Abstract
Background:
Over many years, numerous products have been suggested for the relief of dentin hypersensitivity (DH). Calcium sodium phosphosilicate is one desensitizing agent that has remineralizing potential. Available in toothpaste and mouthwash delivery vehicle, this study was carried out to compare the effectiveness on dentinal hypersensitivity (using the Airblast test and Cold test) and on tooth remineralization (using DIAGNOdent pen) in a 4-week period.
Materials and Methods:
Out of the 45 patients screened, 28 patients who fulfilled the inclusion criteria and who willingly signed the consent form were selected and were randomly allocated into two groups – toothpaste and mouthwash. The tooth numbers and specific site of dentinal hypersensitivity for every patient were noted and the air blast test, cold test, and DIAGNOdent scores were recorded at baseline visit. The patients were instructed about the use of the product and were asked to come on the 30th day for re-evaluation.
Results:
Within-group comparison showed a significant reduction (P < 0.05) in the air blast score (toothpaste – 68.53% and mouth rinse – 48.52%), cold test score (toothpaste – 56.38% and mouth rinse – 38.87%), and DIAGNOdent score (toothpaste – 20.35% and mouth rinse – 9.49%). In-between group comparison showed no statistically significant difference (P > 0.05).
Conclusion:
Desensitizing mouthwash is as effective as toothpaste in reducing DH with a fair remineralization potential comparable with that of the toothpaste.
Keywords: Calcium-sodium-phospho-silicate, DIAGNOdent, dentinal hypersensitivity, desensitizing mouthwash, desensitizing toothpaste, remineralization
Introduction
Dentine hypersensitivity (DH) is a transient and sharp dental pain condition resulting immediately on stimulation of exposed dentin and resolving on stimulus removal.[1] The worldwide prevalence of DH ranges between 4% and 52%.[2] This wide variability among papers is probably due to differences in the study populations and the methodology employed.[3] In regard to the pain pathogenic mechanism, several theories have been proposed. By far the most widely accepted theory is the hydronymic theory proposed by Brannstrom et al.[4,5] According to this theory, DH is caused by shifts in the fluid located inside the open dentin tubules. This movement would trigger pulp nerve fibers type Aα and C, causing pain symptoms to the patient. Regardless of the etiology of dentin exposure, one feature that appears to be in common is open dentinal tubules which provide a direct link between the external environment and the tooth pulp. Over many years, numerous products have been suggested for the relief of DH, working on treatment mechanisms to reduce the stimulus-induced fluid flow in the dentinal tubules and consequent nociceptor activation in the pulp/dentin border area.[6] In fact, various agents with remineralization potential are recommended in the long-lasting treatment of DH. Toothpaste is the most common vehicle for desensitizing agents. The current knowledge supports the use of dentifrices with arginine, strontium acetate, stannous fluoride, and calcium sodium phosphosilicate for the treatment of DH;[6] however, other active agents are still a subject of debate such as casein derivatives,[7,8] oxalates,[9] or potassium nitrate.[10,11] Few of these agents have also been evaluated as a mouthwash with favorable results, although the evidence supporting its efficacy is low and nonconclusive.
The choice of the desensitizing agent delivery vehicle should be in accordance with the Grossman criteria for an ideal desensitizing agent, i.e. with long-term effects, nonirritant to pulp, painless, and easy to apply and should not stain the tooth. This study was thus undertaken to scientifically validate the prescription of a desensitizing mouthwash over a desensitizing toothpaste and also to evaluate and compare the remineralizing potential of the two products using DIAGNOdent pen.
Materials and Methods
Study design
This study is a parallel, double-blinded, randomized clinical trial (the examiner and statistician were blinded) conducted in SDM College of Dental Sciences and Hospital (SDMCDSH), Dharwad. The ethical approval to carry out this study was obtained from the Institutional Review Board – SDMCDSH. Informed consent was taken from all the eligible subjects who agreed to participate in this study. The study was conducted over a period of 2 months from July to August 2017.
Sample size estimation
The sample size was calculated using G power 3.1.9.2 Software (Franz Faul, University Kiel, Germany) using data from a study conducted earlier.[12] The calculated sample size was 13 in each group. Keeping into account 20% regression rate, the sample size was extrapolated to 15 in each group (n = 30; NA = 15 and NB = 15).
p = Significance level at 5%.
β = 0.2 (at 80% power).
Inclusion/exclusion criteria
Adult subjects (age >18 years) with a chief complaint of tooth sensitivity were invited in this study. The subjects were screened to rule out any periodontal destruction, carious lesion, and gingival recession that could have led to tooth sensitivity. Further, only the subjects who exhibited an air blast score >1 and a DIAGNOdent pen score >12 (showing early demineralization) were selected for this study. It was made sure that the subjects were not using any desensitizing agent before the participation in this study. Subjects with a history of any systemic illness such as diabetes, hypertension, or any form of medications such as anti-inflammatory drugs, analgesics, or antibiotics were also excluded from this study. The subjects were enrolled in this study only after they willingly signed the informed consent form.
Methodology
All the subjects enrolled in this study underwent oral prophylaxis and were given the same brand toothpaste (Colgate cavity protection) and a soft-bristled toothbrush for the purpose of standardization. The subjects were asked to use the given toothpaste and toothbrush for a washout period of 7 days and return to the clinic for baseline evaluation of their dental health status.
Tactile, cold, and evaporative air stimuli are recommended as reliable tests for DH. Since DH may be different for different stimuli, it is recommended that at least two hydrodynamic stimuli should be used. The least severe stimulus should be applied first.[11] The interval between stimulus applications should be specified in the protocol and be of sufficient duration to minimize interactions between stimuli. In accordance with the aforementioned guideline, in the present study, the tests used to measure DH are air blast test and cold test. Air blast test was done 5 min before the cold test to minimize the interaction in between the stimuli. The site on the tooth surface from which the air blast test reading was measured was noted down. The same site was used to measure the subsequent cold test reading as well as the DIAGNOdent pen reading. In the follow-up visit also, same tooth and same site were measured for the two DH tests and tooth demineralized status test.
At the baseline visit, the subjects were first evaluated for air blast test, then cold test, and then the DIAGNOdent pen test. For each subject, a minimum of two teeth were scored for the aforementioned tests. The scores were obtained from the facial, cervical one-third of the tooth surface. The tooth number was noted for each subject to aid in the re-evaluation of the same tooth at the follow-up visit.
Air blast test
A blast of air was directed onto the cervical one-third area of the tooth for 1–2 s using a standard dental unit syringe from a distance of 10 mm, while the adjacent teeth were isolated using cotton rolls.
Cold test
Dry ice (used for chairside cold testing) sprayed on to a small cotton pellet was kept onto the tooth surface for 2–3 s.
The aforementioned stimuli tests were applied in the order stated above, with a minimum 5 min gap between the application of different stimuli. For all stimuli tests, subject response was recorded on the following scale:
0 – No significant discomfort or awareness of stimulus
1 – Discomfort but no severe pain
2 – Severe pain during application of stimulus
3 – Severe pain during and after application of stimulus.
For each individual, the cold test score and air blast test score was obtained by adding score for each tooth and then dividing by the total number of teeth examined.
DIAGNOdent pen test
The tooth surface was cleaned and dried. The tip of the Kavo DIAGNOdent pen was placed perpendicular to the long axis of the tooth at the cervical area of each tooth examined for the DH tests before. The reading on the pen was noted for each tooth. Summing up all the teeth demineralized scores as recorded by the DIAGNOdent pen per tooth and dividing by the total number of teeth examined, provided the demineralized status of the affected teeth for that subject.
For carrying out research on desensitizing agents, the study protocol, clinical measurements, data collection, and documentation should be in accordance with the FDA and European guidelines for good clinical practice.[11] According to the guidelines, the subjects should be randomized to the treatment groups using a recognized randomization process. The method of minimization[11] or one of its variants is used to randomly allocate the subjects into the various groups while ensuring the desired balance among the treatment groups. Likewise, in this study also, randomization was done following the principle of minimization.
Following the baseline examination for DH (using air blast test and cold test) and demineralized status of affected teeth (using DIAGNOdent pen scoring), the selected subjects were randomly assigned to one of the two groups:
Group A: Sensodent K-Plus toothpaste
Group B: Sensodent K-Plus toothpaste mouthwash.
These assignments were done by a person other than the chief investigator. The subjects were balanced into the above two groups based on their baseline visit air blast scores (primary outcome variable). The same person also gave the instructions regarding the usage of their respective products.
Instructions to patients in Group A
The patients were asked to apply a small amount of the allotted toothpaste on the affected site of the tooth surface. After 2 min (as per the manufacturer's instructions), the subjects were asked to brush their teeth normally using a pea-sized amount of the same toothpaste on the soft-bristled toothbrush using the modified bass technique. The patients were asked to practice this twice a day – in the morning after waking up and at night just before sleeping. At the baseline visit, the brushing technique was demonstrated to all the subjects.
Instructions to patients in Group B
The patients were asked to brush their teeth normally using a pea-sized amount of the given Colgate cavity protection toothpaste on the soft-bristled toothbrush using the modified Bass technique. After 30 min of brushing, the subjects were asked to take 10 mL of the allotted mouthwash and rinse their mouth thoroughly for 30 s (as per manufacturer's instructions). The patients were asked to practice this twice a day – in the morning after waking up and at night just before sleeping.
The subjects were recalled after 30 days usage of the assigned products and were evaluated by the same calibrated dental examiner for DH status (using air blast test and cold test) and demineralized status of the affected teeth (using DIAGNOdent pen).
Statistical analysis
The data were entered into the computer (MS-Office 2007, Excel data sheet). The data were subjected to statistical analysis using the statistical package (SPSS version 20.0). Statistical significance was recorded at P < 0.05. Shapiro–Wilk test was performed to test normality of the data. Nonparametric tests were used (Wilcoxon Signed-rank test and Mann–Whitney U-test) for within group and in-between group analysis.
Results
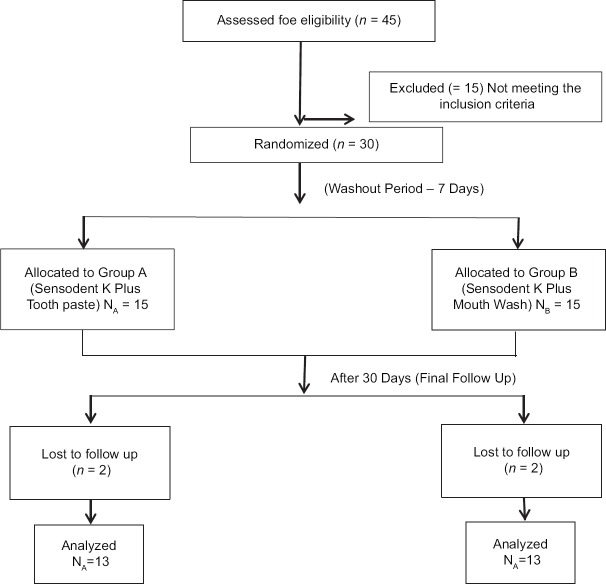
Out of the 45 subjects assessed for eligibility, thirty who fulfilled the inclusion criteria were randomly allocated to the two groups – 15 in Group A (Toothpaste) and 15 in Group B (Mouthwash). Two subjects were lost in follow-up in each group; thus, 13 in each group (total 26) were analyzed at the end of the study [Figure 1].
Figure 1.
Consort flow diagram showing the distribution of the study subjects through each stage of the trial
There was no statistically significant difference in mean age between the groups (34 and 32.33 years, respectively). At baseline, no significant differences were detected among the two groups with respect to mean air blast score, cold test score, and DIAGNOdent Pen Score.
Figures 2-4 shows the mean air blast test scores, mean cold test scores and mean DIAGNOdent Pen scores respectively in the two groups.
Figure 2.
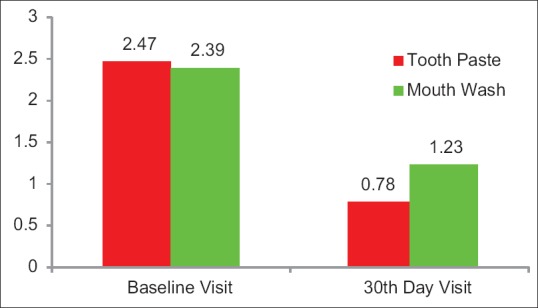
The mean air blast test scores at baseline visit in Group A and in Group B was 2.47 and 2.39 respectively. After 30 days use of the allotted product, the airblast test scores in Group A and Group B was 0.78 and 1.23 respectively
Figure 4.
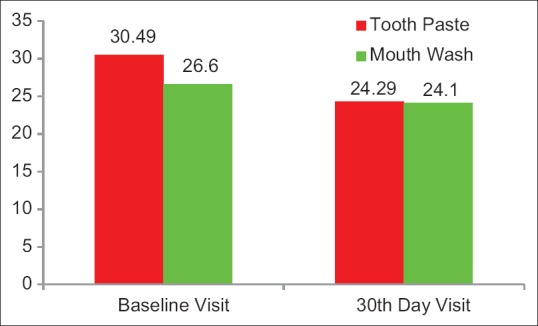
The mean DIAGNOdent Pen test scores at baseline visit in Group A and in Group B were 30.49 and 26.66, respectively. After 30 days use of the allotted product, DIAGNOdent pen test scores in Group A and Group B were 24.49 and 24.1, respectively
Figure 3.
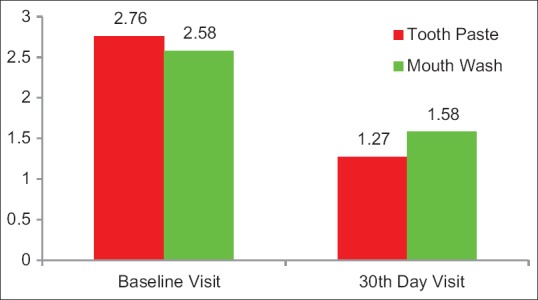
The mean cold test scores at baseline visit in Group A and in Group B was 2.76 and 2.58, respectively. After 30 days use of the allotted product, the cold test scores in Group A and Group B were 1.27 and 1.58, respectively
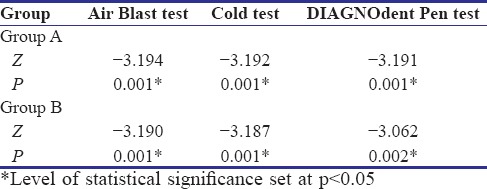
Wilcoxon Signed-rank test shows a highly statistically significant difference (P < 0.01) after a 30-day use of the allotted product in air blast test, cold test, and even the DIAGNOdent pen scores in Group A as well as in Group B [Table 1].
Table 1.
Wilcoxon signed-rank test for air blast test, cold test, and DIAGNOdent pen test in Group A and Group B
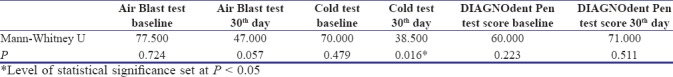
There was no statistically significant difference in between the two groups at baseline for air blast test, cold test, and DIAGNOdent pen scores. Mann–Whitney U-test showed a statistically significant difference only in the 30th-day cold test score [Table 2].
Table 2.
Mann-Whitney U-test for air blast test, cold test, and DIAGNOdent test at baseline visit and 30th-day visit in between Group A and Group B
Discussion
Dentinal hypersensitivity is a significant clinical problem. The effectiveness of a variety of available treatment agents for DH will help the clinician decide the best way to manage this oral pain condition of his/her patients. The desensitizing agent chosen in this study was calcium sodium phosphor silicate that is delivered in two vehicles – toothpaste and mouthwash. Calcium sodium phosphosilicate, originally developed as a regenerative bone material, has been shown to be effective at physically occluding dentinal tubules.[13,14] Clinical evaluations of calcium sodium phosphosilicate for the treatment of DH have recorded statistically significant and clinically positive results.[15,16] It has been shown that this innovative bioactive glass-containing technology occludes dentinal tubules and resists acid challenge.
The remineralizing potential of the desensitizing agent dispensed in the toothpaste form (Group A) and as a mouthwash (Group B) was tested using the DIAGNOdent pen – a laser fluorescence-based instrument introduced in 1998. Once the tip of the DIAGNOdent pen is contacted to the tooth surface, the laser energy penetrates the tooth surface and is absorbed by the surrounding tooth material, and fluorescence within the infra-red spectrum occurs. The emitted fluorescence is collected by the tip and is carried back to the photodiode detector, and the reading gets displayed as a nominal value of 0–99. The underlying principle is that the de-mineralized tooth structure emits a stronger fluorescence and thus is expressed as a higher number readout by the device.[17] In this study, the inclusion criteria stated a DIAGNOdent pen test value >12 as more than 12 signifies early de-mineralization according to the manufacturer's instructions.
There cannot be any direct comparisons of this study with the previous studies owing to different follow-up periods and different evaluation methods. In a study done by Acharya et al. using Sensodent K toothpaste, 48% reduction in visual analog scale scores over a 4-week period was noted and it was also found to be statistically significant.[18] A meta-analysis done on the effectiveness of desensitizing mouthwash concluded statistically significant reduction in sensitivity scores when DH was assessed by means of patients' self-reported pain experience.[2]
This double-blinded randomized clinical trial showed that there is a statistically significant reduction in DH in both the test groups (toothpaste and mouthwash) when used over a period of 30 days. Moreover, both the groups also showed a statistically significant remineralization potential over a period of 30 days. Future studies are recommended to evaluate the long-term efficacy of these products with longer follow up periods and a larger sample size.
Conclusion
In this study, no statistically significant difference was noted in the desensitizing and remineralizing potential of calcium sodium phosphor-silicate when delivered in toothpaste or on mouthwash form. Thus, the choice of which drug delivery vehicle should be prescribed to our patients will depend upon what is easier to use for the patient so that better patient compliance can be attained.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bartold PM. Dentinal hypersensitivity: A review. Aust Dent J. 2006;51:212–8. [PubMed] [Google Scholar]
- 2.Molina A, García-Gargallo M, Montero E, Tobías A, Sanz M, Martín C, et al. Clinical efficacy of desensitizing mouthwashes for the control of dentin hypersensitivity and root sensitivity: A systematic review and meta-analysis. Int J Dent Hyg. 2017;15:84–94. doi: 10.1111/idh.12250. [DOI] [PubMed] [Google Scholar]
- 3.Rees JS, Addy M. A cross-sectional study of dentine hypersensitivity. J Clin Periodontol. 2002;29:997–1003. doi: 10.1034/j.1600-051x.2002.291104.x. [DOI] [PubMed] [Google Scholar]
- 4.Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987;14:280–4. doi: 10.1111/j.1600-051x.1987.tb01533.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoshiyama M, Masada J, Uchida A, Ishida H. Scanning electron microscopic characterization of sensitive vs.insensitive human radicular dentin. J Dent Res. 1989;68:1498–502. doi: 10.1177/00220345890680110601. [DOI] [PubMed] [Google Scholar]
- 6.West NX, Seong J, Davies M. Management of dentine hypersensitivity: Efficacy of professionally and self-administered agents. J Clin Periodontol. 2015;42(Suppl 16):S256–302. doi: 10.1111/jcpe.12336. [DOI] [PubMed] [Google Scholar]
- 7.Azarpazhooh A, Limeback H. Clinical efficacy of casein derivatives: A systematic review of the literature. J Am Dent Assoc. 2008;139:915–24. doi: 10.14219/jada.archive.2008.0278. [DOI] [PubMed] [Google Scholar]
- 8.Talioti E, Hill R, Gillam DG. The efficacy of selected desensitizing OTC products: A Systematic review. ISRN Dent 2014. 2014 doi: 10.1155/2014/865761. 865761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunha-Cruz J, Stout JR, Heaton LJ, Wataha JC Northwest PRECEDENT. Dentin hypersensitivity and oxalates: A systematic review. J Dent Res. 2011;90:304–10. doi: 10.1177/0022034510389179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulsen S, Errboe M, Lescay Mevil Y, Glenny AM. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database Syst Rev. 2006;3:CD001476. doi: 10.1002/14651858.CD001476.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808–13. doi: 10.1111/j.1600-051x.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz K. A new treatment alternative for sensitive teeth: A desensitizing oral rinse. J Dent. 2013;41(Suppl 1):S1–11. doi: 10.1016/j.jdent.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Hench LL, Andersson O. Bioactive glasses. In: Hench LL, Wilson J, editors. Introduction to Bioceramics. Singapore: World Scientific; 1993. pp. 45–7. [Google Scholar]
- 14.Andersson OH, Kangasniemi I. Calcium phosphate formation at the surface of bioactive glass in vitro . J Biomed Mater Res. 1991;25:1019–30. doi: 10.1002/jbm.820250808. [DOI] [PubMed] [Google Scholar]
- 15.Pradeep AR, Sharma A. Comparison of clinical efficacy of a dentifrice containing calcium sodium phosphosilicate to a dentifrice containing potassium nitrate and to a placebo on dentinal hypersensitivity: A randomized clinical trial. J Periodontol. 2010;81:1167–73. doi: 10.1902/jop.2010.100056. [DOI] [PubMed] [Google Scholar]
- 16.Du Min Q, Bian Z, Jiang H, Greenspan DC, Burwell AK, Zhong J, et al. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (novamin) for the treatment of dentin hypersensitivity. Am J Dent. 2008;21:210–4. [PubMed] [Google Scholar]
- 17.Bamzahim MS. Evaluation of the DIAGNOdent method for detection and quantification of carious lesions: In vitro and in vivo studies. Institutionen för Odontologi/Department of Odontology. 2005 [Google Scholar]
- 18.Acharya AB, Surve SM, Thakur SL. A clinical study of the effect of calcium sodium phosphosilicate on dentin hypersensitivity. J Clin Exp Dent. 2013;5:e18–22. doi: 10.4317/jced.50955. [DOI] [PMC free article] [PubMed] [Google Scholar]