Abstract
Background:
To enhance the bioactivity of hydroxyapatite (HA), various ions have been incorporated into its porous structure such as zinc. Zinc has shown to have a stimulatory effect on osteoblastic cells. This study attempts to evaluate the efficacy of an indigenously prepared zinc incorporated nanohydroxyapatite (ZINH) bone graft in the treatment of intrabony defects.
Materials and Methods:
A split-mouth study, which consists of 11 systemically healthy subjects with 45 sites, were randomly treated with ZINH or with nanoHA alone. Plaque index, gingival index, gingival bleeding index, pocket depth (PD) and clinical attachment level (CAL) were assessed at baseline, 3, 6, 9, and 12 months. Bone probing depth (BPD) and radiographic parameters were assessed at baseline, 6, and 12 months. Statistical analysis used was student's t-test and one-way analysis of variance.
Results:
At 12 months, PD and BPD reduction was more in test (4.37 ± 0.989 mm and 3.36 ± 0.446 mm) than control (2.81 ± 0.084 mm and 2.15 ± 0.159 mm). Gain in CAL for test (3.08 ± 0.148 mm) was higher than control (2.33 ± 0.278 mm). Furthermore amount and percentage of bone fill was higher in test (1.92 ± 0.702 mm, 54.7 ± 20.286, respectively) than control (1.38 ± 0.650 mm, 40.2 ± 20.972, respectively). Statistically significant improvements in all parameters were seen in the test sites at 12 months.
Conclusion:
ZINH bone graft can be considered as a prospective bone regenerative material.
Keywords: Bone regeneration, chronic periodontitis, clinical and radiographic parameters, intrabony defects, nanohydroxyapatite bone graft, zinc nanohydroxyapatite
Introduction
Periodontitis is a chronic inflammatory disease of the supportive tissues of the teeth which results in progressive destruction of the periodontal ligament and alveolar bone with increased probing depth formation, recession, or both.[1]
The ultimate aim of any periodontal treatment is to attempt to create a functional periodontium similar to the one lost as a result of periodontitis. Even with advanced technological methods creating a paradigm shift in dentistry, regeneration of the lost alveolar bone still remains one of the greatest challenges to overcome.[2] Due to a difference in the reparative capabilities among periodontal tissues and the variety of materials available, the complete regeneration of the periodontium has become almost impossible to achieve.[3]
One of the most extensively used methods for restoration of the lost attachment is the use of bone grafts.[2] But there is not a single graft material that is ideal and can be considered a gold standard in the treatment of intrabony defects.[4] The selection for this “magic filler” relies on factors such as tissue variability, defect size, cost, ethical considerations and other associated complications.[4]
The chemical similarity of synthetic hydroxyapatite (HA) and natural bone mineral has led to its use as a bone grafting material. Synthetic HA though well known for its ability to bond with bone tissue, is limited by lower solubility, slow rate of bone binding ability and inhibitions of bacteria to adhere to the surface.[5,6] This is a major drawback as patient recovery is hindered, since infection can lead to surgical failure.[6,7] The crystal structure of HA is porous and can easily accommodate substitutions by various ions. Some beneficial ions that have enhanced the properties of HA by improving rate and quality of bone tissue repair[8] are silver, silicon,[5] citric acid,[9] etc. Among various other elements that can be incorporated, zinc (Zn) seems to be a potential candidate. Zinc is the most abundant trace metallic element found in the body with 85% present in muscle and bone.[8] Zinc has proven to possess a direct stimulatory effect on osteoblastic cells in vitro while bone resorption was inhibited.[10] It also reduces bacterial load, thus proving its antimicrobial properties.[10,11] Many studies have demonstrated that zinc significantly improved the bioactivity of HA. Therefore, zinc-HA can be a new generation of materials for bone tissue engineering.
In this study, we have investigated the bone regenerative properties of an indigenously prepared Zinc-nano HA bone graft in comparison to nano-HA alone in the surgical management of intrabony defects in chronic periodontitis patients. To the best of our knowledge, zinc HA (ZnHA) has not been used as a regenerative material in the treatment of chronic periodontitis patients.
Materials and Methods
Patient selection
In this 12-month follow-up split-mouth[12] (to avoid intrapatient variability), randomized, interventional study, a total of 12 systemically healthy subjects [Figure 1] with chronic periodontitis (7 males and 5 females; age range: 35–55 years; mean age of 39.27 ± 6.182 years) were selected from the outpatient section of the department of periodontology. The study was conducted from August 2016 to September 2017. The research protocol was submitted to the Institutional Ethical Committee and Review Board. The study fulfilled the requirements of the “Declaration of Helsinki” as adopted by the 18th World Medical assembly in 1975 and revised in Edinburgh (2000). After ethical approval, verbal and written informed consent was obtained from all the subjects for participation in the study. Subjects who had ≥20 remaining teeth with contralateral intrabony pockets measuring ≥6 mm, having radiographic evidence of vertical bone loss were included in the study. Subjects were excluded from the study if they were pregnant/lactating women, allergic to zinc or on antibiotic therapy in the previous 6 months. Subjects who have undergone any periodontal therapy in the past 6 months, smokers and subjects who were medically compromised or under therapy that may alter the probability of soft tissue and bone healing were also excluded. The trail has been registered under Clinical Trials registry, India; Ref no: CTRI/2017/09/009714.
Figure 1.
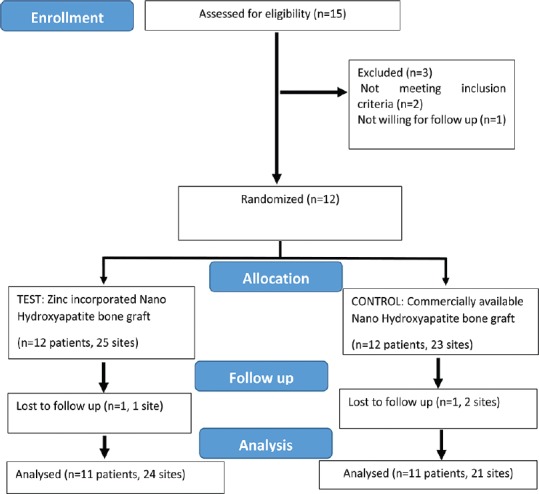
Consort flow chart for patient enrolment, allocation, follow-up, and analysis
Presurgical therapy
Before surgery, each patient was given oral hygiene instructions. A full-mouth supra- and subgingival scaling and root planing procedure were performed under local anesthesia. At reevaluation, the oral hygiene maintenance was evaluated by the use of clinical indices, and only subjects showing optimal oral hygiene were scheduled for the surgical procedure. The sites were randomly assigned using the computer software-generated randomization method to test and control groups. The test sites were treated with Zinc incorporated nano-hydroxyapatite (ZINH) and the control sites were treated with only nano-HA. All pre- and post-treatment clinical parameters were recorded by an examiner who was masked to the type of treatment received by the subjects while another clinician provided treatment to both groups.
Clinical measurements
The clinical parameters measured at baseline, 3, 6, 9, and 12 months were full-mouth plaque index (PI),[13] gingival index (GI),[14] gingival bleeding index (BI),[15] probing depth (PD), and clinical attachment level (CAL). Site-specific PD and CALs were checked at baseline, 3, 6, 9, and 12 months, and bone probing depth (BPD) was checked at baseline, 6, and 12 months.
PD, CAL, and BPD measurements were recorded with periodontal probe (UNC PCP-15 periodontal probe, Hu-Freidy, Chicago, IL, USA) and standardized using customized acrylic stents that were grooved in the area of defect to provide reproducible insertion axis [Figure 2a], Using the apical margin of the customized acrylic stent as the fixed reference point (FRP), the clinical measurements were made of the associated bony defect.[16] Only one site representing the same deepest point of the defect was included.
Figure 2.
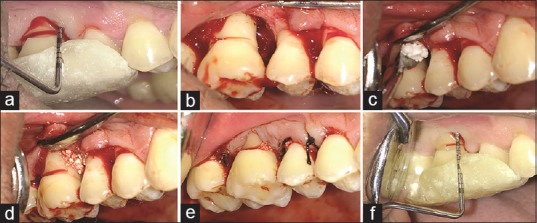
Clinical photographs showing test site with respect to 14, 15, and 16. (a) Preoperative probing. (b) Visualization of the defect. (c and d) Placement of zinc-incorporated nanohydroxyapatite bone graft. (e) Sutures placed. (f) Probing after 12 months
PD = (FRP to BOP [Base of pocket]) – (FRP to GM [gingival margin])
CAL = (FRP to BOP) – (FRP to CEJ)
BPD (bone probing depth) = (FRP to BOD [base of defect, under local anesthesia]) – (FRP to GM).
Radiographic measurements
Site-specific radiographic parameters such as amount of defect fill (ADF), percentage of defect fill (PDF), and change in alveolar crestal level (ALR) were measured at baseline, 6, and 12 months. For radiographs, paralleling technique was used with conventional Kodak E Speed IOPAR films of size 2, with an X-ray mesh gauge with markings at 1 mm2 [Figure 3a]. The XCP Rinn holder was used to provide projection standardization. The exposed films were processed with an automatic processor. As per manufactures guidelines, fresh developer and fixer solutions were prepared.
Figure 3.
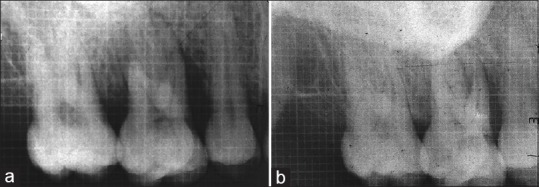
(a) Radiographic bone fill in test site at baseline and (b) radiographic bone fill in test site at 12 months
The cementoenamel junction was taken as the FRP for radiographic parameters. The base of defect (BOD) was defined as the most coronal point where the periodontal ligament space showed continuous width. If no periodontal ligament could be identified, the point where projection of the alveolar crest crossed the root surface was noted. The alveolar crest level (ALV) was taken as the crossing of the silhouette of the alveolar crest with the root surface. In case of several bony contours, the most apical crossing of the root was defined as the BOD and the most coronal as the alveolar crest.
Defect depth (Dd) = (FRP to BOD) – (FRP to ALV)
Changes in Alveolar crest level (ALR) = (FRP to ALV at baseline)–(FRP to ALV at recall interval)
ADF = Initial defect depth − defect depth at recalled time interval
Percentage of defect fill = (ADF/baseline defect depth) ×100
Preparation of the zinc-incorporated nanohydroxyapatite bone graft
For the preparation of the ZINH bone graft calcium hydroxide [Ca(OH)2], zinc nitrate hexahydrate [Zn(NO3)2.6H2O], and orthophosphoric acid [H3PO4] were used, out of which Zn(NO3)2.6H2O was used as the source of zinc. Zinc in >1.2 wt% has been proven to toxic to the cells.[17] Taking this into consideration and during synthesis of zinc into the HA lattice, there is only partial incorporation of the zinc crystals,[11] 1.5 wt% of zinc was selected for this study. With the assumption that Zn would partially be incorporated in the sites of calcium ions, the reagents were mixed keeping the (Ca + Zn)/P molar ratio as 1.67. Calcium hydroxide and zinc nitrate hexahydrate were mixed and under constant stirring, orthophosphoric acid was added to it drop by drop. The pH of the solution was maintained by adding aqueous ammonia. After all the reagents were mixed, the solution was allowed to age for 2 weeks. After aging, to remove any excess of water, the sample were heated at 400°C for 8.5 h. After grinding, the samples were sent for sterilization.
Sterilization
The samples were exposed to Co-60 gamma radiation using gamma chamber (GC)-1200 under laboratory conditions. The GC was calibrated with standard Co-60 source. The dose rate of GC-1200 was 9.0 kGy/h and the samples were exposed in the total doses range from 25 to 27 kGy.[18]
The indigenous ZINH bone graft thus prepared was subjected to different physiochemical tests, such as X-ray powder diffraction (XRD) analysis, scanning electron microscopy (SEM) analysis, and Fourier transform-infrared spectroscopy (FTIR). The corresponding XRD peaks confirmed the incorporation of zinc in HA. SEM analysis revealed ZINH has rod-like structure which is around 10–20 nm in diameter and that the nanoparticles tend to agglomerate due to their nanometric dimensions. Further, FTIR was conducted to check the molecular confirmations of nano-HA.
Surgical procedure
Intraoral antisepsis was performed with a 0.12% chlorhexidine digluconate rinse, and an iodine solution was used to carry out extra-oral antisepsis. After the administration of local anesthesia, buccal and lingual sulcular incisions were made using Bard Parker knife with blade no. 12 and full thickness mucoperiosteal flaps were reflected [Figure 2a]. Meticulous defect debridement and root planing were carried out using area-specific curettes (Gracey curettes, Hu-Friedy) [Figure 2b]. The defect sites in test quadrant were then filled with ZINH bone graft, [Figure 2c and d] while control quadrants were filled with commercially available nano-HA bone graft. Flaps were repositioned and secured using sling and interrupted (4-0) black braided silk sutures [Figure 2e]. The surgical sites were protected with a noneugenol periodontal dressing (Coe-Pak, GC America, Chicago, IL, USA).
Postoperative care
Suitable antibiotics and analgesics (amoxicillin 500 mg thrice daily for 5 days and a combination of paracetamol [500 mg]) + aceclofenac (100 mg) thrice daily for three days) were prescribed. Chlorhexidine digluconate rinses (0.12%) twice daily for 1 week were also prescribed. Periodontal dressing and sutures were removed 1 week postoperatively and oral hygiene measures were reinforced.
Postsurgical measurements
Subjects were recalled to evaluate oral hygiene maintenance and all clinical parameters at 3, 6, 9, and 12 months. BPD and radiographic parameters were evaluated at 6 and 12 months.
Primary and secondary outcome
To evaluate the efficacy of ZINH compared to nano-HA alone, by evaluating the clinical parameters and bone fill, over a period of 3, 6, 9, and 12 months.
Statistical analysis
Data were entered in Microsoft Excel and analyzed using SPSS version 10.0.5 (SPSS Inc., IBM, Armonk, NY, USA) package. The results were averaged (mean ± standard deviation) for continuous data and number and percentage for dichotomous data are presented in tables and graphs. Normality assumption of the data was tested using Shaipro–Wilk's test. In this study, data were normally distributed and parametric tests were used for comparison. Paired t-test was applied to assess the statistical significance between time points within each group for clinical and radiographic parameters. One-way analysis of variance was used to test the difference between groups. In all the above tests, P < 0.05 was considered statistically significant.
Results
A total of 11 subjects (45 sites) out of 12 completed the study. One patient (3 sites) did not return for follow up examinations [Figure 1]. All treated cases showed uneventful postoperative healing. The descriptive statistics have been summarized in Table 1.
Table 1.
Descriptive statistics of demographic data

The full-mouth PI, GI, GBI, PD, and CAL are summarized in Table 2. A statistically significant (P < 0.001) improvement was observed in all the parameters over a period of 12 months [Figure 2f].
Table 2.
Full-mouth reduction in clinical parameters
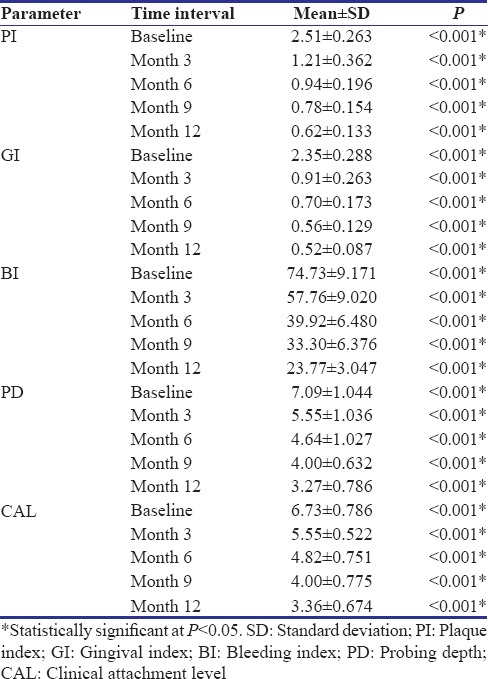
Table 3 summarizes the intergroup comparison of site specific PD, CAL, and BPD.
Table 3.
Sitespecific pocket probing depth, clinical attachment level, bone probing depth, amount of defect fill, percentage defect fill, and alveolar crestal level
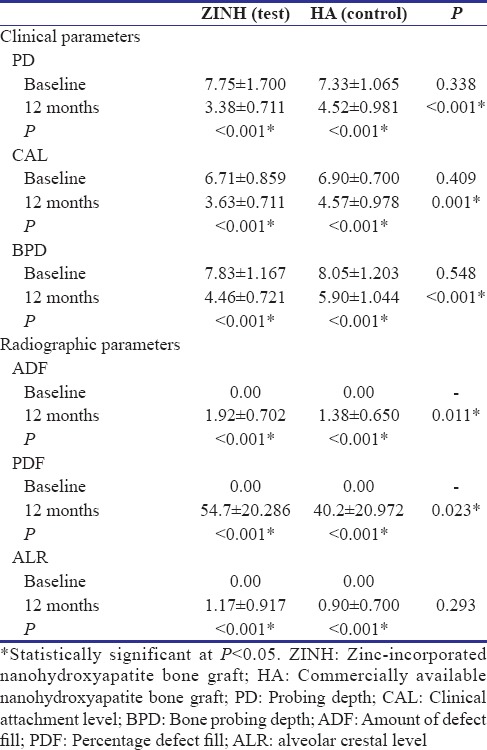
No statistically significant difference was found at baseline for PD (P = 0.338) and CAL (P = 0.409). At 6 months, PD of test group was not statistically significant (P = 0.596) while CAL was statistically significant (P = 0.001) as compared to control. At 12 months, the PD, CAL, and BPD of test group as compared to the control group and was statistically significant (P < 0.001).
At the end of 12 months, the radiographic parameters showed better results in test than in control group. The ADF and PDF were statistically more in test site than in control sites (P = 0.011 and P = 0.023, respectively). The ALR between test and control was statistically insignificant (P = 0.293) [Table 3] [Figure 3b].
A comparison was also done based on the type of defect that was found after open flap debridement. The defects were classified as two walled, three walled and combined defects (defects which had three walls apically while one of two walled coronally). All three types of defects were present in both the groups. The distribution of which is shown in Table 4.
Table 4.
Intergroup comparison of site-specific percentage of bone fill at various time intervals, according to the type of defect (number of walls) present
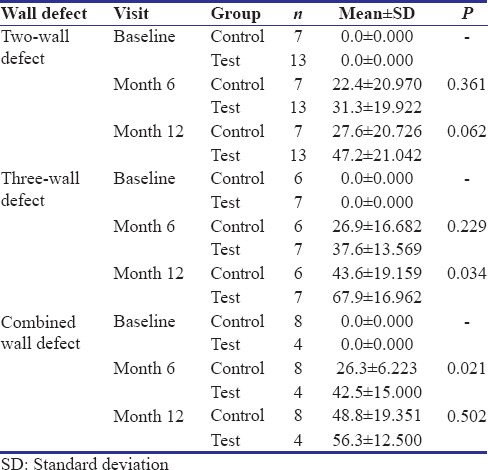
Two-walled defects – At 6 and 12 months the mean difference between control and test sites was statistically nonsignificant (P = 0.361 and P = 0.062, respectively).
Three-walled defects – At 6 months, the difference in test and control was statistically nonsignificant (P = 0.229) while at 12 months it was statistically significant (P = 0.034).
Combined defects – At 6 months, difference in test and control was statistically significant (P = 0.021), while at 12 months the difference was statistically nonsignificant (P = 0.502).
Discussion
The complete regeneration of the periodontium including the formation of a new connective tissue attachment, that is, new cementum with inserting collagen fibers, at the diseased root surfaces is still an unmet objective.[4]
A variety of treatment modalities have been employed to achieve complete regeneration of the alveolar bone. HA is among the most extensively studied materials for this application. However, HA has some inherent disadvantages.[5,6,7] To capitalize on the advantages of HA, it is combined with various types of ions to generate materials with enhanced properties.
In this study, we have formulated a ZINH bone graft and studied its regenerative properties. This indigenously prepared graft has been compared to nano-HA in regeneration of intrabony defects.
On intergroup comparison, the test sites showed greater improvement in clinical and radiographic parameters, which were statistically significant over a period of 12 months. To the best of our knowledge, there have been no clinical trials using ZINH as a regenerative graft. Thus, it was not possible to make a direct comparison with other studies. So far, only animal studies have been reported in literature.
In an in vitro study, 1.6 wt% of zinc was used in the preparation of (ZnHA), in which the cell culture work showed that ZnHA increased the growth of human stem cells and bone cell differentiation markers. In addition, a significant decrease in the number of viable Staphylococcus aureus was observed when in contact with ZnHA, thus confirming its enhanced bioactivity and antimicrobial property.[6]
In another animal study, 0.5% ZnHA was compared to HA, on osseous repair of rabbit's tibia. Cylinders (2 mm × 6 mm) of both materials were implanted. After 28 days, the histologic sections showed ZnHA group had maximum area of new bone, thus concluding its enhanced osteogenic properties at 28 days.[19]
For the preparation of the ZINH bone graft; materials were used, in accordance to an earlier study.[6] Taking into account that a Zn content of >1.2 wt% would result in cytotoxicity,[17] and there would be an incomplete substitution of Zn into the HA crystal lattice during synthesis, the above authors used 1.6 wt% of Zn. In our study, for safety measures, we used 1.5 wt% of zinc to be incorporated into HA with a (Ca + Zn)/P molar ratio of 1.67.[20] XRD analysis, SEM analysis, and FT-IR confirmed the presence of zinc in the porous hydroxyl apatite crystal structure.
At baseline, the clinical parameters showed no significant difference between the groups. Therefore, it was inferred that the observed differences resulted from the direct effect of the treatment modality.
There was reduction in full mouth scores of PI, GI, and BI gradually from baseline to 12 months. This shows that all subjects involved in the study followed given oral hygiene instructions and maintained good gingival health.
In the present study, the clinical parameters showed the following changes at 12 months. The reduction in PD at test and control sites was 4.37 ± 0.989 mm and 2.81 ± 0.084 mm and the gain in CAL was 3.08 ± 0.148 mm and 2.33 ± 0.278 mm, respectively. On intergroup comparison, at 12 months the test sites showed statistically significant improvement in all parameters as compared to the control sites (P < 0.001). In a similar study,[21] synthetic bone graft was compared to open flap debridement in the treatment of intrabony defects, which showed no statistical significance between the two groups in terms of PD, while CAL was statistically increased, at 6 and 9 months. At the end of 12 month follow-up, both PD and CAL showed statistical significant changes.
The reduction in BPD for test and control was 3.36 ± 0.446 mm and 2.15 ± 0.159 mm respectively, which was statistically significant (P < 0.001). To evaluate the formation of bone, surgical reentry was not considered keeping in mind patient acceptability and ethical reasons.
The radiographic parameters, displayed significant increase in bone fill in the test group at the end of 12 months as compared to the control group. This was similar to a study[21] where osseous reentry at the end of 12 months showed a statistically significant bone fill in the test group (synthetic bone graft) as compared to control group (OFD). In our study, it is important to note that the test sites which were treated with ZINH bone graft showed more bone formation as compared to HA alone. This could be due to the reason that zinc has the ability to increase the osteogenic effect by increasing the osteoblast cell proliferation and stimulating alkaline phosphatase activity and collagen synthesis during the proliferation and differentiation phase.[22] Though in vitro studies have shown zinc to act on osteoblasts and osteoclasts, the exact mechanism of these effects is still poorly understood.[22]
The structure of HA is porous and supports osteoblastic cell adhesion, growth, and differentiation, which may lead to new bone deposition by the creeping substitution from the adjacent living bone.[23] Zinc is known to have a stimulatory effect on osteoblastic cells in vivo.[10] Addition of zinc to the HA to be used as a regenerative material has thus proved to have an additive effect on account of the percentage of bone fill that was observed.
Various studies have shown different treatment success rates of bone regeneration in account of the varying morphology of initial bony defects. Previously, it has been reported that,[24] two- and three-wall defects have the highest potential for regeneration when grafted. But in our study, we found that three walled defects had better regenerative capacity as compared to two walled or combined defects. Our study has taken a novel approach while comparing the regenerative capacities of defects according to the number of walls present.
Deep, narrow defects have more potential to regenerate as compared to shallow, wide defects. However, even in best of circumstances, it is impossible to find matched osseous defects. It is hard to control these variables in a clinical investigation, but the potential effects of this variability on the results need to be realized. Randomization may help to control this variable to a certain extent.
One limitation of this study is the inability to assess histologic characteristics of the repaired tissues, which was not possible due to ethical reasons. But increase in the radiodensity in the defect, signifies that the use of bone graft resulted in resolution of the intrabony defect.
Also, the present study has been restricted to linear method of radiographic evaluation of defect fill. Future studies should incorporate advanced digital imaging facilities, with larger sample sizes for better results.
Conclusion
Within the limitations of the study, it has been demonstrated that, the novel Zinc incorporated HA (ZINH) bone graft, resulted in statistically significant improvement in the clinical and radiographic parameters in the treatment of intrabony defects over a period of 12 months.
Future directions
Clearly, it can be stated that the incorporation of zinc opens the opportunity to expand and improve the family of bioactive glasses such as HAs to develop new materials with enhanced performance for bone regeneration. It can be thus be considered as a prospective material for bone regeneration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors thank P. S. Jagannatha, statistician, Rajajinagar, Bangalore, India, for carrying out all required statistics. No funding was received for this study. The authors report no conflicts of interest related to this study.
References
- 1.Newman MG, Takei H, Klokkevold PR, Carranza FA. Classification of diseases and conditions affecting the periodontium. In: Michael GN, Henry HT, Perry RK, Fermin AC, editors. Carranza's Clinical Periodontology. 12th ed. Elsevier Saunders: Elsevier Health Sciences; 2012. pp. 45–67. [Google Scholar]
- 2.Brunsvold MA, Mellonig JT. Bone grafts and periodontal regeneration. Periodontol 2000. 1993;1:80–91. [PubMed] [Google Scholar]
- 3.Kumar A, Lal N, Singhal R, Rastogi P. Comparative evaluation of periosteum as a barrier membrane with and without an alloplastic bone graft in periodontal osseous defects: A 9 months follow-up study. J Indian Soc Periodontol. 2014;18:493–6. doi: 10.4103/0972-124X.138706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao RT, Murakami S, Beirne OR. The use of biologic mediators and tissue engineering in dentistry. Periodontol 2000. 2009;50:127–53. doi: 10.1111/j.1600-0757.2008.00287.x. [DOI] [PubMed] [Google Scholar]
- 5.Friederichs RJ, Chappell HF, Shepherd DV, Best SM. Synthesis, characterization and modelling of zinc and silicate co-substituted hydroxyapatite. J R Soc Interface. 2015;12:20150190. doi: 10.1098/rsif.2015.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thian ES, Konishi T, Kawanobe Y, Lim PN, Choong C, Ho B, et al. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J Mater Sci Mater Med. 2013;24:437–45. doi: 10.1007/s10856-012-4817-x. [DOI] [PubMed] [Google Scholar]
- 7.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–9. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 8.Tapiero H, Tew KD. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed Pharmacother. 2003;57:399–411. doi: 10.1016/s0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 9.Dayashankar CP, Deepika PC, Siddaramaiah B. Clinical and radiographic evaluation of citric acid-based nano hydroxyapatite composite graft in the regeneration of intrabony defects – A randomized controlled trial. Contemp Clin Dent. 2017;8:380–6. doi: 10.4103/ccd.ccd_213_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharjee P, Begam H, Chanda A, Nandi SK. Animal trial on zinc doped hydroxyapatite: A case study. J Asian Ceram Soc. 2014;2:44–51. [Google Scholar]
- 11.Webster TJ, Massa-Schlueter EA, Smith JL, Slamovich EB. Osteoblast response to hydroxyapatite doped with divalent and trivalent cations. Biomaterials. 2004;25:2111–21. doi: 10.1016/j.biomaterials.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Hujoel PP, Moulton LH. Evaluation of test statistics in split-mouth clinical trials. J Periodontal Res. 1988;23:378–80. doi: 10.1111/j.1600-0765.1988.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 13.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 14.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 15.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–35. [PubMed] [Google Scholar]
- 16.Clark DC, Chin Quee T, Bergeron MJ, Chan EC, Lautar-Lemay C, de Gruchy K, et al. Reliability of attachment level measurements using the cementoenamel junction and a plastic stent. J Periodontol. 1987;58:115–8. doi: 10.1902/jop.1987.58.2.115. [DOI] [PubMed] [Google Scholar]
- 17.Ito A, Ojima K, Naito H, Ichinose N, Tateishi T. Preparation, solubility, and cytocompatibility of zinc-releasing calcium phosphate ceramics. J Biomed Mater Res. 2000;50:178–83. doi: 10.1002/(sici)1097-4636(200005)50:2<178::aid-jbm12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Singh D, Singh A. Radiation sterilization of tissue allografts: A review. World J Radiol. 2016;8:355–69. doi: 10.4329/wjr.v8.i4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calasans-Maia M, Calasans-Maia J, Santos S, Mavropoulos E, Farina M, Lima I, et al. Short-term in vivo evaluation of zinc-containing calcium phosphate using a normalized procedure. Mater Sci Eng C Mater Biol Appl. 2014;41:309–19. doi: 10.1016/j.msec.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Xiao X, Liu R, Chen C, Huang L. Structural characterization of zinc-substituted hydroxyapatite prepared by hydrothermal method. J Mater Sci Mater Med. 2008;19:797–803. doi: 10.1007/s10856-007-3213-4. [DOI] [PubMed] [Google Scholar]
- 21.Froum SJ, Weinberg MA, Tarnow D. Comparison of bioactive glass synthetic bone graft particles and open debridement in the treatment of human periodontal defects. A clinical study. J Periodontol. 1998;69:698–709. doi: 10.1902/jop.1998.69.6.698. [DOI] [PubMed] [Google Scholar]
- 22.Seo HJ, Cho YE, Kim T, Shin HI, Kwun IS. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr Res Pract. 2010;4:356–61. doi: 10.4162/nrp.2010.4.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohgushi H, Dohi Y, Tamai S, Tabata S. Osteogenic differentiation of marrow stromal stem cells in porous hydroxyapatite ceramics. J Biomed Mater Res. 1993;27:1401–7. doi: 10.1002/jbm.820271107. [DOI] [PubMed] [Google Scholar]
- 24.Tonetti MS, Prato GP, Cortellini P. Factors affecting the healing response of intrabony defects following guided tissue regeneration and access flap surgery. J Clin Periodontol. 1996;23:548–56. doi: 10.1111/j.1600-051x.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]


