Abstract
Schizophrenia is associated with impairment in a range of cognitive functions. Neuroimaging studies have reported lower, but also higher, task-induced activation accompanying impaired performance. Differences in task-load and the ability of people with schizophrenia (PSZ) to stay engaged in the cognitive operations probed appear to underlie such discrepancies. Similarly, task-induced deactivation of the default mode network (DMN) was weaker in PSZ relative to healthy control subjects (HCS) in most studies, but some reported greater deactivation. An inability to stay engaged in the cognitive operations could account for these discrepancies, too, as it would lead to more time off-task and consequently less deactivation of DMN functions. The present study employed a change detection paradigm with small to moderate set sizes (SSs) of 1, 2, and 4 items. Task training prior to fMRI scanning abolished the group difference in no-response trials. Task-positive regions of interest (ROIs) displayed greater activation with increasing SS in both groups. PSZ showed greater activation relative to HCS at SSs 1 and 2. DMN ROIs displayed greater deactivation with increasing SS in PSZ, but not in HCS, and PSZ tended to hyperdeactivate DMN regions at SS 4. No hypodeactivation was observed in PSZ. In conclusion, when minimizing differences in task-engagement, PSZ tend to over-recruit task-positive regions during low-load operations, and hyperdeactivate DMN functions at higher load, perhaps reflecting heightened non-specific vigilance or effort when dealing with cognitive challenges. This speaks against an inability to down-regulate task-independent thought processes as a primary mechanism underlying cognitive impairment in schizophrenia.
Keywords: schizophrenia, fMRI, default network, cognitive, working memory, load
1. Introduction
Schizophrenia is associated with impairment in a range of cognitive functions (Fioravanti et al., 2012; Schaefer et al., 2013). Accompanying activation differences in task-specific brain areas have been studied extensively. Recently, the focus has widened to include task-related deactivations. A relatively consistent set of brain regions has been shown to deactivate during various external processing tasks relative to rest. These areas, termed the default mode network (DMN) of resting brain function, are thought to subserve stimulus-independent thought processes such as mind-wandering when the individual is not engaged in external stimulus processing (Gusnard and Raichle, 2001; Buckner et al., 2008). Down-regulation of DMN activity appears to be necessary for successful cognitive performance (e.g. Daselaar et al., 2004; Weissman et al. 2006; Eichele et al., 2008).
Functional Magnetic Resonance Imaging (fMRI) studies of cognition reported less pronounced task-induced DMN deactivation in people with schizophrenia (PSZ) than in healthy control subjects (HCS; Pomarol-Clotet et al., 2008; Whitfield-Gabrieli et al., 2009; Hasenkamp et al., 2011; Schneider et al., 2011; Salgado-Pineda et al., 2011; Nygard et al., 2012; Dreher et al., 2012; Anticevic et al., 2013; Madre et al., 2013; Fryer et al. 2013; Haatveit et al. 2016). Such findings led to suggestions that an inability to down-regulate task-independent thought processes may contribute to cognitive impairment in PSZ. There are, however, discrepant reports of DMN hyperdeactivation in PSZ (Harrison et al., 2007; Hahn et al., 2016), with support also from a study employing constrained principle component analysis (Metzak et al. 2012). These three studies have in common that performance of PSZ displayed no clear impairment relative to HCS, whereas PSZ performed significantly worse in most studies reporting hypodeactivation. Hahn et al. (2016) employed extensive task practice prior to scanning, which eliminated the group difference in the number of no-response trials and appeared to equate the time spent “off-task”. This suggests that difficulty managing the cognitive operations and maintaining task engagement may have contributed to findings of DMN hypodeactivation in other studies.
These considerations are reminiscent of the neuroimaging literature on working memory (WM). While early studies overwhelmingly supported lower prefrontal cortex (PFC) activation in PSZ, several subsequent studies reported equal or even greater activation (Manoach, 2003; Ragland et al., 2007). This may reflect the inverted-U relationship between WM load and PFC activation. Greater load increases activation, but when it exceeds an individual’s capacity, activation decreases (Goldberg et al., 1998; Callicott et al., 1999). Thus, when imposing task demands manageable for HCS but exceeding the capacity of PSZ, PFC activation would be lower in PSZ. Indeed, across studies, larger performance deficits of PSZ tend to be associated with greater hypofrontality (Van Snellenberg et al., 2006). In contrast, greater activation can be observed in PSZ at lower WM loads, which are manageable but more challenging to PSZ than to HCS (Manoach, 2003).
The present fMRI study compared WM load-dependent activation of typical task-positive regions, and deactivation of the DMN, between PSZ and HCS. Load was varied as the number of items maintained in a Change Detection Task (CDT). Reduced WM capacity in PSZ has been shown in similar paradigms (Lee and Park, 2005; Gold et al., 2006); however, by keeping the set size (SS) modest and providing extensive practice prior to scanning, we aimed at evoking at most minimally impaired performance and comparable task engagement in PSZ and HCS. We hypothesized that, under these conditions, both activation of task-positive and deactivation of DMN regions would be equal or greater in PSZ relative to HCS, but not reduced.
2. Materials and Methods
2.1. Participants
Twenty-two medicated (detail in Supplement) outpatients meeting DSM-IV criteria for schizophrenia (N=21) or schizoaffective disorder (N=1), and 20 HCS, all right-handed, completed this study. Data from four HCS and one PSZ were excluded because they appeared to be asleep during parts of the scan. Demographic information of the remaining participants is summarized in Table 1. Groups did not differ in age, sex, ethnicity, or parental education. PSZ tended to have fewer years of education (P<0.1) and scored lower on neuropsychological tests of cognition. Because blood pressure can affect BOLD responses to neuronal activation (Wang et al., 2006), we compared groups on systolic (P>0.6) and diastolic blood pressure (P>0.3).
Table 1.
| PSZ (N=21) | HCS (N=16) | Statistic P-value |
|
|---|---|---|---|
| Age | 36.2 ± 11.0 (range 20–53) |
36.5 ± 14.5 (range 19–53) |
t(35)=.06 P=0.95 |
| Male : Female | 13 : 8 | 11 : 5 | χ2=0.19 P=0.67 |
| Afr Am : Cauc : Other | 6 : 14 : 1 | 4 : 11 : 1 | χ2=0.09 P=0.96 |
| Education (years) | 12.8 ± 2.7 | 14.1 ± 1.7 | t(35)=1.76 P=0.088 |
| Parental Education (yrs) a | 13.1 ± 3.0 | 14.3 ± 2.0 | t(35)=1.40 P=0.17 |
| Estimated IQ bf | 104.1 ± 13.2 | 115.6 ± 7.3 | t(35)=3.12 P<0.005 |
| MCCB cf | 37.9 ± 13.1 | 53.2 ± 6.2 | t(35)=4.30 P<0.001 |
| WRAT 4 df | 97.8 ± 14.8 | 110.3 ± 13.0 | t(35)=2.67 P<0.02 |
| WTAR e | 101.0 ± 18.7 | 111.6 ± 9.7 | t(35)=2.06 P<0.05 |
| Smokers : Non-smokers | 6 : 15 | 2 : 14 | χ2=1.38 P>0.2 |
| FTND of current smokers f | 3.5 ± 2.4 | 3.5 ± 2.8 | t(6)=0.24 P>0.8 |
| Systolic blood pressure (mm Hg) g | 125.2 ± 12.0 | 122.9 ± 15.9 | t(35)=0.49 P>0.6 |
| Diastolic blood pressure (mm Hg) g | 76.2 ± 7.6 | 73.8 ± 9.8 | t(35)=0.87 P>0.3 |
Average over maternal and paternal education
Based on vocabulary and matrix reasoning subscales of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)
Composite score on the MATRICS Consensus Cognitive Battery (Nuechterlein and Green 2006)
Wide Range Achievement Test (Wilkinson and Robertson, 2006)
Wechsler Test of Adult Reading (Wechsler, 2001)
Fagerstrom Test for Nicotine Dependence (Heatherton et al., 1991)
taken during screening
Participants provided informed consent for a protocol approved by the Institutional Review Boards of the University of Maryland, Baltimore, and the National Institute on Drug Abuse – Intramural Research Program.
2.2. Procedure
During an initial training session, participants received task instructions, performed the full-length tasks on a PC, and completed neuropsychological testing. Training performance data from one PSZ was lost due to recording errors. Scans were always performed on a separate day to avoid exhaustion. Participants performed two tasks while undergoing fMRI: first a stimulus detection task, reported elsewhere (Hahn et al., 2016), then the CDT described below. An anatomical scan was obtained between tasks. The CDT was preceded by four blocks of a visuomotor control task, not reported here.
2.3. The CDT
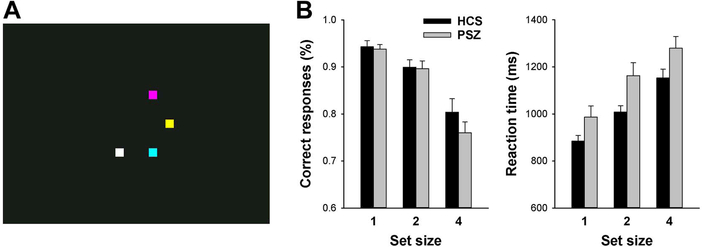
The design was first described by Luck and Vogel (1997). Participants viewed a 200-ms encoding array of 1, 2, or 4 colored squares, circles, or triangles (each participant was allocated one shape) against a black background (Figure 1A). After a 1100-ms blank-screen delay, one of the shape stimuli reappeared for 2000 ms. The task was to make an index finger response if the shape that had been presented at this location changed color, and a middle finger response if the color stayed the same (50% probability). Trials were separated by a 1000-ms intertrial interval. The larger a participant’s WM capacity, the more items are encoded, and the greater the probability of a correct response. While ceiling effects are likely at SS 1 and 2, SS 4 somewhat exceeds the capacity of most participants and thus is sensitive to group differences in WM capacity. Dependent variables were accuracy, reflecting the percentage of correct choices (chance being 50%), average reaction time over all responses, and no-response trials, reflecting the percentage of trials in which participants failed to respond.
Figure 1: Task display and performance.
(A) Screen shot of a change detection task encoding array with SS 4. (B) Average (±SEM) accuracy and reaction time in people with schizophrenia (PSZ) and healthy control subjects (HCS).
The task was presented in 26-s blocks of 6 trials each (3 change, 3 no-change). All trials within a block were of the same SS (block design analysis). Eight scan runs (each 178 s, or 89 TRs) were separated by rest periods during which the scanner was turned off. Each run contained one block of each SS, one 26-s rest block during which participants fixated a central cross, and two blocks in which either one relevant and one irrelevant, or two relevant and two irrelevant shapes were presented. These two conditions tapped specific hypotheses about mechanisms of selective attention in PSZ and were modeled but not further analyzed for the purposes of this study.
2.4. Magnetic resonance imaging
A 3 Tesla Siemens Tim Trio scanner (Erlangen, Germany) acquired whole-brain EPI images for measuring T2*-weighted BOLD effects [4-mm oblique (30º) axial slices, 64×64 matrix, FOV=22×22 cm, TR=2 s, TE=27 ms]. An axial T1-weighted image (MPRAGE) provided anatomical reference (1-mm3 voxels, TR=1.9 s, TE=3.51 ms, FA=9º).
Data were processed using AFNI (Cox, 1996). Each volume was registered to a base volume. For each subject, a composite motion index was calculated from the six motion correction parameters (Yang et al., 2005). These scores did not differ between HCS and PSZ [t(32)=1.66, P>0.1]. TRs with >0.5 mm displacement or >0.5° rotation relative to the preceding TR were censored out of the time series. The number of censored TRs did not differ between groups [t(35)=0.60, P>0.5]. The time series was analyzed by voxel-wise multiple regression. Five 26-s boxcar regressors, corresponding to blocks with 1, 2, and 4 item trials, and to the two blocks involving distractors (see 2.3), were convolved with a model hemodynamic response function. The six motion parameter curves were included as regressors of no interest. All correlations between any of three regressors of interest and any of the motion regressors were near zero (all Ps >0.9), and the groups did not differ in average stimulus-correlated motion [t(35)=0.50, P>0.6]. For each subject, the voxel-wise average amplitude of signal change produced by each of the three SSs relative to rest blocks was determined. These maps were re-sampled to a 1-μL resolution, converted to a standard coordinate system (Talairaque and Tournoux, 1988), and spatially blurred.
Second-level analyses:
For visualization of SS-dependent activity within PSZ and within HCS, a Gaussian 5-mm root mean square (RMS) isotropic kernel was adopted. Whole-brain voxel-wise multiple linear regression was performed within each separate group, with SS as the regressor of interest. Voxel-wise P<0.001 combined with a 438-μL clustersize threshold yielded overall P<0.05 based on Monte Carlo simulations.
For direct statistical groups comparisons based on pre-defined regions of interest (ROIs), a 10-mm RMS kernel was applied to minimize the impact of morphological differences between PSZ and HCS (e.g., Palaniyappan et al., 2012; Mueller et al., 2012) and reduce the risk of group differences in regional activation resulting from comparing non-corresponding anatomical areas. ROIs were defined by an independent study, to avoid bias through “double dipping” (Kriegeskorte et al., 2009). Seven-mm-diameter spheres were centered on peak foci of the task-positive (16 ROIs) and DMN (13 ROIs) networks identified by Fox et al. (2005; page 9676) based on spontaneous correlations and anticorrelations at rest. Thus, ROI definition was not biased by specific task demands. Activity was averaged within each ROI. Task-positive and DMN ROIs were analyzed by two separate three-factor ANOVAs (group × SS × ROI).
3. Results
3.1. CDT performance
Performance during fMRI (Figure 1B) was analyzed by two-factor ANOVA (group × SS). Accuracy decreased with increasing SS [main effect of SS: F(2,70)=66.1; P<0.001], but did not differ between PSZ and HCS [main effect of group: F(1,35)=0.69, P>0.4]. HCS tended to outperform PSZ at SS 4, but the group × SS interaction was not significant [F(2,70)=1.30, P=0.28]. Reaction time slowed with increasing SS [F(2,70)=94.3, P<0.001], and was slower in PSZ relative to HCS [F(1,35)=4.61, P=0.039], but there was no interaction [F(2,70)=0.81, P>0.4]. No-response trials displayed no main effects or interaction.
To test whether pretraining had contributed to equating performance between groups, performance in the training session was analyzed and displayed the same patterns: significant main effects of SS on both variables, and a trend toward a group × SS interaction on accuracy [F(2,68)=2.89, P=0.063], with HCS outperforming PSZ at SS 4. However, PSZ had more no-response trials than HCS during training [group main effect: F(1,34)=9.82, P=0.004], but not in the subsequent MRI session [F(1,35)=0.02, P>0.8]. In a direct comparison of effects between sessions by three-factor ANOVA (group × SS × session), there was indeed a group × session interaction on no-response trials [F(1,34)=8.7, P=0.006].
3.2. fMRI
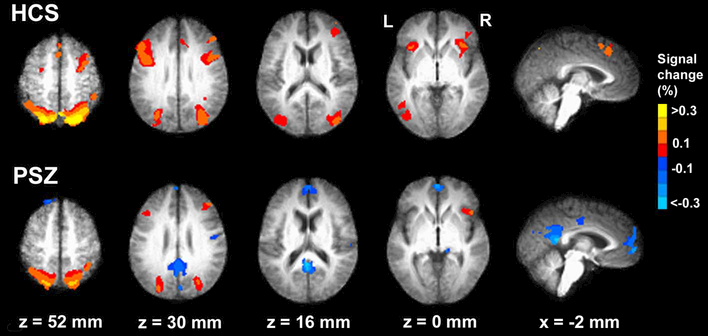
Figure 2 visualizes linear effects of SS within each separate group. Significant clusters identified within each group are listed in Supplementary Tables S1 and S2. Regions displaying step-wise more activity with larger SS are represented by warm colors. These regions were without exception task-positive. Regions displaying step-wise less activity with larger SS are represented by cold colors. These clusters were all task-negative and converged with typical DMN regions.
Figure 2: Within-group effects.
Brain regions responding to working memory load in healthy control subjects (HCS) and in participants with schizophrenia (PSZ). Regions displaying increased BOLD signal with larger SS are drawn in warm colors. Region displaying decreased signal with larger SS are drawn in cold colors. Group activation maps are overlaid onto anatomical scans in Talairach space, averaged separately over all 16 HCS, and over all 21 PSZ.
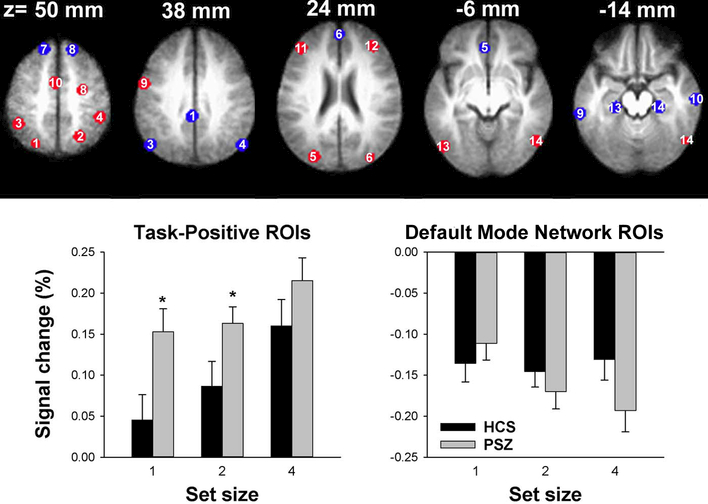
Figure 3 and Table 2 display the spherical ROIs used for statistical comparison of SS-dependent activity between HCS and PSZ.
Figure 3: ROI-based group comparison.
7-mm diameter spheres centered on peak foci of the task-positive (red) and default mode (blue) networks identified by Fox et al. (2005), overlaid onto the average of all 37 anatomical scans in Talairach space. The numbering corresponds to ROIs in Table 2. The graphs display average (±SEM) BOLD activity over task-positive and over default mode network ROIs in healthy controls subjects (HCS) and people with schizophrenia (PSZ) for each SS. *P<0.05 in independent-samples t-test.
Table 2.
ROIs representing 7-mm-diameter spheres centered on peak foci of the task-positive and task-negative/default mode networks identified by Fox et al. (2005)
| Region | Side | Center of Mass (mm) | |||
|---|---|---|---|---|---|
| LR | PA | IS | |||
| Task-positive | |||||
| 1 | Superior parietal lobule, intraparietal sulcus | L | −23 | −66 | 46 |
| 2 | Superior parietal lobule, intraparietal sulcus | R | 25 | −58 | 52 |
| 3 | Inferior parietal lobule, intraparietal sulcus | L | −42 | −44 | 49 |
| 4 | Inferior parietal lobule, intraparietal sulcus | R | 47 | −37 | 52 |
| 5 | Superior occipital gyrus, cuneus | L | −26 | −80 | 26 |
| 6 | Superior occipital gyrus, cuneus | R | 35 | −81 | 29 |
| 7 | Superior precentral gyrus (frontal eye field) | L | −24 | −12 | 61 |
| 8 | Superior precentral gyrus (frontal eye field) | R | 28 | −7 | 54 |
| 9 | Inferior precentral gyrus | L | −54 | 0 | 35 |
| 10 | Medial frontal gyrus (supplementary motor area) | L/R | −2 | 1 | 51 |
| 11 | Middle frontal gyrus | L | −40 | 39 | 26 |
| 12 | Middle frontal gyrus | R | 38 | 41 | 22 |
| 13 | Middle occipital gyrus | L | −47 | −69 | −3 |
| 14 | Middle occipital gyrus | R | 54 | −63 | −8 |
| 15 | Insula | L | −45 | 5 | 8 |
| 16 | Insula | R | 45 | 4 | 14 |
| Task-negative / default mode network | |||||
| 1 | Posterior cingulate gyrus | L/R | −2 | −36 | 37 |
| 2 | Retrosplenial cortex | L/R | 3 | −51 | 8 |
| 3 | Angular gyrus | L | −47 | −67 | 36 |
| 4 | Angular gyrus | R | 53 | −67 | 36 |
| 5 | Rostral anterior cingulate gyrus | L/R | −3 | 39 | −2 |
| 6 | Medial prefrontal gyrus | L/R | 1 | 54 | 21 |
| 7 | Superior frontal gyrus | L | −14 | 38 | 52 |
| 8 | Superior frontal gyrus | R | 17 | 37 | 52 |
| 9 | Middle & inferior temporal gyrus | L | −61 | −33 | −15 |
| 10 | Middle & inferior temporal gyrus | R | 65 | −17 | −15 |
| 11 | Parahippocampal gyrus | L | −22 | −26 | −16 |
| 12 | Parahippocampal gyrus | R | 25 | −26 | −14 |
| 13 | Cerebellar tonsils | L/R | 7 | −52 | −44 |
3.2.1. Task-positive ROIs:
Consistent with Fox et al. (2005)’s classification, the average activity within most ROIs was task-positive [t(36)>2.03, P<0.05], exceptions being left and right superior occipital gyri and right insula, whose activity did not differ from zero. These three ROIs were excluded from further analysis. The remaining 13 ROIs were analyzed by three-factor ANOVA (group × SS × ROI). A main effect of SS [F(2,70)=14.8, P<0.001] reflected greater activation with larger SS. The main effect of group narrowly failed significance [F(1,35)=4.05, P=0.052]. Figure 3 illustrates that PSZ tended to recruit task-positive regions more, especially at SS 1 and 2, resulting in smaller effects of SS relative to HCS. Supplementary Figure S1, showing graphs for individual ROIs, suggests that this pattern originated in parietal and occipital, but not frontal ROIs. However, neither the group × SS [F(2,70)=1.23, P=0.27] nor the group × SS × ROI interaction [F(24,840)=1.09, P>0.3] was significant.
3.2.2. DMN ROIs:
Average activity across groups and SSs was confirmed to be task-negative within each ROI [t(36)<−2.3, P<0.03] except the cerebellar tonsils whose activity did not differ from zero. The retrosplenial ROI (BA 29 and 30) displayed large and extremely variable negative values, probably due to its location at the intersection of the Vein of Galen and internal sagittal sinus. The cerebellar and retrosplenial ROIs were excluded from further analysis. The remaining 11 ROIs were analyzed by three-factor ANOVA. There was a significant group × SS interaction [F(2,70)=3.21, P<0.05], which was followed by one-factor ANOVA within each group. As shown in Figure 3, PSZ displayed step-wise greater deactivation with larger SS, supported by a main effect of SS [F(2,40)=6.73, P=0.003]. HCS showed no SS-dependent modulation [F(2,30)=0.18, P>0.8]. There was no group × SS × ROI interaction [P>0.5].
Graphs for individual ROIs are shown in Supplementary Figure S2. The apparent outlier status of activity patterns in inferior temporal ROIs prompted exploratory analyses of individual ROIs (see Supplement).
3.3. Correlations
We tested whether recruitment of task-positive ROIs at SS 1 (at which PSZ displayed greater activation as compared with HCS) was associated with cognitive performance, psychiatric symptoms, or antipsychotic medication in PSZ. There were no significant Pearson correlations with BPRS, SANS, or LOFS total scores, chlorpromazine equivalents (Andreasen et al., 2010), or CDT accuracy at SS 4 (as proxy for WM capacity). However, over-recruitment correlated with slower RT at SS 1 (R=0.56, P=0.009) and SS 4 (R=0.61, P=0.004), and with lower MATRICS Consensus Cognitive Battery (MCCB) composite scores (R=−0.51, P=0.017).
No significant correlations were identified between DMN hyperdeactivation at SS 4 and accuracy or RT at SS 4, MCCB composite scores, BPRS, SANS, LOFS, or chlorpromazine equivalents.
4. Discussion
Our aim was to compare WM load-dependent activation of task-positive regions and deactivation of the DMN between PSZ and HCS while minimizing group differences in task engagement. No-response trials were more frequent in PSZ in the initial training session, but no longer in the MRI session, suggesting that pre-training remediated group differences in task engagement that otherwise would have influenced the fMRI results.
As expected, frontoparietal task-positive ROIs displayed increasing activation with greater WM load. A trend suggested greater activation in PSZ, especially at smaller SSs, consistent with findings of increased frontoparietal activation in other WM paradigms at lower load (Manoach et al., 1999; Callicott et al., 2000; 2003; Karch et al., 2009; Ettinger et al., 2011). This supports a leftward shift of the load-dependent inverted U-shaped activation curve in schizophrenia (Manoach, 2003). The present study only probed its putative ascending arm, at which task load was clearly manageable for both groups, but was probably more resource-consuming for PSZ due to cognitive impairment associated with the disorder. The latter was supported by significant correlations between over-engagement of task-positive ROIs at low load and impaired performance on several cognitive indices.
Considering the above studies reporting PFC over-recruitment in PSZ at manageable load, it was perhaps surprising that the middle frontal ROIs displayed little SS-dependent activation and no over-recruitment in PSZ. Results by Callicott et al. (2003) suggested significant heterogeneity between prefrontal areas with regard to load-dependent recruitment in PSZ relative to HCS. The present study probed limited PFC regions; the middle frontal ROIs may not have represented the generally more dorsal locations displaying over-recruitment in these previous studies. However, other studies of WM, controlling for task engagement by limiting analyses to epochs of correct performance, similarly found no difference in load-dependent dorsolateral PFC recruitment between PSZ and HCS (Johnson et al. 2006; Walter et al. 2007; Eryilmaz et al., 2016).
In task-negative DMN ROIs, both groups displayed robust task-induced deactivation of the DMN; however, only PSZ displayed increasing deactivation with greater WM load. In HCS, deactivation did not differ between SSs. While numerous studies reported load-dependent DMN deactivation in healthy participants (e.g., Kim et al., 2009; Ceco et al., 2015; Piccoli et al., 2015), those employing visual CDT paradigms similar to ours, with brief stimulus encoding periods, did not observe effects of SS in typical DMN regions (Todd and Marois, 2004; 2005; Ambrose et al., 2016). The color CDT is by and large not conducive to active rehearsal and is only minimally affected by performance of a secondary attention-demanding task in the retention period as long as the stimulus modality differs (Woodman et al., 2001; Woodman and Luck, 2001). Thus, the CDT may vary the number of items stored in WM without significantly varying the engagement of attentional or executive control resources. The SS-dependent DMN deactivation seen in PSZ, then, may reflect an increasing engagement of non-specific vigilance or effort in an attempt to accommodate the increasing WM storage challenges. Given the absence of correlations, this did not appear to benefit task performance or cognitive ability; therefore, it may not be an adaptive strategy.
DMN deactivation in PSZ was never lower than in HCS, consistent with our hypothesis that when minimizing differences in task-engagement by making task-demands manageable, the ability to down-regulate DMN functions is unimpaired in PSZ. This speaks against an inability to down-regulate task-independent thought processes as a primary mechanism causative of cognitive impairment in schizophrenia. Task-induced DMN deactivation even tended to be greater in PSZ relative to HCS, especially at the largest SS. Given that greater processing demands are associated with greater DMN deactivation (McKiernan et al., 2003, 2006; Pallesen et al., 2009; Hayden et al., 2010), this phenomenon may reflect more effortful processing in PSZ. A previous study employing a stimulus detection paradigm of low difficulty with task pre-training (Hahn et al., 2016) reported DMN hyperdeactivation in PSZ in response to cue-induced attentional focusing, not accompanied by over-recruitment of task-positive regions. This prompted the suggestion that DMN hyperdeactivation reflected greater non-specific effort. In the present study, a trend towards hyperdeactivation was seen at higher load, again in the absence of group differences in task-positive ROI engagement. This adds to the notion that exaggerated down-regulation of DMN function in PSZ may constitute an independent mechanism of attempting to deal with challenging (but not unmanageable) task demands.
The use of a block-design did not allow dissociating activity related to WM encoding and maintenance vs. retrieval and decision-related processes. Future studies will aim at expanding the present findings by employing an event-related design and larger sample. Future studies will also test a wider range of SSs that includes WM load conditions that far exceed participants’ capacity and may result in task-engagement “breaking off”. This will enable conclusions about modulation of task-positive regions on the descending arm of the putative load - activation dose-response curve, and may help reconcile reports of DMN hypodeactivation in schizophrenia with the hyperdeactivation found by our group. We expect hypodeactivation exclusively at supra-capacity SSs, accompanied by significant performance deficits in PSZ relative to HCS.
Supplementary Material
References
- Andreasen NC, 1984. The Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa. [Google Scholar]
- Anticevic A, Repovs G, Barch DM, 2013. Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophr. Bull 39 (1) 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad. Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, Goldberg TE, Weinberger DR, 2000. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb. Cortex 10 (11) 1078–1092. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR,1999. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb. Cortex 9 (1) 20–26. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR, 2003. Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. Am. J. Psychiatry 160 (12) 2209–2215. [DOI] [PubMed] [Google Scholar]
- Ceco M, Gracely JL, Fitzcharles MA, Seminowicz DA, Schweinhardt P, Bushnell MC, 2015. Is a responsive default mode network required for successful working memory task performance? J. Neurosci 35 (33) 11595–11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29 (3) 162–173. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R, 2004. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage 23 (3) 921–927. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Koch P, Kohn P, Apud J, Weinberger DR, Berman KF, 2012. Common and differential pathophysiological features accompany comparable cognitive impairments in medication-free patients with schizophrenia and in healthy aging subjects. Biol. Psychiatry 71 (10) 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, von Cramon DY, Ullsperger M, 2008. Prediction of human errors by maladaptive changes in event-related brain networks. Proc. Natl. Acad. Sci. USA 105 (16) 6173–6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Lipton PA, 2008. Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus 18 (12) 1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eryilmaz H, Tanner AS, Fei Ho N Nitenson AZ, Silverstein NJ, Petruzzi LJ, Goff DC, Monoach DS, Roffman JL, 2016. Disrupted working memory circuitry in schizophrenia: disentangling fMRI markers of core pathology vs other aspects of impaired performance. Neuropsychopharmacology 41 (9) 2411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Williams SCR, Fannon D, Premkumar P, Kuipers E, Moller HJ, Kumari V, 2011. Functional magnetic resonance imaging of a parametric working memory task in schizophrenia: relationship with performance and effects of antipsychotic treatment. Psychopharmacology 216 (1) 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti M, Bianchi V, Cinti ME, 2012. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. Bmc. Psychiatry 12 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 102 (27) 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Woods SW, Kiehl KA, Calhoun VD, Pearlson GD, Roach BJ, Ford JM, Srihari VH, McGlashan TH, Mathalon DH, 2013. Deficient suppression of default mode regions during working memory in individuals with early psychosis and at clinical high-risk for psychosis. Front. Psychiatry 4: Article 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ, 2006. Intact attentional control of working memory encoding in schizophrenia. J. Abnorm. Psychol 115 (4) 658–673. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Berman KF, Fleming K, Ostrem J, Van Horn JD, Esposito G, Mattay VS, Gold JM, Weinberger DR, 1998. Uncoupling cognitive workload and prefrontal cortical physiology: A PET rCBF study. Neuroimage 7 (4) 296–303. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, 2001. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci 2 (10) 685–694. [DOI] [PubMed] [Google Scholar]
- Haatveit B, Jensen J, Alnæs D, Kaufmann T, Brandt CL, Thoresen C, Andreassen OA, Melle I, Ueland T, Westlye LT, 2016. Reduced load-dependent default mode network deactivation across executive tasks in schizophrenia spectrum disorders. Neuroimage Clin. 12 (12) 389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Harvey AN, Gold JM, Fischer BA, Keller WR, Ross TJ, Stein EA, 2016. Hyperdeactivation of the default mode network in people with schizophrenia when focusing attention in space. Schizophr. Bull. 42 (5) 1158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Yucel M, Pujol J, Pantelis C, 2007. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr. Res 91 (1–3) 82–86. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, James GA, Boshoven W, Duncan E, 2011. Altered engagement of attention and default networks during target detection in schizophrenia. Schizophr. Res 125 (2–3) 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk AB, Carpenter WT Jr., Strauss JS, 1975. Diagnostic criteria and five-year outcome in schizophrenia. A report from the International Pilot Study of schizophrenia. Arch. Gen. Psychiatry 32 (3) 343–347. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML, 2010. Cognitive control signals in posterior cingulate cortex. Front. Hum. Neurosci 4: Article 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, 1991. The Fagerstrom Test for Nicotine Dependence - A Revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict 86 (9) 1119–1127. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA, Pearlson GD, 2006. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol. Psychiatry 60 (1) 11–21. [DOI] [PubMed] [Google Scholar]
- Karch S, Leicht G, Giegling I, Lutz J, Kunz J, Buselmeier M, Hey P, Sporl A, Jager L, Meindl T, Pogarell O, Moller HJ, Hegerl U, Rujescu D, Mulert C, 2009. Inefficient neural activity in patients with schizophrenia and nonpsychotic relatives of schizophrenic patients: Evidence from a working memory task. J. Psychiatr. Res 43 (15) 1185–1194. [DOI] [PubMed] [Google Scholar]
- Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, Ford JM, Gollub RL, White T, Wible C, Belger A, Bockholt HJ, Clark VP, Lauriello J, O’Leary D, Mueller BA, Lim KO, Andreasen N, Potkin SG, Calhoun VD, 2009. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum. Brain Mapp. 30 (11) 3795–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI, 2009. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci 12 (5) 535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Park S, 2005. Working memory impairments in schizophrenia: A meta-analysis. J. Abnorm. Psychol 114 (4) 599–611. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK, 1997. The capacity of visual working memory for features and onjunctions. Nature 390 (6657) 279–281. [DOI] [PubMed] [Google Scholar]
- Madre M, Pomarol-Clotet E, McKenna P, Radua J, Ortiz-Gil J, Panicali F, Goikolea JM, Vieta E, Sarro S, Salvador R, Amann BL, 2013. Brain functional abnormality in schizo-affective disorder: an fMRI study. Psychol. Med 43 (1) 143–153. [DOI] [PubMed] [Google Scholar]
- Manoach DS, 2003. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr. Res 60 (2–3) 285–298. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S, 1999. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol. Psychiatry 45 (9) 1128–1137. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR, 2006. Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage 29 (4) 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR, 2003. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J. Cogn. Neurosci 15 (3) 394–408. [DOI] [PubMed] [Google Scholar]
- Metzak PD, Riley JD, Wang L, Whitman JC, Ngan ETC, Woodward TS, 2012. Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophr. Bull. 38 (4) 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Keeser D, Reiser MF, Teipel S, Meindl T, 2012. Functional and structural MR imaging in neuropsychiatric disorders, Part 2: Application in Schizophrenia and Autism. Am. J. Neuroradiol 33 (10) 2033–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulderink TA, Gitelman DR, Mesulam MM, Parrish TB, 2002. On the use of caffeine as a contrast booster for BOLD fMRI studies. Neuroimage. 15 (1) 37–44 [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, 2006. MATRICS consensus cognitive battery, manual. Los Angeles: MATRICS Assessment Inc. [Google Scholar]
- Nygard M, Eichele T, Loberg EM, Jorgensen HA, Johnsen E, Kroken RA, Berle JO, Hugdahl K, 2012. Patients with schizophrenia fail to up-regulate task-positive and down-regulate task-negative brain networks: an fMRI study using an ICA analysis approach. Front. Hum. Neurosci 6: Article 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorman DR, 1962. The Brief Psychiatric Rating Scale. Psychol. Rep 799–812. [Google Scholar]
- Palaniyappan L, Balain V, Liddle PF, 2012. The neuroanatomy of psychotic diathesis: A meta-analytic review. J. Psychiatr. Res 46 (10) 1249–1256. [DOI] [PubMed] [Google Scholar]
- Pallesen KJ, Brattico E, Bailey CJ, Korvenoja A, Gjedde A, 2009. Cognitive and emotional modulation of brain default operation. J. Cogn. Neurosci 21 (6) 1065–1080. [DOI] [PubMed] [Google Scholar]
- Piccoli T, Valente G, Linden DEJ, Re M, Esposito F, Sack AT, Di Salle F, 2015. The default mode network and the working memory network are not anti-correlated during all phases of a working memory task. PLOS One 10 (4) e0123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, Guerrero A, Ortiz-Gil J, Sans-Sansa B, Capdevila A, Cebamanos JM, McKenna PJ, 2008. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol. Med 38 (8) 1185–1193. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Yoon J, Minzenberg MJ, Carter CS, 2007. Neuroimaging of cognitive disability in schizophrenia: Search for a pathophysiological mechanism. Int. Rev. Psychiatry 19 (4) 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Pineda P, Fakra E, Delaveau P, McKenna PJ, Pomarol-Clotet E, Blin O, 2011. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophr. Res 125 (2–3) 101–109. [DOI] [PubMed] [Google Scholar]
- Schaefer J, Giangrande E, Weinberger DR, Dickinson D, 2013. The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizophr. Res 150 (1) 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider FC, Royer A, Grosselin A, Pellet J, Barral FG, Laurent B, Brouillet D, Lang F, 2011. Modulation of the default mode network is task-dependant in chronic schizophrenia patients. Schizophr. Res 125 (2–3) 110–117. [DOI] [PubMed] [Google Scholar]
- Talairaque J, Tournoux P, 1988. Co-planar stereotaxic atlas of the human brain. Thieme, New York. [Google Scholar]
- Todd JJ, Marois R, 2004. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature 428 (6984) 751–754. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R, 2005. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn. Affect. Behav. Neurosci 5 (2) 144–155. [DOI] [PubMed] [Google Scholar]
- Van Snellenberg JX, Torres IJ, Thornton AE, 2006. Functional neuroimaging of working memory in schizpohrenia: task performance as a moderating variable. Neuropsychology 20 (5) 497–510. [DOI] [PubMed] [Google Scholar]
- Walter H, Vasic N, Höse A, Spitzer M, Wolf RC, 2007. Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: Evidence from event-related fMRI. Neuroimage 35 (4) 1551–1561. [DOI] [PubMed] [Google Scholar]
- Wang R, Foniok T, Wamsteeker JI, Qiao M, Tomanek B, Vivanco RA, Tuor UI, 2006. Transient blood pressure changes affect the functional magnetic resonance imaging detection of cerebral activation. Neuroimage 31 (1) 1–11. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1999. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D, 2001. Wechsler Test of Adult Reading (WTAR). The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG, 2006. The neural bases of momentary lapses in attention. Nat. Neurosci 9 (7) 971–978. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ, 2009. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. USA 106 (4) 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ, 2006. Wide Range Achievement Test (WRAT) 4. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Woodman GF, Luck SJ, 2004. Visual search is slowed when visuospatial working memory is occupied. Psychon. Bull. Rev 11 (2) 269–274. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Vogel EK, Luck SJ, 2001. Visual search remains efficient when visual working memory is full. Psychol. Sci 12 (3) 219–224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.