Abstract
Background:
The Institute of Medicine emphasizes care timeliness as an important quality metric. We assessed treatment timeliness in stage I-IIIA lung cancer patients deemed eligible for curative intent therapy and analyzed the relationship between time to treatment (TTT) and timely treatment (TT) with survival.
Methods:
We retrospectively reviewed consecutive cases of stage I-IIIA lung cancer deemed eligible for curative intent therapy at the VA San Diego Healthcare System between 10/2010–4/2017. We defined TTT as days from chest tumor board to treatment initiation and TT using guideline recommendations. We used multivariable (MVA) Cox proportional hazards regressions for survival analyses.
Results:
In 177 veterans, the median TTT was 35 days (29 days for chemoradiation, 36 for surgical resection, 42 for definitive radiation). TT occurred in 33% or 77% of patients when the most or least timely guideline recommendation was used, respectively. Patient characteristics associated with longer TTT included other cancer history, high simplified comorbidity score, stage I disease, and definitive radiation treatment. In MVA, TTT and TT [HR 0.53 (95% CI 0.27, 1.01) for least timely definition] were not associated with OS in stage I-IIIA patients, or disease-free survival in subgroup analyses of 122 stage I patients [HR 1.49 (0.62, 3.59) for least timely definition].
Conclusion:
Treatment was timely in 33–77% of veterans with lung cancer deemed eligible for curative intent therapy. TTT and TT were not associated with survival. The time interval between diagnosis and treatment may offer an opportunity to deliver or improve other cancer care.
Keywords: Time-to-treatment, Disease-free survival, Overall survival, Lung cancer, Curative intent treatment
1. Introduction
The goals for lung cancer therapy are to achieve a cure where possible and/or palliate and reduce symptom burden and improve quality of life, depending on the stage and patients’ fitness to tolerate therapy [1,2]. The theoretical harms of treatment delay in patients with early stage lung cancer include a potential for disease progression to more advanced stage where curative treatment is no longer possible; in those with advanced stage and high symptom burden, delays in treatment could unnecessarily lead to prolonged suffering [3]. As such, the Institute of Medicine (IOM) emphasizes timeliness of care as an important health care quality metric, with the goal to reduce waits and sometimes harmful delays for both those who receive and those who give care [4]. Clinical opinion-based guidelines by the American College of Chest Physicians (ACCP) [5], British Thoracic Society (BTS) [6], and the RAND Corporation [7] are available to emphasize and facilitate timely lung cancer care: the ACCP recommends that surgery should occur within 4–8 weeks of referral and radiation within 4 weeks where complex treatment planning is needed [5]; the BTS recommends delays of no more than 8 weeks for surgery, 7 weeks for radiotherapy, and 4 weeks for chemotherapy [6]; and the RAND Corporation within 6 weeks of the diagnosis date [7].
The impact of treatment timeliness on clinical outcomes is unclear based on available evidence [3]. To examine the relationship between time to treatment (TTT) and survival in lung cancer, several factors should be considered, including the target population (stage of disease and comorbidities), intent of treatment delivered (curative or palliative), and precise definitions of start time and outcomes. TTT is longer in patients with early stage disease [8] (partly due to the lack of symptoms) and therefore can be associated with better prognosis. In addition, timely treatment (TT) for advanced stage lung cancer has a lower probability of prolonging overall survival (OS); a more appropriate target population is those with early stage lung cancer who can be cured with treatment. The TTT should start at the time of diagnosis and complete staging since any delay that leads to disease progression/upstaging will not be recognized until appropriate clinical staging is performed, and survival time calculated from that point forward.
Up to 50% of patients with non-small cell lung cancer (NSCLC) are diagnosed with stage I-IIIA disease [9], the treatment of which is typically aimed at achieving a cure through a combination of lung cancer resection surgery, definitive radiation, or combined chemoradiation in eligible patients. In this project, we aim to more carefully examine the relationship between the diagnosis to treatment interval and survival in lung cancer, considering the factors as laid out above. We hypothesize that in patients with stage I-IIIA lung cancer eligible for curative intent therapy, TTT, including treatment delay as defined by clinical guidelines, is associated with worse OS. We also hypothesize that in a subgroup of patients with stage I lung cancer undergoing curative intent treatment, treatment delay is associated with worse disease-free survival (DFS) following treatment.
2. Methods
2.1. Clinical setting
The VA San Diego Healthcare System (VASDHS) Pulmonary Section evaluates most of the cases of suspected lung cancer in a diagnostic clinic. All cases are presented in a weekly multidisciplinary chest tumor board (CTB) consisting of medical and radiation oncologists, nurse-coordinators, pathologists, pulmonologists, radiologists, and thoracic surgeons to establish the diagnosis, stage, and treatment plans. Thereafter, a board-certified physician/pulmonologist who presented the patient to the CTB enters a note in the format of a history and physical examination in the medical record system and refers patients accordingly. Treatment by medical oncologists and thoracic surgeons is delivered within the VASDHS, and by radiation oncologists through outside referral. Patients treated with surgical resection or definitive radiation are typically followed by the VASDHS Pulmonary Section for surveillance; those with signs concerning for disease progression/recurrence are routinely represented to the CTB for additional treatment recommendations. To ensure quality and timely care, the Section keeps an ongoing list of cases of suspected lung cancer since October 2010. In addition, the VA healthcare system has an integrated medical record system and keeps accurate vital records for its patients. All these factors facilitated the reliability and completeness of the data used for this study.
2.2. Study design & patient selection
The VASDHS Institutional Review Board approved this study (Protocol #H170091). We retrospectively reviewed consecutive cases of lung cancer diagnosed and managed from 10/2010 to 4/2017 and included stage I-IIIA lung cancer patients deemed eligible by the CTB for curative intent therapy (i.e. lung cancer resection surgery, definitive radiation, or concurrent chemoradiation). We defined TTT (primary predictor) as time from CTB to initiation of treatment, and OS as the primary outcome. We also assessed DFS in a pre-specified subgroup analysis of stage I patients. We used the ACCP’s definition for disease recurrence/progression following treatment: having the same histology and systemic metastasis, same histology in different lobes and presence of N2/N3 involvement, or disease recurrence (including locoregional recurrence) as determined by the CTB after < 2-year intervals [10].
2.3. Confounders
We collected baseline clinical characteristics from CTB notes and included variables previously known to be associated with survival in lung cancer: age, sex, race/ethnicity, smoking status/tobacco exposure, performance status, history of lung cancer in parents or siblings, comorbidities, tumor size, clinical stage, histology, and treatment type. We used the simplified comorbidity score (SCS) to incorporate the effect of comorbidities and their respective weights on survival: tobacco consumption (7 points), diabetes mellitus (5), renal insufficiency (4), respiratory comorbidity (1), cardiovascular comorbidity (1), neoplastic comorbidity (1), and alcoholism (1); a SCS > 9 has been reported to be associated with worse survival in patients with stage I-IV NSCLC [11]. We also included lung function [forced expiratory volume in 1 s (FEV1), diffusion capacity of the lung for carbon monoxide (DLCO), and total lung capacity (TLC)] where available.
2.4. Statistical analyses
We summarized continuous variables as means and standard deviations or medians and ranges, and for categorical variables counts and percentages, and defined clinical stage using the 7th edition of the American Joint Committee on Cancer TNM staging system. We recorded and analyzed TTT as a continuous (days) and categorical (timely or not) variable. We defined TT using combined (ACCP, BTS, RAND Corporation) guideline recommendations [5–7]: most timely – for all treatment modalities, within 4 weeks (28 days) of CTB, and least timely – for surgical resection, within 8 weeks (56 days); radiation therapy – 7 weeks (49 days), or chemoradiation, within 6 weeks (42 days) of CTB.
We used the Kaplan-Meier method to estimate OS and DFS, log-rank test to compare the survival distributions between groups, and univariable (UVA) and multivariable (MVA) Cox proportional hazards regressions to examine the relationship between TTT or TT and survival. We used stepwise backward selection using a p-value cutoff < 0.15 for all clinical variables associated with survival in UVAs for MVAs, HR’s and 95% confidence intervals (CI’s) to summarize the effect size, and defined statistical significance as p < 0.05 in two-tailed tests. We forced our predictors (TTT and TT) into the MVA models regardless of statistical significance and corrected for multiple pairwise comparisons for treatment type where applicable by multiplying the resulting p-values by the number of comparisons. All data were managed using REDCap electronic data capture tools hosted at the VA Information Resource Center [12]; and analyzed using IBM® SPSS® Statistics software version 24.0.
3. Results
3.1. Characteristics of Included Patients, primary predictor, and outcome
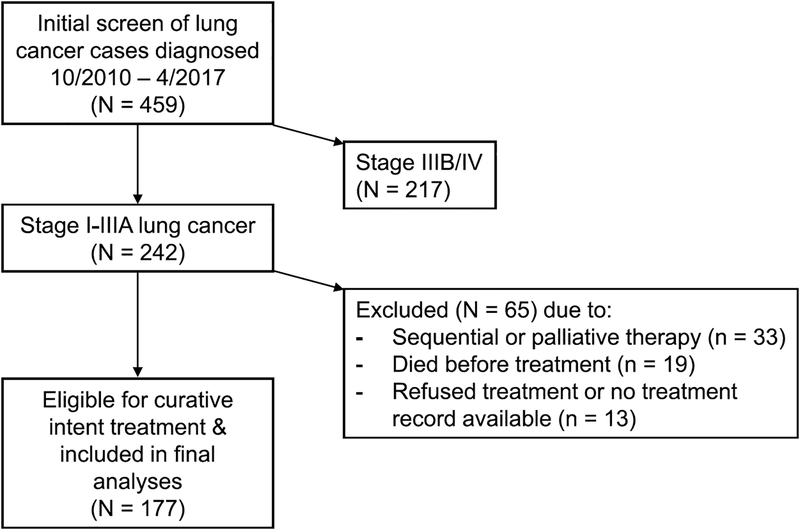
We included 177 stage I-IIIA patients eligible for curative intent therapy (Fig. 1); baseline characteristics are as in Table 1. The median TTT was 35 days for all treatment modalities (29 days for chemoradiation, 36 for surgical resection, and 42 for definitive radiation). Depending on the definition of timeliness used, TT occurred in 33% (using the most timely) or 77% (least timely definition) of patients (Table 1). Patient characteristics associated with longer TTT included other cancer history, high SCS, stage I disease, and definitive radiation treatment (Table 2). The median follow-up time was 25 months (interquartile range, IQR 12–40); death occurred in 75 patients (42%). The median overall survival time (and 95% CI’s) was 42 months (25–59); and 54 months (36–73) for stage I disease, 31 (20–42) stage II, and 25 (20–29) stage IIIA (p = 0.04 by Log-rank pooled over strata).
Fig. 1.
Flow diagram of included patients.
Table 1.
Patient characteristics.
| Patient Characteristic (N = 177) | Value |
|---|---|
| Age, years, mean (SD) | 68.7 (8.2) |
| Male sex, n (%) | 169 (96) |
| Race, n (%) | |
| Black/African American | 15 (9) |
| White | 142 (80) |
| Other/declined | 20 (11) |
| Symptom at presentation, n (%) | |
| Chest pain/cough/dyspnea | 58 (33) |
| Hemoptysis | 8(5) |
| Other | 5(3) |
| None | 106 (60) |
| ECOG PSa < 2, n (%) | 144 (81) |
| Family history of lung cancer, n (%) | 21 (12) |
| Smoking status, n (%) | |
| Current | 100 (57) |
| Former | 70 (40) |
| Never | 7 (4) |
| Pack yearsa, mean (SD) | 50.3 (26.6) |
| Respiratory comorbidities, n (%) | |
| COPD/asthma | 123 (69) |
| Any respiratory comorbidityc | 134 (76) |
| Cardiovascular comorbidities, n (%) | |
| CAD | 54 (31) |
| Arrhythmia | 23 (13) |
| HF | 19 (11) |
| Any cardiovascular comorbidityc | 136 (77) |
| Other cancer, n (%) | 56 (32) |
| Alcohol dependence, n (%) | 21 (12) |
| DM, n (%) | 36 (20) |
| CKD, n (%) | 17 (10) |
| SCS, mean (SD) | 10.1 (3.1) |
| SCS > 9, n (%) | 77 (44) |
| Psychiatric illness, n (%) | |
| Anxiety/Depression/PTSD | 49 (28) |
| Bipolar/Schizophrenia | 8 (5) |
| Any psychiatric illness | 57 (32) |
| Pulmonary function, mean (SD) | |
| FEV1/FVCa, % | 60.5 (14.8) |
| FEV1, % predicteda | 71.5 (23.0) |
| TLC, % predicteda | 107.3 (18.6) |
| DLCO % predicteda | 75.4 (22.0) |
| Ventilatory defectsb, n (%) | |
| Obstructive | 113 (64) |
| Restrictive | 9 (5) |
| DLCO limitation | 91 (51) |
| Lesion sizea, cm, mean (SD) | 2.8 (1.8) |
| Histology, n (%) | |
| Adenocarcinoma | 78 (44) |
| Squamous cell carcinoma | 42 (24) |
| Small cell carcinoma | 10 (6) |
| Other or NSCLC, NOS | 17 (10) |
| Presumed | 30 (17) |
| Clinical stage, n (%) | |
| IA | 90 (51) |
| IB | 32 (18) |
| IIA | 13 (7) |
| IIB | 11 (6) |
| IIIA | 31 (18) |
| Primary treatment, n (%) | |
| Surgical resection | 59 (33) |
| SBRTd | 64 (36) |
| XRTd | 14 (8) |
| Chemoradiation | 40 (23) |
| Time to treatment | |
| All modalities | |
| TTT, days, median (IQR) | 35 (24–55) |
| Most TT, n (%) | 59 (33) |
| Least TT, n (%) | 136 (77) |
| Surgical resection | |
| TTT, days, median (IQR) | 36 (17–51) |
| Most TT, n (%) | 24 (41) |
| Least TT, n (%) | 46 (78) |
| SBRT/XRT | |
| TTT, days, median (IQR) | 42 (29–67) |
| Most TT, n (%) | 17 (22) |
| Least TT, n (%) | 54 (69) |
| Chemoradiation | |
| TTT, days, median (IQR) | 29 (21–36) |
| Most TT, n (%) | 18 (45) |
| Least TT, n (%) | 36 (90) |
CAD = coronary artery disease; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; DLCO = diffusion capacity of the lung for carbon monoxide; DM = diabetes mellitus; ECOG PS = Eastern Cooperative Oncology Group Performance Status; FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity; HF = heart failure; IQR = interquartile range; NOS = not otherwise specified; NSCLC = non-small cell lung cancer; PTSD = post-traumatic stress disorder; SBRT = stereotactic body radiotherapy; SCS = simplified comorbidity score; SD = standard deviation; TT = timely treatment; TTT = time to treatment; TLC = total lung capacity; XRT = radiotherapy.
Missing value (n): ECOG PS (1), Pack years (2), FEV1/FVC (5), FEV1% predicted (5), TLC % predicted (57), DLCO % predicted (17), Lesion size(1).
Defined as FEV1/FVC < 0.7 for obstructive ventilatory defect, TLC % predicted < 80 for restrictive, and DLCO % predicted < 80 for DLCO limitation; missing values were assumed to be normal.
As defined by the SCS: for respiratory comorbidity, history of tuberculosis, pleural effusion or pneumonia, asthma, pulmonary embolism, chronic hypoxemia, and/or COPD (any); for cardiovascular comorbidity: congestive HF, CAD, severe valvular disease, arrhythmia requiring treatment, cerebrovascular disease, hypertension, and/or peripheral vascular disease (any).
Grouped and analyzed as definitive radiation (SBRT/XRT).
Table 2.
Associations of patient characteristics and time to treatment.
| Patient Characteristic (N = 177) | Mean Difference,a days (95% CI) |
|---|---|
| Age (> 70/≤70) | −10.2 (−25.3, 4.99) |
| Sex (F/M) | −3.06 (−32.5, 26.43) |
| Race (Nonwhite/White) | 20.9 (−3.50, 45.2) |
| Symptomatic (N/Y) | −2.66 (−15.2, 9.83) |
| ECOG (≥ 2/< 2) | −5.46 (−21.2, 10.2) |
| Family history (N/Y) | 6.06 (−12.9, 25.0) |
| Pack years (> 30/≤30) | −4.45 (−18.9, 10.0) |
| DM (N/Y) | 5.01 (−10.2, 20.2) |
| CKD (N/Y) | 2.80 (−18.0, 23.6) |
| COPD (N/Y) | −3.25 (−16.5, 9.98) |
| Any respiratory comorbidity (N/Y) | −9.24 (−23.5, 4.98) |
| CAD (N/Y) | 6.69 (−23.7, 2.76) |
| Any CVD comorbidity (N/Y) | 3.94 (−10.6, 18.4) |
| Other cancer history (N/Y) | −17.4 (−34.4, −0.47) |
| Alcohol dependence (N/Y) | −25.8 (−66.2, 14.6) |
| SCS > 9 (N/Y) | −13.6 (−26.9, −0.25) |
| Any psychiatric illness (N/Y) | 8.43 (−1.97, 18.8) |
| Ventilatory defect (N/Y) | |
| Obstructive | −4.06 (−16.8, 8.68) |
| Restrictive | 4.29 (−23.6, 32.2) |
| DLCO limitation | −0.53 (−12.8, 11.7) |
| Tumor size < 2 cm | −3.34 (−16.0, 9.30) |
| SCLC (N/Y) | 13.2 (6.45, 58.6) |
| Stage I (N/Y) | −13.7 (−26.8, −0.62) |
| Treatment categoryb | |
| Surgery vs. SBRT/XRT | −17.8 (−34.7, −0.86) |
| Surgery vs. chemoradiation | 8.95 (−1.93, 19.8) |
| SBRT/XRT vs. Chemoradiation | 26.7 (10.4, 43.1) |
CAD = coronary artery disease; CI = confidence interval; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CTB = chest tumor board; CVD = cardiovascular disease; DLCO = diffusion capacity of the lung for carbon monoxide; DM = diabetes mellitus; ECOG PS = Eastern Cooperative Oncology Group Performance Status; FEV1 = forced expiratory volume in 1 s; SBRT = stereotactic body radiotherapy; SCLC = small cell lung cancer; SCS = simplified comorbidity score; TLC = total lung capacity; XRT = radiotherapy.
Bolded variables: statistically significant, p < 0.05.
Independent-samples tests (t-test for equality of means and Levene’s test for equality of variances).
Independent-samples Kruskal-Wallis test for nonparametric distribution, p < 0.001.
3.2. Univariable and multivariable cox regressions
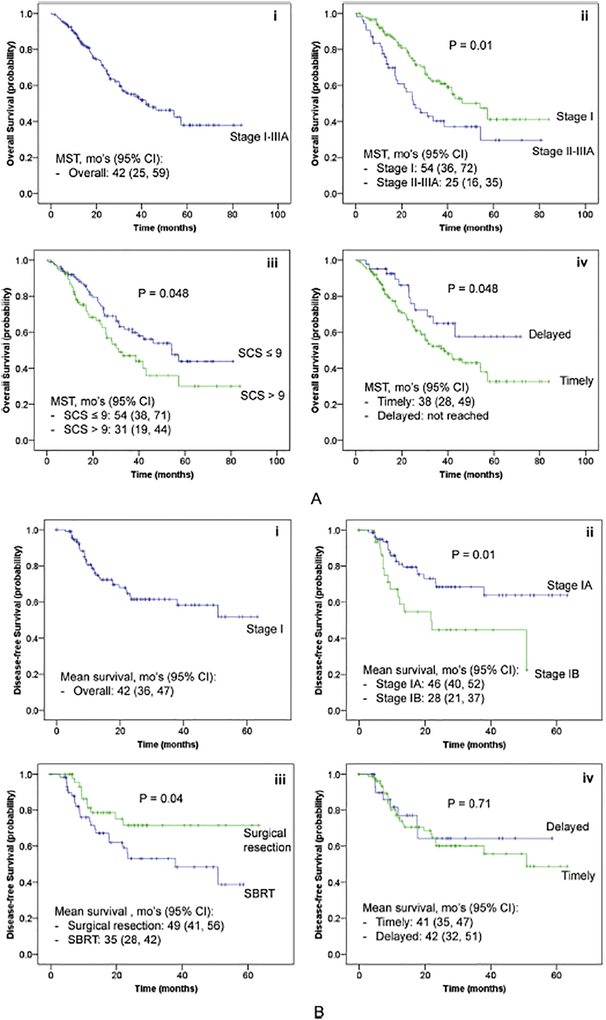
In UVA, tobacco exposure (pack years), SCS (points), DLCO % predicted, tumor size/stage I disease, and treatment modality were associated with OS (Table 3). TTT and TT [either most or least timely (Fig. 2A)] were not associated with better OS. In MVA, higher DLCO % predicted and treatment category were associated with OS; TTT was not associated with OS (HR 1.00 for each day, 95% CI 0.99, 1.01) (Table 4A – Model 1). When categorized as most or least TT, there was a paradoxical trend towards better OS in patients with delayed treatment as defined by the least timely definition (HR 0.53, 95% CI 0.27–1.01) (Table 4A – Model 2).
Table 3.
Univariable cox regression analyses of survival.
| Variable | HR (95% CI) | |
|---|---|---|
| OS (Stage I-IIIA) | DFS (Stage I) | |
| Age, per year | 1.02 (0.99, 1.04) | 1.02 (0.98, 1.06) |
| Sex (F/M) | 0.42 (0.10, 1.72) | 1.16 (0.28, 4.86) |
| Race (Nonwhite/White) | 1.09 (0.63, 1.88) | 1.17 (0.45, 3.03) |
| Symptomatic (N/Y) | 0.88 (0.56, 1.40) | 1.67 (0.76, 3.67) |
| ECOG PS (≥ 2/<2) | 1.21 (0.70, 2.11) | 1.68 (0.81, 3.50) |
| Family history (N/Y) | 1.29 (0.62, 2.69) | 0.21 (0.03, 1.51) |
| Each pack year | 1.01 (1.00, 1.02) | 1.02 (1.01, 1.03) |
| DM (N/Y) | 0.94 (0.54, 1.66) | 1.47 (0.60, 3.49) |
| CKD (N/Y) | 0.50 (0.25, 1.01) | 0.63 (0.22, 1.80) |
| COPD (N/Y) | 0.74 (0.44, 1.22) | 0.65 (0.29, 1.43) |
| Any respiratory comorbidity (N/Y) | 0.65 (0.37, 1.14) | 0.40 (0.14, 1.13) |
| CAD (N/Y) | 0.99 (0.60, 1.64) | 2.5 (1.05, 6.12) |
| HF (N/Y) | 0.70 (0.36, 1.37) | 1.62 (0.50, 5.28) |
| Any CVD comorbidity (N/Y) | 0.89 (0.51, 1.54) | 1.46 (0.64, 3.35) |
| Other cancer history (N/Y) | 0.82 (0.51, 1.33) | 0.74 (0.37, 1.47) |
| Alcohol dependence (N/Y) | 0.76 (0.40, 1.44) | 0.77 (0.30, 1.99) |
| Comorbidity score | ||
| SCS, per point | 1.08 (1.01, 1.15) | 1.03 (0.93, 1.14) |
| SCS > 9 (N/Y) | 0.64 (0.40, 1.00) | 0.88 (0.45, 1.70) |
| Any psychiatric illness (N/Y) | 0.79 (0.49, 1.28) | 0.86 (0.43, 1.71) |
| Pulmonary function (per % pred) | ||
| FEV1 | 0.99 (0.98, 1.00) | 0.99 (0.98, 1.01) |
| TLC | 1.00 (0.98, 1.01) | 1.01 (0.99, 1.04) |
| DLCO | 0.99 (0.97, 1.00) | 0.98 (0.97, 1.00) |
| Ventilatory defect (N/Y) | ||
| Obstructive | 0.85 (0.53, 1.36) | 0.71 (0.34, 1.49) |
| Restrictive | 0.92 (0.37, 2.28) | 1.30 (0.18, 9.54) |
| DLCO limitation | 0.67 (0.42, 1.07) | 0.54 (0.27, 1.07) |
| Tumor size, per cm | 1.21 (1.09, 1.34) | 1.41 (1.05, 1.89) |
| SCLC (N/Y) | 0.87 (0.35, 2.16) | 0.50 (0.12, 2.11) |
| Clinical stagea | 1.82 (1.15, 2.88) | 0.43 (0.22, 0.85) |
| Treatment category | ||
| Surgical resection | 0.32 (0.17, 0.59) | ref |
| SBRT/XRT | 0.57 (0.34, 0.97) | 2.07 (1.02, 4.24) |
| Chemoradiation | ref | N/A |
| Time to treatment | ||
| TTT, per day | 1.00 (0.99, 1.00) | 1.00 (0.99, 1.01) |
| Most TT (N/Y) | 0.95 (0.60, 1.52) | 1.11 (0.54, 2.26) |
| Least TT (N/Y) | 0.54 (0.29, 1.01) | 0.86 (0.39, 1.90) |
CAD = coronary artery disease; CI = confidence interval; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CTB = chest tumor board; CVD = cardiovascular disease; DFS = disease-free survival; DLCO = diffusion capacity of the lung for carbon monoxide; DM = diabetes mellitus; ECOG PS = Eastern Cooperative Oncology Group Performance Status; F = female; FEV1 = forced expiratory volume in 1 s; HF = heart failure; HR = hazard ratio; M = male; OS = overall survival; SBRT = stereotactic body radiotherapy; SCLC = small cell lung cancer; SCS = simplified comorbidity score; TLC = total lung capacity; TT = timely treatment; TTT = time to treatment; XRT = radiotherapy.
Bolded variables: statistically significant, p < 0.05.
Categorized as stage I (N/Y) for stage I-IIIA patients, or stage IA/IB for stage I patients.
Fig. 2.
A: Kaplan-Meier Estimates* of Overall Survival in Stage I-IIIA Patients. Legend: Kaplan-Meier estimates of OS in i) all stage I-IIIA lung cancer eligible for curative intent therapy, stratified by ii) clinical stage, iii) simplified comorbidity score, and iv) least TT. *P-values from Log-rank tests, pooled over strata. CI = confidence interval; mo’s = months; MST = median survival time; OS = overall survival; SCS = simplified comorbidity score; TT = timely treatment. B: Kaplan-Meier Estimates*† of Disease-free Survival in Stage I Patients. Legend: Kaplan-Meier estimates of DFS in i) all stage I lung cancer eligible for curative intent therapy, stratified by ii) clinical stage, iii) treatment type, and iv) least TT. *P-values from Log-rank tests, pooled over strata. †Median survival time not reached in all groups (> 26 months). CI = confidence interval; DFS = disease-free survival; mo’s = months; SBRT = stereotactic body radiotherapy; TT = timely treatment.
Table 4A.
Multivariable cox regression analyses* of overall survival in stage I-IIIA patients.
| Model 1† | ||
| Variable | HR (95% CI) | P-value |
| DLCO, per 10% predicted | 0.86 (0.76, 0.98) | 0.02 |
| Treatment category** | ||
| Surgery | 0.32 (0.16, 0.62) | 0.003 |
| SBRT/XRT | 0.40 (0.21, 0.76) | 0.02 |
| Chemoradiation | ref | Ref |
| TTT, per day | 1.00 (0.99, 1.01) | 0.56 |
| Model 2‡ | ||
| Variable | HR (95% CI) | P-value |
| Each pack year | 1.01 (1.00, 1.02) | 0.11 |
| DLCO, per 10% predicted | 0.86 (0.75, 0.98) | 0.02 |
| Treatment category** | ||
| Surgery | 0.36 (0.18, 0.69) | 0.006 |
| SBRT/XRT | 0.39 (0.21, 0.73) | 0.009 |
| Chemoradiation | ref | ref |
| Least TT (N/Y) | 0.53 (0.27, 1.01) | 0.054 |
Stepwise backward selection including baseline characteristics with p < 0.15: smoking history (pack year), SCS, DLCO % predicted, stage I/II-IIIA disease, treatment category.
P-values corrected for 3 pairwise comparisons.
Overall model P < 0.001; no significant interaction between DLCO and treatment category (p=0.95).
Overall model P < 0.001; no significant interaction between DLCO and pack year (p=0.37) or timely treatment and treatment category (p=0.73).
3.3. Pre-specified subgroup analyses of stage I patients
In subgroup analyses of 122 stage I patients, 52 (43%) underwent surgical resection and 9 (7%) adjuvant therapy. The median TTT for the entire stage I subgroup was 37 days (36 days for surgical resection and 41 days for definitive radiation). TT occurred in 30–74% of patients, depending on the definition of timeliness used. The median follow-up time was 26 months (IQR 13–40), with 35 patients (29%) having disease recurrence/progression and 44 (36%) deaths. The median DFS time was not reached (> 26 months); the mean DFS time was 42 months (Fig. 2B). UVA and MVA results for DFS are shown in Tables 3 and 4B, respectively. TTT (Table 4B – Model 1) and TT [either most or least timely (Table 4B – Model 2)] were not associated with DFS.
Table 4B.
Multivariable cox regression analyses* of disease-free survival in stage I patients.
| Model 1† | ||
| Variable | HR (95% CI) | P-value |
| Each pack year | 1.02 (1.00, 1.03) | 0.01 |
| CAD (N/Y) | 3.25 (1.25, 8.44) | 0.02 |
| Stage IA/IB | 0.32 (0.15, 0.66) | 0.002 |
| Surgical resection (N/Y) | 2.26 (1.07, 4.78) | 0.03 |
| TTT, per day | 1.00 (0.99, 1.01) | 0.74 |
| Model 2 | ||
| Each pack year | 1.02 (1.01, 1.03) | 0.008 |
| CAD (N/Y) | 3.50 (1.35, 9.07) | 0.01 |
| Stage IA/IB | 0.30 (0.14, 0.63) | 0.002 |
| Surgical resection (N/Y) | 2.35 (1.11, 4.94) | 0.03 |
| Least TT (N/Y) | 1.49 (0.62, 3.59) | 0.37 |
CAD = coronary artery disease; CI = confidence interval; DLCO = diffusion capacity of the lung for carbon monoxide; HR = hazard ratio; SBRT = stereotactic body radiotherapy; SCS = simplified comorbidity score; TT = timely treatment; TTT = time to treatment; XRT = radiotherapy.
Stepwise backward selection including baseline characteristics with p < 0.15: family history of lung cancer, smoking history (pack year), any respiratory comorbidity, CAD, DLCO % predicted, stage, surgical resection.
Overall final model P < 0.001; no significant interaction between CAD and pack year (p = 0.06) or CAD and surgical treatment (p = 0.22).
4. Discussion
In consecutive lung cancer patients with stage I-IIIA disease eligible for curative intent therapy at the VASDHS from 2010 to 2017, TTT and TT were not associated with better OS, or DFS in stage I patients. To the best of our knowledge, our study is the first to examine the relationship between TTT/TT and DFS in lung cancer.
Previous studies have examined the relationship between TTT and OS in lung cancer. For instance, Gould and coworkers [13] analyzed 129 consecutive patients with NSCLC (29% stage I-II and 33% stage III disease) diagnosed at the VA Palo Alto Healthcare System from 2002 to 2003 (median diagnosis-to-treatment time 22 days) and found that OS was paradoxically worse in patients treated within 84 days compared to > 84 days after initial suspicion (HR 1.6, 95% CI 1.3–1.9) [13]. Olsson and coworkers [14] systematically reviewed published studies from 1995 to 2007 and found that in 15 studies with multivariable survival analyses, eight reported no association between timely care and OS, four reported an association between delayed care and better OS, and three an association between timely care and better OS. However, the definition of timely care varied greatly, including in the three studies that showed associations between timely care and better OS. In one study [15], delayed care was defined as time from initial tumors missed on screening but identified one year later (hence at least a one-year delay to treatment), and in the other two [16,17], the time interval most accurately reflected time-to-diagnosis, and not TTT.
Recently, Nadpara and coworkers [18] analyzed 16,747 elderly lung cancer patients included in the Surveillance, Epidemiology, and End Results (SEER) database from 2002 to 2007 (median diagnosis to NSCLC treatment time 27 days), and found that delayed care was again paradoxically associated with better survival (HR 0.68, 95% CI 0.66–0.71) in MVA. When stratified by lung cancer stage, there was a trend towards better survival in stage I/II patients receiving timely care, however the results were not statistically significant. Notably, the author excluded patients with a prior history of malignancy (11,846 patients, 15% of the patients screened). In a similar study, Gomez and coworkers [19] analyzed SEER data on 28,732 patients with histologically confirmed NSCLC from 2004 to 2007 (median diagnosis-to-treatment time 27 days), and found that in MVA, TT was associated with better survival (HR 0.86, 95% 0.80–0.91) in patients with localized disease. In those with regional disease, there was a trend towards worse survival with TT (HR 1.05, 95% CI 0.99–1.11), and in distant disease TT was again paradoxically associated with worse survival (HR 1.35, 95% CI 1.28–1.42) [19]. Most recently, Yang and coworkers [20] analyzed 4984 patients with stage IA squamous cell carcinoma included in the National Cancer Database from 2006 to 2011 (median diagnosis-to-treatment time 38 days) and found that in MVA, there was a 13% increased hazard of overall death in those who had surgery 38 days or later after diagnosis (HR 95% CI 1.02–1.25, p = 0.02). Notably, there was no significant difference in the pathologic tumor or nodal stage between timely and delayed lobectomy [20].
Compared to our study, a few notable differences exist for interpretation. Studies by Nadpara [18] and Gomez [19] and coworkers excluded patients with other cancers (32% of patients in our study), and Gomez [19] and Yang [20] and coworkers excluded patients with presumed lung cancer who tend to have high comorbidity burden and poor prognosis (17% in our study). Since TTT tends to be longer in patients with a high comorbidity burden [14], excluding sicker patients increases the chance of false-positive findings. In addition, none of the studies included tobacco exposure and lung function (notably DLCO % predicted [21,22]) as covariates which are important prognostic variables in lung cancer. Last, lung cancer-specific survival including DFS, a more logical outcome to investigate the significance of timeliness of lung cancer treatment, was not assessed in any of these studies.
Another logical outcome is tumor upstaging associated with treatment delay. To this end, Liberman and coworkers [23] analyzed 256 patients who underwent lung cancer resection surgery (89% with pathologic stage I-IIIA) at the Montreal General Hospital between 1993 and 2002 (median surgical visit to operation time 82 days), and found that preoperative delay was not associated with surgical stage categories. Clinical stage was not available in this study to assess the proportions of patients upstaged at the time of surgery. Similarly, Maiga and coworkers [24] analyzed 197 lung cancer patients who had a tissue diagnosis and subsequently underwent surgical resection (median diagnosis to surgery time 53 days) at the VA Tennessee Valley Healthcare System from 2005 to 2015, and in univariable analyses, found no significant correlation between time to resection with tumor progression, defined as tumor growth > 0 mm between radiographic and pathologic tumor size; this definition is unlikely to be sensitive to small/subtle changes in tumor growth. In our subgroup analysis of stage I patients, 52 (43%) underwent surgical resection, 18 of whom (35%) were upstaged at the time of surgery; there was no difference in TTT in those upstaged versus those not upstaged (mean 42 vs. 33 days, respectively, t-test for equality of means p = 0.25).
Partly due to the observational nature of available studies, our understanding of the impact of delayed care on lung cancer outcomes is incomplete. However, one can make references to the causal relationship between TTT/TT and survival (biologically caused by disease progression) with some key considerations: consistency and strength of association, linearity/dose-response relationship, and plausibility of alternative explanations. Based on our review, there is an inconsistent and weak association between TT and survival (HR’s are consistently < 2 in patients with delayed care where there are positive associations); no available study describes a dose-response relationship between TTT and survival. In addition, there are alternative explanations for positive associations including for the results from the study by Yang and coworkers [20], in which patients who are healthier and/or with better lung function may have received lobectomy sooner than those who are sicker and/or with worse lung function.
While TTT/TT were not associated with improved survival in our study, one should be careful in not assuming that extending the period between diagnosis and treatment beyond the limits of timeliness as defined by practice guidelines will have no impact on survival. Our findings should not be interpreted as a “ticket” to extend workups or delay treatment beyond recommended limits or lessen a diligence to keep timeliness within these limits as a sensible quality measure. In addition, efforts to improve timeliness should be made with careful considerations of modifiable/nonmodifiable factors. In a systematic analysis, institutional factors explained only < 1% of the variations in treatment times [25]. Overcoming patient self-blame and stigma [26] and facilitating positive coping [27] strategies may play an important role in improving timeliness. Moreover, the time interval between diagnosis and treatment may offer an opportunity to deliver or improve other quality cancer care. In this cancer continuum, the IOM highlights needs in patient care planning, psychosocial support, prevention of treatment related effects, and family caregiver support [28]. Development and implementation of health services including cancer rehabilitation may decrease treatment related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes [29].
The prevalence COPD (64% confirmed by lung function testing) was higher in our study compared to others [30,31] including in veterans (53–56%) [32], possibly due to higher tobacco and/or environmental/occupational exposure or referral bias. The prevalence of diabetes (20%) and other cancer (32%) were somewhat comparable to other veteran populations (26% and 21%, respectively) [32]. Congestive heart failure (HF, 11%) was similarly prevalent compared to lung cancer patients included in the Nebraska Cancer Registry (13%) [30] and SEER database (12%) [31].
Like previous studies, our study reports the likelihood of receiving timely care decreasing with high comorbidity burden, NSCLC (compared to SCLC) histology, and early stage disease [14,18,19,33]. Radiation was associated with the longest TTT compared to other treatment modalities, likely due to outside referral. Like existing literature, tobacco exposure, comorbidity burden, DLCO % predicted, tumor size, stage, and treatment type were associated with survival in our study. Unlike previous studies, HF [30] and SCLC histology were not associated with survival, likely due to a small sample size. Timely treatment (least timely definition) was paradoxically significantly/borderline associated with worse OS, likely due to confounding/residual confounding effects of stage and/or comorbidities. Not having a history of CAD or lung cancer resection surgery as treatment was associated with worse DFS, possibly due to survivor bias and/or confounding effects of stage.
Our study is limited by its retrospective, single-institutional nature; these findings may not be generalizable due to all patients included from the VA healthcare system. Also, the definition of TTT starting at the time of weekly CTB conferences does not include preceding time including time of first imaging to cancer diagnosis, tissue diagnosis to appropriate staging, and diagnosis to CTB presentation which may be delayed and potentially influence outcomes. In addition, we did not have information on the reason for treatment delay and lung cancer-specific mortality. Last, our sample size may not have adequate power to detect a significant association between TTT and survival. Our study also has strengths. First, we maximized the accuracy of the data collected by extracting patient characteristics including comorbidities that were entered into the electronic medical record system by board-certified physicians. Second, we included many of the variables previously known to be associated with survival in lung cancer, including tobacco exposure and lung function which tend to be missing in large national databases. Third, the VA healthcare system keeps accurate and up-to-date vital records of its patients, allowing for accurate OS analyses. Fourth we assessed DFS, a more logical outcome in a subgroup of stage I patients.
We conclude that in a single-institutional analysis of stage I-IIIA lung cancer patients deemed eligible for curative intent therapy, treatment was timely in 33% or 77% of veterans, depending on the guideline recommendation used. Treatment delay was not associated with overall survival in stage I-IIIA, or disease-free survival in stage I patients.
Acknowledgments
Funding
Duc Ha, MD was supported indirectly by the NIH Loan Repayment Program for Extramural Clinical Research, L30CA208950, from the NCI; and directly by an Institutional National Research Service Award 1T32HL134632-01, from the NIH/NHLBI; and Postdoctoral Fellowship, PF-17-020-01-CPPB, from the American Cancer Society.
Abbreviations:
- ACCP
American College of Chest Physicians
- BTS
British Thoracic Society
- CAD
coronary artery disease
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CTB
chest tumor board
- DD
diastolic dysfunction
- DFS
disease-free survival
- DLCO
diffusion capacity of the lung for carbon monoxide
- FEV1
forced expiratory volume in 1 s
- HF
heart failure
- HR
hazard ratio
- IOM
Institute of Medicine
- IQR
interquartile range
- mo’s
months
- MST
median survival time
- MVA
multivariable regression analysis
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PH
pulmonary hypertension
- SBRT
stereotactic body radiotherapy
- SCLC
small cell lung cancer
- SCS
simplified comorbidity score
- SEER
Surveillance Epidemiology and End Results
- TLC
total lung capacity
- TT
timely treatment
- TTT
time-to-treatment
- UVA
univariable regression analysis
- VASDHS
VA San Diego Healthcare System
Footnotes
Conflicts of interest
All authors declare no conflict of interest exists.
References
- [1].Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ, Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd Ed: American College of Chest Physicians Evidence-based Clinical Practice Guidelines. Chest, 143 2013. e166S–90S. [DOI] [PubMed] [Google Scholar]
- [2].Donington J, Ferguson M, Mazzone P, et al. , American college of chest physicians and society of thoracic surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer, Chest 142 (2012) 1620–1635. [DOI] [PubMed] [Google Scholar]
- [3].Ost DE, Jim Yeung SC, Tanoue LT, Gould MK, Clinical and organizational factors in the initial evaluation of patients with lung cancer: diagnosis and management of lung cancer, 3rd Ed: American College of Chest Physicians Evidence-based Clinical Practice Guidelines. Chest, 143 2013. e121S–41S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Institute of medicine, Committee on Quality of Healthcare in America. Crossing the Quality Chasm: a New Health System for the 21st century, National Academy Press, washington, DC, 2001. [Google Scholar]
- [5].Alberts WM, Bepler G, Hazelton T, Ruckdeschel JC, Williams JH Jr., American college of chest physicians, Lung Canc. Pract. org. chest 123 (2003) 332S–7S. [DOI] [PubMed] [Google Scholar]
- [6].BTS recommendations to respiratory physicians for organising the care of patients with lung cancer, The lung cancer working party of the british thoracic society standards of care committee, Thorax 53 (1) (1998) S1–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Asch SM, Kerr EA, Hamilton EG, Reifel JL, McGlynn EA, Quality of care for oncologic condition and HIV: a review of the literature and quality indicators, Lung Cancer. RAND (2000) 133–171. [Google Scholar]
- [8].Powell AA, Schultz EM, Ordin DL, et al. , Timeliness across the continuum of care in veterans with lung cancer, J. Thorac. Oncol 3 (2008) 951–957. [DOI] [PubMed] [Google Scholar]
- [9].Dinan MA, Curtis LH, Carpenter WR, et al. , Stage migration, selection bias, and survival associated with the adoption of positron emission tomography among medicare beneficiaries with non-small-cell lung cancer, 1998–2003, J. Clin. Oncol 30 (2012) 2725–2730. [DOI] [PubMed] [Google Scholar]
- [10].Kozower BD, Larner JM, Detterbeck FC, Jones DR, Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd Ed: American College of Chest Physicians Evidence-based Clinical Practice Guidelines. Chest, 143 2013. e369S–99S. [DOI] [PubMed] [Google Scholar]
- [11].Colinet B, Jacot W, Bertrand D, et al. , A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: description and comparison with the charlson’s index, Br. J. Canc 93 (2005) 1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inf 42 (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gould MK, Ghaus SJ, Olsson JK, Schultz EM, Timeliness of care in veterans with non-small cell lung cancer, Chest 133 (2008) 1167–1173. [DOI] [PubMed] [Google Scholar]
- [14].Olsson JK, Schultz EM, Gould MK, Timeliness of care in patients with lung cancer: a systematic review, Thorax 64 (2009) 749–756. [DOI] [PubMed] [Google Scholar]
- [15].Kashiwabara K, Koshi S, Itonaga K, Nakahara O, Tanaka M, Toyonaga M, Outcome in patients with lung cancer found on lung cancer mass screening roentgenograms, but who did not subsequently consult a doctor, Lung Canc 40 (2003) 67–72. [DOI] [PubMed] [Google Scholar]
- [16].Buccheri G, Ferrigno D, Lung cancer: clinical presentation and specialist referral time, Eur. Respir. J 24 (2004) 898–904. [DOI] [PubMed] [Google Scholar]
- [17].Kanashiki M, Satoh H, Ishikawa H, Yamashita YT, Ohtsuka M, Sekizawa K, Time from finding abnormality on mass-screening to final diagnosis of lung cancer, Oncol. Rep 10 (2003) 649–652. [PubMed] [Google Scholar]
- [18].Nadpara P, Madhavan SS, Tworek C, Guideline-concordant timely lung cancer care and prognosis among elderly patients in the United States: a population-based study, Cancer Epidemiol 39 (2015) 1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gomez DR, Liao KP, Swisher SG, et al. , Time to treatment as a quality metric in lung cancer: staging studies, time to treatment, and patient survival, Radiother. Oncol 115 (2015) 257–263. [DOI] [PubMed] [Google Scholar]
- [20].Yang CJ, Wang H, Kumar A, et al. , Impact of timing of lobectomy on survival for clinical stage IA lung squamous cell carcinoma, Chest 152 (2017) 1239–1250. [DOI] [PubMed] [Google Scholar]
- [21].Ferguson MK, Dignam JJ, Siddique J, Vigneswaran WT, Celauro AD, Diffusing capacity predicts long-term survival after lung resection for cancer, Eur. J. Cardio. Thorac. Surg 41 (2012) e81–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brunelli A, Dinesh P, Woodcock-Shaw J, Littlechild D, Pompili C, Ninety-day mortality after video-assisted thoracoscopic lobectomy: incidence and risk factors, Ann. Thorac. Surg 104 (2017) 1020–1026. [DOI] [PubMed] [Google Scholar]
- [23].Liberman M, Liberman D, Sampalis JS, Mulder DS, Delays to surgery in non-small-cell lung cancer, Can. J. Surg 49 (2006) 31–36. [PMC free article] [PubMed] [Google Scholar]
- [24].Maiga AW, Deppen SA, Pinkerman R, et al. , Timeliness of care and lung cancer tumor-stage progression: how long can we wait? Ann. Thorac. Surg 104 (2017) 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schultz EM, Powell AA, McMillan A, et al. , Hospital characteristics associated with timeliness of care in veterans with lung cancer, Am. J. Respir. Crit. Care Med 179 (2009) 595–600. [DOI] [PubMed] [Google Scholar]
- [26].Scott N, Crane M, Lafontaine M, Seale H, Currow D, Stigma as a barrier to diagnosis of lung cancer: patient and general practitioner perspectives, Prim. Health Care Res. Dev 16 (2015) 618–622. [DOI] [PubMed] [Google Scholar]
- [27].Ellis J, Lloyd Williams M, Wagland R, Bailey C, Molassiotis A, Coping with and factors impacting upon the experience of lung cancer in patients and primary carers, Eur. J. Canc. Care 22 (2013) 97–106. [DOI] [PubMed] [Google Scholar]
- [28].Committee on Improving the Quality of Cancer Care, Addressing the Challenges of an Aging Population, Board on Health Care Services, Institute of Medicine, 2013. [Google Scholar]
- [29].Silver JK, Baima J, Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes, Am. J. Phys. Med. Rehabil 92 (2013) 715–727. [DOI] [PubMed] [Google Scholar]
- [30].Islam KM, Jiang X, Anggondowati T, Lin G, Ganti AK, Comorbidity and survival in lung cancer patients, Cancer Epidemiol. Biomark. Prev 24 (2015) 1079–1085. [DOI] [PubMed] [Google Scholar]
- [31].Edwards BK, Noone AM, Mariotto AB, et al. , Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer, Cancer 120 (2014) 1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang S, Wong ML, Hamilton N, Davoren JB, Jahan TM, Walter LC, Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans, J. Clin. Oncol 30 (2012) 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yorio JT, Xie Y, Yan J, Gerber DE, Lung cancer diagnostic and treatment intervals in the United States: a health care disparity? J. Thorac. Oncol 4 (2009) 1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]