Abstract
Aim:
This study evaluated the effect of staining and subsequent bleaching on the color stability and surface roughness of three resin composites.
Materials and Methods:
One hundred and eight customized plastic disks from a plastic mold with an outer diameter of 10 mm and thickness of 1.02 mm were fabricated. Samples were divided into three groups of 36 specimens each – Group I (Estelite® α – Supranano), Group II (Filtek™ Z250 XT – Nanohybrid), and Group III (Filtek™ P90–Silorane). Subsequently, baseline surface color and roughness values were tabulated using spectrophotometer and profilometer, respectively. The specimens were then subjected to staining with coffee and turmeric for a period of 3 h/day for 40 days and following this, bleaching of the samples was done using a bleaching agent carbamide peroxide gel 15% for 8 h/day for 14 consecutive days.
Results:
Estelite® α performed better by maintaining color stability with minimal roughness compared to Filtek™ P90 and Filtek™Z250 XT. Turmeric had a greater potential to stain composites compared to coffee in all the groups.
Keywords: Color stability, nanohybrid, silorane, Supranano, surface roughness
Introduction
Resin composites have become the material of choice for esthetic restorations involving both anterior and posterior teeth due to their improved physical and adhesive properties.[1] Surface roughness, surface gloss, and color are the most important esthetic elements of a restoration.[2] It is reported that saliva, food components, and beverages may affect the esthetics and integrity of dental composites,[3] leading to its degradation in the oral environment. The maintenance of color stability of resin composites is influenced by various intrinsic and extrinsic factors.[4] Alteration of matrix or filler components of resin composites and incomplete polymerization constitute intrinsic factors leading to discoloration. Extrinsic factors may include adsorption and absorption of stains on exposure to various beverages, tobacco, and other food color additives.[5]
Continuous research and development in the field of material science have led to the invention of newer composite materials with variations in their material structure.[6] One such composite is the SupraNano, Estelite® α (Tokuyama, Japan) which contains Supra-Nano spherical fillers, that apparently help in enhancing the optical and physical properties of these materials. Yet another advancement in the material-based technology witnessed the introduction of Silorane-based composites, which is based on a change in the matrix system of the composite resin thus exhibiting improvements in their physical, mechanical, and chemical aspects; such as reduced polymerization shrinkage, high fracture toughness, bond strength, low water sorption, high light ambient stability, and a high resistance to external staining.[7,8]
The usage of at-home bleaching products is on the rise due to its easy availability and the enhanced esthetic demand of patients. As these agents come in contact with various restorative materials, their influence on the physical and optical properties of these materials, needs to be evaluated.[9,10,11,12] Current literature has limited studies evaluating the effects of bleaching agents on the color stability of newer resin composites stained with commonly used food commodities in an Indian household.
Hence, the aim of this study was to compare and evaluate the effect of staining of three resin composites namely, Supra-nano filled composite (Estelite® α), silorane-based composite (Filtek™ P90, and Nano-hybrid composite (Filtek™ Z250 XT), in two solutions, coffee, and turmeric and the effect of subsequent bleaching using 15% carbamide peroxide gel on the color stability and surface roughness of these resin composites using spectrophotometer and profilometer, respectively.
Materials and Methods
A total of 108 plastic disks were prepared from a plastic mold and custom modified such that each disk had an outer diameter of 10 mm and a thickness of 1.02 mm, calibrated using a Vernier caliper (Aerospace digital Vernier caliper, India). The disks were randomly divided into three groups depending on the composite material used namely, Supra-nano filled composite (Estelite® α, Group 1), silorane-based composite (Filtek™ P90, Group 2, and Nano-hybrid composite (Filtek™ Z250 XT Group 3). All samples were placed on frosted micro-slides (Blue Star, Polar Industrial Corporation, Mumbai, India) and filled with each of the testing material (n = 36). After filling the disk with the respective composite material to a slight excess, a microscopic cover glass (Blue Star, Polar Industrial Corporation, Mumbai, India) was placed over the top of the uncured composite, and a load of 500 g was applied for 20 s to obtain a flat surface. The specimens were cured for 40 s each from both directions using a visible light curing unit with an intensity of 1470 mW/cm2 (EliparTM 250, 3M ESPE Dental products, US). The specimens prepared were finished and polished with coarse, medium, fine and superfine aluminum oxide discs (Sof-Lex® system, 3M ESPE). All specimens were stored in distilled water for 24 h. Subsequently, a baseline color and surface roughness value at the end of the polishing procedure was measured for all specimens.
Staining of the samples
The three groups of composites were then randomly subdivided into two sub-groups each (n = 18) depending on the staining agent, namely coffee (Subgroup A) and turmeric (Subgroup B) respectively. The specimens were immersed in 20 ml of freshly prepared respective staining solutions (coffee or turmeric) for 3 h daily over a 40-day test period and were stored at 37°C in artificial saliva after staining. The samples were then rinsed in distilled water for 1 min and blot dried and subsequently evaluated for color change and surface roughness.
Bleaching of the samples
All specimens of subgroups A and B were subsequently bleached using 15% carbamide peroxide, Opalescence Potassium nitrate & fluoride (PF)-(Ultradent) for 8 h per day for 14 consecutive days. The bleaching agent was applied with the help of a cotton applicator on the same surface of the sample each time and were stored in dark containers to simulate at-home bleaching at 37°C. At the end of 14 days, specimens were rinsed with distilled water for 1 min and blot dried before color change and surface roughness measurements.
Color change measurement
Data color 650-spectrophotometer was used to detect color changes of all specimens. Color measurements were taken at three occasions, namely baseline, after 40 days, and after bleaching for a period of 14 days using the CIELAB color space (Commission Internationale de I'Eclairage L*, a*, b*) system.
Color changes were calculated using the formula:
ΔE = _ΔL2+ Δa2+ Δb2_1/2
The calculations of ΔE were as follows:
ΔE1= ([ΔL2− ΔL1] 2+ [Δa2− Δa1] 2+ [Δb2− Δb1] 2) 1/2
ΔE2= ([ΔL3− ΔL2] 2+ [Δa3− Δa2] 2+ [Δb3− Δb2] 2) 1/2
ΔE3= ([ΔL3− ΔL1] 2+ [Δa3− Δa1] 2+ [Δb3− Δb1] 2) 1/2
Surface roughness measurement
The average roughness of the samples was evaluated using a SJ.201P Mitutoyo profilometer (Mitutoyo Corporation, Tokyo, Japan) after preparation, after staining and after bleaching, using a standardized jig. The profilometer tip touched each specimen and ran a distance of 10 mm for 12 readings, following a clockwise radial direction, and an average was then obtained.
Statistical analysis
Data analysis was carried out using the SPSS version 10.5 (SPSS Inc., Chicago, IL, USA). The results for each parameter were analyzed using one-way analysis of variance. Normality assumption of data was analyzed using Shapiro–Wilks test. If normality assumption was not met, comparison between the groups was carried out by the nonparametric test.
Results
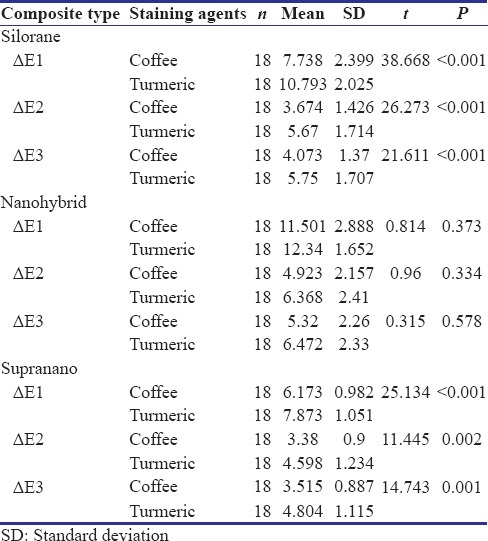
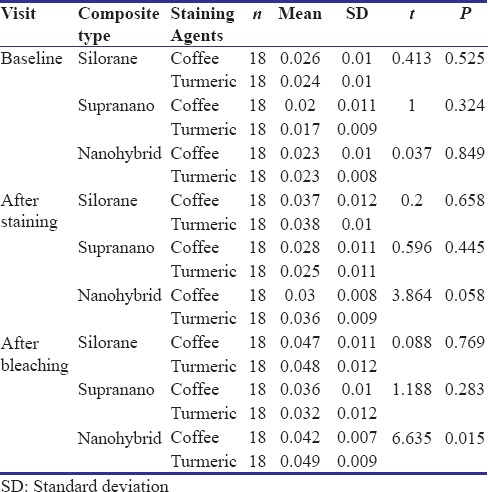
Results from the study showed significant changes in color (P < 0.05) in all the groups of composites after staining and bleaching (Table 1 and Graph 1). Increase in color change was noted after staining in all groups of composites, with nanohybrid composite showing the highest change in ΔE and supra-nano composite showing the least. Turmeric had a greater potential to stain composites compared to coffee in all the groups. Decrease in color of stained composite specimens was seen after bleaching. A significant difference (P < 0.05) in surface roughness of all composites was present after staining and a further increase in roughness was noted after bleaching (Table 2 and Graph 2). No significant difference between coffee and turmeric on surface roughness was noted in all the tested composites. Supra-nano composite performed better by maintaining color stability and minimum roughness compared to silorane-based composite and nanohybrid composite.
Table 1.
Means and standard deviations for color change (ΔE) of the two staining agents at different time intervals in the composites studied
Graph 1.
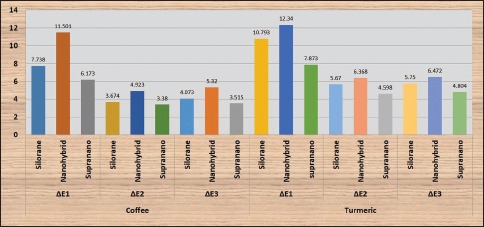
Comparison of the means for color change (ΔE) in the composites at the different experimental stages using the two staining agents
Table 2.
Comparison of the mean surface roughness of the two staining agents on the composites studied at different time intervals
Graph 2.
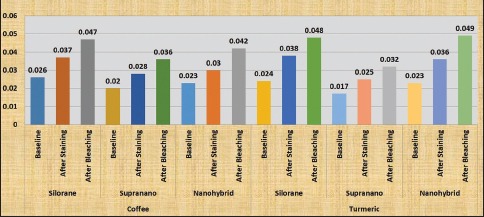
Comparison of the mean surface roughness of the composites at the different experimental stages using the two staining agents
Discussion
Resin-based composites (RBCs) typically consist of a methacrylate-based resin matrix (mass fraction of about 25%–30%), glass or ceramic fillers (mass fraction of about 70%–75%) and a filler-matrix coupling agent.[13] Under conditions of the oral cavity, RBCs are exposed to saliva, food components, and beverages.[14] To ensure excellent esthetics, it is necessary for tooth-colored materials to maintain intrinsic color stability and a resistance to surface staining. However, over time, composite restorations do acquire external stains from various food items and get internally discolored. This could be attributed to the biphasic nature of the material consisting of a matrix and fillers in its structure. In addition to this, degradation can also occur due to the hydrolytic breakdown of bond between the silane and filler particles and/or the resin matrix which is again dependent on the hydrophilicity of the polymers and the cross-linking density of the composite network.[14,15]
The present study analyzed the effects of staining the respective composites using coffee and turmeric. It was noticed that the change in color was significantly higher for turmeric for all the composite groups. This can be attributed to the high colorant nature of turmeric due to the presence of curcumin.[4] Conjugated diarylhepnoids in curcumin is responsible for the orange color and higher staining ability of turmeric. Coffee also exhibited considerable staining of all specimens. The less polar colorants and water-soluble polyphenols in coffee such as tannin, caffeine, and caffeinic acid might have penetrated deep into the material, possibly because such colorants are more compatible with polymer matrices thus leading to staining of the composites in the present study.[16,17] The staining period was kept at 3 h per day since an average person spends approximating 60–180 min per day eating and drinking thus simulating possible staining susceptibility of restorations. The specimens were customized to a specific dimension to obtain a significant comparison of penetration of staining agents and record color change and surface roughness.
The type of resin matrix has shown to play an important role in stain susceptibility of composites.[15] In the present study, the Nanohybrid composite, Filtek™ Z250 XT was affected the most with the highest color change. This could be attributed to the structure of this composite which mainly consists of bisphenol A glycol dimethacrylate, urethane dimethacrylate, and triethylene glycol dimethacrylate (TEGDMA). The presence of the ethoxy group in TEGDMA which is hydrophilic in nature may lead to increased water sorption,[18] allowing penetration of water into the matrix or filler-matrix interface and hence increased susceptibility to environmental stains. In addition, the lesser amount of fillers in the composition of Filtek™ Z250 XT could have led to a greater color variation observed in the present study. Filtek™P90 also exhibited a significant discoloration which could be attributed to the loss of fillers. The abrasion of the softer resin might cause a lack of support for the filler, which finally detaches from the matrix, resulting in a concavity in the surface allowing the penetration of stains.[19] In the Supranano group, Estelite® α, changes in surface discoloration was found to be lesser, possibly due to decreased filler size (0.2 μm) and spherical shape of the filler.
Discolored composite restorations maybe replaced or repaired by finishing and polishing of the restorations or bleaching. The interaction between the bleaching agent and restorative material and its role in reversal of staining is of clinical interest. Thus, this study also evaluated the effect of bleaching using 15% carbamide peroxide gel on the color stability and surface integrity of the stained composites.
This study demonstrated a more intense attack of carbamide peroxide on the surface of nanohybrid as compared to silorane-based composites. The exact mechanism of how bleaching regimens affect restorative material is not clear, but presumably, this may be due to the breakdown of carbamide peroxide into hydrogen peroxide and urea in aqueous solution, with hydrogen peroxide being the active bleaching agent, that may penetrate the surface of restorative materials. This in turn could also weaken the bond between the staining agent and composite causing the elimination of the stain.[10]
A significant increase in the surface roughness value was observed from baseline to after staining in all the groups of composites stained with coffee and turmeric. Surface roughness of a composite is altered by a spectrum of factors such as the filler size and shape, chemical integration of the fillers and matrix, staining agent, the occlusal load, and the finishing and polishing procedures employed for final restoration.[20] The supra-nano filled composites, Estelite® α (0.2 μm) and Nanohybrid Composites, Filtek™ Z250 XT (20 nm) have reduced particle size compared to the silorane-based composite, Filtek™ P90 (0.47 μm). Finer particles lead to an increase in filler surface area. Estelite® α exhibited better properties than Filtek™ Z250XT due to its spherical nanofillers although the Filtek™ Z250 XT had broad particle distribution and higher filler loading. The supra-nano filled composite exhibited minimal change in surface roughness following bleaching, among all the tested composite groups.
The impact of bleaching agents on surface roughness of restorative materials could be considered concentration dependent with studies proving an increase in surface roughness noted between 20% and 35% carbamide peroxide.[21] It was claimed that this difference could be due to the chemical degradation of resin matrix by the concentration or repeated application of peroxide. In addition, if the bleaching agent degraded the coupling agent of resin composites, the resultant roughness would be exaggerated.[21,22,23]
From a clinical point of view, the present research indicated that the restorative materials must be selected depending on the color stability of these materials and patients' habits, as these factors greatly influence the discoloration potential of the restoration. When confronted with discolored composite restoration, bleaching can be considered as an alternate option.
Conclusion
Within the limitations of the present study, following conclusions maybe drawn:
Nanohybrid-Filtek™ Z250 XT showed the highest change in color while Supranano-Estelite® α showed the least
Bleaching of the specimens could reverse the effect of staining in all the groups. Nanohybrid composite, Filtek™ Z250 XT showed greatest reversal followed by Silorane-based Filtek™ P90 and Supranano filled composite, Estelite® α
The supra-nano filled Estelite® α performed better by maintaining color stability and minimal roughness compared to the nanohybrid composite Filtek™ P90 and Silorane-based Filtek™ Z250 XT.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hervás-García A, Martínez-Lozano MA, Cabanes-Vila J, Barjau-Escribano A, Fos-Galve P. Composite resins. A review of the materials and clinical indications. Med Oral Patol Oral Cir Bucal. 2006;11:E215–20. [PubMed] [Google Scholar]
- 2.Yap AU, Sau CW, Lye KW. Effects of finishing/polishing time on surface characteristics of tooth-coloured restoratives. J Oral Rehabil. 1998;25:456–61. doi: 10.1046/j.1365-2842.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein RE, Garber DA, Schwartz CG, Goldstein CE. Patient maintenance of esthetic restorations. J Am Dent Assoc. 1992;123:61–7. doi: 10.14219/jada.archive.1992.0027. [DOI] [PubMed] [Google Scholar]
- 4.Lee SY, Huang HM, Lin CY, Shih YH. Leached components from dental composites in oral simulating fluids and the resultant composite strengths. J Oral Rehabil. 1998;25:575–88. doi: 10.1046/j.1365-2842.1998.00284.x. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Bakr N, Han L, Okamoto A, Iwaku M. Color stability of compomer after immersion in various media. J Esthet Dent. 2000;12:258–63. doi: 10.1111/j.1708-8240.2000.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 6.Musanje L, Ferracane JL, Ferracane LL. Effects of resin formulation and nanofiller surface treatment on in vitro wear of experimental hybrid resin composite. J Biomed Mater Res B Appl Biomater. 2006;77:120–5. doi: 10.1002/jbm.b.30400. [DOI] [PubMed] [Google Scholar]
- 7.Joshi P, Chitinis R. Silorane composite system. Sci J. 2008;2:1–5. [Google Scholar]
- 8.Lien W, Vandewalle KS. Physical properties of a new silorane-based restorative system. Dent Mater. 2010;26:337–44. doi: 10.1016/j.dental.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Cooley RL, Burger KM. Effect of carbamide peroxide on composite resins. Quintessence Int. 1991;22:817–21. [Google Scholar]
- 10.Bailey SJ, Swift EJ., Jr Effects of home bleaching products on composite resins. Quintessence Int. 1992;23:489–94. [PubMed] [Google Scholar]
- 11.Canay S, Cehreli MC. The effect of current bleaching agents on the color of light-polymerized composites in vitro . J Prosthet Dent. 2003;89:474–8. doi: 10.1016/S0022391303001689. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Lee YK, Lim BS, Rhee SH, Yang HC. Effect of tooth-whitening strips and films on changes in color and surface roughness of resin composites. Clin Oral Investig. 2004;8:118–22. doi: 10.1007/s00784-004-0275-2. [DOI] [PubMed] [Google Scholar]
- 13.Fong H, Dickens SH, Flaim GM. Evaluation of dental restorative composites containing polyhedral oligomeric silsesquioxane methacrylate. Dent Mater. 2005;21:520–9. doi: 10.1016/j.dental.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Bagheri R, Burrow MF, Tyas M. Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J Dent. 2005;33:389–98. doi: 10.1016/j.jdent.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Oysaed H, Ruyter IE. Water sorption and filler characteristics of composites for use in posterior teeth. J Dent Res. 1986;65:1315–8. doi: 10.1177/00220345860650110601. [DOI] [PubMed] [Google Scholar]
- 16.Um CM, Ruyter IE. Staining of resin-based veneering materials with coffee and tea. Quintessence Int. 1991;22:377–86. [PubMed] [Google Scholar]
- 17.Madhyastha PS, Naik DG, Kotian R, Srikant N, Bhat KM. Effect of staining solutions on color stability of silorane & methacrylate restorative material. Int J Biomed Sci. 2015;11:29–34. [Google Scholar]
- 18.da Silva MA, Fardin AB, de Vasconcellos RC, Santos Lde M, Tonholo J, da Silva JG, Jr, et al. Analysis of roughness and surface hardness of a dental composite using atomic force microscopy and microhardness testing. Microsc Microanal. 2011;17:446–51. doi: 10.1017/S1431927611000250. [DOI] [PubMed] [Google Scholar]
- 19.Pires-de-Souza Fde C, Garcia Lda F, Hamida HM, Casemiro LA. Color stability of composites subjected to accelerated aging after curing using either a halogen or a light emitting diode source. Braz Dent J. 2007;18:119–23. doi: 10.1590/s0103-64402007000200006. [DOI] [PubMed] [Google Scholar]
- 20.Ertaş E, Güler AU, Yücel AC, Köprülü H, Güler E. Color stability of resin composites after immersion in different drinks. Dent Mater J. 2006;25:371–6. [PubMed] [Google Scholar]
- 21.Bahannan SA. Effects of different bleaching agent concentrations on surface roughness and microhardness of esthetic restorative materials. Saudi J Dent Res. 2015;6:124–8. [Google Scholar]
- 22.Türkün LS, Türkün M. Effect of bleaching and repolishing procedures on coffee and tea stain removal from three anterior composite veneering materials. J Esthet Restor Dent. 2004;16:290–301. doi: 10.1111/j.1708-8240.2004.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 23.Atali PI, Topbasi FB. The effect of different bleaching methods on the surface roughness and hardness of resin composites. J Dent Oral Hyg. 2011;3:10–7. [Google Scholar]


