Abstract
Aim:
This study evaluated the antibiofilm efficacy of calcium hydroxide-based sealer incorporated with chitosan nanoparticles (CS-NPs) and zinc oxide nanoparticles (ZnO-NPs) against two strains of Enterococcus faecalis (ATCC 29212, OG1RF).
Materials and Methods:
The materials tested were Apexit Plus sealer in the commercial unmodified form and two modified forms with CS-NP and ZNO-NP, respectively. Crystal violet assay and confocal laser scanning microscopy (CLSM) study were used to assess the bacterial viability of biofilms grown in wells of microtiter plate and glass slides, respectively. Two E. faecalis strains (ATCC 29212, OG1RF) were used for the study.
Results:
The crystal violet assay done on E. faecalis strain ATCC 29212 showed a significant decrease in the optical density (OD) value with ZNO-NP-incorporated calcium hydroxide sealer when compared with CS-NP. In the case of E. faecalis strain OG1RF, only ZNO-NP-incorporated calcium hydroxide-based sealer showed reduction in the OD value. In CLSM study done on E. faecalis strain ATCC 29212, only ZNO-NP-incorporated calcium hydroxide-based sealer showed reduction in the thickness of biofilm. No groups of OG1RF strain showed reduction in the thickness of biofilm.
Conclusion:
The incorporation of nanoparticles (ZnO and CS) into calcium hydroxide-based sealers significantly enhances the antibiofilm efficiency against E. faecalis strain ATCC 29212 but has questionable effectiveness against E. faecalis strain OG1RF. The present study demonstrates that ZNO-NP shows better antibiofilm efficacy than CS-NP against both strains of E. faecalis.
Keywords: Calcium hydroxide-based sealer, chitosan nanoparticles, confocal laser scanning microscope, crystal violet assay, Enterococcus faecalis, zinc oxide nanoparticles
Introduction
Endodontic infection is a biofilm mediated one and persistent apical periodontitis due to the intraradicular biofilm is one of the main reasons for the failure of endodontic treatment.[1,2,3] Although the current chemomechanical aids can reduce the intracanal microbial load, the complete eradication has not yet been attained. Bacterial biofilm resistance to the most commonly used intracanal disinfectants is due to inherent microbiological factors and the complex anatomy of the root canal system. It was reported that almost 35% of the root canal surfaces remain untouched by root canal files, regardless of the filing method.[1] The bacteria present in the dentinal tubules are inaccessible to the various chemomechanical aids due to their limited penetrability into the dentinal tubules. Most of the sealers and obturating materials have limited antimicrobial activity, and they lose their antibacterial property within 1 week.[1] All these factors contribute to persistence of bacterial biofilm within the root canal system.
The antimicrobial effect of calcium hydroxide sealer due to its high pH and release of hydroxyl ions is well known, but the potential disadvantage is that it cannot irradiate Enterococcus faecalis biofilms which will ultimately result in treatment failure.[4] Facultative anaerobes, especially E. faecalis, were the most commonly isolated microorganisms in cases of symptomatic root-filled teeth.[5] Hence, addition of other antibacterial nanoparticulate agents which are effective against E. faecalis biofilms can be considered to improve the antibacterial efficacy of endodontic sealers. Nanoparticles are those particles with diameter of 100 nm or less and possess properties that are very different compared to their bulk counterparts. Barros et al. already proved that addition of quaternary ammonium polyethylenimine nanoparticles improved the antibiofilm effect of AH Plus and pulp canal sealer on E. faecalis strains ATCC 29212 and RW35.[6] Although recent studies have been done on antimicrobial activity of nanoparticles, its effect on bacterial biofilm when incorporated with endodontic sealers particularly calcium hydroxide sealer has not been consistently studied. Shrestha et al. in 2010 already proved that zinc oxide nanoparticles (ZNO-NP) and chitosan nanoparticles (CS-NP) have antibiofilm activity against E. faecalis strains in planktonic and biofilm forms.[1] Hence, they can be used as potential antibacterial agents to modify the efficacy of calcium hydroxide sealer, but its synergistic effects on bacterial biofilm are yet to be studied.
Although several studies have reported the antimicrobial activity of some endodontic sealers, most investigators used either direct contact test or the agar diffusion test.[6] Both methods have significant limitations. Hence, in the present study, colorimetric crystal violet assay and confocal laser scanning microscopy (CLSM) were used to analyze the antibiofilm efficacy of nanoparticle-incorporated sealers. Although crystal violet assay is a widely used method which allows rapid quantification of biofilm bacteria, it is sometimes difficult to interpret because the absorbance/optical density (OD) measured is a reflection of the number of bacteria and is not a true indicator of the extracellular polymeric substances in the biofilm structure.[7] CLSM is a preferred tool for studying biofilm because of its ability to assess viability profile, spatial distribution of biofilm bacteria, and three-dimensional (3D)-reconstructed image of entire biofilm.[4]
The purpose of the present study was to evaluate the effects of a calcium hydroxide-based sealer, unmodified or loaded with ZNO-NP and CS-NP at 20% w/w concentration, on biofilms formed by two E. faecalis strains.
Materials and Methods
Materials tested
Endodontic sealer tested was Apexit Plus (Ivoclar Vivadent, Liechtenstein). CS-NP and ZNO-NP were synthesized in Biogenix Research Center, Thiruvananthapuram, using ionic gelation method and sol–gel method, respectively.[8,9] The synthesized nanoparticles (20 mg) were added into freshly mixed sealer (100 mg) in a vial containing phosphate-buffered saline (PBS) and it was mixed by vortexing. Thereafter, samples were kept for incubation at 37°C and overnight and used for the experiment.
Specimen preparation for crystal violet assay
Two strains of E. faecalis ATCC29212 (ATCC, USA) and OG1RF (ATCC, USA) were inoculated separately into brain–heart infusion broth (BHI, HiMedia, India) and were incubated for 24 h at 37°C. After standardization of bacterial suspension to 1 McFarland scale, 200-μL aliquots of the bacterial suspension were distributed in wells of a 96-well microtiter plate (Polystyrene Tarsons, India), totaling 40 wells, and incubated for 24 h at 37°C. The content of each well was aspirated, and the wells were rinsed three times with PBS to remove loosely attached cells. The adhering bacteria on the wells of the microtiter plate had formed the biofilm and were used for analyzing by crystal violet assay. The wells were divided into groups depending on the different types of samples to be added into the wells. With confidence level of 95 and power of 90%, the sample size was calculated as 5 for each group. The wells were divided into the following groups [Table 1] for each strain of E. Faecalis (ATCC 29212 and OG1RF).
Table 1.
Sample distribution among different groups

Two hundred microliter of sealer extracts from Group 1, Group 2, and Group 3 [Table 1] was distributed into 5 wells for each group and for each strain for 120 min at 37°C. After removal of the sealer samples, each well was washed three times with PBS, and adhering bacteria were stained with 50 μL of 0.1% crystal violet stain and were incubated for 20 min at room temperature. Excess stain was rinsed off by washing with distilled water. After washing, the plates were overturned and air-dried, and the dye bound to the adhering cells was solubilized with 200 μL of dimethyl sulfoxide for 20 min.
Biofilm mass remaining after treatment with nanoparticle-incorporated sealer was quantified by the absorbance (590 nm) of the crystal violet solution which was measured using an enzyme-linked immunosorbent assay reader (ELISA, Agilent Cary 60, USA) in the form of OD value at Biogenix Research Center, Thiruvananthapuram. For the positive control, saline was used instead of the test substance. For the negative control, sterile culture broth was used. The OD measured is the reflection of the amount of bacterial biofilm present on the microtiter plate after sealer application.
Specimen for confocal laser microscopy
Two strains of E. faecalis strain ATCC 29212 and OG1RF were inoculated into the BHI broth and were incubated for 24 h at 37°C. After the standardization to 1 McFarland scale, 200-μL aliquots of the bacterial suspension were distributed in the wells of the 16-well microtiter plate in which the glass coverslips (Tarsons, India) were placed at the base, totaling 40 wells. Then, the microtiter plates were placed in the incubator for 1 week at 37°C. Fresh medium of broth was replenished every 48 h to provide a constant supply of nutrients and to remove dead bacterial cells. After 1 week, the microtiter plates were removed from the incubator for analyzing under confocal laser microscope (CLSM, Nikon, Japan) in Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram.
Two hundred microliter of sealer extracts of Group 1, Group 2, and Group 3 [Table 1] was added into the wells containing the glass coverslips and incubated at 37°C for 24 h. After this period, sealer extracts were removed from the wells, and glass coverslips with biofilms were washed gently twice with sterile deionized water. Biofilm on coverslip was stained with 20 μl of fluorescein diacetate (FDA, Sigma Aldrich, USA) and propidium iodide (PI, Sigma Aldrich, USA) stain and incubated in the dark for 10 min. This stain differentiates the viable and nonviable bacterial cells. Biofilm structures are then viewed under a CLSM. CLSM was equipped with two lasers, argon ion and HeNe, emitting excitation wavelengths at 488 and 543 nm and long pass 500–523 and 622–722 nm emission filter settings for green and red signals, respectively. CLSM was coupled to a Z-stage piezo-controller, and the objective used was a ×60 oil immersion. CLSM was used to measure the thickness of the biofilm before and after application of nanoparticle sealer. The optical sections of biofilm structure were also recorded, and 3D reconstruction was done using NIS element software.
Statistical analysis
The antibiofilm efficacy of the test materials by crystal violet assay and CLSM against two E. faecalis strains was compared by Kruskal–Wallis and Mann–Whitney test. A statistical significance level of 5% (P < 0.05) was established for all analysis.
Results
Assessment of antibacterial efficacy by crystal violet assay
The microtiter plate assay using crystal violet done on 1-day-old biofilm of E. faecalis strains ATCC 29212 and OG1RF provides information on the effects of the materials on the biofilm biomass. The mean OD obtained for the test materials against two E. faecalis strains was demonstrated in Figure 1. Crystal violet assay against E. faecalis strain ATCC 29212 showed that Group 1 showed statistically significant (P < 0.05) reduction in OD (0.4817) than Group 2 (0.6198) and Group 3 (0.7865) when compared with positive control. Crystal violet assay against E. faecalis strain OG1RF showed that Group 1 showed the highest reduction in OD (0.6252). Group 2 (0.7245) and Group 3 (0.7570) show minimal or no reduction in OD when compared with positive control. When the two strains of E. faecalis were compared, Group 1 showed a better reduction in OD (0.4817) of E. faecalis ATCC 29212 strain compared to E. faecalis strain OG1RF (0.6252) which was statistically significant (P < 0.05).
Figure 1.

Effects of root canal sealers on biofilms from two Enterococcus faecalis strains. Microtiter-plate crystal violet antibiofilm assay
Assessment of antibacterial efficacy by CLSM
CLSM study on 1-week-old biofilm of E. faecalis strain ATCC 29212 [Figure 2] revealed that Group 1 showed a maximum reduction in the thickness of biofilm (24.8 μm) when compared to other groups (P < 0.05), Group 2 had less reduction in the thickness of biofilm (29.6 μm), and Group 3 showed no reduction in the thickness of the biofilm (45.6 μm).
Figure 2.

Effects of sealers on bacterial cell viability and biofilm thickness from two Enterococcus faecalis strains. CLSM study
Confocal laser microscopic study on 1-week-old biofilm of E. faecalis strain OG1RF [Figure 2] revealed no significant reduction in the thickness of biofilms in all the groups.
Assessment of biofilm structure after nanoparticulate treatment
Figure 3 shows the CLSM images of the bacterial biofilm before and after the nanoparticulate treatment. Biofilm structure in positive control consists of both live (green) and dead (red) bacterial cells in a multilayered architecture, and the number of live bacterial cells was observed to be higher as compared to dead cells. After treatment with ZNO-NP and CS-NP, the biofilm architecture was altered, thickness reduced significantly, and there was reduction in viable bacterial cells case of E. faecalis strain ATCC 29212. However, in case of E. faecalis strain OG1RF, sealer or nanoparticulate inoculated sealer could not disrupt the biofilm structure.
Figure 3.
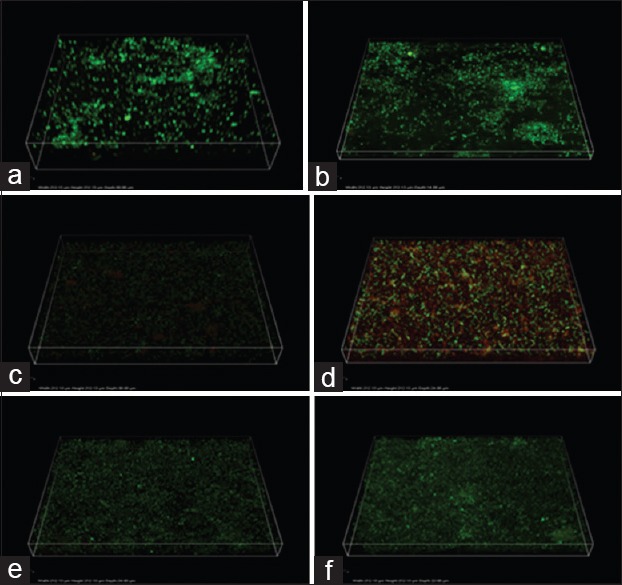
The three-dimensional CLSM reconstruction of Enterococcus faecalis strain ATCC 29212 (a) and OG1RF (b), after treatment with antibacterial zinc oxide nanoparticles against ATCC 29212 (c) and OG1RF (d), after treatment with chitosan nanoparticles against ATCC 29212 (e) and OG1RF (f)
Discussion
The biofilm mode of growth is a microbial strategy to survive in most environments and endure adverse conditions.[10] This study was conducted to evaluate the improvement of antibiofilm and antibacterial efficacy of sealers incorporated with ZNO-NP and CS-NP against two strains of E. faecalis.
In this study, E. faecalis was used because it is the most resistant to majority of the intracanal disinfecting agents and one of the most prevalent species in root canals of the teeth with posttreatment disease.[11,12] The only limitation of calcium hydroxide sealer is the inability to eradicate E. faecalis biofilms.[4] The two strains were used in this study and were shown to form biofilms in all experiments. E. faecalis strain ATCC 29212 is isolated from the urine and has been commonly used in most of the endodontic in vitro studies, whereas the other strain OG1RF is an endodontic isolate from a root canal-treated tooth with persistent disease and forms more resistant or aggressive biofilm compared to the ATCC 29212.[1,13,14] Moreover, different isolates of E. faecalis diverge in their abilities to form biofilms, and this may help explain the present results.
There are only very limited number of studies assessing the antibiofilm efficacy of endodontic sealers because it is very difficult to adapt the available methods to do the same.[15] One of the studies evaluated the activity of seven different sealers against E. faecalis biofilms formed on bovine dentine to simulate clinical conditions, whereas three studies utilized direct contact and membrane-restricted antibiofilm assays.[6,15,16,17] Another study used CLSM to evaluate the antibiofilm efficacy and bacterial viability.[1] In the present study, antibiofilm activity was evaluated against monospecies biofilms of E. faecalis strains after 24 h by crystal violet assay and after 1 week by CLSM.
Although calcium hydroxide-based sealers have a good antimicrobial activity,[18,19] its effect on E. faecalis is limited because of its ability to tolerate high pH due to its efficient proton-pump system.[20,21] Hence, in the present study, an attempt is made by modifying calcium hydroxide-based sealer (Apexit Plus) to improve its antimicrobial property.
Various studies showed that addition of nanoparticles into the root canal sealers improves its antibiofilm activity.[1,22,23] Nanoparticles are a natural, incidental, or manufactured material containing particles in an unbound state or as an aggregate and agglomerate whose external dimensions are in the range of 1–100 nm. The antibacterial nanoparticles have greater surface area and charge density, which enable them to achieve a higher degree of interaction with the negatively charged surface of bacterial cells and have the ability to disrupt the extracellular polymerase matrix. Nanoparticles have a broad spectrum of antimicrobial activity and have lesser chance to induce microbial resistance when compared to antibiotics.[1] CS-NP is derived from chitin. It is one of the most abundant biopolymers in nature and can be found in the exoskeletons of crustaceans. CS-NPs are biocompatible and positively charged, so they interact with negatively charged bacterial cell wall causing disruption of the cell wall leading to leakage of intracellular components and cell death.[24,25,26,27,28] ZNO-NPs are metallic and they are effective in inhibiting Gram-positive, Gram-negative bacteria and highly resistant bacterial spores due to the generation of free radicals. ZNO-NPs are known to cause membrane damage as a result of lipid peroxidation by the reactive oxygen species (ROS) such as superoxide (O2) and hydroxyl radicals (OH). The antibacterial activity depends on the surface area and concentration; the higher the concentration and the surface area, better is the antibacterial activity of the ZnO-NP.[29]
Confocal laser scanning microscopy and crystal violet assay are used to analyze the antibiofilm efficacy of nanoparticle-incorporated calcium hydroxide sealers. CLSM is one of the preferred tools for biofilm analysis technique that is restricted till 200-μm-thick biofilm structures. CLSM creates a thin plane of focus in which out-of-focus light will be blocked, either conventionally by optical barriers or by applying the physics of light absorption. These optical sections can then be stacked by software to generate a 3D-reconstructed image of the entire biofilm. The CLSM images can be used to determine the thickness and distribution of cells in a biofilm structure.[7,30] It uses a fluorescent dye combination of FDA and PI. FDA is taken up by viable bacterial cells which convert the nonfluorescent FDA into the green fluorescent metabolite fluoresce which serves as an indicator for viable bacterial cells. In contrast, the nuclei staining red dye PI cannot pass through a viable bacterial cell membrane. It reaches the nucleus by passing through disordered areas of dead cell membranes and reacts with the DNA of the cell. Hence, the dead cells are seen in red color. Hence, it determines the viability profile, architecture, and spatial distribution of cells in the biofilm.[7] Crystal violet assay is widely used method to study the bacterial biofilm. It is a colorimetric assay technique, which is based on the dye uptake by the bacterial cells in the biofilm. The dye uptake is measured using the ELISA reader. This is an easy technique which allows for the rapid quantification of the biofilm bacteria.[7] Despite the high reproducibility and rapid quantification of biofilm reduction, the semi-quantitative method stains both viable and dead cells as well as the biofilm matrix. Consequently, it cannot be used specifically to evaluate bacterial viability.
Hence, the present study showed that addition of nanoparticles (ZnO and CS) has improved the antibiofilm efficacy of calcium hydroxide-based sealer (Apexit Plus) against E. faecalis strain ATCC 29212 in both crystal violet assay and CLSM study. ZNO-NP-incorporated sealer showed the maximum reduction of OD and biofilm thickness when compared to other groups against E. faecalis ATCC 29212. This is in accordance with the study done by Shreshtha et al. in 2010, who proved that ZNO-NP had greater antibiofilm efficiency compared to the CS-NP.[1] Kishen et al. in 2008 checked the antibacterial property of CS-NP and ZNO-NP-incorporated zinc oxide eugenol-based sealer, and they concluded that ZNO-NP incorporated in the sealer had greater antibacterial activity than CS-NP.[15] However, when E. faecalis strain OG1RF was considered, only ZNO-NP-incorporated sealer showed slight reduction in OD in crystal violet assay. CLSM study showed minimal or no improvement in antibiofilm efficacy in case of nanoparticles (ZnO and CS) incorporated sealer.
However, in this study, CS-NP incorporated in calcium hydroxide-based sealers had no significant reduction in biofilm compared to the positive control, especially in case of E. faecalis strain OG1RF. This could be due to higher pH of the calcium hydroxide-based sealer. Kong et al. in 2010 showed that chitosan had stronger inhibitory effect at low pH and the antibacterial activity is reduced with increase in the pH.[31]
When the two strains are compared, Apexit Plus incorporated with ZNO-NP showed better reduction of OD in E. faecalis strain ATCC 29212 compared to E. faecalis strain OG1RF. Apexit Plus incorporated with CS-NP also showed better reduction of OD in E. faecalis strain ATCC 29212 compared to the strain OG1RF.
Conclusion
Addition of ZNO-NP has effectively increased the antibiofilm efficacy of calcium hydroxide-based sealer against E. faecalis strain ATCC 29212 and OG1RF, whereas CS-NP showed effective antibiofilm activity only against E. faecalis strain ATCC 29212. If having antibiofilm effects is considered relevant for endodontic sealers, improvements in this ability are required and should be a target for future research efforts.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shrestha A, Shi Z, Neoh KG, Kishen A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J Endod. 2010;36:1030–5. doi: 10.1016/j.joen.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Friedman S, Mor C. The success of endodontic therapy – Healing and functionality. J Calif Dent Assoc. 2004;32:493–503. [PubMed] [Google Scholar]
- 3.Nair PN. On the causes of persistent apical periodontitis: A review. Int Endod J. 2006;39:249–81. doi: 10.1111/j.1365-2591.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 4.Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35:221–8. doi: 10.1046/j.1365-2591.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 5.Pinheiro ET, Gomes BP, Ferraz CC, Sousa EL, Teixeira FB, Souza-Filho FJ, et al. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J. 2003;36:1–1. doi: 10.1046/j.1365-2591.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- 6.Barros J, Silva MG, Rôças IN, Gonçalves LS, Alves FF, Lopes MA, et al. Antibiofilm effects of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. J Endod. 2014;40:1167–71. doi: 10.1016/j.joen.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Kishen A, Haapasalo M. Biofilm models and methods of biofilm assessment. Endod Topics. 2012;22:58–78. [Google Scholar]
- 8.Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res. 2004;339:2693–700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Hasnidawani JN, Azlina HN, Norita H, Bonnia NN, Ratim S, Ali ES. Synthesis of ZnO nanostructures using Sol-Gel method. Procedia Chem. 2016;19:211–6. [Google Scholar]
- 10.Donlan RM, Costerton JW. Biofilms: Survival mechanism of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundqvist G, Figdor D, Persson S, Sjögren U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:86–93. doi: 10.1016/s1079-2104(98)90404-8. [DOI] [PubMed] [Google Scholar]
- 12.Rôças IN, Siqueira JF., Jr Characterization of microbiota of root canal-treated teeth with posttreatment disease. J Clin Microbiol. 2012;50:1721–4. doi: 10.1128/JCM.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray BE, Singh KV, Ross RP, Heath JD, Dunny GM, Weinstock GM, et al. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J Bacteriol. 1993;175:5216–23. doi: 10.1128/jb.175.16.5216-5223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishen A, George S, Kumar R. Enterococcus faecalis-mediated biomineralized biofilm formation on root canal dentine in vitro . J Biomed Mater Res A. 2006;77:406–15. doi: 10.1002/jbm.a.30622. [DOI] [PubMed] [Google Scholar]
- 15.Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod. 2008;34:1515–20. doi: 10.1016/j.joen.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Faria-Júnior NB, Tanomaru-Filho M, Berbert FL, Guerreiro-Tanomaru JM. Antibiofilm activity, pH and solubility of endodontic sealers. Int Endod J. 2013;46:755–62. doi: 10.1111/iej.12055. [DOI] [PubMed] [Google Scholar]
- 17.Kayaoglu G, Erten H, Alaçam T, Ørstavik D. Short-term antibacterial activity of root canal sealers towards Enterococcus faecalis. Int Endod J. 2005;38:483–8. doi: 10.1111/j.1365-2591.2005.00981.x. [DOI] [PubMed] [Google Scholar]
- 18.Mickel AK, Nguyen TH, Chogle S. Antimicrobial activity of endodontic sealers on Enterococcus faecalis. J Endod. 2003;29:257–8. doi: 10.1097/00004770-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Reyhani MF, Ghasemi N, Eskandarinejad M, Milani AS, Mokhtari H, Tootunchian K. Antimicrobial effects of Apexit Plus, Epiphany, MTA Fillapex and Dorifill sealers on Enterococcus faecalis at different time intervals. IJSRIT. 2015;2:10–5. [Google Scholar]
- 20.Upadya M, Shrestha A, Kishen A. Role of efflux pump inhibitors on the antibiofilm efficacy of calcium hydroxide, chitosan nanoparticles, and light-activated disinfection. J Endod. 2011;37:1422–6. doi: 10.1016/j.joen.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Love RM. Enterococcus faecalis – A mechanism for its role in endodontic failure. Int Endod J. 2001;34:399–405. doi: 10.1046/j.1365-2591.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 22.Abramovitz I, Beyth N, Weinberg G, Borenstein A, Polak D, Kerler-Shvero D, et al. In vitro biocompatibility of endodontic sealers incorporating antibacterial nanoparticles. J Nanomater. 2012;2012:1–9. [Google Scholar]
- 23.Kesler Shvero D, Abramovitz I, Zaltsman N, Perez Davidi M, Weiss EI, Beyth N, et al. Towards antibacterial endodontic sealers using quaternary ammonium nanoparticles. Int Endod J. 2013;46:747–54. doi: 10.1111/iej.12054. [DOI] [PubMed] [Google Scholar]
- 24.Jung BO, Kim CH, Choi KS, Lee YM, Kim JJ. Preparation of amphiphilic Chitosan and their antimicrobial activities. J Appl Polym Sci. 1999;72:1713–9. [Google Scholar]
- 25.Liu XF, Guan Y L, Yang D.Z, Li Z, Yao K D. Antibacterial action of Chitosan and Carboxymethylated Chitosan. J Appl Polym Sci. 2001;79:1324–35. [Google Scholar]
- 26.Muzzarelli R, Tarsi R, Filippini O, Giovanetti E, Biagini G, Varaldo PE, et al. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob Agents Chemother. 1990;34:2019–23. doi: 10.1128/aac.34.10.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Z, Neoh KG, Kang ET, Wang W. Antibacterial and mechanical properties of bone cement impregnated with Chitosan nanoparticles. Biomaterials. 2006;27:2440–9. doi: 10.1016/j.biomaterials.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 28.Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules. 2003;4:1457–65. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 29.Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18:6679–86. [Google Scholar]
- 30.Zapata RO, Bramante CM, de Moraes IG, Bernardineli N, Gasparoto TH, Graeff MS, et al. Confocal laser scanning microscopy is appropriate to detect viability of Enterococcus faecalis in infected dentin. J Endod. 2008;34:1198–201. doi: 10.1016/j.joen.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int J Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]


