management many years after the primary treatment. In line with this observation, in the phase III randomized ASCENDE-RT trial, the 5-year cumulative incidence of grade 3 or more GU events was 18.4% for LDR-BT versus 5.2% for standard 78 Gy IMRT (P < 0.001). Along the same lines, the 5-year cumulative incidence of grade 3 GI events was 8.1% for LDR-BT versus 3.2% for standard EBRT (P = 0.124).14 Protocols using protons or SBRT have demonstrated very low incidence of severe side effects although these comparisons of technology need to be corroborated in a head-to-head clinical trial.
Furthermore, it is widely believed that younger PCa patients are at greater risk of toxicity after radiotherapy; therefore, large randomized trials with long-term follow-up are required to see if new radiotherapy treatments help all patients with PCa.
Our systematic review demonstrates that biochemical control is achieved in 80–100% of cases when using directed dose escalation to the IDN. The summary outcomes presented in Table 1 and Fig. 3 compared well with the historical EBRT trials and other radiation series that used different modalities and have mature follow-up. Supplementary Table 2. However, despite these encouraging results and because of the variability of patient selection, use of ADT, and length of follow-up, it is difficult to preclude definitive statements regarding efficacy of the boost techniques.
King et al56 demonstrated the favorable therapeutic ratio obtained in a consortium of patients from phase 2 whole prostate SBRT trials who were treated between 2003 and 2011 at eight institutions. Five-year bDFS was achieved in 95, 84, and 81% of low-, intermediate-, and high-risk patients, respectively. The use of ADT and SBRT dose did not significantly affect bDFS even after stratifying by risk group. In a recent randomized trial that included 218 intermediate- to high-risk patients and that compared EBRT + BT boost (35.75 Gy in 13 fractions followed by a HDR-BT boost of 2 × 8.5 Gy in 24 h) versus EBRT (55 Gy in 20 fractions), there was a significant improvement in bDFS for EBRT + HDR-BT, with a median time to relapse of 116 months compared with 74 months for EBRT alone. The 5-, 7-, and 10-year bDFS estimates were 75%, 66%, and 46% for EBRT + HDR-BT boost compared with 61%, 48%, and 39% for EBRT alone (log rank P = 0.04), with no significant difference in side effects. In univariate and multivariate analysis, treatment arm and risk category were significant covariates for risk of biochemical relapse as was the use of ADT13.
Although our systematic review exhaustively investigated different aspects of patient selection, treatment delivery, and outcome, there were areas that could not be evaluated and therefore constitute the limitations of this study. Nevertheless, it is pertinent to discuss these limitations and controversies in an attempt to improve future trial designs. Owing to the heterogeneity of patient selection, we were unable to determine the disease outcome by risk category. The EBRT series reported were comprised of primarily intermediate- to high-risk patients; the BT series included a higher proportion of low-risk patients. It should be thus highlighted that according to the natural history of PCa, dose escalation RT should be delivered to patients who are likely to benefit from active treatment, whereas men with clinically insignificant disease should be monitored carefully by active surveillance.
Specifically, the patients targeted with whole-gland dose irradiation and focal boost should be those with multifocal disease but a clinically significant nodule localized in one area of the prostate. This dominant nodule has been reported to be responsible for local recurrences and drives the natural history of the disease15, 16, 17, 18, 57, 58. In our systematic review, most of the studies used pretreatment MRI as criteria to define the IDN. Although one investigator41 in our review used TRUS, this method has been reported to be inaccurate for localizing disease,59 and less information is available on PET/CT imaging22. In general, it is accepted that imaging in the form of a high-quality mpMRI reported by expert radiologists may have the performance required to localize significant areas of PCa. Evidence is building to show that an area deemed negative on mpMRI stands a 95% probability of having no clinically significant disease as defined by the presence of any Gleason pattern 4 and/or a lesion volume of ≥0.5 ml60, 61. Nevertheless, the diagnostic accuracy of mpMRI to detect IDNs is still a matter of debate and cannot be a solid prerequisite to rationally target these lesions with higher doses of radiation. Therefore, mpMRI should be accompanied by US fusion-targeted biopsy sampling that will allow the detection and biological characterization of the IDN.62 Furthermore, the use of MRI-based radiotherapy planning is still a matter of debate because of the interobserver variability in GTV contouring and concerns about geometric distortions from the MRI system and the patient to be imaged63, 64
In our review, we assumed that most investigators aimed at treating all known visible areas of cancer. We could not obtain information regarding lesions that could deliberately be excluded from the boost area and thus could have been underdosed. This could be the situation in the case where multiple nodules exist in close contact with organs at risk, and thus, the investigator may deliberately decide not to boost them to avoid overdosage of healthy tissue. Indeed, nowadays, it is difficult to ascertain which of the prostate nodules has clinical significance and is likely to have an impact on life expectancy. It is difficult to ascertain if the EBRT dose delivered to the whole gland is sufficient65, 66, 67 to eliminate these tumors. Thus, this area will require further clinical studies to be able to define the biology of prostate tumors that require dose escalation treatment and the radiotherapy dose constraints for the OARs that may limit the delivery of radiation to several dominant nodules. Clinicians have abandoned the use of posttreatment prostate biopsies, but this may constitute the only method to increase our knowledge on the biology of the IDN. As clinicians, we should aim at a better stratification of patients far beyond the current use of clinical prognostic factors. This will allow offering our patients an individualized cancer treatment with local therapy alone or combination with systemic therapy. To explore this, the Radiation Therapy Oncology Group has performed immunohistochemical markers on tissue samples from patients treated in phase III radiotherapy trials68 (with and without ADT). Immunohistochemical-based assessment of protein cell surface expression for p53, p16, Cox-2, PKA, Ki-67, MDM2, BCL2, and Bax were analyzed. Both Ki-67 and bcl2/bax were independently related to early relapse. Another approach is to study the somatic tumor genetics on tissue derived from pretreatment and posttreatment biopsies. The laboratory of Bristow et al has identified c-MYC, NKX3.1, PTEN, STAR, and HSD17B2 as adverse prognostic factors after EBRT69, 70, 71. Novel gene signatures reflective of the underlying biology of PCa progression are also being developed in biopsy material and radical prostatectomy specimens (i.e., Myriad Genetics Prolaris Score, Genome Health OncotypeDx, Genomic Prostate Score, GenomeDx Biosciences Decipher, Nuclear Factor kappa B (NF-kB)–activated recurrence predictor 2172). Careful monitoring of tumor vascularization, hypoxia, DNA damage markers (i.e., Ku70), the development of serum biomarkers of CYP17A1, and antigen receptor activity will be crucial to identify those patients likely to respond to ADT and RT as well as new combined modality combinations.73
Another important limitation of our series was that it was not possible to determine the EBRT dose target coverage per lesion. It is possible in EBRT today to perform heterogeneous planning to mimic BT dosimetry. In that scenario, tumoricidal “hot spots” are deliberately located within the tumor while a dose fall-off bath covers the periphery of the lesion. In that way, the dose to the periphery of the tumor or the prostate may be compromised to respect conservative rectal or urethra dose constraints. Investigators have proposed to manipulate urethra doses not to exceed a maximum of 110% of the prescribed dose although this raises concerns about reducing cancer control for tumors that are too close to the urethra74, 75, 76. In addition, with the constant physiological movement of the bladder and rectum, the urethra is a vulnerable organ that may easily get into the high-dose irradiation area. Therefore, controlling the exact location of the prostate and the IDN by tracking intraprostatic fiducial markers during IGRT sessions is an obvious method to improve EBRT delivery.
Among all series, there was variability in terms of use of ADT, which may affect the GTV definition. For instance, it is uncertain whether reducing the IDN radiation volume based on neoadjuvant ADT response may expose the patients to target missing and subsequent risk of recurrence. Our review also highlights the heterogeneity in the administration of ADT in most series. Despite the strong level-1 scientific evidence supporting the use of ADT to conventional EBRT in intermediate- and high-risk patients even in the context of conventional dose escalation up to 78 Gy, only 27.8% of the patients in our series received ADT. This underutilization of ADT was recently highlighted by Ong et al77 who prospectively evaluated 1,806 PCa patients treated in the population-based Prostate Cancer Outcome Registry Victoria. They reported that one in five men with high-risk PCa and one in two with unfavorable intermediate-risk PCa did not receive ADT with RT. It is possible that in our series, patients have declined standard ADT in the hopes that experimental higher dose radiation to the prostate could provide equal disease control with better QoL and especially sexual QoL compared with the addition of ADT. Based on the current evidence, it is difficult to rule out that dose escalation to the IDN could provide a benefit on local tumor control in the same magnitude than the addition of ADT.
Last but not least, our systematic review revealed relatively few studies with patient-reporting outcomes for assessing toxicity. Future trials should incorporate global health and prostate-specific QoL questionnaires to be able to capture the patient's experience with these new treatments.
Supplementary Table 3 shows prospective registered clinical trials that may provide further evidence to implement this technique in the future11, 42, 46, 78, 79, 80.
The most appropriate radiation dose level, dose constraints, the size of margins, lymph node treatment, and whether neoadjuvant or adjuvant ADT provides any benefit are variables yet to be determined. These caveats should be taken into account before drawing definitive conclusions.
5. Conclusion
Keeping in mind the limitations of this systematic review, there are encouraging results for focal dose escalation to the IDN with acceptable short- to medium-term side effects and biochemical disease control rates. However, owing to the heterogeneity of the studies included, there are many confounding factors limiting the scope of this review. Considering that the clinical endpoint in the studies was biochemical recurrence, the use and duration of ADT administration should be carefully considered before driving definitive conclusions. Randomized trials following similar hypofractionated regimens with sufficient follow-up are needed before this technique can be generally recommended. Therefore, patients who intend to be treated with a dose escalation to the IDN should be enrolled in clinical trials.
Conflicts of interest
None to be declared.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.prnil.2018.03.005.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Forest plots presenting the grade 1–2 toxicity: A) acute gastrointestinal, B) acute genitourinary, C) late gastrointestinal, D) late genitourinary with their calculated 95% confidence interval from the included articles where this could be retrieved.
figs1.
figs2.
figs3.
figs4.
References
- 1.Ferlay J., Steliarova-Foucher E., Lortet-Tieulent J., Rosso S., Coebergh J.W., Comber H. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer Oxf Engl 1990. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration. Fitzmaurice C., Dicker D., Pain A., Hamavid H., Moradi-Lakeh M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godtman R.A., Holmberg E., Khatami A., Stranne J., Hugosson J. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Göteborg randomised population-based prostate cancer screening trial. Eur Urol. 2013;63(1):101–107. doi: 10.1016/j.eururo.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 4.Viani G.A., Stefano E.J., Afonso S.L. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74(5):1405–1418. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 5.Pickett B., Vigneault E., Kurhanewicz J., Verhey L., Roach M. Static field intensity modulation to treat a dominant intra-prostatic lesion to 90 Gy compared to seven field 3-dimensional radiotherapy. Int J Radiat Oncol Biol Phys. 1999;44(4):921–929. doi: 10.1016/s0360-3016(98)00502-1. [DOI] [PubMed] [Google Scholar]
- 6.van Lin E.N.J.T., Fütterer J.J., Heijmink S.W.T.P.J., van der Vight L.P., Hoffmann A.L., van Kollenburg P. IMRT boost dose planning on dominant intraprostatic lesions: gold marker-based three-dimensional fusion of CT with dynamic contrast-enhanced and 1H-spectroscopic MRI. Int J Radiat Oncol Biol Phys. 2006;65(1):291–303. doi: 10.1016/j.ijrobp.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 7.Zaider M., Zelefsky M.J., Lee E.K., Zakian K.L., Amols H.I., Dyke J. Treatment planning for prostate implants using magnetic-resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys. 2000;47(4):1085–1096. doi: 10.1016/s0360-3016(00)00557-5. [DOI] [PubMed] [Google Scholar]
- 8.Ellis R.J., Zhou H., Kim E.Y., Fu P., Kaminsky D.A., Sodee B. Biochemical disease-free survival rates following definitive low-dose-rate prostate brachytherapy with dose escalation to biologic target volumes identified with SPECT/CT capromab pendetide. Brachytherapy. 2007;6(1):16–25. doi: 10.1016/j.brachy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Pouliot J., Kim Y., Lessard E., Hsu I.-C., Vigneron D.B., Kurhanewicz J. Inverse planning for HDR prostate brachytherapy used to boost dominant intraprostatic lesions defined by magnetic resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys. 2004;59(4):1196–1207. doi: 10.1016/j.ijrobp.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 10.Pollack A., Zagars G.K., Starkschall G., Antolak J.A., Lee J.J., Huang E. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53(5):1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 11.Morris W.J., Tyldesley S., Rodda S., Halperin R., Pai H., McKenzie M. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Sathya J.R., Davis I.R., Julian J.A., Guo Q., Daya D., Dayes I.S. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(6):1192–1199. doi: 10.1200/JCO.2005.06.154. [DOI] [PubMed] [Google Scholar]
- 13.Hoskin P.J., Rojas A.M., Bownes P.J., Lowe G.J., Ostler P.J., Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2012;103(2):217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Rodda S., Tyldesley S., Morris W.J., Keyes M., Halperin R., Pai H. ASCENDE-RT: An Analysis of Treatment-Related Morbidity for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost with a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):286–295. doi: 10.1016/j.ijrobp.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Arrayeh E., Westphalen A.C., Kurhanewicz J., Roach M., 3rd, Jung A.J., Carroll P.R. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. Int J Radiat Oncol Biol Phys. 2012;82(5):e787–793. doi: 10.1016/j.ijrobp.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cellini N., Morganti A.G., Mattiucci G.C., Valentini V., Leone M., Luzi S. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: implications for conformal therapy planning. Int J Radiat Oncol Biol Phys. 2002;53(3):595–599. doi: 10.1016/s0360-3016(02)02795-5. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed H.U., Hindley R.G., Dickinson L., Freeman A., Kirkham A.P., Sahu M. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012;13(6):622–632. doi: 10.1016/S1470-2045(12)70121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pucar D., Hricak H., Shukla-Dave A., Kuroiwa K., Drobnjak M., Eastham J. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69(1):62–69. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 19.Huang C.C., Deng F.-M., Kong M.X., Ren Q., Melamed J., Zhou M. Re-evaluating the concept of “dominant/index tumor nodule” in multifocal prostate cancer. Virchows Arch Int J Pathol. 2014;464(5):589–594. doi: 10.1007/s00428-014-1557-y. [DOI] [PubMed] [Google Scholar]
- 20.Sciarra A., Barentsz J., Bjartell A., Eastham J., Hricak H., Panebianco V. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. Eur Urol. 2011;59(6):962–977. doi: 10.1016/j.eururo.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Chang J.H., Joon D.L., Lee S.T., Gong S.J., Scott A.M., Davis I.D. Histopathological correlation of (11)C-choline PET scans for target volume definition in radical prostate radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2011;99(2):187–192. doi: 10.1016/j.radonc.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Maurer T., Eiber M., Schwaiger M., Gschwend J.E. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13(4):226–235. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 23.Simmons L.A.M., Autier P., Zát’ura F., Braeckman J., Peltier A., Romic I. Detection, localisation and characterisation of prostate cancer by prostate HistoScanning(TM) BJU Int. 2012;110(1):28–35. doi: 10.1111/j.1464-410X.2011.10734.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y., Chen Y., Qi T., Jiang J., Qi J., Yu Y. Prostate cancer detection with real-time elastography using a bi-plane transducer: comparison with step section radical prostatectomy pathology. World J Urol. 2014;32(2):329–333. doi: 10.1007/s00345-012-0922-1. [DOI] [PubMed] [Google Scholar]
- 25.Zelefsky M.J., Kollmeier M., Cox B., Fidaleo A., Sperling D., Pei X. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84(1):125–129. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 26.Xie Y., Djajaputra D., King C.R., Hossain S., Ma L., Xing L. Intrafractional motion of the prostate during hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(1):236–246. doi: 10.1016/j.ijrobp.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klayton T., Price R., Buyyounouski M.K., Sobczak M., Greenberg R., Li J. Prostate bed motion during intensity-modulated radiotherapy treatment. Int J Radiat Oncol Biol Phys. 2012;84(1):130–136. doi: 10.1016/j.ijrobp.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zelefsky M.J., Cohen G., Zakian K.L., Dyke J., Koutcher J.A., Hricak H. Intraoperative conformal optimization for transperineal prostate implantation using magnetic resonance spectroscopic imaging. Cancer J Sudbury Mass. 2000;6(4):249–255. [PubMed] [Google Scholar]
- 29.DiBiase S.J., Hosseinzadeh K., Gullapalli R.P., Jacobs S.C., Naslund M.J., Sklar G.N. Magnetic resonance spectroscopic imaging-guided brachytherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2002;52(2):429–438. doi: 10.1016/s0360-3016(01)02609-8. [DOI] [PubMed] [Google Scholar]
- 30.De Meerleer G., Villeirs G., Bral S., Paelinck L., De Gersem W., Dekuyper P. The magnetic resonance detected intraprostatic lesion in prostate cancer: planning and delivery of intensity-modulated radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2005;75(3):325–333. doi: 10.1016/j.radonc.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Singh A.K., Guion P., Sears-Crouse N., Ullman K., Smith S., Albert P.S. Simultaneous integrated boost of biopsy proven, MRI defined dominant intra-prostatic lesions to 95 Gray with IMRT: early results of a phase I NCI study. Radiat Oncol Lond Engl. 2007;2:36. doi: 10.1186/1748-717X-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonteyne V., Villeirs G., Speleers B., De Neve W., De Wagter C., Lumen N. Intensity-modulated radiotherapy as primary therapy for prostate cancer: report on acute toxicity after dose escalation with simultaneous integrated boost to intraprostatic lesion. Int J Radiat Oncol Biol Phys. 2008;72(3):799–807. doi: 10.1016/j.ijrobp.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Ares C., Popowski Y., Pampallona S., Nouet P., Dipasquale G., Bieri S. Hypofractionated boost with high-dose-rate brachytherapy and open magnetic resonance imaging-guided implants for locally aggressive prostate cancer: a sequential dose-escalation pilot study. Int J Radiat Oncol Biol Phys. 2009;75(3):656–663. doi: 10.1016/j.ijrobp.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Miralbell R., Mollà M., Rouzaud M., Hidalgo A., Toscas J.I., Lozano J. Hypofractionated boost to the dominant tumor region with intensity modulated stereotactic radiotherapy for prostate cancer: a sequential dose escalation pilot study. Int J Radiat Oncol Biol Phys. 2010;78(1):50–57. doi: 10.1016/j.ijrobp.2009.07.1689. [DOI] [PubMed] [Google Scholar]
- 35.Schick U., Popowski Y., Nouet P., Bieri S., Rouzaud M., Khan H. High-dose-rate brachytherapy boost to the dominant intra-prostatic tumor region: hemi-irradiation of prostate cancer. Prostate. 2011;71(12):1309–1316. doi: 10.1002/pros.21347. [DOI] [PubMed] [Google Scholar]
- 36.Ellis R.J., Zhou H., Kaminsky D.A., Fu P., Kim E.Y., Sodee D.B. Rectal morbidity after permanent prostate brachytherapy with dose escalation to biologic target volumes identified by SPECT/CT fusion. Brachytherapy. 2007;6(2):149–156. doi: 10.1016/j.brachy.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Ellis R.J., Kaminsky D.A., Zhou E.H., Fu P., Chen W.D., Brelin A. Ten-year outcomes: the clinical utility of single photon emission computed tomography/computed tomography capromab pendetide (Prostascint) in a cohort diagnosed with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):29–34. doi: 10.1016/j.ijrobp.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 38.Wong W.W., Schild S.E., Vora S.A., Ezzell G.A., Nguyen B.D., Ram P.C. Image-guided radiotherapy for prostate cancer: a prospective trial of concomitant boost using indium-111-capromab pendetide (ProstaScint) imaging. Int J Radiat Oncol Biol Phys. 2011;81(4):e423–429. doi: 10.1016/j.ijrobp.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 39.Pinkawa M., Piroth M.D., Holy R., Klotz J., Djukic V., Corral N.E. Dose-escalation using intensity-modulated radiotherapy for prostate cancer - evaluation of quality of life with and without (18)F-choline PET-CT detected simultaneous integrated boost. Radiat Oncol Lond Engl. 2012;7:14. doi: 10.1186/1748-717X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ippolito E., Mantini G., Morganti A.G., Mazzeo E., Padula G.D., Digesù C. Intensity-modulated radiotherapy with simultaneous integrated boost to dominant intraprostatic lesion: preliminary report on toxicity. Am J Clin Oncol. 2012;35(2):158–162. doi: 10.1097/COC.0b013e318209cd8f. [DOI] [PubMed] [Google Scholar]
- 41.Myers M.A., Hagan M.P., Todor D., Gilbert L., Mukhopadhyay N., Randolf J. Phase I/II trial of single-fraction high-dose-rate brachytherapy-boosted hypofractionated intensity-modulated radiation therapy for localized adenocarcinoma of the prostate. Brachytherapy. 2012;11(4):292–298. doi: 10.1016/j.brachy.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Aluwini S., van Rooij P., Hoogeman M., Kirkels W., Kolkman-Deurloo I.-K., Bangma C. Stereotactic body radiotherapy with a focal boost to the MRI-visible tumor as monotherapy for low- and intermediate-risk prostate cancer: early results. Radiat Oncol Lond Engl. 2013;8:84. doi: 10.1186/1748-717X-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schild M.H., Schild S.E., Wong W.W., Vora S.A., Silva A.C., Silva A.M. Early Outcome of Prostate Intensity Modulated Radiation Therapy (IMRT) Incorporating a Simultaneous Intra-Prostatic MRI Directed Boost. OMICS J Radiol. 2014;3(4) doi: 10.4172/2167-7964.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez-Iturriaga A., Casquero F., Urresola A., Ezquerro A., Lopez J.I., Espinosa J.M. Dose escalation to dominant intraprostatic lesions with MRI-transrectal ultrasound fusion High-Dose-Rate prostate brachytherapy. Prospective phase II trial. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2016;119(1):91–96. doi: 10.1016/j.radonc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 45.King M.T., Nasser N.J., Mathur N., Cohen G.N., Kollmeier M.A., Yuen J. Long-term outcome of magnetic resonance spectroscopic image-directed dose escalation for prostate brachytherapy. Brachytherapy. 2016;15(3):266–273. doi: 10.1016/j.brachy.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundahl N., De Meerleer G., Villeirs G., Ost P., De Neve W., Lumen N. Combining high dose external beam radiotherapy with a simultaneous integrated boost to the dominant intraprostatic lesion: Analysis of genito-urinary and rectal toxicity. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2016;119(3):398–404. doi: 10.1016/j.radonc.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 47.Kotecha R., Djemil T., Tendulkar R.D., Reddy C.A., Thousand R.A., Vassil A. Dose-Escalated Stereotactic Body Radiation Therapy for Patients With Intermediate- and High-Risk Prostate Cancer: Initial Dosimetry Analysis and Patient Outcomes. Int J Radiat Oncol Biol Phys. 2016;95(3):960–964. doi: 10.1016/j.ijrobp.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Uzan J., Nahum A.E., Syndikus I. Prostate Dose-painting Radiotherapy and Radiobiological Guided Optimisation Enhances the Therapeutic Ratio. Clin Oncol R Coll Radiol G B. 2016;28(3):165–170. doi: 10.1016/j.clon.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Garibaldi E., Delmastro E., Gabriele D., Bresciani S., Russo F., Di Dia A. Clinical and technical feasibility of ultra-boost irradiation in Dominant Intraprostatic Lesion by Tomotherapy: preliminary experience and revision of literature. Panminerva Med. 2016;58(1):16–22. [PubMed] [Google Scholar]
- 50.Brenner D.J., Hall E.J. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43(5):1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 51.Fowler J.F., Ritter M.A., Chappell R.J., Brenner D.J. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56(4):1093–1104. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 52.Pinkawa M., Attieh C., Piroth M.D., Holy R., Nussen S., Klotz J. Dose-escalation using intensity-modulated radiotherapy for prostate cancer–evaluation of the dose distribution with and without 18F-choline PET-CT detected simultaneous integrated boost. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2009;93(2):213–219. doi: 10.1016/j.radonc.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Hannan R., Tumati V., Xie X.-J., Cho L.C., Kavanagh B.D., Brindle J. Stereotactic body radiation therapy for low and intermediate risk prostate cancer-Results from a multi-institutional clinical trial. Eur J Cancer Oxf Engl 1990. 2016;59:142–151. doi: 10.1016/j.ejca.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 54.Mariados N., Sylvester J., Shah D., Karsh L., Hudes R., Beyer D. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;92(5):971–977. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 55.Ma J.L., Hennessey D.B., Newell B.P., Bolton D.M., Lawrentschuk N. Radiotherapy-related complications presenting to a urology department - a more common problem than previously thought? BJU Int. January 2018 doi: 10.1111/bju.14145. [DOI] [PubMed] [Google Scholar]
- 56.King C.R., Freeman D., Kaplan I., Fuller D., Bolzicco G., Collins S. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2013;109(2):217–221. doi: 10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 57.Bott S.R.J., Ahmed H.U., Hindley R.G., Abdul-Rahman A., Freeman A., Emberton M. The index lesion and focal therapy: an analysis of the pathological characteristics of prostate cancer. BJU Int. 2010;106(11):1607–1611. doi: 10.1111/j.1464-410X.2010.09436.x. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed H.U. The index lesion and the origin of prostate cancer. N Engl J Med. 2009;361(17):1704–1706. doi: 10.1056/NEJMcibr0905562. [DOI] [PubMed] [Google Scholar]
- 59.Washington S.L., Bonham M., Whitson J.M., Cowan J.E., Carroll P.R. Transrectal ultrasonography-guided biopsy does not reliably identify dominant cancer location in men with low-risk prostate cancer. BJU Int. 2012;110(1):50–55. doi: 10.1111/j.1464-410X.2011.10704.x. [DOI] [PubMed] [Google Scholar]
- 60.Puech P., Potiron E., Lemaitre L., Leroy X., Haber G.P., Crouzet S. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology. 2009;74(5):1094–1099. doi: 10.1016/j.urology.2009.04.102. [DOI] [PubMed] [Google Scholar]
- 61.Villers A., Puech P., Mouton D., Leroy X., Ballereau C., Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol. 2006;176(6 Pt 1):2432–2437. doi: 10.1016/j.juro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Gomez-Iturriaga A., Casquero F., Lopez J.I., Urresola A., Ezquerro A., Buscher D. Transperineal biopsies of MRI-detected aggressive index lesions in low- and intermediate-risk prostate cancer patients: Implications for treatment decision. Brachytherapy. 2017;16(1):201–206. doi: 10.1016/j.brachy.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Adjeiwaah M., Bylund M., Lundman J.A., Karlsson C.T., Jonsson J.H., Nyholm T. Quantifying the Effect of 3T Magnetic Resonance Imaging Residual System Distortions and Patient-Induced Susceptibility Distortions on Radiation Therapy Treatment Planning for Prostate Cancer. Int J Radiat Oncol Biol Phys. October 2017 doi: 10.1016/j.ijrobp.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 64.Rischke H.C., Nestle U., Fechter T., Doll C., Volegova-Neher N., Henne K. 3 Tesla multiparametric MRI for GTV-definition of Dominant Intraprostatic Lesions in patients with Prostate Cancer–an interobserver variability study. Radiat Oncol Lond Engl. 2013;8:183. doi: 10.1186/1748-717X-8-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu W., Laitinen S., Khan S., Vihinen M., Kowalski J., Yu G. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15(5):559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van der Kwast T.H. The trade-off between sensitivity and specificity of clinical protocols for identification of insignificant prostate cancer. Eur Urol. 2012;62(3):469–471. doi: 10.1016/j.eururo.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 67.Grasso C.S., Wu Y.-M., Robinson D.R., Cao X., Dhanasekaran S.M., Khan A.P. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollack A., Kwon D., Walker G., Khor L.Y., Horwitz E.M., Buyyounouski M.K. Prospective Validation of Diagnostic Tumor Biomarkers in Men Treated With Radiotherapy for Prostate Cancer. J Natl Cancer Inst. 2017;109(2):1–8. doi: 10.1093/jnci/djw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zafarana G., Ishkanian A.S., Malloff C.A., Locke J.A., Sykes J., Thoms J. Copy number alterations of c-MYC and PTEN are prognostic factors for relapse after prostate cancer radiotherapy. Cancer. 2012;118(16):4053–4062. doi: 10.1002/cncr.26729. [DOI] [PubMed] [Google Scholar]
- 70.Locke J.A., Zafarana G., Malloff C.A., Lam W.L., Sykes J., Pintilie M. Allelic loss of the loci containing the androgen synthesis gene, StAR, is prognostic for relapse in intermediate-risk prostate cancer. Prostate. 2012;72(12):1295–1305. doi: 10.1002/pros.22478. [DOI] [PubMed] [Google Scholar]
- 71.Locke J.A., Zafarana G., Ishkanian A.S., Milosevic M., Thoms J., Have C.L. NKX3.1 haploinsufficiency is prognostic for prostate cancer relapse following surgery or image-guided radiotherapy. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18(1):308–316. doi: 10.1158/1078-0432.CCR-11-2147. [DOI] [PubMed] [Google Scholar]
- 72.Dal Pra A., Locke J.A., Borst G., Supiot S., Bristow R.G. Mechanistic Insights into Molecular Targeting and Combined Modality Therapy for Aggressive, Localized Prostate Cancer. Front Oncol. 2016;6:24. doi: 10.3389/fonc.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McGrath S., Christidis D., Perera M., Hong S.K., Manning T., Vela I. Prostate cancer biomarkers: Are we hitting the mark? Prostate Int. 2016;4(4):130–135. doi: 10.1016/j.prnil.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen L.N., Suy S., Uhm S., Oermann E.K., Ju A.W., Chen V. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol Lond Engl. 2013;8:58. doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fuller D.B., Naitoh J., Lee C., Hardy S., Jin H. Virtual HDR CyberKnife treatment for localized prostatic carcinoma: dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys. 2008;70(5):1588–1597. doi: 10.1016/j.ijrobp.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 76.Vainshtein J., Abu-Isa E., Olson K.B., Ray M.E., Sandler H.M., Normolle D. Randomized phase II trial of urethral sparing intensity modulated radiation therapy in low-risk prostate cancer: implications for focal therapy. Radiat Oncol Lond Engl. 2012;7:82. doi: 10.1186/1748-717X-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ong W.L., Foroudi F., Evans S., Millar J. Large institutional variations in use of androgen deprivation therapy with definitive radiotherapy in a population-based cohort of men with intermediate- and high-risk prostate cancer. BJU Int. 2017;120(Suppl 3):35–42. doi: 10.1111/bju.13969. [DOI] [PubMed] [Google Scholar]
- 78.Michalski J.M., Yan Y., Watkins-Bruner D., Bosch W.R., Winter K., Galvin J.M. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87(5):932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zietman A.L., DeSilvio M.L., Slater J.D., Rossi C.J., Jr., Miller D.W., Adams J.A. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. J Am Med Assoc. 2005;294(10):1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 80.Katz A.J., Kang J. Quality of Life and Toxicity after SBRT for Organ-Confined Prostate Cancer, a 7-Year Study. Front Oncol. 2014;4:301. doi: 10.3389/fonc.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.