Abstract
Background:
The chemomechanical plaque control measures are helpful in controlling dental plaque and thus caries, especially in pediatric age group.
Aim:
This study aims to compare effectiveness of herbal mouthrinse containing Terminalia chebula to that of 0.2% chlorhexidine against children's salivary mutans streptococci levels.
Settings and Design:
A double-blind, randomized, controlled study design will be framed for conducting this study.
Methods:
A total of 45 participants were randomly categorized in Group 1, Group 2, or Group 3 (control group, experimental group, or negative control). Baseline unstimulated saliva was collected. All the participants were instructed regarding the use of mouthrinse for 2 weeks. After 2 weeks, again unstimulated saliva was collected. After collection, saliva samples were sent for microbiological analysis.
Statistical Analysis:
The mean colony-forming units (CFU/ml) were determined. Paired t-test, ANOVA test, and post hoc test were applied for statistical analysis.
Results:
Statistically significant difference in CFU count has been observed in 0.2% chlorhexidine and Oratreat groups at 15 days as compared to baseline (P < 0.001). At 15 days, reduction in CFU count has seen more in Oratreat group as compared to 0.2% chlorhexidine group, and the difference is statistically significant (P < 0.001).
Conclusion:
0.2% chlorhexidine and Oratreat mouthwash reduce the salivary Streptococcus mutans count. Oratreat herbal mouthwash has proved to be better as compared to 0.2% chlorhexidine mouthwash.
Keywords: Chlorhexidine, dental caries, herbal, mouthwash, Terminalia chebula
Introduction
Modern concepts consider caries as an interaction between genetic and environmental factors in which social, behavioral, psychological, and biological factors are expressed in a highly complex interactive manner. The removal of plaque is utmost important to control dental caries that is commonly maintained by mechanical methods. However, in children, factors such as lack of dexterity and individual motivation and monitoring limit the effectiveness of toothbrushing. Children also experience difficulty in maintaining adequate plaque control, particularly at interproximal sites, which necessitates the use of chemotherapeutic agents for control of plaque.[1]
Colonization of tooth surfaces by bacteria is an important etiological factor in the most common oral diseases – dental caries, gingivitis, and destructive periodontal diseases. The literature is replete with studies establishing Streptococcus mutans as a major player in the formation of pit and fissure caries in the primary, mixed, and permanent dentition and that the amount of S. mutans in the saliva is related to the number of colonized surfaces. Therefore, decreasing the concentration of S. mutans in the oral cavity would have a great benefit with respect to decreasing the incidence of dental caries.[2]
Among the chemotherapeutic agents used in mouthwashes, chlorhexidine is the “gold standard” or positive control for comparison with other substances due to its proven efficiency.[1] Chlorhexidine (0.2%) mouthrinse has also shown antibacterial efficacy. Rindom, Briner, and Loe found a reduction of 30%–50% in the population of S. mutans[3] after rinsing with 10 ml of 0.2% chlorhexidine mouthrinse once daily. Although effective, 0.2% chlorhexidine has certain side effects such as brown discoloration of the teeth, oral mucosal erosion, and bitter taste. Hence, there is need of an alternative mouthrinse that could negate all the side effects of chlorhexidine but yet effective equivalent to it.[1]
Various herbal extracts such as Terminalia chebula, Acacia catechu, Glycyrrhiza glabra root, Foeniculum vulgare fruit, and Piper cubeba are known to provide therapeutic benefits in the oral cavity. Nayak et al. showed that Terminalia has effect on reduction of S. mutans count in saliva.[4] Sasaki et al. stated that G. glabra root inhibits periodontitis.[5] According to Gazi, Acacia has an antiplaque feature.[6] Aneja et al. showed antimicrobial effect against selected bacterial and other fungal pathogens of P. cubeba.[7]
The concentration of mutans streptococci inhibition in the oral cavity would have great benefit in reducing the incidence of pit and fissure caries. The second window of infectivity of mutans streptococci is observed in the mixed dentition age group (6–12 years).[8] Thus, keeping in mind the potential antimicrobial effects of T. chebula and other herbal products, the need of the present study is to compare and evaluate the effects of herbal mouthrinse containing the above-mentioned ingredients (Oratreat, Cadila GA/540-A) on salivary mutans streptococci levels in children. The aim of the study is to evaluate the effect of herbal and 0.2% chlorhexidine mouthrinses on S. mutans in children aged 7–8 years.
The null hypothesis was set for this study as no difference will be observed in the antimicrobial efficacy of herbal (Oratreat) and chlorhexidine mouthrinse on salivary S. mutans.
Methods
A double-blind, randomized, controlled study design was framed for conducting this study. Ethical approval was taken from the university ethical committee for the study (SVIEC/ON/DENT/SRP/15098). Prior written permission and informed consent were obtained from the participant's parents.
Minimum sample size required for the statistically significant result was determined using formula – N = 2 × (Zalfa/2 = Z1− β)2/(m1 − m2/σ)2. As per the formula, the sample size of 15 participants per group was determined.
Children between the age groups of 7 and 8 years, children with at least one restored decayed and/or missing tooth (decayed, missing, and filled teeth [DMFT]/dmft ≥1), who are not taking any orthodontic treatment, and whose parents had given written informed consent for study were included in the study. Children with mild-to-severe gingival inflammation, who requires special health care and presence of any other systemic medical conditions, were excluded from the study.
A total of 98 participants who reported to the Department of Paedodontics and Preventive Dentistry were examined out of which 45 participants were selected for the study based on selection criteria [Figure 1]. All the participants were thoroughly examined by principal investigator on their first visit. Complete oral prophylaxis was carried out for all the participants using sterile hand instruments. After prophylaxis, oral hygiene instructions were given to all the participants. Modified bass technique was demonstrated, and each participant was provided toothbrush and toothpaste on the first visit for use. After oral prophylaxis, unstimulated saliva was collected in a sterile disposable container for the baseline data.
Figure 1.
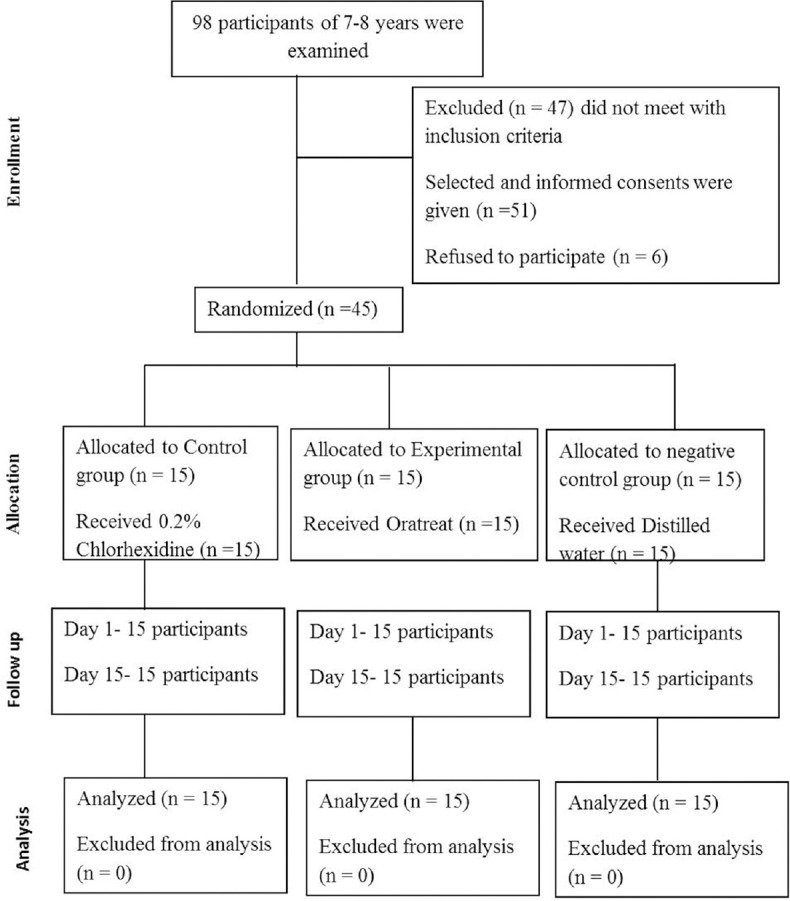
The CONSORT diagram showing the flow of participants through each stage of a randomized controlled study
All 45 participants were categorized as Group 1, Group 2, or Group 3 based on the randomization sheet which has been prepared in advance. The label for each mouthrinse group as 1, 2, or 3 was done by co-investigator. List of blinded groups was sealed in an envelope which had been opened only after completion of the study. Based on the grouping, the person who has labeled the groups dispensed the material accordingly. Participants and principal investigator did not know in which group they belong negative control, control group, or experimental group.
Group 1 – Control group: Conventional 0.2% chlorhexidine (HAA, Cadila S-MNB/09/41)
Group 2 – Experimental group: Herbal mouthwash (Oratreat, Cadila GA/540-A)
Group 3 – Negative control group: Distilled water.
All the participants were instructed regarding the use of mouthrinse (undiluted 2.5 ml mouthrinse for 1 min twice a day) for 2 weeks. After 2 weeks, again unstimulated saliva was collected in a sterile disposable container. Thus, a total of two saliva samples were taken from each individual.
After containing saliva both on the 1st day and after 15 days, all samples had been sent for microbiological analysis. Each saliva sample was vortexed vigorously for 30 s to ensure a representative mixture throughout the sample before plating. The mitis salivarius bacitracin agar (MSBA, HiMedia) media was used in this study for culturing salivary S. mutans. The plates were then incubated at 37°C for 48 h under 5%–10% CO2. To avoid bias, all plates were processed and examined by the principal investigator. The colony count of each plate was recorded, and the mean colony-forming units (CFU/ml) were determined. Paired t-test, ANOVA test, and post hoc tests had been applied for statistical analysis.
Results
This study was conducted as a double-blinded, randomized, controlled study. A total of 45 participants met the inclusion criteria in this study, and all the participants were equally divided into three groups. Table 1 shows the distribution of samples in both the groups at baseline and at day 15. No dropout had occurred in any groups in this study.
Table 1.
Distribution of samples

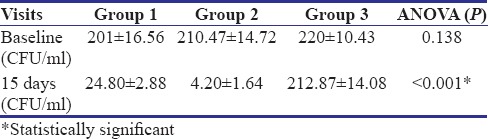
Table 2 shows a comparison of CFU count per ml of all three groups at baseline and after 15 days. Baseline CFU scores are 201, 210.47, and 220 CFU/ml for Group 1 (control group), Group 2 (experimental group), and Group 3 (negative control group), respectively. On 15 days follow-up visit, the count was reduced to 24.80, 4.20, and 212.87 CFU/ml, respectively. Statistically significant reduction in CFU count has been observed in Group 1 and Group 2 at 15 days as compared to that of baseline (P < 0.001).
Table 2.
Intragroup comparison of colony-forming unit count of three groups
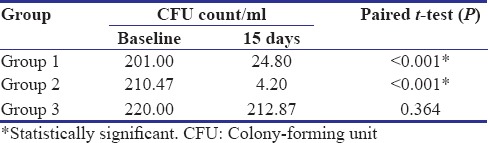
Table 3 shows intergroup comparison of CFU/ml count of all three groups at baseline and at 15 days. At baseline, the intergroup comparison shows a statistically insignificant difference in CFU count of all three groups. At 15-day follow-up, statistically significant difference is seen between Group 1 and Group 2 (P = 0.002), between Group 1 and Group 3 (P < 0.001), and also between Group 2 and Group 3 (P < 0.001). Figure 2 shows colonies of salivary S. mutans at baseline and after 15 days of all 3 groups.
Table 3.
Intergroup comparison of colony-forming unit count of three groups at baseline and 15 days
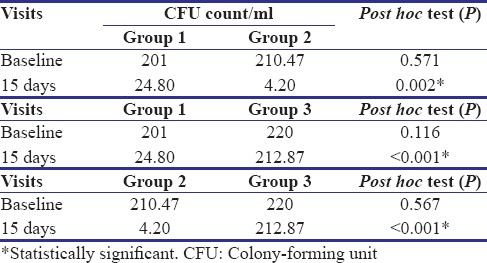
Figure 2.
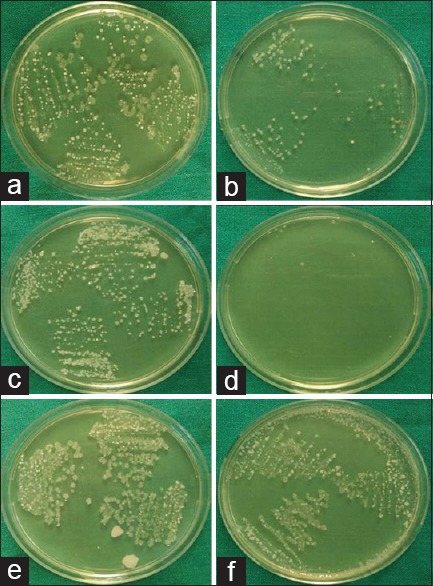
Colonies of Streptococcus mutans of Group 1%–0.2% chlorhexidine group (control group) at baseline (a) and after 15 days (b) colonies of Streptococcus mutans of Group 2 – Oratreat herbal mouthwash group (experimental group) at baseline (c) and after 15 days (d) colonies of Streptococcus mutans of Group 3 – distilled water group (negative control) at baseline (e) and after 15 days (f)
Table 4 shows intergroup comparison of CFU count of all three groups using ANOVA test. At baseline, the values are 201 ± 16.56, 210.47 ± 14.72, and 220 ± 10.43 CFU/ml of Group 1, Group 2, and Group 3, respectively, and the difference is statistically insignificant (P = 0.138). On the 15th day, the colony count is 24.80 ± 2.88, 4.20 ± 1.64, and 212.87 ± 14.08 CFU/ml for Group 1, Group 2, and Group 3, respectively, with statistically significant difference (P < 0.001).
Table 4.
Intergroup comparison of colony-forming units/ml with ANOVA test
Discussion
Many children have inadequate oral and general health because of active and uncontrolled dental caries. It is the single most common chronic childhood disease. Owing to its nonlife threatening nature and ubiquitousness, its significance in overall human health has minimized. Initiation of dental caries and the microbial composition of plaque have generally involved either S. mutans or Lactobacilli. Children with high dmft have increased S. mutans count. As a result, variety of antiplaque agents has been examined for their ability to control S. mutans.[1]
The surface of the oral cavity is constantly colonized by microorganisms. One milliliter of whole saliva may contain more than 200 million organism representing more than 250 different species. Streptococcus constitutes an essential part of the microflora which constantly colonizes the mucous membrane and the teeth. The streptococci in the oral cavity comprise Streptococcus sanguis, Streptococcus mitis, Streptococcus salivarius, Streptococcus intermedius, and other streptococci of which mutans streptococci, especially Streptococcus mutans and Streptococcus sobrinus, are maximum.[3]
All the children who had enrolled in this study were between 7 and 8 years of age, which falls under mixed dentition age group. Age is a critical factor in subject selection for many reasons, of which the most important is the number of tooth surfaces at risk. Since the intervention in the present study is a mouthrinse, younger children might face difficulties to use it.[9] Participants with age of 7–8 years were chosen because they were entering a period of high caries activity, with permanent teeth erupting in the oral cavity.[10]
Unstimulated saliva was used for the microbial analysis in the present study as it has been reported by Rupesh et al. that the unstimulated saliva represents the basal salivary flow rate.[2] Sterile disposable containers were used for collection of unstimulated saliva for microbial analysis in this study.
In contrast to most other bacteria, mutans streptococci can grow in an environment with a high sucrose concentration and are resistant to a particular antibiotic, bacitracin, the most commonly used selective medium that is MSBA. Hence, in the present study, selective media MSBA was used for incubation of salivary S. mutans.[11] The technique which was adopted in this study for agar plating and colony counting was similar to that suggested by Wan et al.[12] The similar technique was also used by Rupesh et al. for culturing S. mutans in their study.[2]
This study was designed to simulate a realistic home regimen in which the participants rinsed for 60 s twice daily while continuing their normal twice-daily toothbrushing routine. In this context, it is noteworthy that the reductions in salivary mutans streptococci in this study occurred in addition to the effects of daily toothbrushing.
The results of the present study showed that there is a definite decrease in the salivary mutans streptococci levels with both the herbal and chlorhexidine mouthwash even within 15 days of regular practice of the adjunctive oral hygiene measures.
The results of Group 1, i.e., 0.2% chlorhexidine mouthwash showed statistically highly significant reduction of salivary S. mutans count after 15 days (24.80 ± 2.88 CFU/ml) as compared to that of baseline level (201 ± 16.56 CFU/ml). In the previous studies done by Sekino et al. evaluated that daily use of chlorhexidine mouthrinse as an adjunct to careful mechanical tooth cleaning reduces the number of microorganisms that could be detected in saliva sample.[13] Schiott et al. evaluated that the number of S. mutans present in saliva decreased significantly by treatment with chlorhexidine.[14]
The high efficacy of chlorhexidine could be due to its immediate bactericidal action during the time of application followed by a prolonged bacteriostatic action due to adsorption at the tooth surface.[15] The adsorbed chlorhexidine is gradually released for up to 24 h.[16]
The herbal mouthwash Oratreat contains A. catechu, A. catechu bark, T. chebula, G. glabra root, P. cubeba, F. vulgare fruit, Ficus benghalensis bark, Ficus religiosa, Ficus glomerata bark, Quercus infectoria, Symplocos racemosa, and Elettaria cardamomum, and each of this ingredient has antimicrobial efficacy for some or the other oral pathogen. Various studies had proven the individual efficacy of above-mentioned ingredient, but none of the studies have compared the combined effect of these all ingredient.[4,5,6,7] Hence, the herbal mouthwash Oratreat which contains all above-mentioned ingredients was chosen as an experimental group in this study.
One of the ingredients of Oratreat is T. chebula. It has been reported that T. chebula exerts a range of therapeutic activities including antibacterial, antiviral, antifungal, and antioxidant activities.[17] Jagtap and Karkera reported inhibition of growth of S. mutans 90 min postrinsing with T. chebula.[18] T. chebula contains 30%–40% tannins which are hydrolyzable type including chebulic acid, chebulagic acid, corilagin, and gallic acid. It also contains fructose, succinic acid, and amino acid. Tannins are believed to be majorly responsible for the antibacterial action.[9] These tannins might have been released into saliva as its concentration decreased over a period of time exerting prolonged action and has advantages such as cost-effectiveness and good safety margin.[4]
In this study, Group 2, i.e., herbal mouthwash Oratreat has also shown statistically highly significant reduction in the salivary S. mutans count at 15 days (4.20 ± 1.64 CFU/ml) as compared to that of baseline (210.47 ± 14.72 CFU/ml). A similar result has also been shown in the study done by Mehta et al. where the test herbal mouthwash had better antimicrobial effect on salivary S. mutans than chlorhexidine.[19]
Group 3 where distilled water has been used as negative control showed no difference in the salivary streptococcus level at baseline (220 ± 10.43 CFU/ml) to that of 15 days (212.87 ± 14.08 CFU/ml), which proves that normal saline has no effect against salivary S. mutans count and it acts as a negative control.
Intergroup comparison was done with post hoc and ANOVA tests. Intergroup comparisons revealed that there were no significant differences in the salivary mutans streptococci levels between the groups at baseline. This implies that the groups were statistically equivalent before the start of treatment.
Statistically significant difference was observed in salivary S. mutans count at 15 day between Group 1 and Group 2 (P = 0.002). This shows that the antibacterial efficacy of herbal mouthwash Oratreat, which contains T. chebula, is better than that of 0.2% chlorhexidine mouthwash. A similar result has also been shown in the study done by Nayak et al. where the test herbal mouthwash had a better antimicrobial effect on salivary S. mutans than 0.2% chlorhexidine.[9]
However, Lakade et al. in their study stated that 0.2% chlorhexidine showed a greater reduction of mutans streptococcus count than combination mouthrinse.[1] Similarly, Sharma et al. found that 0.2% chlorhexidine was most effective than combination mouthwash containing 0.03% triclosan and 0.05% sodium fluoride.[20]
Both the 0.2% chlorhexidine and Oratreat herbal mouthwashes have shown statistically highly significant difference in their efficacy in reducing salivary S. mutans when compared to negative control group, i.e., distilled water (P < 0.001). This shows that both the control and experimental mouthwashes are highly efficient against salivary S. mutans.
Both the mouthrinses were well accepted by the participants of the study; none of the side effects like irritation, taste alteration and staining of teeth or soft tissues was reported by participants; the rinses appear safe for long-term oral use and are worthy of further study for potential applications in the practice of pediatric and preventive dentistry. However, community-based research should be done to check the efficacy of Oratreat mouthwash and cross-over study design should be done to evaluate the efficacy of Oratreat mouthwash against salivary S. mutans.
Conclusion
The present study concludes that herbal mouthwash Oratreat has superior antimicrobial efficacy showing no side effects when compared to 0.2% chlorhexidine mouthwash against salivary S. mutans in children of 7–8 years' age group, vulnerable to dental caries due to the second window of infectivity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lakade LS, Shah P, Shirol D. Comparison of antimicrobial efficacy of chlorhexidine and combination mouth rinse in reducing the mutans streptococcus count in plaque. J Indian Soc Pedod Prev Dent. 2014;32:91–6. doi: 10.4103/0970-4388.130780. [DOI] [PubMed] [Google Scholar]
- 2.Rupesh S, Winnier JJ, Nayak UA, Rao AP, Reddy NV. Comparative evaluation of the effects of an alum-containing mouthrinse and a saturated saline rinse on the salivary levels of Streptococcus mutans. J Indian Soc Pedod Prev Dent. 2010;28:138–44. doi: 10.4103/0970-4388.73780. [DOI] [PubMed] [Google Scholar]
- 3.Gupta N, Bhat M. Comparative evaluation of 0.2 percent chlorhexidine and magnetized water as a mouth rinse on Streptococcus mutans in children. Int J Clin Pediatr Dent. 2011;4:190–4. doi: 10.5005/jp-journals-10005-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nayak SS, Kumar BR, Ankola AV, Hebbal M. The efficacy of Terminalia chebula rinse on Streptococcus mutans count in saliva and its effect on salivary pH. Oral Health Prev Dent. 2010;8:55–8. [PubMed] [Google Scholar]
- 5.Sasaki H, Suzuki N, Alshwaimi E, Xu Y, Battaglino R, Morse L, et al. 18β-glycyrrhetinic acid inhibits periodontitis via glucocorticoid-independent nuclear factor-κB inactivation in interleukin-10-deficient mice. J Periodontal Res. 2010;45:757–63. doi: 10.1111/j.1600-0765.2010.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazi MI. The finding of antiplaque features in Acacia arabica type of chewing gum. J Clin Periodontol. 1991;18:75–7. doi: 10.1111/j.1600-051x.1991.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 7.Aneja KR, Joshi R, Sharma C, Aneja A. Antimicrobial efficacy of fruit extracts of two Piper species against selected bacterial and oral fungal pathogens. Braz J Oral Sci. 2010;9:421–6. [Google Scholar]
- 8.Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: Evidence for a discrete window of infectivity. J Dent Res. 1993;72:37–45. doi: 10.1177/00220345930720010501. [DOI] [PubMed] [Google Scholar]
- 9.Nayak SS, Ankola AV, Metgud SC, Bolmal U. Effectiveness of mouthrinse formulated from ethanol extract of Terminalia chebula fruit on salivary Streptococcus mutans among 12 to 15 year old school children of Belgaum city: A randomized field trial. J Indian Soc Pedod Prev Dent. 2012;30:231–6. doi: 10.4103/0970-4388.105016. [DOI] [PubMed] [Google Scholar]
- 10.Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65:1028–37. [PubMed] [Google Scholar]
- 11.Gold OG, Jordan HV, van Houte J. A selective medium for Streptococcus mutans. Arch Oral Microbiol. 1973;18:1357–64. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- 12.Wan AK, Seow WK, Walsh LJ, Bird PS. Comparison of five selective media for the growth and enumeration of Streptococcus mutans. Aust Dent J. 2002;47:21–6. doi: 10.1111/j.1834-7819.2002.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 13.Sekino S, Ramberg P, Uzel NG, Socransky S, Lindhe J. Effect of various chlorhexidine regimens on salivary bacteria and de novo plaque formation. J Clin Periodontol. 2003;30:919–25. doi: 10.1034/j.1600-051x.2003.00420.x. [DOI] [PubMed] [Google Scholar]
- 14.Schiott CR, Briner WW, Kirkland JJ, Löe H. Two years oral use of chlorhexidine in man. III. Changes in sensitivity of the salivary flora. J Periodontal Res. 1976;11:153–7. doi: 10.1111/j.1600-0765.1976.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins S, Addy M, Newcombe RG. Dose response of chlorhexidine against plaque and comparison with triclosan. J Clin Periodontol. 1994;21:250–5. doi: 10.1111/j.1600-051x.1994.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 16.Fardal O, Turnbull RS. A review of the literature on use of chlorhexidine in dentistry. J Am Dent Assoc. 1986;112:863–9. doi: 10.14219/jada.archive.1986.0118. [DOI] [PubMed] [Google Scholar]
- 17.Chattopadhyay RR, Bhattacharyya SK. Terminalia chebula: An update. Pharmacognosy Rev. 2007;1:1516. [Google Scholar]
- 18.Jagtap AG, Karkera SG. Potential of the aqueous extract of Terminalia chebula as an anticaries agent. J Ethnopharmacol. 1999;68:299–306. doi: 10.1016/s0378-8741(99)00058-6. [DOI] [PubMed] [Google Scholar]
- 19.Mehta S, Pesapathy S, Joseph M, Tiwari PK, Chawla S. Comparative evaluation of a herbal mouthwash (Freshol) with chlorhexidine on plaque accumulation, gingival inflammation, and salivary Streptococcus mutans growth. J Int Soc Prev Community Dent. 2013;3:25–8. doi: 10.4103/2231-0762.115717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma U, Jain RL, Pathak A. A clinical assessment of the effectiveness of mouthwashes in comparison to toothbrushing in children. J Indian Soc Pedod Prev Dent. 2004;22:38–44. [PubMed] [Google Scholar]


