Abstract
Pulmonary arterial hypertension (PAH) is a common complication of a congenital heart defect (CHD). Recent studies suggest metformin may be a potential drug to improve cardiac function in PAH. A pilot study was conducted to investigate the efficacy of short-term treatment with a combination regimen consisting of bosentan and metformin in PAH-CHD patients as compared with bosentan monotherapy in a prospective, randomised study.
Patients with PAH-CHD were randomised to receive bosentan (initially at 62.5 mg twice daily for 4 weeks and then 125 mg twice daily) for 3 months with or without the combination treatment of metformin (500 mg twice daily).
93 patients were enrolled to bosentan monotherapy (n=48) or bosentan/metformin combination treatment (n=45). After 3 months, both treatments significantly improved World Health Organization functional class, 6-min walking distance (6MWD), N-terminal pro-brain natriuretic peptide and right heart haemodynamic parameters. The improvements in 6MWD and pulmonary vascular resistance index were significantly greater in patients treated with combination therapy than in those who received monotherapy (mean±sd 95±136 versus 48±119 m (p=0.017) and −1.8±1.2 versus −1.2±1.3 Wood units per m2 (p<0.001), respectively). Pulmonary endothelin (EDN)1 was significantly decreased after combination therapy (p=0.006). However, plasma EDN1 levels were not affected.
Combination therapy with bosentan and metformin in PAH-CHD patients provides improvements in important outcomes such as exercise capacity and pulmonary haemodynamics, compared with bosentan alone.
Short abstract
This study investigated, for the first time, the efficacy of a combination regimen consisting of bosentan and metformin in patients with pulmonary arterial hypertension associated with congenital heart defect as compared with bosentan monotherapy http://ow.ly/n4nJ30kT98d
Introduction
Pulmonary arterial hypertension (PAH) is a common complication of a congenital heart defect (CHD), and leads to significant morbidity and mortality [1, 2]. PAH-CHD develops as a result of increased pulmonary vascular resistance (PVR) due to chronic volume and pressure overload of the pulmonary circulation, mainly in patients diagnosed late or in patients who did not have access to cardiovascular care and surgical management as infants, particularly in developing countries [3]. Currently, there are no curative treatments for PAH-CHD other than heart–lung transplantation, which has been limited by the shortage of organ donors and long waiting times. Therefore, management focuses on slowing down the progress of the PAH and improving quality of life and heart function before transplantation proceeds.
The pathogenesis of PAH-CHD is complex and involves multiple biochemical signalling pathways including the endothelin (EDN), nitric oxide and prostacyclin pathways. Therefore, combining drugs that target more than one of these pathways may offer additional benefits over endothelin receptor antagonist (e.g. bosentan) monotherapy. While bosentan may help to prevent World Health Organization (WHO)/New York Heart Association functional class deterioration, such as by reducing dyspnoea and improving cardiopulmonary haemodynamic variables, there is little evidence to demonstrate it improves mortality outcomes [4–6]. Metformin is a well-known blood glucose-lowering drug for type 2 diabetes that has been shown to reduce cardiovascular disease risks in those patients [7] and, in experimental studies, has shown promise in improving function in PAH models [8, 9].
Previous studies suggest the cardiovascular protective effects of metformin may not be solely attributed to its anti-hyperglycaemic properties and may involve its actions on improving lipid metabolism, oxidative stress, inflammatory response, and endothelial and vascular smooth muscle cell (VSMC) functions [10, 11]. There is also evidence that it can attenuate cardiac fibrosis and retard cardiac hypertrophy during hypertensive stress [12, 13]. At a cellular level, it is noted that metformin may improve mitochondrial respiration and ATP synthesis in myocardial cells [14]. The positive effects of metformin on vascular reactivity have been demonstrated in normoinsulinaemic patients [15] and in response to vasodilator agents in diabetic rats [16]. Interestingly, metformin has been shown to reverse the development of experimental PAH [8, 9]. For example, Agard et al. [8] demonstrated in rats with hypoxia- and monocrotaline-induced PAH that metformin retarded pulmonary vascular proliferation and remodelling, as well as improved pulmonary artery vasoreactivity. While these studies suggest clinical potential, there have been no studies in humans to demonstrate translational efficacy of metformin in PAH-CHD. Therefore, the current study aimed to investigate the efficacy of short-term treatments with a combination regimen consisting of bosentan and metformin in PAH-CHD patients compared with bosentan monotherapy, and also explore the possible mechanism of action of metformin in those patients.
Methods
This prospective, randomised study was approved by the Institutional Ethics Committee of Nanchang University on Human Research (Nanchang, China) and informed consent was obtained from each patient. Consecutive adult patients (18–65 years of age) first diagnosed with PAH-CHD at our hospital between May 2016 and December 2017 were enrolled.
The inclusion criteria of this study were patients defined as being at intermediate risk with 5–10% estimated 1-year mortality according to the 2015 European Society of Cardiology/European Respiratory Society/International Society of Heart and Lung Transplantation guidelines [17]. Exclusion criteria were patients who progressed to pulmonary-to-systemic or bidirectional shunting (Eisenmenger's syndrome), developed PAH after defect correction, were diagnosed with type 1/2 diabetes or impaired glucose tolerance (defined as blood glucose of ≥7.8 mmol·L−1 or more but <11.1 mmol·L−1 after a 2-h oral glucose tolerance test), or had abnormal liver/kidney function tests. All patients underwent right heart catheterisation for a definitive diagnosis of PAH, and to assess the severity of haemodynamic impairment before and after treatments. Other assessments included self-assessment of symptoms, WHO functional class, 6-min walking distance (6MWD), N-terminal pro-brain natriuretic peptide (NT-proBNP), and echocardiography for structural and functional measurements of right heart function. Plasma NT-proBNP and EDN1 were measured by human-specific ELISAs as per the manufacturers' instructions (Life Technologies, Carlsbad, CA, USA).
Treatment
All enrolled patients were randomised to receive an endothelin receptor antagonist (bosentan, initially at 62.5 mg twice daily for 4 weeks and then 125 mg twice daily) for 3 months with or without the combination treatment of metformin (500 mg twice daily). Liver function tests were performed every 2 weeks during the first 2 months and monthly thereafter. Patients who could not tolerate bosentan at 125 mg twice daily were down-titrated to 62.5 mg twice daily.
After 3 months treatments, all patients underwent right heart catheterisation and acute vasoreactivity testing, and operability was assessed by our paediatric heart surgery team. Selected patients underwent operations with biventricular circulation. The criteria for shunt closure based on the baseline right heart catheterisation and acute vasoreactivity testing were a baseline PVR index <6 Wood units (WU) per m2 and PVR/systemic vascular resistance (SVR) ratio <0.3 [18, 19]. In patients with a baseline PVR index between 6 and 9 WU·m−2 and a PVR/SVR ratio of 0.3–0.5, acute vasoreactivity testing was performed. If there was a 20% decrease in PVR index and PVR/SVR ratio, or final results of PVR index <6 WU·m−2 and PVR/SVR ratio <0.3, patients were eligible for the operation. Additional criteria included the type of defect, age and the pulmonary/systemic flow ratio. Lung needle biopsy specimens were collected from different areas of either the left or right lung before cardiopulmonary bypass intraoperatively and immediately placed in liquid nitrogen for further analysis.
Quantitative real-time PCR
Total RNA was isolated from lung samples using Trizol (Life Technologies). The quantity and integrity of RNA were determined using a NanoDrop spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA) and an Bioanalyzer RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA, USA), respectively. Isolated RNA was treated with DNAase I (Life Technologies). Single-stranded complementary DNA (cDNA) was synthesised from 1 μg RNA using a Transcriptor first-strand cDNA synthesis kit (Roche, Basel, Switzerland) according to the manufacturer's protocol. Real-time PCR analysis was carried out using predesigned PrimeTime quantitative PCR assays (Integrated DNA Technologies, Coralville, IA, USA) on a Lightcycler 480 (Roche). The gene expression levels of endothelial NO synthase (eNOS; NOS3, Hs.PT.58.27740527), inducible NO synthase (NOS2, Hs.PT.58.14740388), neuronal NO synthase (NOS1, Hs.PT.58.24422037), EDN1 (Hs.PT.58.28278183.g), EDN2 (Hs.PT.58.20315946), endothelin receptor type A (EDNRA, Hs.PT.58.3235504) and endothelin receptor type B (EDNRB, Hs.PT.58.40275179) were normalised to two housekeeping genes (glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin (ACTB)) by subtracting the geometric mean cycle threshold (Ct) of housekeeping genes from the Ct for the gene of interest to produce a ΔCt value. The ΔCt for each treatment sample was compared with the mean ΔCt for vehicle-treated samples using the relative quantification 2-ΔΔCt method to determine fold change [20].
Statistical analysis
All normally distributed data are presented as mean±sd unless stated otherwise. Group means were compared using Student's t-test. Categorical variables were compared using the Chi-squared or Fisher's exact test. An ANCOVA, with the main outcome at 3 months follow-up as the dependent variable and baseline value and treatment group as independent variables, was used to estimate the mean effect in each treatment arm, considering the potential baseline differences [21]. Analysis was conducted using SPSS Statistics 24 (IBM, Armonk, NY, USA). A p-value of <0.05 was accepted as statistically significant.
Results
Baseline characteristics
During the study period, 93 patients were enrolled: 48 were randomised to bosentan monotherapy, and 45 to the combination treatment of bosentan and metformin. There were no significant differences between the two groups in terms of baseline characteristics and haemodynamic parameters (table 1). The mean age of all participants was 31.2 years and 62.4% were female. The majority of patients (82.8%) developed PAH associated with a ventricular septal defect. 92.5% of participants had WHO functional class III symptoms. None of the patients had been treated with any PAH-specific drugs (e.g. sildenafil or bosentan) prior to the study.
TABLE 1.
Baseline characteristics in bosentan monotherapy and bosentan/metformin combination therapy groups
| Bosentan | Bosentan/metformin | p-value# | |
| Patients n | 48 | 45 | |
| Age years | 32.5±13.2 | 28.6±10.4 | 0.119 |
| Females n (%) | 32 (66.7%) | 26 (57.8%) | 0.399 |
| BMI kg·m−2 | 22.4±5.4 | 23.6±6.4 | 0.331 |
| Heart defects n (%) | 0.663 | ||
| ASD | 7 (14.6%) | 5 (11.1%) | |
| VSD | 39 (81.2%) | 38 (84.4%) | |
| PDA | 0 (0%) | 1 (2.2%) | |
| TAPVR | 2 (4.2%) | 1 (2.2%) | |
| WHO functional class n (%) | 0.761 | ||
| II | 4 (8.3%) | 3 (6.7%) | |
| III | 44 (91.7%) | 42 (93.3%) | |
| 6MWD m | 315±125 | 329±122 | 0.586 |
| NT-proBNP ng·L−1 | 626±253 | 746±319 | 0.046 |
| Peak TRV m·s−1 | 3.5±0.7 | 3.9±1.2 | 0.051 |
| RA area cm2 | 30±8 | 33±9 | 0.092 |
| Mean PAP mmHg | 55±12 | 61±18 | 0.060 |
| RAP mmHg | 18±8 | 16±7 | 0.204 |
| PAWP mmHg | 8.9±3.3 | 8.8±3.6 | 0.889 |
| PVR index WU·m−2 | 6.8±0.9 | 6.6±0.9 | 0.287 |
| PVR/SVR ratio | 0.38±0.13 | 0.41±0.16 | 0.322 |
| Cardiac index L·min−1·m−2 | 2.2±0.3 | 2.3±0.2 | 0.635 |
| SvO2 | 68±9% | 70±9% | 0.287 |
Data are presented as mean±sd unless otherwise stated. BMI: body mass index; ASD: atrial septal defect; VSD: ventricular septal defect; PDA: patent ductus arteriosus; TAPVR: total anomalous pulmonary venous return; WHO: World Health Organization; 6MWD: 6-min walking distance; NT-proBNP: N-terminal pro-brain natriuretic peptide; TRV: tricuspid regurgitation velocity; RA: right atrial; PAP: pulmonary arterial pressure; RAP: right atrial pressure; PAWP: pulmonary arterial wedge pressure; PVR: pulmonary vascular resistance; WU: Wood unit; SVR: systemic vascular resistance; SvO2: mixed venous oxygen saturation.#: Chi-squared test or t-test as appropriate.
Treatment outcomes
All patients survived and had the follow-up visit at 3 months. Four patients in the monotherapy group and three patients in the combination therapy group did not tolerate bosentan at 125 mg twice daily, suffering moderate to severe liver dysfunction and/or elevated aminotransferases greater than three times the upper limit of normal, and were down-titrated to 62.5 mg twice daily. One patient stopped metformin at 2 weeks of treatment due to severe diarrhoea and was excluded from statistical analysis. One patient in the monotherapy group needed hospitalisation due to pneumonia at 6 weeks.
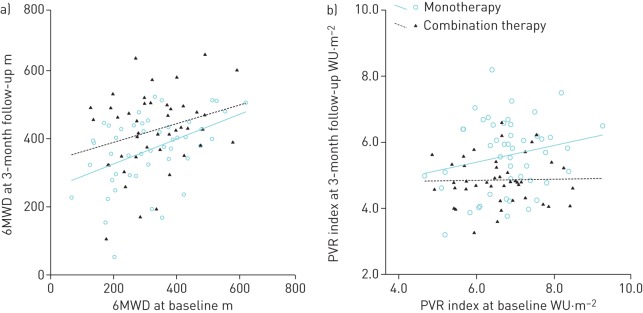
Fasting blood glucose and haemoglobin A1c levels and lipid profile were not affected by either bosentan monotherapy or bosentan/metformin combination therapy for 3 months (data not shown). Three patients in the bosentan monotherapy group and two patients in the combination therapy group did not have improvements in any observational parameters. Compared with baseline, WHO functional class, 6MWD, NT-proBNP and right heart haemodynamic variables all improved with bosentan monotherapy and bosentan/metformin combination therapy (table 2). The improvement in 6MWD from treatment initiation to the 3-month follow-up was significantly greater in patients treated with combination therapy than in those who received monotherapy (95±136 versus 48±119 m, p=0.017) (table 3 and figure 1a). The improvement in PVR index in combination therapy was also significantly greater than in monotherapy group (−1.8±1.2 versus−1.2±1.3 WU·m−2, p<0.001) (table 3 and figure 1b). Interestingly, the PVR/SVR ratio in the combination therapy group, but not in the monotherapy group, demonstrated a significant reduction in acute vasoreactivity when challenged with NO during right heart catheterisation (0.31±0.11 versus 0.25±0.15, p<0.05). There were no differences in plasma EDN1 levels between the two groups after treatment (2.25±0.91 versus 2. 18±1.12 pg·mL−1, p>0.05).
TABLE 2.
Baseline characteristics and haemodynamic changes from baseline to 3 months evaluation in the bosentan monotherapy group versus the bosentan/metformin combination therapy group
| Variables | Bosentan# | Bosentan/metformin¶ | ||||
| Baseline | 3 months | p-value+ | Baseline | 3 months | p value+ | |
| BMI kg·m−2 | 22.4±5.4 | 23.1±6.2 | 0.557 | 23.5±6.1 | 22.5±5.4 | 0.397 |
| WHO functional class n (%) | <0.001 | <0.001 | ||||
| II | 4 (8.3%) | 22 (45.8%) | 3 (6.8%) | 28 (63.6%) | ||
| III | 44 (91.7%) | 26 (54.2%) | 41 (93.2%) | 16 (36.4%) | ||
| 6MWD m | 315±125 | 368±103 | 0.026 | 324±121 | 426±111 | <0.001 |
| NT-proBNP ng·L−1 | 626±253 | 438±189 | <0.001 | 743±316 | 568±269 | 0.006 |
| Peak TRV m·s−1 | 3.5±0.7 | 3.1±0.6 | 0.003 | 3.9±1.2 | 3.2±0.9 | 0.003 |
| RA area cm2 | 30±8 | 28±9 | 0.253 | 32±9 | 31±8 | 0.583 |
| Mean PAP mmHg | 55±12 | 43±9 | <0.001 | 60 ±18 | 40±10 | <0.001 |
| RAP mmHg | 18±8 | 11±7 | <0.001 | 16±6 | 10±6 | <0.001 |
| PAWP mmHg | 8.9±3.3 | 9.2±3.8 | 0.681 | 8.7±3.5 | 8.9±3.4 | 0.786 |
| PVR index WU·m−2 | 6.8±0.9 | 5.6±1.1 5.3±1.3§ |
<0.001 | 6.6±0.9 | 4.8±0.8 4.4±1.5§ |
<0.001 |
| PVR/SVR ratio | 0.38±0.13 | 0.28±0.16 0.26±0.18§ |
<0.001 | 0.41±0.17 | 0.31±0.11 0.25±0.15§ |
<0.001 |
| Cardiac index L·min−1·m−2 | 2.2±0.3 | 2.5±0.4 | <0.001 | 2.3±0.2 | 2.6±0.4 | <0.001 |
| SvO2 | 68±9% | 70±8% | 0.253 | 71±9% | 72±9% | 0.604 |
Data are presented as mean±sd unless otherwise stated. BMI: body mass index; WHO: World Health Organization; 6MWD: 6-min walking distance; NT-proBNP: N-terminal pro-brain natriuretic peptide; TRV: tricuspid regurgitation velocity; RA: right atrial; PAP: pulmonary arterial pressure; RAP: right atrial pressure; PAWP: pulmonary arterial wedge pressure; PVR: pulmonary vascular resistance; WU: Wood unit; SVR: systemic vascular resistance; SvO2: mixed venous oxygen saturation. #: n=48; ¶: n=44; +: Chi-squared test or t-test as appropriate; §: results of acute vasoreactivity testing with nitric oxide.
TABLE 3.
Differences in treatment effects between the bosentan monotherapy and bosentan/metformin combination therapy groups
| Variables | Change from baseline mean±sd |
Treatment effect mean change (95% CI) |
p-value# | |
| Bosentan | Bosentan/metformin | |||
| 6MWD m | 48±119 | 95±136 | 52 (10–95) | 0.017 |
| NT-proBNP ng·L−1 | −176±325 | −183±415 | −141 (−236– −47) | 0.094 |
| Mean PAP mmHg | −12±15 | −20±19 | −3 (−7–1) | 0.145 |
| PVR index WU·m−2 | −1.2±1.3 | −1.8±1.2 | −0.8 (−1.2– −0.4) | <0.001 |
| PVR/SVR ratio | −0.07±0.19 | −0.11±0.19 | −0.10 (−0.63–0.43) | 0.148 |
| Cardiac index L·min−1·m−2 | 0.3±0.5 | 0.3±0.4 | 0.11 (−0.61–0.28) | 0.27 |
6MWD: 6-min walking distance; NT-proBNP: N-terminal pro-brain natriuretic peptide; PAP: pulmonary arterial pressure; PVR: pulmonary vascular resistance; WU: Wood unit; SVR: systemic vascular resistance.#: ANCOVA adjusted for a baseline covariate.
FIGURE 1.
a) 6-min walking distance (6MWD) and b) pulmonary vascular resistance (PVR) index at baseline and 3-month follow-up in each group using fitted lines. The estimated difference between the groups from ANCOVA is the vertical distance between the two lines.
After 3 months of treatment, 40 out of 48 patients on monotherapy and 38 out of 44 patients on combination therapy were eligible for operations with biventricular circulation. There were no significant differences in surgical data and postoperative evolution (table 4). All the patients who underwent surgical interventions continued treatment and were alive at the 1-month follow-up.
TABLE 4.
Peri- and postoperative characteristics in the bosentan monotherapy and bosentan/metformin combination therapy groups
| Bosentan | Bosentan/metformin | |
| Patients undergoing operation n (%) | 40 (83.3%) | 38 (86.4%) |
| CPB time min | 48±13 | 56±13 |
| Cross-clamping time min | 26±9 | 24±8 |
| Hospital mortality | 0 (0%) | 0 (0%) |
| Mechanical ventilation time h | 18±8 | 16±6 |
| Length of ICU stay h | 36±9 | 28±6 |
| Length of hospital stay days | 12±6 | 10±4 |
| Infection | 4 (10%) | 6 (15.8%) |
| Reintubation | 3 (7.5%) | 2 (5.3%) |
| Tracheotomy | 1 (2.5%) | 0 (0%) |
| Arrhythmia | 12 (30%) | 9 (23.7%) |
| Neurologic complication | 2 (5%) | 3 (7.9%) |
Data are presented as mean±sd or n (%). CPB: cardiopulmonary bypass; ICU: intensive care unit.
Safety
Patients in both treatment groups reported typical PAH symptoms or signs with similar frequency. These were thought not to be associated with increased bosentan- or metformin-related side-effects. The most commonly observed drug related symptoms were facial flushing (monotherapy/combination therapy n=4/6), headache (n=12/8), gastrointestinal disturbance (n=3/7) and swelling of the legs (n=5/3).
Pulmonary gene expression after treatments
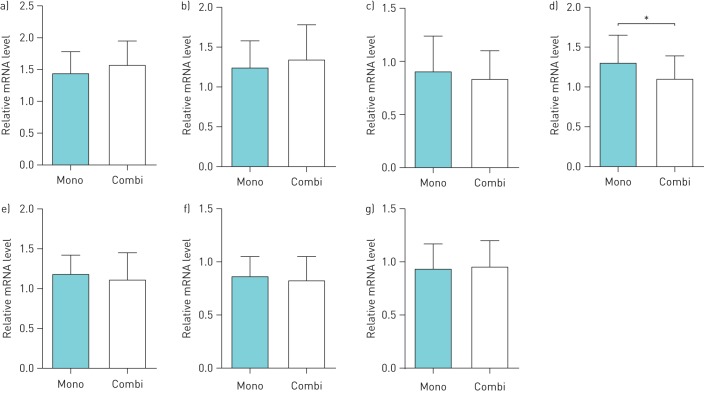
To determine the effect of metformin on pulmonary vascular tone, we first measured pulmonary gene expression related to NO and EDN pathways. Compared with bonsentan monotherapy, bosentan/metformin combination therapy significantly decreased EDN1 expression in the lung (p=0.006, figure 2). However, there were no significant differences in the gene expression of NOS3, NOS2, NOS1, EDN2, EDNRA or EDNRB between the two treatment groups (figure 2).
FIGURE 2.
Pulmonary mRNA expression of a) endothelial nitric oxide synthase (NOS3), b) inducible NO synthase (NOS2), c) neuronal NO synthase (NOS1), d) endothelin 1 (EDN1), e) endothelin 2 (EDN2), f) endothelin receptor type A (EDNRA) and g) endothelin receptor type B (EDNRB) after bosentan monotherapy (Mono) and bosentan/metformin combination therapy (Combi) for 3 months. Groups were compared using Student's t-test. Expression was normalised to the geometric mean of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin (ACTB). Data are presented as mean±sd. *: p<0.05.
Discussion
Our study describes a novel pharmacological combination treatment for PAH-CHD treatment before selected surgical shunt closure. Either monotherapy or combination therapy significantly improved WHO functional class, 6MWD, NT-pro BNP and right heart haemodynamic parameters. Patients who received bosentan/metformin combination therapy experienced additional improvements in exercise capacity (6MWD), PVR index and vascular reactivity responses to NO. This suggests metformin added to bosentan therapy may confer additional benefits, in terms of future survival and operability, in PAH-CHD patients with intermediate risk.
In the present study, for the first time, we demonstrated that metformin combined with bosentan significantly decreased pulmonary EDN1 expression in PAH-CHD patients compared with bosentan monotherapy. Decreased circulating EDN1 levels after bosentan or metformin treatments were described in previous studies [22–25]. However, no additional effects of metformin in plasma EDN1 levels were observed when comparing the monotherapy with combination therapy groups in our study.
EDN1 is a strong vasoconstrictor and proliferative cytokine, and is one of key mediators of PAH development. Both plasma EDN1 levels and EDN1 expression in the lungs were elevated in patients with PAH and correlated with raised PVR [26–29]. Furthermore, the clinical application of bosentan, and its beneficial effects on exercise capacity and haemodynamics, as well as the time to clinical worsening in PAH patients, also emphasise the important role of EDN1 in PAH.
Indeed, it is reported that activation of AMP-activated protein kinase (AMPK) by metformin suppresses EDN1-induced pulmonary artery smooth muscle cell (SMC) proliferation in rats [30], and inhibits EDN1 expression at the transcriptional and translational level in the aorta [31]. Moreover, SMC-specific EDN1 deletion attenuates hypoxia-induced increases in pulmonary vascular tone and structural remodelling, whereas exposure to selective EDN1 receptor antagonists had no effects [32], suggesting that local EDN1 expression may play an independent role in modulating vascular tone. Decreased pulmonary EDN1 expression might be a major contributor to the beneficial effects of metformin treatment observed in the present study.
Metformin lowers glucose levels and improves insulin sensitivity, and has been recently described as a pleiotropic molecule [33]. The action of metformin in AMPK activation, which has been well documented in previous studies [34, 35], is suggested to be a significant pathway target for pulmonary hypertension [9]. AMPK is a heterotrimeric enzyme that is expressed in many tissues, including the lungs and vasculature, and plays a central role in in cellular and organ metabolism [36]. NO, a potent vasodilator and a suppressor of SMC proliferation, is important in the modulation of the PVR [37]. Activation of AMPK by metformin stimulates NO synthesis in aortic endothelial cells by increasing phosphorylation and activation of eNOS [38, 39]. In our study, the mRNA levels of NOS3 were not affected by metformin treatment. Consistent results were demonstrated in the study by Sartoretto et al. [16] that metformin treatment improves vascular reactivity by increasing eNOS activity but not eNOS expression. Other mechanisms, however, might be involved in the effect of metformin on vascular function. For example, a direct effect of metformin on VSMC function, in terms of tyrosine kinase activity, glucose transport and intracellular calcium, has also been suggested by previous studies [40]. Moreover, metformin has shown an endothelium-independent effect on potentiating phenylephrine-induced AMPK phosphorylation and promoting vasorelaxation in endothelium-denuded rat aortic rings [41].
A limitation of this study is that it was designed to assess short-term use of a combination therapy on improving pulmonary haemodynamics and 1-month outcomes in patients with PAH-CHD. Long-term data are currently unavailable for survival/prognosis and safety evaluations. Due to limited tissue size from a lung needle biopsy, protein analysis was not able to be carried out alongside with the study of gene expression.
Perspectives
In conclusion, our study shows that combination therapy with bosentan and metformin in PAH-CHD patients may provide improvements in important outcomes such as exercise capacity and pulmonary haemodynamics, compared with bosentan alone. Further evaluation of this approach in a large multicentre randomised controlled study is warranted, with a longer period of follow-up.
Footnotes
Conflict of interest: None declared.
Support statement: The authors gratefully acknowledge the financial support from The Limingzhang Sciences Foundation (2014-778903). S. Liao is funded under an NHMRC Development Grant. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Barst RJ, Ivy DD, Foreman AJ, et al. Four- and seven-year outcomes of patients with congenital heart disease-associated pulmonary arterial hypertension (from the REVEAL Registry). Am J Cardiol 2014; 113: 147–155. [DOI] [PubMed] [Google Scholar]
- 2.Kaemmerer H, Gorenflo M, Hoeper M, et al. Pulmonalarterielle Hypertonie bei angeborenen Herzfehlern: Problemstellung und Versorgungslage [Pulmonary arterial hypertension in patients with congenital heart disease: current issues and health care situation]. Dtsch Med Wochenschr 2013; 138: 1247–1252. [DOI] [PubMed] [Google Scholar]
- 3.Krieger EV, Leary PJ, Opotowsky AR. Pulmonary hypertension in congenital heart disease: beyond Eisenmenger syndrome. Cardiol Clin 2015; 33: 599–609. [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Palazzini M, Manes A. Pulmonary arterial hypertension: from the kingdom of the near-dead to multiple clinical trial meta-analyses. Eur Heart J 2010; 31: 2080–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galie N, Rubin L, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008; 371: 2093–2100. [DOI] [PubMed] [Google Scholar]
- 6.Galie N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006; 114: 48–54. [DOI] [PubMed] [Google Scholar]
- 7.Abbasi F, Chu JW, McLaughlin T, et al. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism 2004; 53: 159–164. [DOI] [PubMed] [Google Scholar]
- 8.Agard C, Rolli-Derkinderen M, Dumas-de-La-Roque E, et al. Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br J Pharmacol 2009; 158: 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean A, Nilsen M, Loughlin L, et al. Metformin reverses development of pulmonary hypertension via aromatase inhibition. Hypertension 2016; 68: 446–454. [DOI] [PubMed] [Google Scholar]
- 10.Gundewar S, Calvert JW, Jha S, et al. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 2009; 104: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meaney E, Vela A, Samaniego V, et al. Metformin, arterial function, intima-media thickness and nitroxidation in metabolic syndrome: the Mefisto study. Clin Exp Pharmacol Physiol 2008; 35: 895–903. [DOI] [PubMed] [Google Scholar]
- 12.Cittadini A, Napoli R, Monti MG, et al. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes 2012; 61: 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao H, Ma X, Feng W, et al. Metformin attenuates cardiac fibrosis by inhibiting the TGFβ1–Smad3 signalling pathway. Cardiovasc Res 2010; 87: 504–513. [DOI] [PubMed] [Google Scholar]
- 14.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med 2002; 137: 25–33. [DOI] [PubMed] [Google Scholar]
- 15.Romualdi D, Costantini B, Selvaggi L, et al. Metformin improves endothelial function in normoinsulinemic PCOS patients: a new prospective. Hum Reprod 2008; 23: 2127–2133. [DOI] [PubMed] [Google Scholar]
- 16.Sartoretto JL, Melo GA, Carvalho MH, et al. Metformin treatment restores the altered microvascular reactivity in neonatal streptozotocin-induced diabetic rats increasing NOS activity, but not NOS expression. Life Sci 2005; 77: 2676–2689. [DOI] [PubMed] [Google Scholar]
- 17.Galiè N, Humbert M, Vachiery JL, et al. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 18.Lopes AA, O'Leary PW. Measurement, interpretation and use of haemodynamic parameters in pulmonary hypertension associated with congenital cardiac disease. Cardiol Young 2009; 19: 431–435. [DOI] [PubMed] [Google Scholar]
- 19.Kozlik-Feldmann R, Hansmann G, Bonnet D, et al. Pulmonary hypertension in children with congenital heart disease (PAH-CHD, PPHVD-CHD). Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016; 102: Suppl. 2, ii42–ii48. [DOI] [PubMed] [Google Scholar]
- 20.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008; 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 21.Vickers AJ, Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ 2001; 323: 1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawashiri SY, Ueki Y, Terada K, et al. Improvement of plasma endothelin-1 and nitric oxide in patients with systemic sclerosis by bosentan therapy. Rheumatol Int 2014; 34: 221–225. [DOI] [PubMed] [Google Scholar]
- 23.Diamanti-Kandarakis E, Spina G, Kouli C, et al. Increased endothelin-1 levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapy. J Clin Endocrinol Metab 2001; 86: 4666–4673. [DOI] [PubMed] [Google Scholar]
- 24.Kocer D, Bayram F, Diri H. The effects of metformin on endothelial dysfunction, lipid metabolism and oxidative stress in women with polycystic ovary syndrome. Gynecol Endocrinol 2014; 30: 367–371. [DOI] [PubMed] [Google Scholar]
- 25.Orio F Jr, Palomba S, Cascella T, et al. Improvement in endothelial structure and function after metformin treatment in young normal-weight women with polycystic ovary syndrome: results of a 6-month study. J Clin Endocrinol Metab 2005; 90: 6072–6076. [DOI] [PubMed] [Google Scholar]
- 26.Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 1993; 328: 1732–1739. [DOI] [PubMed] [Google Scholar]
- 27.Cacoub P, Dorent R, Maistre G, et al. Endothelin-1 in primary pulmonary hypertension and the Eisenmenger syndrome. Am J Cardiol 1993; 71: 448–450. [DOI] [PubMed] [Google Scholar]
- 28.Stewart DJ, Levy RD, Cernacek P, et al. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 1991; 114: 464–469. [DOI] [PubMed] [Google Scholar]
- 29.Jankowich MD, Wu WC, Choudhary G. Association of elevated plasma endothelin-1 levels with pulmonary hypertension. Mortality, and heart failure in African American individuals: the Jackson Heart Study. JAMA Cardiol 2016; 1: 461–469. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Liu L, Zhang Y, et al. Activation of AMPK inhibits pulmonary arterial smooth muscle cells proliferation. Exp Lung Res 2014; 40: 251–258. [DOI] [PubMed] [Google Scholar]
- 31.Tang ST, Su H, Zhang Q, et al. Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-κB/IκBα system through the activation of AMP-activated protein kinase. Int J Mol Med 2016; 37: 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim FY, Barnes EA, Ying L, et al. Pulmonary artery smooth muscle cell endothelin-1 expression modulates the pulmonary vascular response to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 2015; 308: L368–L377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia 2013; 56: 1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou MH, Kirkpatrick SS, Davis BJ, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem 2004; 279: 43940–43951. [DOI] [PubMed] [Google Scholar]
- 36.Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol 2015; 33: 1–7. [DOI] [PubMed] [Google Scholar]
- 37.Huber LC, Bye H, Brock M. The pathogenesis of pulmonary hypertension – an update. Swiss Med Wkly 2015; 145: w14202. [DOI] [PubMed] [Google Scholar]
- 38.Morrow VA, Foufelle F, Connell JM, et al. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem 2003; 278: 31629–31639. [DOI] [PubMed] [Google Scholar]
- 39.Davis BJ, Xie Z, Viollet B, et al. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 2006; 55: 496–505. [DOI] [PubMed] [Google Scholar]
- 40.Dominguez LJ, Davidoff AJ, Srinivas PR, et al. Effects of metformin on tyrosine kinase activity, glucose transport, and intracellular calcium in rat vascular smooth muscle. Endocrinology 1996; 137: 113–121. [DOI] [PubMed] [Google Scholar]
- 41.Pyla R, Osman I, Pichavaram P, et al. Metformin exaggerates phenylephrine-induced AMPK phosphorylation independent of CaMKKβ and attenuates contractile response in endothelium-denuded rat aorta. Biochem Pharmacol 2014; 92: 266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]