Abstract
Introduction:
Bacteria residing in the oral cavity penetrate freely through the resultant fissures under the fillings, which might lead to the development of secondary caries. Nowadays dentistry, different nanotechnological materials with antibacterial activity are being developed for oral cavity disinfection.
Aim:
This study aims to investigate the antibacterial activity of a new cavity disinfectant NanoCare Plus Silver Gold® (NanoCare) in comparison to (0.2%) chlorhexidine (CHX) gluconate disinfectant against Streptococcus mutans growth and colony count using Agar well diffusion method and contact test, respectively, and also, to assess the nanoparticles (NPs) size distribution and Zeta potential of a NanoCare disinfectant.
Materials and Methods:
A total of 36 samples of cavity disinfectants were divided into two main groups (G) (n = 18); G1: NanoCare, and G2: CHX. Each group was subdivided into two subgroups (C) (n = 9) according to the antimicrobial test that the samples were subjected to.
Results:
A chlorohexidine group demonstrated the higher mean inhibition zone values than NanoCare group where P ≤ 0.05, as well both groups had a significant decrease in bacterial colony count where P ≤ 0.001. The particles size distribution in NanoCare sample was found that 99% of particles number with an average size of 29.07 nm and 1% was 136.7 nm, while the Zeta potential value was −6.5 mV.
Conclusions:
NanoCare cavity disinfectant displayed good antibacterial property against S. mutans. The innovative incorporation of NPs into this cavity disinfectant could be used to improve the antimicrobial capacity of the material and help to decrease secondary caries risk.
Keywords: Antibacterial effect, cavity disinfectant, chlorhexidine, gold nanoparticles, silver nanoparticles, Streptococcus mutans
Introduction
The most common problem of preventive dentistry is the formation of microfissures between tooth tissues and the filling. Even the greatest precision applied during restoration, work performance cannot guarantee that the material will perfectly and permanently adhere to dentine. The causes of leaks formation are polymerization shrinkages of materials used for fillings and chewing forces.[1] Bacteria residing in oral cavity penetrate freely through the resultant fissures under the fillings, which might lead to the development of secondary caries. Nowadays, dentistry is based on new materials and advanced technologies.[2] However, the treatment of deep carious lesions remains dubious. For initiation and progression of dental caries, presence of a biofilm is necessary. Silver-based preparations that incorporated into various materials have been shown to be effective in inhibiting biofilm formation.[3] At nanoscale, nanosilver particles exhibit remarkably unusual physical, chemical, and biological properties.[4] Recently, a new disinfecting agent for use within dentistry has been introduced to the market: NanoCare Plus Silver Gold® (NanoCare) (Dental Nanotechnology, Katowice, Poland), as the manufacturer states; the material comprises of silver nanoparticles (AgNPs) and small amount of gold nanoparticles (AuNPs). Yet, its exact characteristics are restricted.[5] According to manufacturer's specification for NanoCare, its practical application covers antibacterial and antifungal effects and physical parameters enhancement of restorative materials.[6] Moreover, it is a biocompatible material and probably may be used in deep cavities restoration.[7] However, there is a lack of studies regarding NanoCare efficacy against one of the cariogenic bacteria, especially Streptococcus mutans, hence it could be a promising dental material that allows the elimination of secondary caries problem. Chlorhexidine (CHX) is considered to be the gold standard for the same purpose of using NanoCare.[8] However, it has numerous side-effects such as staining, alteration of taste sensation, and parotid duct stenosis.[9,10,11] Therefore, the present study was conducted to investigate the antibacterial activity of a new disinfecting agent NanoCare against S. mutans growth in comparison to CHX gluconate (0.2%) and assess the NPs size of a NanoCare disinfectant.
Materials and Methods
Study design
A total of 36 samples of cavity disinfectants were divided into two main groups (G) (n = 18) as following; G1: NanoCare and G2: CHX. Each group was subdivided into two subgroups (C) (n = 9) according to the antimicrobial test that the samples were subjected to.
Antibacterial test
Microorganism and culture condition
Carious dentine of serotype (c) strain of S. mutans ATCC 25175 (Microbiological Resources Centre, Cairo MIRCEN, Egypt) was used throughout the study. Bacteria were cultured overnight at 37°C in the brain–heart infusion broth (BHI, Merck KGaA 64271 Darmstadt, Germany) and used as inoculum. About 0.5 ml of suspension of inoculums having 106 S. mutans/ml was estimated through McFarland standard (Densimat, BioMerieux, France) and used as working microbial solution before each experiment.[12]
Agar well diffusion method
Mitis salivarius-bacitracin agar was poured into sterile Petri dishes (15 ml each) and 50 μl of S. mutans (106 colony-forming uni [CFU]/ml) were dispersed on the surface of each agar plate. There were nine plates, in all the plates, two wells were punctured, and then one well was filled with aliquots of 100 μl of NanoCare and the other with 0.2% CHX digluconate (Sigma-Aldrich Prod. No. C9394, Germany). After incubation at 37°C for 24 and 48 h, the inhibition zones that were displayed by NanoCare group and CHX group against microbial growth were estimated.[13]
Contact test
Different dilutions for commercial product NanoCare were prepared along with standard CHX solution. The range of dilution tested was 1:5–1:100 ratio (diluting fluid: Commercial product tested). To prepare 1:5 dilution; 0.5 ml of tested product were added to 9.5ml of distilled water to start with dilution at 1:5 in a sterile test tube using an automatic micropipette. Then to form dilution at 1:10; 1 ml of tested product were added to 9 ml of distilled water and so on dilution was carried out till the last tube was kept of 10 ml of 100% concentration of respective NanoCare, and the same experiment was repeated for CHX digluconate.
One milliliter of bacterial suspension, S. mutans (1.2 × 104 CFU/ml) which was previously estimated using 0.5 McFarland standard (bacterial count 1.5 × 107) and serial diluted, was added to each dilution of NanoCare containing tube and the same for CHX solution. The contact time was kept constant 1 min for all dilutions of NanoCare and CHX under test. After that, 1 ml of each contact solution was transferred to 9 ml of standard neutralizer, and finally, 0.1 ml of this suspension was transferred on Tryptic Soy Agar Media (TSA; Difco Laboratories, Detroit, MI, USA). All the plates (triplicate for each dilution) were incubated for 24 h at 37°C. After incubation, the bacterial colonies were counted from the most suitable plates for each dilution in comparison with the counts of S. mutans (without test agents).[14]
The evaluation of particle size distribution and zeta potential
The particle size distribution by number as well as intensity and zeta potential of NanoCare samples were measured using a Zeta-Sizer Ver. 7.04 instrument (Nano-ZS, Malvern Instruments Ltd., UK). For measuring zeta potential, the samples were diluted (5 times) by deionized water just before assessment.[15]
Statistical analysis
The mean and standard deviation (SD) values were calculated for each group in each test. Data were explored for normality using Kolmogorov–Smirnov and Shapiro–Wilk tests. Data showed parametric (normal) distribution. Independent sample t-test was used to compare between two groups in nonrelated samples. Paired sample t-test was used to compare between two groups in related samples. The significance level was set at P ≤ 0.05. Statistical analysis was performed with IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp, USA.
Results
Antibacterial result
Inhibition zones result (agar well diffusion method)
The inhibition zones result of different incubation periods (after 24 and after 48 h) for the two cavity disinfectants (NanoCare and CHX) were assessed against S. mutans growth. The means and SDs values for the inhibition zones (mm) were displayed in [Table 1].
Table 1.
The mean±standard deviation values of inhibition zone results of the two disinfectant agents against the profile growth of Streptococcus mutans after different incubation period
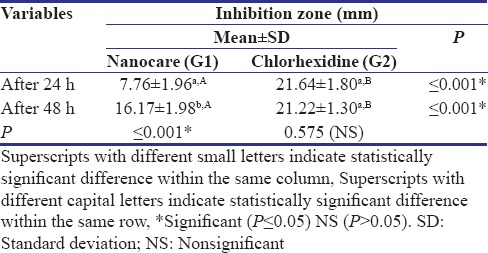
Concerning the effect of time on inhibition zones exhibited by each disinfectant, the NanoCare group showed a statistically significant increase in mean values of inhibition zones diameter from 24 h (7.76 ± 1.96) to 48 h (16.17 ± 1.98), where P ≤ 0.001. For the CHX group, no statistically significant change was found in mean values of inhibition zones diameter through the incubation period from 24 h (21.22 ± 1.30) to 48 h (21.64 ± 1.80) where P = 0.575.
Comparison between the two disinfectant agents in each time period against S. mutans growth revealed a statistical significance difference (P ≤ 0.05). A CHX group demonstrated the higher mean inhibition zone values (21.64 ± 1.80, 21.22 ± 1.30) than NanoCare group (7.76 ± 1.96, 16.17 ± 1.98) after 24 h and after 48 h, respectively.
Counting bacterial colonies result (contact test)
A statistically significant difference in S. mutans colony counts was found between controls, NanoCare, and CHX groups where P ≤ 0.001. In addition, there was a reduction in Streptococcus colony counts at different dilutions for each disinfectant (CHX group and NanoCare group) (P ≤ 0.001) compared to control group. Furthermore, a statistically significant difference was found between NanoCare and CHX groups where P ≤ 0.001. The highest mean value was found in control (282.67 × 103 ± 2.52 × 103) followed by NanoCare (206.14 × 103 ± 43.60 × 103), the lowest mean value was found in CHX (11.28 × 103 ± 6.11 × 103) [Figure 1].
Figure 1.
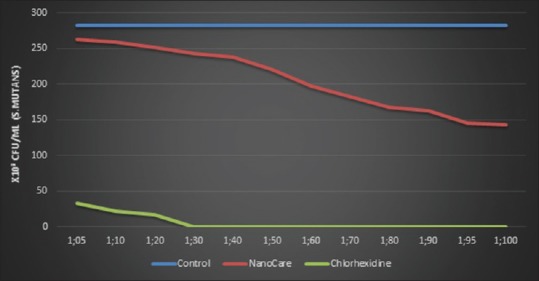
Line chart representing counts of Streptococcus mutans were expressed as a number of colony-forming units/ml at different dilutions for each cavity disinfectant (NanoCare and Chlorhexidine) and comparison with the counts of Streptococcus mutans (without disinfectant)
Particle size distribution by number and zeta potential measurements
Figure 2 shows the particles size distribution in NanoCare sample measured using a Zeta-Sizer Ver. 7.04 instrument. The data revealed that 99% of particles number with average size of 29.07 nm ± 7.244 nm and 1% of the total particle had an average size of 136.7 ± 60.89 nm. Furthermore, the Zeta potential value of NanoCare was revealed an average −6.5 ± 8.73 mV [Figure 3].
Figure 2.
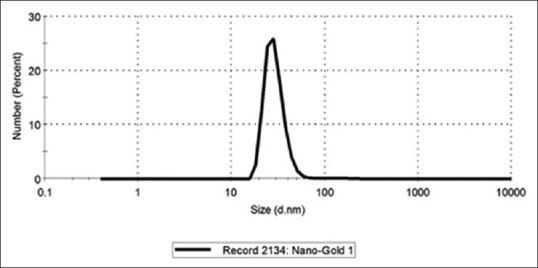
Size distribution by number
Figure 3.
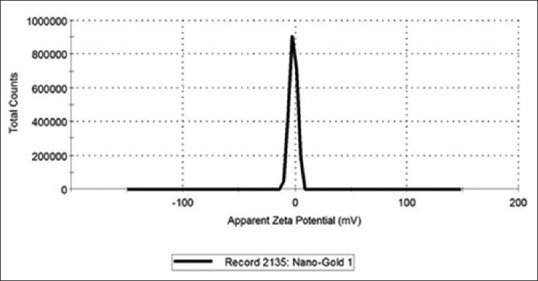
Zeta potential measurement
Discussion
Dental caries is the most frequent infectious disease affecting the tooth's hard tissues. Its development and further progression are caused by Gram-positive bacteria, especially Streptococcus and Lactobacillus, which proliferate in the acidic environment of the dental plaque (pH ≤5.0) and are important etiological factors in caries formation.[16,17]
The operative treatment methods focus on the removal of the infected tissue and its reconstruction with a restorative dental material, e.g., polymer resin. However, the seal between the tooth's tissues and the filling material is never ideal; and therefore, the infection beneath the restoration is considered a great threat to the pulp and more than half of the cavity restorations are replaced due to bacterial microleakage.[2,16]
The bacteria may survive underneath the restorations up till 139 days producing toxins and other destructive products of their metabolism. Thus, the treatment efficacy depends on bacteria clearance, proper seal (elimination of the microleakage), and possibly, antibacterial properties of restorative materials.[2,16] Sadly, not all of the dental materials possess antibacterial properties. Therefore, the concept of toileting of the cavity is gaining wider acceptance with a variety of commercially available dentin disinfectants launched into the market.[2] These dentin disinfectants are (among others) CHX. NanoCare was selected as a surface pretreatment material to be investigated in this study as a suggested newly innovated cavity disinfectant with a strong antibacterial property.[18]
Following the manufacturer's instructions, NanoCare is to be applied on dentin surface after acid etching and before primer and adhesive application with etch-and-rinse adhesive systems. Furthermore, the manufacturer claimed that NanoCare was developed to enhance the physical parameters of restorative materials and improve the adhesion between dentin surface and resin composite.[2,16]
In the present study, we focused on NanoCare to assess its antibacterial activity against Gram-positive cariogenic bacteria in comparison with CHX disinfectant. S. mutans was chosen as the target microorganism because it is a well-known major pathogen of caries.[19] The present counting bacterial colonies and inhibition zones results showed that NanoCare and CHX groups had a significant decrease in bacterial count, where CHX showed significantly higher rate of bacterial inhibition at both time intervals.
Regarding the antimicrobial efficacy of NanoCare, Mohamed Hamouda[20] confirmed that the incorporation of AgNPs into different adhesives was successful in enhancing their physical and antimicrobial properties. Furthermore, several studies reported that the experimental composite adhesive enriched with AgNPs are less prone to bacterial biofilm accumulation on their surfaces without any negative impact on the polymer's physical characteristics.[20,21,22] In addition, a dental literature by Cristóbal et al.[23] concluded that AgNPs present antibacterial activity on S. mutans and this property depends on the size of the particles. This latter study was clarified that the smaller the NP, the more it releases the silver ions (Ag + ions) and their antibacterial effect can be better.[23] According to these studies, the possibility for antibacterial activity of NanoCare disinfectant could be proposed that once bacteria are exposed to NanoCare solution, Ag + ions could be released from AgNPs into the bacterial suspension and interact with bacteria by electrostatic attraction between negative charges of bacterial cell wall/membrane and positive charges of ions. In keeping with the preceding investigation, it could be explained our inhibition zones results of NanoCare disinfectant toward S. mutans growth.
While for CHX, it has frequently been applied to tooth cavities before placing a restoration because of its broad-spectrum antibacterial activity.[24,25] The antibacterial capacity of CHX is owing to a positively charged of its hydrophobic and lipophilic molecule that interacts with phospholipids and lipopolysaccharides in the bacterial cell membrane and enters the cell.[8]
According to the microscopic evaluation of chemical composition of NanoCare that performed by Mackiewicz and Olczak-Kowalczyk,[6] the dominant type of NPs in NanoCare solution was AgNPs. This latter study was in agreement with particle size distribution results of the present study. Therefore, from our study, it was revealed that 99% of particles number in NanoCare disinfectant were AgNPs with an average size of 29.07 nm, while the rest of particles(1%) was AuNPs with average size 136.7 nm. Those mingy content of gold bound to AgNPs creating larger conglomerates as reported by Mackiewicz and Olczak-Kowalczyk.[6] In the conglomerate Au-AgNPs, gold is situated on the surface of a AgNP, thus it may affect the release of silver ions or decrease the efficacy of antimicrobial activity of AgNPs.[6] In addition, Hashimoto et al.[26] reported that the efficacy of AuNPs was size dependent, the smaller the particle size, the higher the potency. As was mentioned above, the average size of AuNPs was 136.7 nm, so the potential antimicrobial activity of AuNPs could be decreased. Hence, all previous investigations could be explained the present lower mean inhibition zone values of NanoCare group than a CHX group after 24 h and after 48 h, respectively. Accordingly, based on our data, we suggest that antibacterial activity of NanoCare disinfectant depends on the type and size of its incorporating NPs.
When using antimicrobial preparations, it is highly important to know whether there is any possibility of the occurrence and spreading of pathogenic bacterial forms resistant to them that would significantly decrease these preparations effectiveness.[27] Accordingly, Ag+ ions that were released from AgNPs presenting in NanoCare might decrease the antimicrobial efficacy of this disinfectant. However, due to the silver ion action on various cellular processes, silver resistance in bacteria is rather seldom; resistant forms of pathogenic bacteria appear more rarely than antibiotic-resistant bacterial forms, and no wide spreading of silver-resistant forms was observed.[27] Furthermore, AgNPs were nondirect sources of toxicity due to their permanent slow release of silver ions into the media. Therefore, NanoCare disinfectant has a potential antimicrobial activity with no chances for either pathogenic bacterial forms resistant or the risk of AgNPs cytotoxicity. Furthermore, previous studies were reported that cytotoxicity difference was associated with differences in NPs diameter.[28,29,30] accordingly, there is a high probability that the larger AuNPs in NanoCare disinfectant showing the least cytotoxicity.
Although the positively charged NPs showed much greater cell uptake than negatively charged NPs because this charge promoted their interactions with the negatively charged cell surface,[31] our Zeta potential results for NanoCare disinfectant revealed negatively charged NPs (−6.5 ± 8.73 mV). This discrepancy between the latter study and our results could be due to NanoCare disinfectant has been designed to interact with the positive catalytic domain (i.e., Zn2+) of matrix metalloproteinases (MMPs).[32,33] Hashimoto et al.[34,35] showed an inhibitory effect of AuNPs on MMPs – 1, 2, 8, 9 through chelation of zinc in their catalytic domain and subsequently its inactivation.
Since we tested only one strain of S. mutans which was a sole target microorganism used in this study because of its well-known pathogenicity in dental caries, the effect may be different in other strains of cariogenic bacteria. However, dental plaque is a complicated ecosystem of approximately 1000 bacterial species.[36] Therefore, use of a microcosm model in the future studies would be preferable.[37] In line with previous studies, compare with bacteria in planktonic culture, bacterial biofilms have dramatically higher resistance to antimicrobial agents, including NPs and antibiotics.[38,39] Therefore, studies the effect of NanoCare disinfectant on cariogenic bacterial biofilm would be noteworthy to explore. Moreover, further studies on the mechanism of NP action incorporating NanoCare disinfectant would help us better understand the function of both Ag and AuNPs affecting on this cariogenic bacterium as well as other strains. Furthermore, a supplemental research must consider to detect the resistance of v to NanoCare disinfectant, in time.
Conclusions
NanoCare cavity disinfectant displayed good antibacterial activity against Gram-positive cariogenic bacteria, S. mutans. The innovative incorporation of AgNPs and a few AuNPs into this cavity disinfectant could improve the antimicrobial capacity of material and might help to decrease secondary caries risk.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the continuous positive emotional support and fruitful advice of late Dr. Mokhtar N. Ibrahim, Professor of Operative Dentistry, Faculty of Dentistry, Ain-Shams University.
References
- 1.Pallesen U, van Dijken JW, Halken J, Hallonsten AL, Höigaard R. A prospective 8-year follow-up of posterior resin composite restorations in permanent teeth of children and adolescents in public dental health service: Reasons for replacement. Clin Oral Investig. 2014;18:819–27. doi: 10.1007/s00784-013-1052-x. [DOI] [PubMed] [Google Scholar]
- 2.Elkassas DW, Fawzi EM, El Zohairy A. The effect of cavity disinfectants on the micro-shear bond strength of dentin adhesives. Eur J Dent. 2014;8:184–90. doi: 10.4103/1305-7456.130596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight GM, McIntyre JM, Craig GG, Mulyani, Zilm PS, Gully NJ, et al. Inability to form a biofilm of Streptococcus mutans on silver fluoride – And potassium iodide-treated demineralized dentin. Quintessence Int. 2009;40:155–61. [PubMed] [Google Scholar]
- 4.Shrestha A, Shi Z, Neoh KG, Kishen A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J Endod. 2010;36:1030–5. doi: 10.1016/j.joen.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Bednarski M, Soska-Czop A, Zarzycka B, Ebert J, Pawlicka H. Nanocare plus silvergold® can eliminate Enterococcus faecalis from dentinal tubules. Dent Med Probl. 2013;50:418–23. [Google Scholar]
- 6.Mackiewicz A, Olczak-Kowalczyk D. Microscopic evaluation of surface topography and chemical composition of nanocare gold. J Stoma. 2014;6:826–40. [Google Scholar]
- 7.Mackiewicz A, Olczak-Kowalczyk D, Grzeczkowicz A, Granicka L, Antosiak-Iwańska M, Godlewska E, et al. Cytotoxicity of nanocare gold® in in vitro assay – Pilot study. Dent Med Probl. 2015;52:167–4. [Google Scholar]
- 8.Cha HS, Shin DH. Antibacterial capacity of cavity disinfectants against Streptococcus mutans and their effects on shear bond strength of a self-etch adhesive. Dent Mater J. 2016;35:147–52. doi: 10.4012/dmj.2015-175. [DOI] [PubMed] [Google Scholar]
- 9.Duss C, Lang NP, Cosyn J, Persson GR. A randomized, controlled clinical trial on the clinical, microbiological, and staining effects of a novel 0.05% chlorhexidine/herbal extract and a 0.1% chlorhexidine mouthrinse adjunct to periodontal surgery. J Clin Periodontol. 2010;37:988–97. doi: 10.1111/j.1600-051X.2010.01609.x. [DOI] [PubMed] [Google Scholar]
- 10.Bagis B, Baltacioglu E, Özcan M, Ustaomer S. Evaluation of chlorhexidine gluconate mouthrinse-induced staining using a digital colorimeter: An in vivo study. Quintessence Int. 2011;42:213–23. [PubMed] [Google Scholar]
- 11.Frank ME, Gent JF, Hettinger TP. Effects of chlorhexidine on human taste perception. Physiol Behav. 2001;74:85–99. doi: 10.1016/s0031-9384(01)00558-3. [DOI] [PubMed] [Google Scholar]
- 12.Jinan RM. Effect of water cinnamon extract on mutans streptococci. MDJ. 2008;5:250–60. [Google Scholar]
- 13.Krumina G, Ratkevicha L, Nikolajeva V, Babarikina A, Babarykin D. Influence of plant extracts on the growth of oral pathogens Streptococcus mutans and Candida albicans in vitro . Proc Estonian Acad Sci. 2015;64:62–7. [Google Scholar]
- 14.Parkar SM, Thakkar P, Shah K. Antimicrobial activity of four commercially available mouthwashes against Streptococcus mutans: An in vitro study. Univ Res J Dent. 2013;3:108–12. [Google Scholar]
- 15.Ghasemi S, Abbasi S. Formation of natural casein micelle nano capsule by means of pH changes and ultrasound. Food Hydrocoll. 2014;42:42–7. [Google Scholar]
- 16.Porenczuk A, Firlej P, Szczepańska G, Kolenda A, Olczak-Kowalczyk D. The laboratory comparison of shear bond strength and microscopic assessment of failure modes for a glass-ionomer cement and dentin bonding systems combined with silver nanoparticles. Acta Bioeng Biomech. 2016;18:59–70. [PubMed] [Google Scholar]
- 17.Eiampongpaiboon T, Chung WO, Bryers JD, Chung KH, Chan DCN. Antibacterial activity of gold-titanates on gram-positive cariogenic bacteria. Acta Biomater Odontol Scand. 2015;1:51–8. doi: 10.3109/23337931.2015.1084883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magdalen M, Szymańska L, Kleczewska J, Bieliński D, Jakubowski W, Sokołowski J. Bactericidal properties of experimental dental composites based on dimethacrylate resins reinforced by nanoparticles. Eur J Chem. 2014;5:419–23. [Google Scholar]
- 19.Kim JS, Shin DH. Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin. Restor Dent Endod. 2013;38:36–42. doi: 10.5395/rde.2013.38.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed Hamouda I. Current perspectives of nanoparticles in medical and dental biomaterials. J Biomed Res. 2012;26:143–51. doi: 10.7555/JBR.26.20120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.das Neves PB, Agnelli JA, Kurachi C, de Souza CW. Addition of silver nanoparticles to composite resin: Effect on physical and bactericidal properties in vitro. Braz Dent J. 2014;25:141–5. doi: 10.1590/0103-6440201302398. [DOI] [PubMed] [Google Scholar]
- 22.Sharma V, Nainan MT, Shivanna V. The effect of cavity disinfectants on the sealing ability of dentin bonding system: An in vitro study. J Conserv Dent. 2009;12:109–13. doi: 10.4103/0972-0707.57634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cristóbal L, Castanon G, Martínez R, Rodríguez J, Marín N, Macías J, et al. Antibacterial effect of silver nanoparticles against Streptococcus mutans. Mater Lett. 2009;63:2603–6. [Google Scholar]
- 24.Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifácio KC, Ito IY, et al. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25:167–71. doi: 10.1016/s0099-2399(99)80135-6. [DOI] [PubMed] [Google Scholar]
- 25.Khademi AA, Mohammadi Z, Havaee A. Evaluation of the antibacterial substantivity of several intra-canal agents. Aust Endod J. 2006;32:112–5. doi: 10.1111/j.1747-4477.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto M, Kawakami H, Kawai K, Imazato S. Effect of particle size of gold nanoparticles on matrix metalloprotease inhibition, cytotoxicity and genotoxicity. J Biomater Tissue Eng. 2017;7:139–46. [Google Scholar]
- 27.Nadtochenko V, Radtsig M, Khme I. Antimicrobial effect of metallic and semiconductor nanoparticles. Nanotechnol Russ. 2010;5:277–89. [Google Scholar]
- 28.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 29.Yildirimer L, Thanh NT, Loizidou M, Seifalian AM. Toxicology and clinical potential of nanoparticles. Nano Today. 2011;6:585–607. doi: 10.1016/j.nantod.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao F, Zhao Y, Liu Y, Chang X, Chen C, Zhao Y, et al. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322–37. doi: 10.1002/smll.201100001. [DOI] [PubMed] [Google Scholar]
- 31.Schlinkert P, Casals E, Boyles M, Tischler U, Hornig E, Tran N, et al. The oxidative potential of differently charged silver and gold nanoparticles on three human lung epithelial cell types. J Nanobiotechnology. 2015;13:1. doi: 10.1186/s12951-014-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G, Men P, Perry G, Smith MA. Nanoparticle and iron chelators as a potential novel Alzheimer therapy. Methods Mol Biol. 2010;610:123–44. doi: 10.1007/978-1-60327-029-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanakit A, Rouffet M, Martin DP, Cohen SM. Investigating chelating sulfonamides and their use in metalloproteinase inhibitors. Dalton Trans. 2012;41:6507–15. doi: 10.1039/c2dt12373h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto M, Sasaki JI, Yamaguchi S, Kawai K, Kawakami H, Iwasaki Y, et al. Gold nanoparticles inhibit matrix metalloproteases without cytotoxicity. J Dent Res. 2015;94:1085–91. doi: 10.1177/0022034515589282. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto M, Kawai K, Kawakami H, Imazato S. Matrix metalloproteases inhibition and biocompatibility of gold and platinum nanoparticles. J Biomed Mater Res A. 2016;104:209–17. doi: 10.1002/jbm.a.35557. [DOI] [PubMed] [Google Scholar]
- 36.ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006;94:1–9. doi: 10.1007/s10266-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 37.McBain AJ. Chapter 4: In vitro biofilm models: An overview. Adv Appl Microbiol. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]
- 38.Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89:1175–86. doi: 10.1177/0022034510377794. [DOI] [PubMed] [Google Scholar]
- 39.Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65:1803–15. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]


